Abstract
Neurotrophins control neuronal survival in a target-derived manner during the period of naturally occurring cell death in development. The specificity of this mechanism has been attributed to a restricted spatio-temporal expression of neurotrophin ligands in target tissues, as well as a selective expression of their cognate tyrosine kinase (Trk) receptors in different neuronal subpopulations. However, several in vitro and in vivo studies of null mutant mice have suggested that neurotrophin 3 (NT3) also signals through the non-preferred TrkB receptor. In this study, we have directly addressed the in vivo preference of NT3 to signal through TrkB or TrkC, by crossing the NT3 knock-in mice (BDNFNT3/NT3 mice) with the TrkB- or TrkC-null mutant mice. We find that TrkB is dispensable, whereas TrkC is required for the neuronal rescue by the NT3 allele in the brain-derived neurotrophic factor- and NT3-dependent cochleovestibular system. Our results show that NT3 maintains survival of cells as well as target innervation only through interactions with TrkC in vivo. TrkB and TrkC receptors are thus not functionally redundant for NT3, even when coexpressed in neurons of the cochleovestibular system.
Keywords: cochlear ganglia, inner ear, promiscuity, trophic factors, vestibular ganglia
Introduction
Analyses of null mutant mice for neurotrophins or their receptors suggest diverse requirements of different neurotrophins for selective neuronal populations (Ernfors, 2001; Huang & Reichardt, 2001). The varying requirements of neurotrophins between subpopulations of sensory neurons are set by the availability of ligands in the target tissues, as well as a selective expression of Trk receptors in different neurons. TrkB and TrkC messenger RNAs are ubiquitously expressed in vestibular ganglion neurons at embryonic and postnatal stages as well as in the adult (Ylikoski et al, 1993), and both brain-derived neurotrophic factor (BDNF) and neurotrophin 3 (NT3) are present in the vestibular sensory epithelia (Ernfors et al, 1992; Pirvola et al, 1992). Yet, ligand- and receptor-null mutant mice show distinct and specific deficits. In the BDNF−/− mice, 80% of the vestibular ganglion neurons are lost, accompanied by a near-complete loss of innervation of the target sensory hair cells at birth, whereas only 56% of the neurons are lost in the TrkB mutant mice. In the NT3−/− and TrkC−/− mice, 34% and 16%, respectively, of the vestibular ganglion neurons are lost. In the cochlea, 87%, 51%, 7% and 15% of cochlear ganglion neurons are lost in the NT3−/−, TrkC−/−, BDNF−/− and TrkB−/− mice, respectively (Ernfors et al, 1994, 1995; Schimmang et al, 1995). In a study by Farinas et al (1998), it was claimed that TrkB can be directly activated by NT3 in primary neurons, both in vitro and in vivo. Recent results using knock-in mice of BDNF and NT3 show that both BDNF and NT3 are redundant in parts of the peripheral nervous system (Coppola et al, 2001; Agerman et al, 2003), but receptor engagement was not addressed in the latter studies. Thus, several reports have drawn attention to the issue of whether ligand redundancy is mirrored by a receptor redundancy. None of the previous studies has directly addressed whether the control of cell death and target innervation by neurotrophins is mediated by preferred or non-preferred receptor signalling in vivo.
In this study, we have taken a genetic approach to determine the in vivo preference of NT3 to signal through either TrkB or TrkC in the vestibular and auditory systems. For this purpose, we used our previously established mice, in which the coding part of the BDNF gene has been replaced by the NT3 gene. In these mice, NT3 can partly and completely rescue neuronal numbers in the vestibular and cochlear systems, respectively, but only a few of the remaining neurons still innervate the sensory epithelia (Agerman et al, 2003). This is consistent with the fact that fibre growth (Tessarollo et al, 2004), short-range innervation and synaptogenesis (Agerman et al, 2003) are largely mediated by BDNF in the inner ear. By crossing the BDNFNT3/NT3 mice with the TrkB- or TrkC-null mutant mice, we separately eliminated each of the two potential signalling pathways of NT3. We have focused our analysis on the cochleovestibular system, as both TrkB and TrkC mRNAs are expressed ubiquitously by the cochlear and vestibular neurons. Therefore, non-promiscuous receptor signalling can directly be identified as such, without being confounded by the restricted presence of receptors in different neuronal subpopulations. Analysis of the BDNF-dependent cochleovestibular system in the two mouse strains, BDNFNT3/NT3 × TrkB−/− and BDNFNT3/NT3 × TrkC−/−, shows exclusive signalling of NT3 through the TrkC receptor.
