Abstract
Target-derived neurotrophins regulate neuronal survival and growth by interacting with cell-surface tyrosine kinase receptors. The p75 neurotrophin receptor (p75NTR) is coexpressed with Trk receptors in long-range projection neurons, in which it facilitates neurotrophin binding to Trk and enhances Trk activity. Here, we show that TrkA and TrkB receptors undergo robust ligand-dependent ubiquitination that is dependent on activation of the endogenous Trk activity of the receptors. Coexpression of p75NTR attenuated ubiquitination of TrkA and TrkB and delayed nerve growth factor-induced TrkA receptor internalization and receptor degradation. These results indicate that p75NTR may prolong cell-surface Trk-dependent signalling events by negatively regulating receptor ubiquitination.
Keywords: Trk, p75NTR, ubiquitin, PC12, neuron
Introduction
The four mammalian neurotrophins comprise a family of related secreted factors required for differentiation, survival, development and death of specific populations of neuronal and non-neuronal cells. The effects of neurotrophins are mediated by binding to TrkA, TrkB and TrkC receptor tyrosine kinases (RTKs) and to the p75 neurotrophin receptor (p75NTR). The Trk receptors have crucial roles in neuronal survival and growth, and are important modulators of synaptic function (Huang & Reichardt, 2003). p75NTR is a component of several distinct cellsurface signalling platforms that function to induce apoptosis and mediate neuronal growth inhibition. p75NTR also acts as a Trk co-receptor that increases the binding specificity and affinity of Trk receptors for neurotrophins (reviewed by Roux & Barker, 2002).
Ligand binding to RTKs initiates receptor internalization through clathrin-coated vesicles (CCVs; Ehlers et al, 1995; Grimes et al, 1996). Once internalized, CCVs shed clathrin and fuse with internal vesicles to form endosomes, from which receptors can be either recycled or targeted to multivesicular bodies for degradation. The spatial complexity of neurons presents a special challenge for receptor regulation, as ligand–receptor complexes formed at neuronal targets have to transduce responses to cell bodies that may be many centimetres away. In most cells, receptors in endosomes remain activated for relatively short periods (Di Fiore & De Camilli, 2001), but in neurons, endosomal Trk receptors remain tyrosine phosphorylated and bound to neurotrophin when they are transported retrogradely (Delcroix et al, 2003; Ye et al, 2003). The retrograde transport of these signalling endosomes allows ligand–receptor interactions, which are initiated in axons and dendrites, to be transduced to the cell body and nucleus, and has a crucial role in neuronal function (Howe & Mobley, 2004).
Conjugation of ubiquitin to many RTKs, or their associated adaptor proteins, is required for appropriate receptor trafficking and degradation (Hicke & Dunn, 2003). The function of RTK ubiquitination has been best studied using the epidermal growth factor receptor (EGFR), and is shown to be required for the regulation of CCV-dependent endocytosis and endosomal trafficking (Dikic, 2003). Other RTKs, including Met, platelet-derived growth factor (PDGF), colonystimulating factor-1 and Ron receptors, are also ubiquitinated; the importance of ubiquitination in RTK regulation has been underscored by the identification of mutations that cause cellular transformation through disruption of RTK ubiquitination (Peschard & Park, 2003).
In this study, we examined Trk receptor ubiquitination in primary neurons, in PC12 cells and in transfected human embryonic kidney (HEK) 293 cells. We found that TrkB undergoes robust ubiquitination in primary cortical neurons treated with brain-derived neurotrophic factor (BDNF), whereas nerve growth factor (NGF)-dependent TrkA ubiquitination in PC12 cells was modest and difficult to detect. We hypothesized that these differences in Trk receptor ubiquitination reflect the expression of p75NTR and, consistent with this, showed that ligand-dependent ubiquitination of TrkA and TrkB is greatly reduced in the presence of p75NTR. When expressed with p75NTR, TrkA internalization and degradation were delayed, and activated TrkA was retained at the cell surface. By reducing Trk ubiquitination, p75NTR may act to prolong Trk signalling events.
