Abstract
Unlike other positive-stranded RNA viruses that use either a 5′-cap structure or an internal ribosome entry site to direct translation of their messenger RNA, calicivirus translation is dependent on the presence of a protein covalently linked to the 5′ end of the viral genome (VPg). We have shown a direct interaction of the calicivirus VPg with the cap-binding protein eIF4E. This interaction is required for calicivirus mRNA translation, as sequestration of eIF4E by 4E-BP1 inhibits translation. Functional analysis has shown that VPg does not interfere with the interaction between eIF4E and the cap structure or 4E-BP1, suggesting that VPg binds to eIF4E at a different site from both cap and 4E-BP1. This work lends support to the idea that calicivirus VPg acts as a novel ‘cap substitute' during initiation of translation on virus mRNA.
Keywords: calicivirus, eIF4E, translation, VPg
Introduction
Protein synthesis can be considered as a three-stage process consisting of initiation, elongation and termination (reviewed by Hershey & Merrick, 2000). In eukaryotic cells, initiation requires the participation of several initiation factors (eIFs) that help to assemble 48S preinitiation complexes on messenger RNA before the assembly of the 80S initiation complex at the initiation codon. The first step in translation of 5′-capped host-cell mRNAs is the binding of the initiation factor eIF4F (Gingras et al, 1999). eIF4F consists of three subunits; eIF4E binds to the cap, whereas eIF4A is an RNA helicase. The largest of the three proteins, eIF4G, acts as a ‘scaffold' and bridges the ribosome to the mRNA through eIF3.
Positive-stranded RNA viruses have evolved to use a wide variety of mechanisms for translation initiation (reviewed by Pe'ery & Mathews, 2000). Several viruses use a 5′-cap-dependent mechanism for initiation, whereas other viruses adopt a cap-independent tactic, the best example being the Picornaviridae, which have an internal ribosome entry site (IRES) element (Belsham & Jackson, 2000). It now seems that caliciviruses may use a novel strategy for translation initiation that probably involves the interaction of initiation factors with VPg, a viral protein covalently linked to the 5′ end of the viral genome.
The human caliciviruses (noroviruses and sapoviruses) are the main cause of non-bacterial gastroenteritis in adults worldwide. In animals, related viruses are responsible for a wider range of symptoms and may cause respiratory, haemorrhagic and vesicular diseases. Earlier work showed that the removal of VPg from feline calicivirus (FCV) mRNA noticeably decreased translation of the genomic RNA, suggesting a role for VPg in protein synthesis (Herbert et al, 1997). More recently, work with Norwalk virus VPg has shown an interaction with eIF3, as well as with other initiation factors (Daughenbaugh et al, 2003), although a functional role for this interaction was not shown.
To understand fully the role of VPg in translation, we have studied its interaction with translation initiation factors. Here, we show that FCV and Lordsdale virus (LDV; a human enteric calicivirus) VPg bind directly to eIF4E and that this interaction is required for translation of calicivirus VPg-linked viral mRNA in vitro. This would thus constitute a novel mechanism by which certain animal RNA viruses use a proteinacious ‘cap substitute' to initiate translation of their mRNAs.
Results
Calicivirus VPg interacts with eIF4E
To understand the role of VPg in translation initiation, we assessed for direct interaction of VPg with the components of the eIF4F complex. Recombinant His-tagged FCV VPg was purified from Escherichia coli and used in in vitro pull-down assays with HeLa S10 extracts (Fig 1A). Western blot analysis showed that eIF4E was pulled down when VPg was coupled to agarose beads (Fig 1A). The remaining components of the eIF4F complex, namely eIF4G and eIF4A, were not detected under the conditions used in this assay (Fig 1A).
Figure 1.

Calicivirus VPg interacts with eIF4E. (A) His-tagged feline calicivirus (FCV) VPg coupled to NTA-agarose beads was incubated with HeLa S10 extracts. Complexes were isolated by lowspeed centrifugation and proteins bound were analysed for the components of the eIF4F complex by western blot. (B) VPg interaction with murine eIF4E and eIF4G, as shown by enzyme-linked immunosorbent assay-based binding assay. Wells were precoated with 1.0 μg of untagged VPg from both FCV and Lordsdale virus (LDV) and incubated with increasing amounts of an Escherichia coli lysate expressing comparable levels of glutathione S-transferase (GST)–eIF4E or GST–eIF4G. Complexes were detected using anti-GST antibodies. OD, optical density.