Results
TrkB and TrkC expression in BDNFNT3/NT3 mice
As any discrepancies in neuronal survival between neurotrophin- and cognate receptor-null mutant mice could be caused by changes in receptor expression as a consequence of eliminating the ligands, we first investigated whether genetically removing BDNF as well as introducing the NT3 allele in place of BDNF affected TrkB and TrkC mRNA expression. Real-time PCR showed that, in the wild-type mice, only the full-length form of TrkC (TrkC-FL) was expressed in the inner ear (Fig 1A), as there was an equal number of copies of extracellular domain (TrkC-all) and TrkC-FL mRNA transcripts (Fig 1B). TrkB-FL transcripts were present at levels similar to those of TrkC-FL in the inner ear. However, unlike TrkC, TrkB-all expression was fivefold that of TrkB-FL, suggesting a 4:1 ratio of truncated to full-length TrkB receptors. Expression of full-length and truncated TrkB and TrkC isoforms did not differ in the wild-type and BDNFNT3/NT3 mice (Fig 1B). These results indicate that there is no misregulation of Trk receptors that could confound interpretations of receptor promiscuity in the present study.
Figure 1.
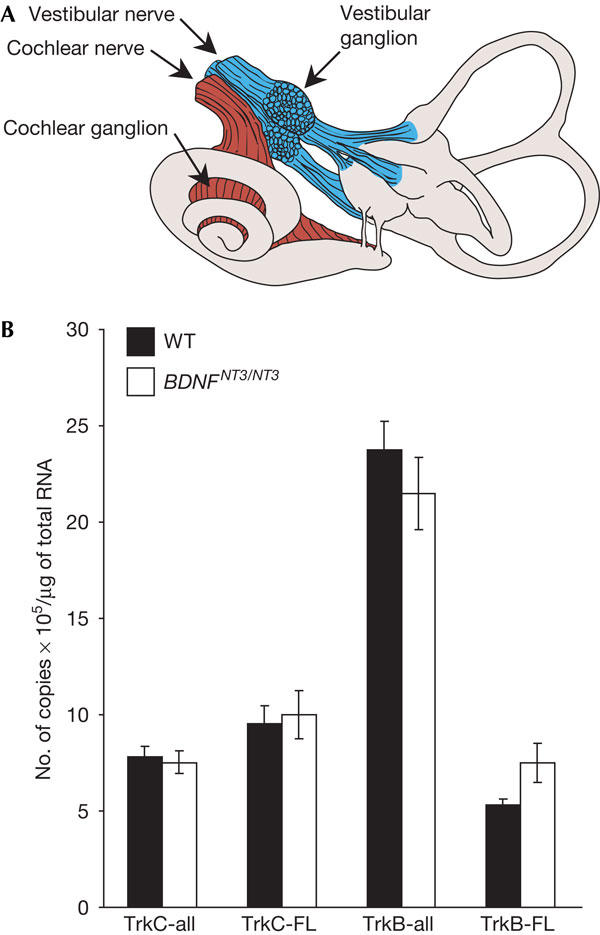
TrkB and TrkC messenger RNA expression in mice of different genotypes. (A) Illustration of the tissue dissected for real-time PCR in (B). (B) Quantitative real-time PCR for TrkB and TrkC measuring both truncated and full-length TrkB or TrkC (TrkB-all and TrkC-all) or only full-length receptors (TrkB-FL and TrkC-FL). The data are presented as copy numbers per microgram RNA. Note that full-length TrkB and TrkC are expressed at similar levels, that there is abundant expression of truncated TrkB and that there are no changes in the expression of any isoforms in the BDNFNT3/NT3 mice compared with the wild-type (WT) mice.
Signalling of NT3 exclusively through TrkC in vivo
Quantitative data based on morphological criteria identifying peripheral neurons obtained from cresyl violet stainings in the vestibular ganglion correlated well with the overt changes in ganglion size (Fig 2; Table 1).