Results And Discussion
TrkB undergoes robust BDNF-dependent ubiquitination
To determine whether Trk receptors undergo ligand-induced ubiquitination, we first examined cortical neurons derived from E16 mouse cortices, which express abundant TrkB but little p75NTR (Bhakar et al, 2003). Cortical neurons that were maintained in vitro for 3 days were exposed to 50 ng/ml BDNF for 5–90 min. TrkB was immunoprecipitated and then immunoblotted using antibodies directed against ubiquitin and phosphotyrosine. P4D1, an antiubiquitin antibody that recognizes both mono- and polyubiquitin, was used for these initial studies. Fig 1A shows that TrkB phosphotyrosine content is rapidly increased by BDNF exposure, peaking by 5 min but still detectable at later time points. TrkB immunoprecipitated from untreated cells is not ubiquitinated, but the ubiquitination content of the receptor is markedly increased in cells exposed to BDNF for 5 min. Receptor ubiquitination levels are maintained 15 and 30 min after BDNF addition but drop to low levels after 90 min of ligand exposure.
Figure 1.

Treatment of mouse cortical neurons with brain-derived neurotrophic factor (BDNF) induces ubiquitination of TrkB. (A) Mouse cortical neurons were treated with 50 ng/ml BDNF for 5–90 min and TrkB was immunoprecipitated and immunoblotted for total ubiquitin and phosphotyrosine (pTyr) content, as indicated. (B) Cortical neurons were treated with BDNF or nerve growth factor (NGF) in the absence or presence of 200 ng/ml K252A for 5 or 15 min, as indicated, and TrkB was immunoprecipitated and analysed as above. (C) Cortical neurons were treated with BDNF (50 ng/ml) for 5–90 min and TrkB was immunoprecipitated. Immunoprecipitates were analysed by immunoblotting for phosphotyrosine, total ubiquitin and polyubiquitin content and for TrkB levels using 4G10, P4D1, FK1 and anti-TrkB (RTB) antibodies, respectively. Experiments in (A–C) were each performed three times, all with identical results.
To determine whether TrkB kinase activity is required for ligand-induced ubiquitination, primary cortical neurons were exposed to BDNF in the presence of K252A, an inhibitor of Trk kinases (Berg et al, 1992). As shown above, TrkB was immunoprecipitated and analysed for ubiquitin and phosphotyrosine content by immunoblot. Fig 1B shows that BDNF-induced TrkB tyrosine phosphorylation and ubiquitination were both strongly attenuated in the presence of K252A, which indicates that TrkB kinase activity is required for BDNF-induced ubiquitination of TrkB in mouse cortical neurons.
Receptor ubiquitination can reflect the addition of monomeric ubiquitin or can reflect polyubiquitination. Recent studies indicate that RTKs, such as EGFR, are predominantly monoubiquitinated at several sites within the intracellular domain (Dikic, 2003). Polyubiquitin chains most commonly consist of ubiquitin linked by lysine 48, and these linkages can be detected on immunoblots using the FK1 antibody (Haglund et al, 2003). To determine the type of ubiquitination that predominates on TrkB, the receptor was immunoprecipitated from primary cortical neurons that were treated with BDNF and analysed for total ubiquitin (using P4D1) and polyubiquitin (using FK1). Ubiquitinated TrkB was readily detected with both antibodies (Fig 1C), which indicates that TrkB is polyubiquitinated in response to ligand binding.
TrkA undergoes modest ligand-dependent ubiquitination
We next examined ligand-dependent ubiquitination of TrkA. For this, we used the PC12 cell line, which expresses TrkA and p75NTR but not TrkB or TrkC (Ip et al, 1993). PC12 cells were treated with 50 ng/ml NGF for 5, 15 or 90 min and lysed, and TrkA was immunoprecipitated and then immunoblotted with antibodies directed against total ubiquitin (P4D1) or phosphotyrosine. Fig 2A shows that TrkA was ubiquitinated in an NGF-dependent manner but, in marked contrast to the robust TrkB ubiquitination observed in cortical neurons, the levels of TrkA ubiquitination in PC12 cells were low and difficult to detect (which probably explains why TrkA ubiquitination was not detected in a previous study that examined TrkA ubiquitination in PC12 cells; Kao et al, 2001). Nonetheless, with long immunoblot exposures, TrkA ubiquitination could be detected using antibodies directed against both total ubiquitin (P4D1) and polyubiquitin (FK1) and was blocked in PC12 cells preincubated with K252A (Fig 2B,C). Thus, TrkA becomes polyubiquitinated after NGF binding.