To show that the observed interaction between FCV VPg and eIF4E was direct and was not bridged by another protein present in the S10 extracts, an enzyme-linked immunosorbent assay (ELISA)-based binding assay (Leonard et al, 2000) was carried out with eIF4F components expressed in E. coli. Recombinant FCV and LDV (a human calicivirus) VPg were purified from E. coli (see the supplementary information online) and were shown to bind directly to glutathione S-transferase (GST)-tagged eIF4E, but not to eIF4G (Fig 1B), eIF4A or poly(A)-binding protein (PABP; data not shown).
VPg–eIF4E interaction is insensitive to cap analogue
To further study the role of VPg–eIF4E interaction in calicivirus translation, VPg-linked mRNA was isolated from virus-infected cells. This RNA was infectious when transfected into cells (data not shown) and produced a translation profile as predicted from the available cleavage map (Sosnovtseva et al, 1999; Fig 2). Translation was dependent on the covalent linkage of VPg to the viral mRNA, as prior treatment of the mRNA with proteinase K markedly decreased protein synthesis, but translation of an in vitro-transcribed control RNA (of the form cap-CAT/EMCV IRES/LUC) was unaffected by the proteinase K treatment (Fig 2).
Figure 2.
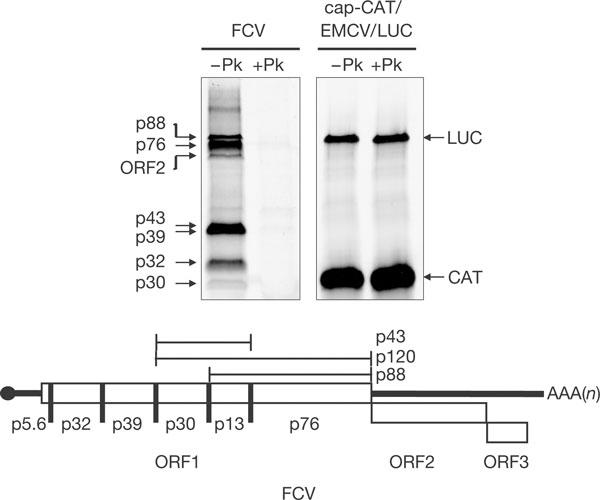
Translation of calicivirus RNA prepared from replication complexes is dependent on VPg. Feline calicivirus (FCV) messenger RNA or in vitro-transcribed control mRNA was treated with proteinase K (+Pk) or mock treated (−Pk) before translation in rabbit reticulocyte lysates. A schematic of the FCV genome is shown (adapted from Sosnovtsev et al, 2002).
Previous data have shown that in vitro translation of FCV VPg-linked RNA was not inhibited by cap analogue (Herbert et al, 1997). Equally, here, translation of FCV mRNA prepared from replication complexes was not inhibited by high concentrations of cap analogue—conditions that inhibited cap-dependent translation, but not IRES-dependent translation, from the CAT/IRES/LUC mRNA (Fig 3A). The interaction of VPg with eIF4E, shown in the ELISA-based binding assay, was also unaffected by high concentrations of cap analogue (Fig 3B), which suggests that the binding sites for VPg and cap are distinct.
Figure 3.