Figure 2.
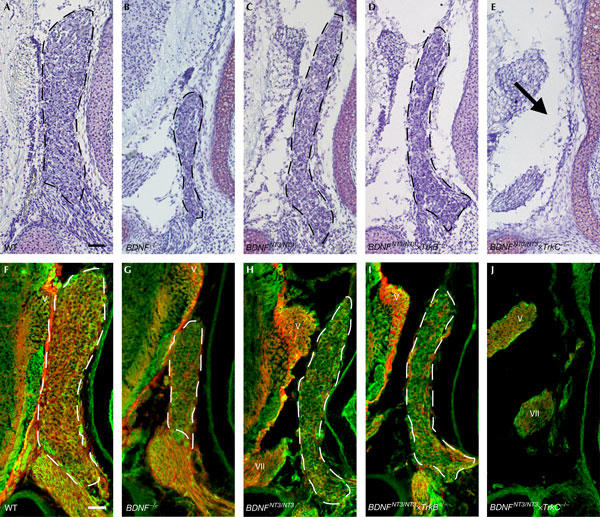
Histological and immunohistochemical analyses of the vestibular ganglion. (A–E) Cresyl violet staining of embryonic day 18 (E18) vestibular ganglion in mice of indicated genotypes. The arrow in (E) indicates the location at which the ganglion is missing in the BDNFNT3/NT3 × TrkC−/− mice. (F–J) Immunohistochemical staining for p75NTR (red) and βIII tubulin (green) of E18 vestibular ganglion in mice of indicated genotypes. WT, wild type; V, fifth cranial nerve; VII, seventh cranial nerve. Scale bars, 50 μm.
Table 1.
Neuronal cell counts in vestibular ganglion
| Mean number of neurons±s.e.m. | Percentage of control±s.e.m. | |
|---|---|---|
| Wild type | 3,823±182 (n=8) | 100±5 |
| BDNF−/− | 854±64 (n=4) | 22±2* |
| BDNFNT3/NT3 | 2,143±248 (n=4) | 56±6*,° |
| BDNFNT3/NT3 × TrkB−/− | 2,136±105 (n=8) | 56±3*,° |
| BDNFNT3/NT3 × TrkC−/− | 0±0 (n=6) | 0±0*,°,‡,§ |
Cryostat sections from embryonic day 18 mice of the indicated genotypes were prepared and stained with cresyl violet. Vestibular ganglion neurons with a clear nucleus and nucleoli were counted in every third section. ANOVA Bonferroni's
*P<0.001 between wild-type and mutant mice,
°P<0.001 between BDNF−/− and other mutant mice,
‡P<0.001 between BDNFNT3/NT3 and BDNFNT3/NT3 × TrkC−/− and
§P<0.001 between BDNFNT3/NT3 × TrkB−/− and BDNFNT3/NT3 × TrkC−/−.
The neuronal loss in the vestibular ganglion of BDNF−/− mice was markedly rescued by the NT3/NT3 alleles in the BDNF locus (22% versus 56% neurons remaining). The rescue of neuronal numbers in the BDNFNT3/NT3 mice was not affected by eliminating signalling through the TrkB receptor, as was seen after introducing a null mutation of the TrkB allele (56% remaining). In contrast, the BDNFNT3/NT3 × TrkC−/− mice showed a complete loss of mature vestibular ganglion neurons (Table 1). In the cochlear ganglion, 75% of the neurons remained in the BDNF−/− mice and 99% in the BDNFNT3/NT3 mice (Agerman et al, 2003). Similar to vestibular neurons, the rescue of cochlear neurons by the NT3/NT3 alleles was not affected in the BDNFNT3/NT3 × TrkB−/− compound mutant mice, whereas no remaining neurons could be found in the BDNFNT3/NT3 × TrkC−/− mice (Table 2).
Table 2.