Figure 2.
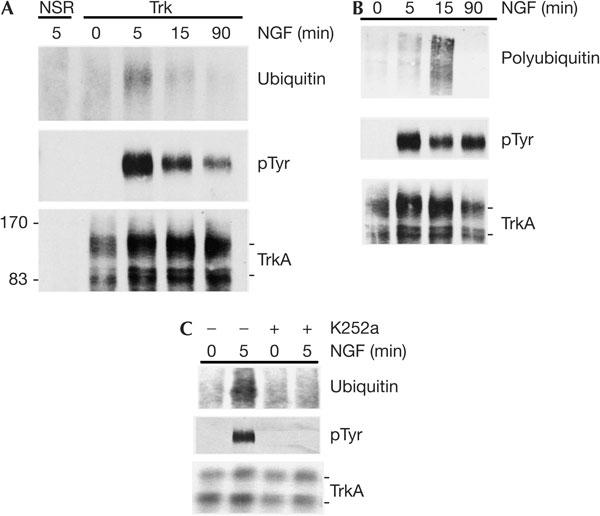
Nerve growth factor (NGF) induces modest TrkA ubiquitination in PC12 cells. (A) PC12 cells were treated with 50 ng/ml NGF for 5–90 min, as indicated, and TrkA was immunoprecipitated and analysed by immunoblotting for total ubiquitin and phosphotyrosine (pTyr) content and for TrkA levels, as indicated. NSR, nonspecific rabbit sera. (B) PC12 cells were treated with 50 ng/ml NGF for 5–90 min and TrkA was immunoprecipitated. Immunoprecipitates were analysed by immunoblotting for polyubiquitin content (using the FK1 antibody), phosphotyrosine content and TrkA levels, as indicated. (C) PC12 cells were treated with NGF in the absence or presence of 200 ng/ml K252A for 5 min and TrkA was immunoprecipitated and analysed by immunoblotting for phosphotyrosine, total ubiquitin and TrkA, as indicated. Experiments in (A,B) were each performed three times, and the experiment in (C) was performed twice, all with identical results.
p75NTR reduces Trk ubiquitination
p75NTR physically interacts with TrkA and alters its activity in vivo (reviewed by Roux & Barker, 2002). Because PC12 cells express abundant p75NTR and primary mouse cortical neurons express much lower amounts, we proposed that Trk receptor ubiquitination is reduced by p75NTR coexpression. To address this, we examined the effect of p75NTR on NGF-induced TrkA ubiquitination in transfected HEK293 cells. Cells were co-transfected with plasmids encoding TrkA and Myc-tagged ubiquitin in the absence or presence of an expression plasmid encoding p75NTR; after stimulation with NGF for 5 or 90 min, ubiquitinated proteins were immunoprecipitated under harsh denaturing conditions using an antibody directed against the Myc epitope tag and were detected by immunoblot. Fig 3A shows that, in the absence of p75NTR, low levels of ubiquitinated TrkA are present and that these increase about fourfold in response to NGF treatment. In HEK293 cells, NGF-induced ubiquitination is maintained for at least 90 min. Strikingly, when p75NTR is coexpressed with TrkA, ubiquitination of TrkA falls below our detection limit, in both the absence and presence of NGF. This p75NTR-induced decrease reflects lower levels of total ubiquitination and polyubiquitination of TrkA (Fig 3B). p75NTR itself shows NGF-dependent ubiquitination, but this is not altered by the coexpression of TrkA (Fig 3A).
Figure 3.