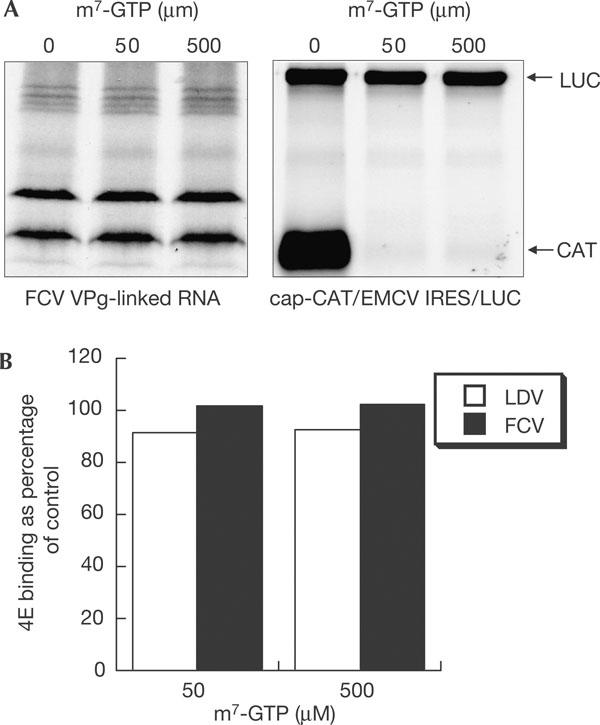
Calicivirus translation and VPg–eIF4E interaction is insensitive to cap analogue. (A) Feline calicivirus (FCV) messenger RNA or control dicistronic mRNA was translated in rabbit reticulocyte lysates in the presence of increasing concentrations of cap analogue. (B) The interaction of VPg with eIF4E in the presence of high concentrations of m7-GTP was analysed using the enzyme-linked immunosorbent assay-based binding assay. Untagged Lordsdale virus (LDV) or FCV VPg (1 μg) was bound to the plate and 10 μg of glutathione S-transferase (GST)–eIF4E-containing lysate was passed over in the presence of increasing amounts of m7-GTP. Bound proteins were detected with anti-GST antibody.
VPg interacts with eIF4E in infected cells
After showing a direct interaction of calicivirus VPg with eIF4E in vitro, we used m7-GTP Sepharose affinity chromatography to pull down eIF4E and associated VPg from infected cells (Fig 4A). Analysis of complexes that are able to bind to cap–Sepharose showed that, in addition to the mature form of VPg (p13), the VPg precursors p43, p88 and p120 also bind to eIF4E (Fig 4A). The putative calicivirus ATPase-RNA helicase (p39) was not purified by cap–Sepharose chromatography, confirming the specific interaction of VPg in its mature and precursor forms with eIF4E in infected cells and also indicating an in vivo interaction between eIF4E and VPg. In addition, neither VPg nor eIF4E was isolated when purifications were carried out using unmodified Sepharose 4B (Fig 4B).
Figure 4.

VPg interacts with eIF4E in infected cells. Feline kidney cells were mock infected or infected with feline calicivirus and extracts were prepared 5 h after infection. Extracts were incubated with either cap–Sepharose (A), as described (Ptushkina et al, 1999), or Sepharose 4B (B) and bound proteins were analysed by Western blot with antisera to VPg, p39 or eIF4E.
4E-BP1 inhibits calicivirus translation
To show the importance of the initiation factor in calicivirus mRNA translation, inactivation of eIF4E was achieved using the eIF4E inhibitor 4E-BP1 (Lawrence & Abraham, 1997). Prior incubation of RRL with purified recombinant 4E-BP1 resulted in a dose-dependent inhibition of both cap-dependent and VPg-dependent translation, with little or no effect on EMCV IRES-mediated translation (cap-independent; Fig 5A). Quantification of the levels of translation showed that VPg-dependent translation was inhibited to similar levels as that seen for cap-dependent translation (Fig 5B), indicating that calicivirus translation is dependent on eIF4E. The addition of exogenous 4E-BP1 as a competitor to the ELISA-based initiation factor capture assay had no effect on the interaction of VPg with eIF4E but significantly decreased the levels of eIF4E captured by immobilized 4E-BP1 (Fig 5C). This would suggest that VPg and 4E-BP1 interact with distinct sites on eIF4E. This was confirmed by our ability to detect a VPg–eIF4E–4E-BP1 complex formed on immobilized VPg (Fig 5D).
Figure 5.