Neuronal cell counts in cochlear ganglion
| Mean number of neurons±s.e.m. | Percentage of control±s.e.m. | |
|---|---|---|
| Wild type | 6,499±198 (n=3) | 100±3 |
| BDNF−/− | 4,872±270 (n=3)a | 75±4a |
| BDNFNT3/NT3 | 6,455±351 (n=3)a | 99±5a |
| BDNFNT3/NT3 × TrkB−/− | 6,434±244 (n=7) | 99±4 |
| BDNFNT3/NT3 × TrkC−/− | 0±0 (n=6) | 0±0*,° |
Cryostat sections from embryonic day 18 mice of the indicated genotypes were prepared and stained with cresyl violet. Cochlear ganglion neurons with a clear nucleus and nucleoli were counted in every third section.
aData from Agerman et al (2003), reproduced with permission.
*P<0.001 between wild-type and BDNFNT3/NT3 × TrkC−/−,
°P<0.001 between BDNFNT3/NT3 × TrkB−/− and BDNFNT3/NT3 × TrkC−/−.
We next used immunohistochemistry for βIII tubulin (Tuj1) to address more directly whether there is a complete absence of neurons in the BDNFNT3/NT3 × TrkC−/− mice (Fig 2F–J). The results were similar to those obtained in the histological analysis, and the marked loss of vestibular neurons in the BDNFNT3/NT3 × TrkC−/− mice (Fig 2J) was confirmed.
Early rescue in BDNFNT3/NT3 mice
Most of the naturally occurring cell death in the vestibular ganglion takes place between embryonic day 13 (E13) and E16, and it is also during this period in development that most of the excessive cell death in the BDNF−/− mice occurs (Ernfors et al, 1995). We examined whether the neuronal rescue in BDNFNT3/NT3 mice coincided with the period of naturally occurring cell death by counting the number of vestibular neurons at E16 and E18. A statistical difference was found in cell numbers between these stages in the wild-type mice, which showed 4,615±234 neurons at E16 (n=6) and 3,823±182 neurons at E18 (n=8; P<0.05). This result shows that close to 20% of the neurons present at E16 have died at E18 in the wild-type mice, indicating that the period of normal cell death continues until E18. Much of the neuronal rescue in the BDNFNT3/NT3 mice had already taken place at E16 (2,019±64 neurons, n=6) and the number of neurons remained unchanged between E16 and E18 in the BDNFNT3/NT3 mice (E18, 2,143±248 neurons, n=4). These data show that there is a rescue of neurons in the BDNFNT3/NT3 mice throughout the period of naturally occurring cell death. We conclude that the neuronal rescue in the BDNFNT3/NT3 mice coincides with the period of excessive neuronal loss in the BDNF−/− mice.
Rescue of innervation is dependent on TrkC signalling
Previous results illustrate that BDNF is the most important trophic factor for a proper terminal innervation and maturation of functional sensory nerve endings in the vestibular organ, but that NT3 expressed from the BDNF locus in BDNFNT3/NT3 mice is sufficient to rescue a minor afferent innervation (Agerman et al, 2003). We studied whether the remaining nerve fibre innervation of the BDNFNT3/NT3 mice was dependent on TrkB or TrkC signalling. We stained for βIII tubulin, which is present in both afferents and efferents of the inner ear (Fig 3A–E; Kim et al, 2001). Semiquantification of the staining (Fig 3F) showed a greater loss in the epithelial layer than in the subepithelial layer of the BDNF−/− mice (Fig 3B). A distinct increase in number and thickness of nerve fibres in the sensory epithelium was found in the BDNFNT3/NT3 mice (Fig 3C) as compared with the BDNF−/− mice, and this effect remained in the BDNFNT3/NT3 × TrkB−/− mice (Fig 3D), showing that the effect of NT3 is independent of TrkB. In agreement with this, nerve fibres were nearly completely absent in both layers in the BDNFNT3/NT3 × TrkC−/− mice (Fig 3E).
Figure 3.