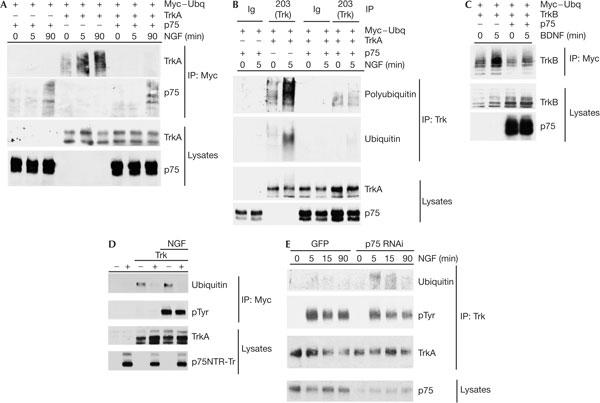
p75 neurotrophin receptor (p75NTR) inhibits ligand-induced Trk receptor ubiquitination. (A) Human embryonic kidney 293 cells were transfected with expression plasmids encoding TrkA and Myc-tagged ubiquitin (Myc–Ubq) in the presence and absence of an expression plasmid encoding p75NTR. At 2 days after the transfection, cells were treated with nerve growth factor (NGF; 50 ng/ml) for 5 or 90 min and then lysed in harsh denaturing conditions. Ubiquitinated proteins were immunoprecipitated (IP) using antibodies directed against the Myc epitope and analysed on immunoblots using antibodies directed against TrkA and p75NTR. Lysate levels of TrkA and p75NTR are shown in the lower panels. (B) Transfections performed as in (A) were followed by immunoprecipitations using an anti-TrkA antibody (203) or using a rabbit antibody control (Ig). Immunoblots for total ubiquitin and polyubiquitin content were performed using P4D1 and FK1 antibodies, respectively. (C) Experiments were performed as in (A), except that the expression plasmid encoding TrkA was replaced with a TrkB expression plasmid and cells were treated with 50 ng/ml brain-derived neurotrophic factor (BDNF) for 5 min. Lysate levels of TrkB and p75NTR are shown in the lower panels. (D) Experiments were performed as in (A), except that the expression plasmid encoding p75NTR was replaced with an expression plasmid encoding a mutant form of p75NTR (p75NTR-Tr) lacking cysteine-rich domains 2–4 and cells were treated with 50 ng/ml NGF for 5 min only. pTyr, phosphotyrosine. (E) The PC12p75kd line and the control PC12 line (stably transfected with green fluorescent protein (GFP) and maintained in G418) were treated with NGF (50 ng/ml) for 5–90 min and TrkA was immunoprecipitated. Immunoprecipitates were analysed by immunoblotting for TrkA levels and for total ubiquitin and phosphotyrosine content, as indicated. p75NTR levels in the two lines are shown in the lower panel. GFP, green fluorescent protein; RNAi, RNA interference. Experiments in (A–F) were each performed three times, all with identical results.
Similar experiments were performed to determine whether p75NTR alters TrkB ubiquitination. In HEK293 cells lacking p75NTR, low levels of ubiquitinated TrkB are present in the absence of ligand and the ubiquitination content of the receptors increases after BDNF treatment (Fig 3C). In the presence of p75NTR, basal and BDNF-induced TrkB ubiquitination is muted considerably. To address whether the p75NTR-dependent reduction in TrkA ubiquitination requires ligand binding to p75NTR, we tested whether a mutant form of p75NTR (p75NTR-Tr) that does not bind to neurotrophin is able to suppress TrkA ubiquitination. Fig 3D shows that coexpression of p75NTR-Tr strongly suppresses p75NTR ubiquitination in the absence and presence of NGF, which indicates that ligand binding to p75NTR is not a prerequisite for this effect. To determine whether p75NTR modulates ubiquitination of non-Trk RTKs, we tested whether p75NTR altered EGFR ubiquitination and found that p75NTR coexpression had no effect on ubiquitination of EGFR in the absence or presence of ligand (see the supplementary information online). This suggests that the effect of p75NTR is specific for Trk receptors.
The effect of p75NTR on Trk ubiquitination in HEK293 cells may reflect high non-physiological expression levels that can be achieved in this line. To test whether physiological levels of p75NTR alter TrkA ubiquitination, we produced a PC12 cell line (PC12p75kd) that stably expressed a small interfering RNA directed against p75NTR messenger RNA. Fig 3E shows that this line had a substantial reduction in p75NTR levels (80% by densitometry) and when treated with NGF, TrkA ubiquitination in the PC12p75kd line was substantially increased above that induced in the control line, which expressed normal levels of p75NTR. We conclude that p75NTR acts to suppress ligand-induced Trk ubiquitination under physiological conditions.