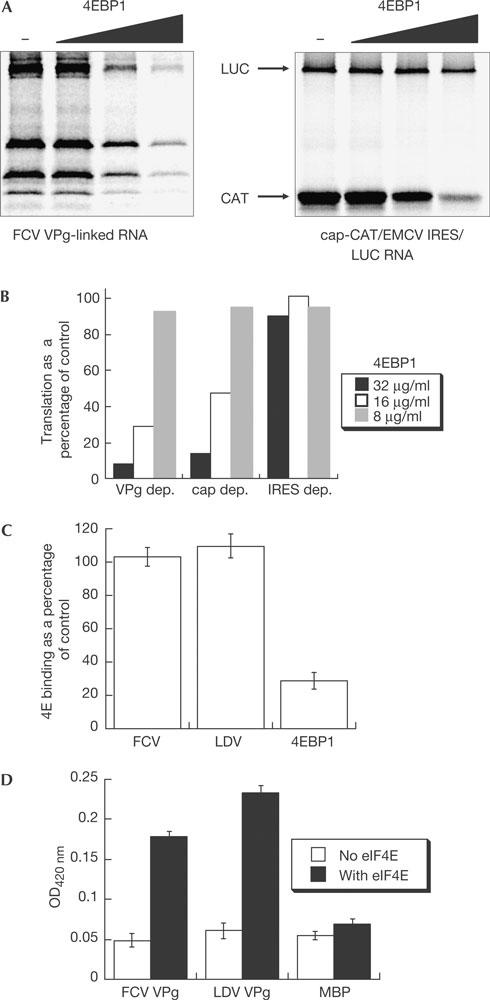
4E-BP1 inhibits calicivirus translation but does not affect VPg–eIF4E interaction. (A) In vitro translation reactions were carried out after preincubation of rabbit reticulocyte lysate with 8, 16 or 32 μg of recombinant 4E-BP1 using either in vitro-transcribed, capped dicistronic messenger RNA (CAT/IRES/LUC) or feline calicivirus (FCV) mRNA. Reactions were analysed by 12.5% SDS–polyacrylamide gel electrophoresis (A) and quantified by phosphorimaging (B). The level of translation is expressed as a percentage of the control reaction. (C) Wells were precoated with 10 μg of untagged FCV and Lordsdale virus (LDV) VPg or 4E-BP1 and incubated with an Escherichia coli lysate expressing GST–eIF4E either in the presence or absence of 5 μg of 4E-BP1. Complexes were detected using anti-eIF4E antisera and expressed as a percentage of 4E bound in the absence of exogenous 4E-BP1. (D) Enzyme-linked immunosorbent assay showing the formation of a VPg–eIF4E–4E-BP1 complex. The assay was set up as in (C), except that maltose-binding protein (MBP) was absorbed to the plate to control for nonspecific 4E-BP1 binding and bound 4EBP1 was detected using anti-His tag antisera. OD, optical density.
Discussion
Our data, and those of Daughenbaugh et al (2003), who previously showed an interaction of Norwalk virus VPg with eIF3, suggest that calicivirus VPg may function as a cap substitute in the initiation of calicivirus translation. Interestingly, eIF4E and also other translation initiation factors were present in purified Norwalk VPg–eIF3 interacting complexes, although the direct interaction between VPg and eIF4E was not investigated (Daughenbaugh et al, 2003). Here, we show a direct interaction between FCV and LDV VPg with eIF4E using in vitro studies and isolation of FCV VPg from infected cells on cap–Sepharose. In this way, VPg may bind to eIF4E and eIF3 in the recruitment of an initiation complex to the viral mRNA.
It seems that FCV p43 is preferentially isolated on cap–Sepharose compared with the mature form of VPg (Fig 4). However, this simply reflects the relative ratios of VPg to p43 seen in an infected cell, as p43 seems to be the main form of VPg found during infection. Quantification using densitometry confirms this (data not shown). The role of precursor–eIF4E interactions in translation is unknown, but it is possible that the high concentrations of VPg-containing precursors in the replication complexes may function to effectively concentrate eIF4E at the sites of replication and translation, in a similar manner to VPgPro from Turnip mosaic virus (TuMV; Leonard et al, 2004).