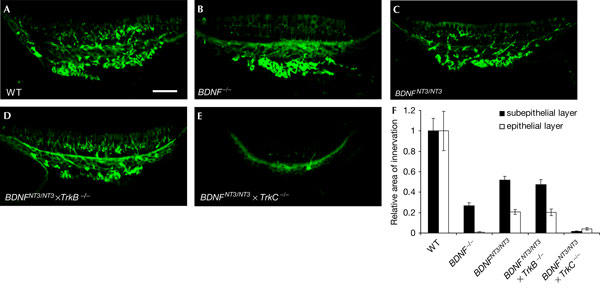
Rescue of innervation in BDNFNT3/NT3 mice occurs independent of TrkB. (A–E) Immunohistochemistry for βIII tubulin, which stains both efferent and afferent innervation in the utricular maculae at embryonic day 18 (E18) of mice of indicated genotypes. (F) Semiquantification of nerve fibres in the epithelial and subepithelial layers of the different genotypes. Note the greater loss of innervation in the epithelial layer in all knockout animals. Data are ±s.e.m. WT, wild type. Scale bars, 50 μm.
A greater rescue of efferent than of afferent innervation
We next examined βIII tubulin and p75NTR double immunohistochemistry at high power to compare possible differences between efferent and afferent innervation. Afferents are known to express both p75NTR and βIII tubulin, whereas efferents express only βIII tubulin. Consistently, p75NTR-positive fibres in the wild-type mice also expressed βIII tubulin, whereas there were many βIII tubulin-expressing fibres that did not stain for p75NTR (supplementary Fig 1A online).
In the subepithelial layer, efferents were abundantly present in all genotypes except the BDNFNT3/NT3 × TrkC−/− mice, which displayed a marked paucity. In contrast, the presence of afferents reflected the number of vestibular ganglion neurons remaining in each genotype (supplementary Fig 1A–E online). In the sensory epithelia, the efferents were very sparse in the BDNF−/− and BDNFNT3/NT3 × TrkC−/− mice, whereas many fibres were present in the BDNFNT3/NT3 and BDNFNT3/NT3 × TrkB−/− mice. However, afferent innervation of the sensory epithelial layer did not correlate with the number of fibres present in the subepithelial layer, as there was an absence in the BDNF−/− and BDNFNT3/NT3 × TrkC−/− mice, and only a minor presence in the BDNFNT3/NT3 and BDNFNT3/NT3 × TrkB−/− mice (supplementary Fig 1A–E online, and summarized in Fig 4). From this, it is clear that NT3 expressed from the BDNF locus is sufficient for afferent axonal growth and projection to the subepithelial layer but not for significant target innervation of hair cells in the sensory epithelia (which depends on BDNF; Agerman et al, 2003).
Figure 4.
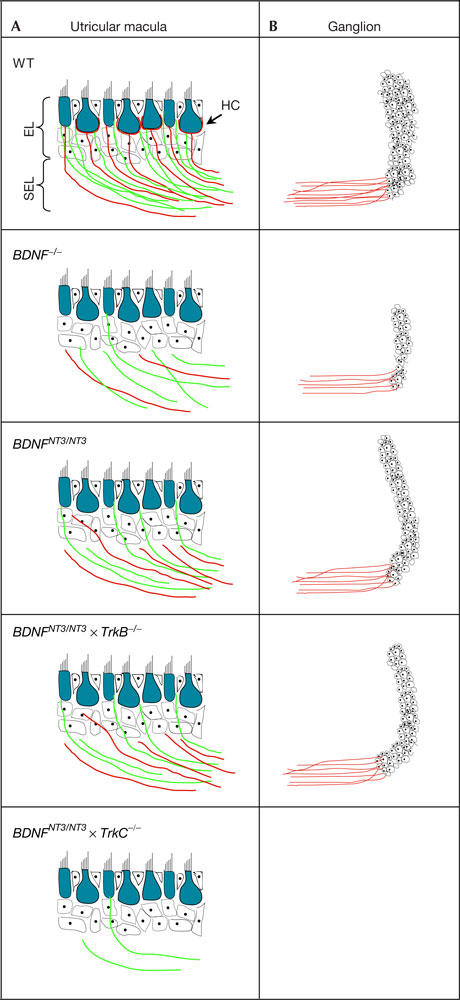
Schematic drawing summarizing target innervation and neuronal survival in all studied genotypes. The illustration of the vestibular sensory epithelia (A) and vestibular ganglia (B), summarizing afferent (red) and efferent (green) innervation of the epithelial layer (EL) and the subepithelial layer (SEL) of the different genotypes. Density of afferent innervation in the subepithelial layer had a direct correlation with ganglion size. Although neurotrophin 3 (NT3) through TrkC activation rescued neurons and nerve fibres in the sensory epithelia, hair-cell (HC) innervation and functional recovery was minor in the BDNFNT3/NT3 mice. The efferents were reduced when the number of afferents decreased. WT, wild type.