p75NTR delays TrkA internalization
The ubiquitination of RTKs is important for their internalization and degradation. To address whether p75NTR alters TrkA internalization, HEK293 cells, which had been transfected either with TrkA alone or with TrkA and p75NTR, were exposed to NGF for 2, 5 or 15 min at 37°C and then subjected to cell-surface biotinylation at 4°C. Cells were lysed, cell-surface proteins were immunoprecipitated and levels of biotinylated TrkA were determined by immunoblot. Fig 4A shows that TrkA is rapidly internalized in cells exposed to NGF, with cellsurface TrkA levels falling below the detection limit in 5 min. In cells coexpressing TrkA and p75NTR, the internalization of TrkA is significantly delayed and reductions in cell-surface levels are observed only after a 15 min NGF exposure. This reduction in the rate of TrkA internalization correlates with a profound increase in the levels of tyrosine-phosphorylated TrkA at the cell surface in cells expressing p75NTR. These data suggest that a p75NTR-dependent decrease in TrkA receptor ubiquitination delays TrkA internalization and prolongs TrkA signalling from the cell surface.
Figure 4.
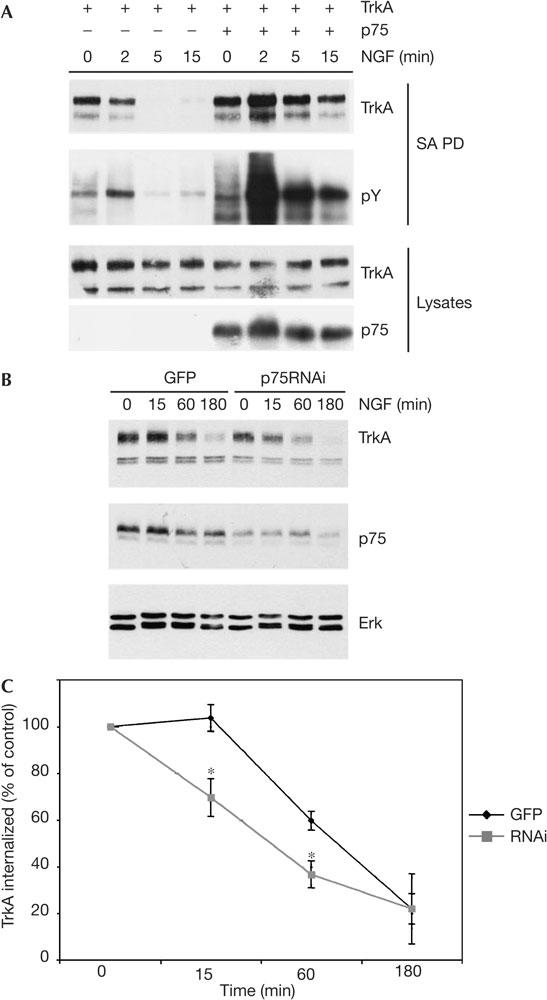
p75NTR inhibits ligand-induced tyrosine kinase (Trk) receptor ubiquitination. (A) Human embryonic kidney 293 cells were transfected with expression plasmids encoding TrkA and p75 neurotrophin receptor (p75NTR), and after 2 days were exposed to nerve growth factor (NGF; 50 ng/ml) at 37°C for 2–15 min, as indicated. Cellsurface proteins were then biotinylated at 4°C, biotinylated proteins were precipitated from lysed cells using streptavidin beads and levels of TrkA and the TrkA phosphotyrosine (pTyr) content were determined by immunoblotting. Lysate levels of TrkA and p75NTR are shown in the lower panels. SA PD, streptavidin pullot. (B) The PC12p75kd line and the control PC12 line were biotinylated at 4°C, exposed to 50 ng/ml NGF and then incubated at 37°C for the durations indicated. Biotinylated proteins were recovered from lysed cells using streptavidin-coated beads and levels of TrkA in the precipitates were determined by immunoblotting using RTA. Lysate levels of p75NTR and Erk are shown in the lower panels. GFP, green fluorescent protein; RNAi, RNA interference. (C) Quantification of data shown in (B) from three separate experiments determined by densitometry, pooled and analysed for significance by one-way analysis of variance. *P<0.05. The experiment in (A) was performed three times, all with identical results.