Given that a VPg-containing complex can be isolated from calicivirus-infected cells using cap–Sepharose, we suggest that the VPg-binding site on eIF4E is distinct from the region known to bind to cap. From this, it could be predicted that expression of VPg during infection would not contribute to the shutting off of host-cell protein synthesis observed in FCV infection (Willcocks et al, 2004). Overexpression of either FCV or LDV VPg in cells was found to affect neither cap-dependent nor EMCV IRES-dependent translation (data not shown). However, in previous work, the addition of recombinant Norwalk virus VPg protein to in vitro translation reactions was shown to inhibit both cap-dependent and IRES-dependent translation (Daughenbaugh et al, 2003). Norwalk virus VPg inhibits both the EMCV and cricket paralysis virus IRES elements, of which the latter does not require any initiation factors for initiation complex formation. Whether the differences between the two studies reflect differences in the in vitro versus in vivo assays or true differences in the function of FCV, LDV and norovirus VPg proteins is not yet known.
Our data imply that VPg binds to eIF4E at a different site from cap, as both can be accommodated on the initiation factor (Fig 4A). Given our observation that 4E-BP1 can inhibit calicivirus translation (Fig 4A), it is possible that the VPg-binding site partially overlaps with the 4E-BP1-binding site on eIF4E. However, further analysis indicated that 4E-BP1 did not affect VPg–eIF4E interaction (Fig 5C) and that a VPg–eIF4E–4E-BP1 complex can be formed in vitro (Fig 5D), indicating that the VPg- and 4E-BP1-binding sites on eIF4E are distinct. It is therefore likely that the inhibition of calicivirus translation observed in the presence of 4E-BP1 is a result of the ability of 4E-BP1 to prevent the binding of eIF4E to eIF4G (Haghighat et al, 1995). This would suggest that a functional eIF4F complex is required for calicivirus translation. Our failure to isolate other components of the eIF4F complex using recombinant VPg (Fig 1A) is probably owing to the stabilization of the eIF4F complex on VPg-linked RNA through the interaction of eIF4G or eIF4A with the viral RNA. Preliminary results confirm that eIF4A can bind to the 5′ end of the FCV genome (data not shown).
We have previously shown that the eIF4GI and eIF4GII cleavage products generated during FCV infection remain associated with eIF4E (Fig 2 in Willcocks et al, 2004), indicating that the interaction of VPg with eIF4E does not affect the eIF4E–4G interaction and further strengthening the hypothesis that an interaction between eIF4E and eIF4G is required for calicivirus translation.
The interaction of the calicivirus VPg protein with translation initiation factors is not unique, as the potyvirus VPg proteins have also been shown to interact with eIF4E (Wittmann et al, 1997; Leonard et al, 2000; Schaad et al, 2000). The importance of this for infection has been shown in mutant Arabidopsis thaliana plants that do not express eIF(iso)4E (Duprat et al, 2002; Lellis et al, 2002). Although these plants had a normal phenotype, they were immune to TuMV. VPg has also been shown to have a role in overcoming viral resistance in plants and the host recessive resistance gene has been identified as encoding eIF4E (Ruffel et al, 2002; Nicaise et al, 2003). Recently, a precursor of VPg (VPgPro) of TuMV was shown to interact with PABP (Leonard et al, 2004); this may lead to viral RNA circularization. Interaction with eIF(iso)4E and PABP suggests that VPgPro may serve as a focal point for translation initiation complex assembly.
Here, we describe the first functional characterization of a viral ‘proteinaceous cap substitute'–initiation factor interaction for any mammalian RNA virus. The calicivirus VPg–eIF4E interaction seems to represent a novel way in which animal RNA viruses recruit the ribosome for translation initiation. Further functional analysis of calicivirus translation is in progress to determine whether all the components of the eIF4F complex are required for initiation. Disruption of this interaction may provide a useful antiviral strategy for the control of this economically important group of viruses.
Methods
HeLa S10 pull-downs. Nuclease-treated S10 extracts were prepared from HeLa S3 cells as described (Barton et al, 1995). The FCV F9 VPg coding gene was amplified by reverse transcription–PCR (RT–PCR) from RNA isolated from infected CRFK cells and cloned into pET28a. Expression and purification were carried out according to the manufacturer's instructions (Novagen, Nottingham, UK). His-tagged FCV VPg was bound to nickel NTA-agarose and incubated with S10; bound proteins were analysed by western blot using antisera to eIF4A, eIF4E and eIF4G, which were gifts from S. Morley (University of Sussex, UK).