To determine whether p75NTR expression also alters TrkA degradation rates, we examined TrkA degradation in normal PC12 cells and in PC12p75kd cells, which have reduced p75NTR expression. Fig 4B shows that NGF-dependent loss of TrkA occurs considerably more rapidly in cells with reduced p75NTR content. When results from several experiments were quantified, a statistically significant acceleration of TrkA degradation was apparent after a 15 and 60 min NGF exposure (Fig 4C). Thus, p75NTR coexpression reduces the rate of TrkA degradation in PC12 cells.
Our study shows that Trk receptors are ubiquitinated in a ligand-dependent manner, that coexpression of p75NTR strongly attenuates TrkA and TrkB ubiquitination, and that p75NTR alters the internalization kinetics and degradation rate of TrkA. Several previous studies have examined aspects of TrkA internalization and have consistently shown that Trk receptors are internalized in minutes after the addition of neurotrophin to p75NTR-expressing cells (Zhang et al, 2000; Jullien et al, 2002; Bronfman et al, 2003). Our study, which examined the early phase of TrkA internalization as a function of p75NTR expression, showed that TrkA internalization is slowed in the presence of p75NTR, but nonetheless is in agreement with these earlier studies because the delay in TrkA internalization evoked by p75NTR is, at best, a few minutes and therefore in the range of resolution of these earlier studies, which did not test directly the effect of p75NTR. Our results are also consistent with two earlier reports that have examined the effect of p75NTR on TrkA receptor dynamics. In Sf9 cells, mammalian TrkA undergoes NGF-induced internalization, which is significantly reduced in the presence of p75NTR (Gargano et al, 1997), in agreement with our studies. Others have shown that NGF that is bound to NGF30, an anti-NGF monoclonal antibody, can bind to and activate TrkA but cannot bind to p75NTR. Interestingly, the NGF:NGF30 complex is internalized more rapidly than NGF alone (Saragovi et al, 1998), which is consistent with the hypothesis that p75NTR normally acts to delay the internalization of TrkA.
A recent report has shown that LRP1, a PDGFR co-receptor, reduces PDGFR ubiquitination and extends the time that the PDGFR spends on the cell surface (Takayama et al, 2005), similar to what we report for p75NTR and TrkA. It is possible that co-receptor interactions may be important for regulating ubiquitination of several types of RTK. The precise mechanisms that these co-receptors use to attenuate RTK ubiquitination remain to be explained, but it is possible that they act in a manner analogous to cytosolic proteins such as Sprouty2, which increases cellsurface retention of the EGFR by inhibiting c-Cbl-mediated ubiquitination of the receptor (Wong et al, 2002). Both p75NTR (Ohrt et al, 2004) and LRP1 (Takayama et al, 2005) interact with c-Cbl, and this could alter the activity of c-Cbl or analogous E3 ligases towards substrates such as TrkA and PDGR.
Endosomes that contain activated TrkA have a crucial role in neurotrophin responses (Howe & Mobley, 2004). Mechanisms that reduce targeting of TrkA to lysosomal compartments that mediate receptor degradation may increase the long-range signalling ability of signalling endosomes. Signalling from the cell surface is also important for a variety of functions, including neuronal growth, morphology and turning (Zhang et al, 2000; Gallo & Letourneau, 2004), and regulation of cellsurface TrkA levels would be expected to have a significant impact on neuronal function. Identification of the Trk ubiquitination machinery and the mechanisms that allow p75NTR to impinge on this process are important next steps that will allow the precise physiological impact of these events to be determined.
Methods
Materials. p75NTR antibody directed against the p75NTR intracellular domain has been previously described (Roux et al, 1999); the 203 antibody directed against TrkA was a kind gift from David Kaplan and the polyclonal anti-TrkB antibody (RTB) was a kind gift from Louis Reichardt. Anti-extracellular signal-regulated kinase 1/2 (ERK1/2), anti-phospho-ERK1/2 and 4G10 anti-phosphotyrosine antibodies were from Upstate (Lake Placid, NY, USA), P4D1 and FK1 antibodies were from Covance (Denver, PA, USA) and Affiniti (Devon, UK), respectively, and anti-EGFR was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Horseradish peroxidase-conjugated antibodies were from Jackson Laboratories (West Grove, PA, USA). All other reagents were from Sigma (Oakville, Ontario, Canada) or Calbiochem (San Diego, CA, USA) unless otherwise indicated.
Cell culture, transfections and RNA interference. HEK293 cells, PC12 cells and primary cortical neurons were cultured as described by Roux et al (1999) and Bhakar et al (2003). The RNA interference (RNAi) used to direct stable knockdown of p75NTR was chosen after screening four p75NTR-directed RNAis and two scrambled RNAs in HEK293 cells overexpressing p75NTR and in PC12 cells. The four p75NTR-directed RNAis all led to significant knockdown of overexpressed and endogenous receptor, whereas the scrambled RNAis (5′-GTCGCGATTACTTATAACGG-3′ and 5′-CCGTTGGAAGGACATCTAG-3′) had no effect on p75NTR levels. Stable PC12 cell lines were created by co-transfecting 1:5 ratio of pSV2neo with a U6 expression plasmid directing production of an RNA hairpin loop containing one of these p75NTRspecific sequences (directed to nucleotides 1,331–1,349 of rat p75NTR; 5′-CCAGAGAGCTGACATTGTG-3′) and selected in G418-containing medium.
Immunoprecipitation, cell-surface biotinylation and sodium dodecyl sulphate–polyacrylamide gel electrophoresis and immunoblots. Immunoblots and most immunoprecipitations were performed as described by Bhakar et al (2003). For immunoprecipitation using harsh denaturing conditions, the final concentration of sodium dodecyl sulphate (SDS) in the lysis buffer was increased to 2% and after boiling for 5 min, lysates were diluted in lysis buffer lacking SDS to produce a final concentration of 0.1% SDS. Other steps were identical to those previously described. For biotinylation, cells were washed twice with ice-cold phosphate-buffered saline (PBS) supplemented with 2 mM MgCl2 and then exposed to PBS supplemented with 0.5 mg/ml succinimidyl-6-(biotinamido) hexanoate NHS-LC biotin (Pierce, Rockford, IL, USA) and 2 mM MgCl2 for 45 min at 4°C. Cells were washed and incubated in ice-cold quenching buffer (Tris-buffered saline, supplemented with 2 mM MgCl2 and 2 mM CaCl2) and either lysed for precipitation (internalization assays) or exposed to NGF at 37°C and then lysed for precipitation (degradation assays) with streptavidin-conjugated beads (Pierce).
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Figure 1
Acknowledgments
We are grateful to Dr T. Fon for providing an expression plasmid for Myc–His ubiquitin. This work was supported by Grant MOP37850 from the Canadian Institute of Health Research. P.A.B. is Investigator of the Canadian Institute of Health Research and D. Auld was a postdoctoral fellow of the Fonds de Recherche en Santé du Québec.
References
- Berg MM, Sternberg DW, Parada LF, Chao MV (1992) K252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. J Biol Chem 267: 13–16 [PubMed] [Google Scholar]
- Bhakar AL, Howell JL, Paul CE, Salehi AH, Becker EB, Said F, Bonni A, Barker PA (2003) Apoptosis induced by p75NTR overexpression requires Jun kinase-dependent phosphorylation of Bad. J Neurosci 23: 11373–11381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronfman FC, Tcherpakov M, Jovin TM, Fainzilber M (2003) Ligand-induced internalization of the p75 neurotrophin receptor: a slow route to the signaling endosome. J Neurosci 23: 3209–3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delcroix JD, Valletta JS, Wu C, Hunt SJ, Kowal AS, Mobley WC (2003) NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron 39: 69–84 [DOI] [PubMed] [Google Scholar]
- Di Fiore PP, De Camilli P (2001) Endocytosis and signaling. An inseparable partnership. Cell 106: 1–4 [DOI] [PubMed] [Google Scholar]
- Dikic I (2003) Mechanisms controlling EGF receptor endocytosis and degradation. Biochem Soc Trans 31: 1178–1181 [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Kaplan DR, Price DL, Koliatsos VE (1995) NGFstimulated retrograde transport of trkA in the mammalian nervous system. J Cell Biol 130: 149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G, Letourneau PC (2004) Regulation of growth cone actin filaments by guidance cues. J Neurobiol 58: 92–102 [DOI] [PubMed] [Google Scholar]