ELISA-based initiation factor capture assay. To assess any direct binding of FCV VPg with the eIF4F complex components and PABP, an ELISA-based binding assay was conducted, as described previously (Leonard et al, 2000). Plasmids encoding GST-fused forms of murine eIF4GI and eIF4E, as well as eIF4A and PABP, were generous gifts from N. Sonenberg (McGill University). Recombinant untagged forms of FCV and LDV VPg proteins were produced using the ubiquitin fusion system previously described (Gohara et al, 1999) and purified using standard chromatographic methods (see the supplementary information online). For assays in which 4E-BP1 was included, recombinant 4E-BP1 (a gift from S. Morley) was preincubated with the eIF4E-containing lysate before binding to immobilized VPg or 4E-BP1. Complexes were detected with either anti-His, to detect bound 4E-BP1, or anti-eIF4E.
VPg-dependent translation assay. Replication complexes were prepared as described (Green et al, 2002) and total RNA was prepared (GenElute, Sigma, Gillingham, UK). Translation reactions were carried out using the Flexi reticulocyte system according to the manufacturer's instructions using 25 μg/ml of RNA (Promega, Southampton, UK). Capped control dicistronic mRNAs containing the EMCV IRES were synthesized in vitro from the plasmid pGEM-CAT/EMCV/LUC (van der Velden et al, 1995). To study the effects of prior treatment with either exogenous cap or 4E-BP1, reticulocyte lysate was preincubated with inhibitor for 15 min at 30°C. Recombinant purified 4E-BP1 was a generous gift from S. Morley.
Isolation of eIF4E complexes using m7-GTP Sepharose. CRFK cells were infected with FCV and eIF4E-associated protein was purified using either m7-GTP Sepharose, as described (Ptushkina et al, 1999), or Sepharose 4B as a control.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
We thank G. Belsham for a critical reading of the manuscript, N. Sonenberg, S. Morley, C. Cameron, P. Lambden and I. Clarke for reagents. I.G.G. and Y.C. are supported by a Wellcome Trust Research Career Development Fellowship to I.G.G. L.O.R. acknowledges support from the Wellcome Trust. J.F.L. is supported by the Natural Sciences and Engineering Research Council of Canada.
References
- Barton DJ, Black EP, Flanegan JB (1995) Complete replication of poliovirus in vitro: preinitiation RNA replication complexes require soluble cellular factors for the synthesis of VPg-linked RNA. J Virol 69: 5516–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsham GJ, Jackson RJ (2000) Translation initiation on picornavirus RNA. In Translational Control of Gene Expression, Sonenberg N, Hershey JWB, Mathews MB (eds) pp 869–900. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press [Google Scholar]
- Daughenbaugh KF, Fraser CS, Hershey JW, Hardy ME (2003) The genome-linked protein VPg of the Norwalk virus binds eIF3, suggesting its role in translation initiation complex recruitment. EMBO J 22: 2852–2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprat A, Caranta C, Revers F, Menand B, Browning KS, Robaglia C (2002) The Arabidopsis eukaryotic initiation factor (iso)4E is dispensable for plant growth but required for susceptibility to potyviruses. Plant J 32: 927–934 [DOI] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N (1999) eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem 68: 913–963 [DOI] [PubMed] [Google Scholar]
- Gohara DW, Ha CS, Kumar S, Ghosh B, Arnold JJ, Wisniewski TJ, Cameron CE (1999) Production of ‘authentic' poliovirus RNA-dependent RNA polymerase (3D(pol)) by ubiquitin-protease-mediated cleavage in Escherichia coli. Protein Expr Purif 17: 128–138 [DOI] [PubMed] [Google Scholar]