- Gargano N, Levi A, Alema S (1997) Modulation of nerve growth factor internalization by direct interaction between p75 and TrkA receptors. J Neurosci Res 50: 1–12 [DOI] [PubMed] [Google Scholar]
- Grimes ML, Zhou J, Beattie EC, Yuen EC, Hall DE, Valletta JS, Topp KS, LaVail JH, Bunnett NW, Mobley WC (1996) Endocytosis of activated TrkA: evidence that nerve growth factor induces formation of signaling endosomes. J Neurosci 16: 7950–7964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I (2003) Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol 5: 461–466 [DOI] [PubMed] [Google Scholar]
- Hicke L, Dunn R (2003) Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol 19: 141–172 [DOI] [PubMed] [Google Scholar]
- Howe CL, Mobley WC (2004) Signaling endosome hypothesis: a cellular mechanism for long distance communication. J Neurobiol 58: 207–216 [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF (2003) Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 72: 609–642 [DOI] [PubMed] [Google Scholar]
- Ip NY, Stitt TN, Tapley P, Klein R, Glass DJ, Fandl J, Greene LA, Barbacid M, Yancopoulos GD (1993) Similarities and differences in the way neurotrophins interact with the trks in neuronal and non-neuronal cells. Neuron 10: 137–149 [DOI] [PubMed] [Google Scholar]
- Jullien J, Guili V, Reichardt LF, Rudkin BB (2002) Molecular kinetics of nerve growth factor receptor trafficking and activation. J Biol Chem 277: 38700–38708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao S, Jaiswal RK, Kolch W, Landreth GE (2001) Identification of the mechanisms regulating the differential activation of the mapk cascade by epidermal growth factor and nerve growth factor in PC12 cells. J Biol Chem 276: 18169–18177 [DOI] [PubMed] [Google Scholar]
- Ohrt T, Mancini A, Tamura T, Niedenthal R (2004) c-Cbl binds to tyrosine-phosphorylated neurotrophin receptor p75 and induces its ubiquitination. Cell Signal 16: 1291–1298 [DOI] [PubMed] [Google Scholar]
- Peschard P, Park M (2003) Escape from Cbl-mediated downregulation: a recurrent theme for oncogenic deregulation of receptor tyrosine kinases. Cancer Cell 3: 519–523 [DOI] [PubMed] [Google Scholar]
- Roux PP, Barker PA (2002) Neurotrophin signaling through the p75 neurotrophin receptor. Prog Neurobiol 67: 203–233 [DOI] [PubMed] [Google Scholar]
- Roux PP, Colicos MA, Barker PA, Kennedy TF (1999) p75 neurotrophin receptor expression is induced in apoptotic neurons after seizure. J Neurosci 19: 6887–6896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saragovi HU, Zheng W, Maliartchouk S, DiGugliemo GM, Mawal YR, Kamen A, Woo SB, Cuello AC, Debeir T, Neet KE (1998) A TrkAselective, fast internalizing nerve growth factor–antibody complex induces trophic but not neuritogenic signals. J Biol Chem 273: 34933–34940 [DOI] [PubMed] [Google Scholar]
- Takayama Y, May P, Anderson RG, Herz J (2005) Low density lipoprotein receptor-related protein 1 (LRP1) controls endocytosis and c-CBL-mediated ubiquitination of the platelet-derived growth factor receptor β(PDGFRβ). J Biol Chem 280: 18504–18510 [DOI] [PubMed] [Google Scholar]
- Wong ES, Fong CW, Lim J, Yusoff P, Low BC, Langdon WY, Guy GR (2002) Sprouty2 attenuates epidermal growth factor receptor ubiquitylation and endocytosis, and consequently enhances Ras/ERK signalling. EMBO J 21: 4796–4808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Kuruvilla R, Zweifel LS, Ginty DD (2003) Evidence in support of signaling endosome-based retrograde survival of sympathetic neurons. Neuron 39: 57–68 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Moheban DB, Conway BR, Bhattacharyya A, Segal RA (2000) Cell surface Trk receptors mediate NGF-induced survival while internalized receptors regulate NGF-induced differentiation. J Neurosci 20: 5671–5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1