- Green KY, Mory A, Fogg MH, Weisberg A, Belliot G, Wagner M, Mitra T, Ehrenfeld E, Cameron CE, Sosnovtsev SV (2002) Isolation of enzymatically active replication complexes from feline calicivirus-infected cells. J Virol 76: 8582–8595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghighat A, Mader S, Pause A, Sonenberg N (1995) Repression of cap-dependent translation by 4e-binding protein-1: competition with p220 for binding to eukaryotic initiation factor-4e. EMBO J 14: 5701–5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert TP, Brierley I, Brown TD (1997) Identification of a protein linked to the genomic and subgenomic mRNAs of feline calicivirus and its role in translation. J Gen Virol 78: 1033–1040 [DOI] [PubMed] [Google Scholar]
- Hershey J, Merrick W (2000) Pathway and Mechanism of Initiation of Protein Synthesis. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory [Google Scholar]
- Lawrence JC Jr, Abraham RT (1997) PHAS/4E-BPs as regulators of mRNA translation and cell proliferation. Trends Biochem Sci 22: 345–349 [DOI] [PubMed] [Google Scholar]
- Lellis AD, Kasschau KD, Whitham SA, Carrington JC (2002) Loss-ofsusceptibility mutants of Arabidopsis thaliana reveal an essential role for eIF(iso)4E during potyvirus infection. Curr Biol 12: 1046–1051 [DOI] [PubMed] [Google Scholar]
- Leonard S, Plante D, Wittmann S, Daigneault N, Fortin MG, Laliberte JF (2000) Complex formation between potyvirus VPg and translation eukaryotic initiation factor 4E correlates with virus infectivity. J Virol 74: 7730–7737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard S, Viel C, Beauchemin C, Daigneault N, Fortin MG, Laliberte JF (2004) Interaction of VPg-Pro of turnip mosaic virus with the translation initiation factor 4E and the poly(A)-binding protein in planta. J Gen Virol 85: 1055–1063 [DOI] [PubMed] [Google Scholar]
- Nicaise V, German-Retana S, Sanjuan R, Dubrana MP, Mazier M, Maisonneuve B, Candresse T, Caranta C, LeGall O (2003) The eukaryotic translation initiation factor 4E controls lettuce susceptibility to the Potyvirus Lettuce mosaic virus. Plant Physiol 132: 1272–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pe'ery T, Mathews M (2000) Viral Translational Strategies and Host Defense Mechanisms. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory [Google Scholar]
- Ptushkina M, von der Haar T, Karim MM, Hughes JM, McCarthy JE (1999) Repressor binding to a dorsal regulatory site traps human eIF4E in a high cap-affinity state. EMBO J 18: 4068–4075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffel S, Dussault MH, Palloix A, Moury B, Bendahmane A, Robaglia C, Caranta C (2002) A natural recessive resistance gene against potato virus Y in pepper corresponds to the eukaryotic initiation factor 4E (eIF4E). Plant J 32: 1067–1075 [DOI] [PubMed] [Google Scholar]
- Schaad MC, Anderberg RJ, Carrington JC (2000) Strainspecific interaction of the tobacco etch virus NIa protein with the translation initiation factor eIF4E in the yeast two-hybrid system. Virology 273: 300–306 [DOI] [PubMed] [Google Scholar]
- Sosnovtsev SV, Garfield M, Green KY (2002) Processing map and essential cleavage sites of the nonstructural polyprotein encoded by ORF1 of the feline calicivirus genome. J Virol 76: 7060–7072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosnovtseva SA, Sosnovtsev SV, Green KY (1999) Mapping of the feline calicivirus proteinase responsible for autocatalytic processing of the nonstructural polyprotein and identification of a stable proteinase-polymerase precursor protein. J Virol 73: 6626–6633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Velden A, Kaminski A, Jackson RJ, Belsham GJ (1995) Defective point mutants of the encephalomyocarditis virus internal ribosome entry site can be complemented in trans. Virology 214: 82–90 [DOI] [PubMed] [Google Scholar]
- Willcocks MM, Carter MJ, Roberts LO (2004) Cleavage of eukaryotic initiation factor eIF4G and inhibition of host-cell protein synthesis during feline calicivirus infection. J Gen Virol 85: 1125–1130 [DOI] [PubMed] [Google Scholar]
- Wittmann S, Chatel H, Fortin MG, Laliberte JF (1997) Interaction of the viral protein genome linked of turnip mosaic potyvirus with the translational eukaryotic initiation factor (iso) 4E of Arabidopsis thaliana using the yeast two-hybrid system. Virology 234: 84–92 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