Discussion
By establishing the BDNFNT3/NT3 × TrkB−/− and BDNFNT3/NT3 × TrkC−/− mice and comparing neuronal survival and target innervation in these mice with those in the BDNFNT3/NT3 and wild-type mice, we have characterized the selectivity of NT3 to its cognate receptor, TrkC, in vivo. Earlier studies (Agerman et al, 2003) had shown a partial rescue of vestibular neuronal numbers in the BDNFNT3/NT3 mice but a failure to rescue the balance defect, presumably a result of the failure of rescued neurons to innervate the hair cells. The present results indicate that NT3 activation of TrkC is not equivalent to BDNF activation of TrkB in supporting hair-cell innervation and recovery of balance. Although trkB and trkC mRNAs are ubiquitous in all neurons of the vestibular ganglion, their presence at the protein level has not been examined. It is possible that the partial neuronal rescue in the BDNFNT3/NT3 mice (56% remaining), as compared with that in the BDNF−/− mice, is the result of neurons expressing both TrkB and TrkC, but responding primarily to BDNF; hence, introducing the NT3/NT3 allele effectively rescues them. Others may express only TrkB protein and therefore are not rescued by NT3. A final population could contain only TrkC, and all neurons are therefore lost in the BDNFNT3/NT3 × TrkC−/− mice.
The issue of whether neurotrophins signal through non-preferred receptors in vivo was raised by the findings that the NT3-null mutant mice in several peripheral ganglia show a more severe neuronal loss than the TrkC receptor-null mutant mice (Klein et al, 1994; Tessarollo et al, 1997; Ernfors, 2001; Huang & Reichardt, 2001). For example, the NT3-null mutant mice lose 85% of the cochlear sensory neurons (Farinas et al, 1994; Ernfors et al, 1995), whereas only a 51% loss has been reported in the TrkC mutant mice (Schimmang et al, 1995). These initial findings were followed up by studies addressing a putative non-preferred receptor signalling by NT3, both in vivo (Farinas et al, 1998; Huang et al, 1999, Coppola et al, 2001) and in vitro (Davies et al, 1995; Kuruvilla et al, 2004). The results from these studies are consistent with biochemical studies indicating that, in addition to TrkC, NT3 can, with lesser affinity, bind to and activate its non-preferred receptors TrkA and TrkB in non-neuronal cell lines (Barbacid, 1994). Although these results only indirectly address the issue of ligand interactions with non-preferred receptors in vivo, they are in agreement with a role for NT3 signalling through its non-preferred receptors, which has become generally accepted as a physiological process participating in the development of the peripheral nervous system.
Other work has, however, pointed to a high degree of receptor specificity in vivo, suggesting little, if any, interaction of neurotrophins with non-preferred Trk receptors (ElShamy & Ernfors, 1996; White et al, 1996; Ernfors, 2001). Consistently, in more recent studies of the TrkC−/− mice, the reported loss of cochlear neurons is 70% (Tessarollo et al, 1997), thus approaching the 85% loss in NT3-deficient mice. The high specificity of NT3 signalling through TrkC in the inner ear suggests a similar high specificity and the lack of TrkB interactions also in other neuronal populations in which NT3 is active. The evidence of NT3 acting through TrkA in sympathetic neurons (Kuruvilla et al, 2004) suggests that the remaining discrepancies in neuronal numbers of, for instance, the sensory trigeminal and dorsal root ganglion could be accounted for by its action through TrkA. Unlike the auditory and vestibular systems, TrkA is also expressed in many neurons of these ganglia. Because several of the analyses that suggested NT3 signalling through TrkB and TrkC were based on the detection of protein or presence of mRNA at cellular resolution and could be confounded by rapidly changing patterns of expression during development, we have taken a genetic approach to determine engagement of preferred versus non-preferred Trk receptors. Thus, in this study, we have, for the first time, in vivo addressed the promiscuity of receptor activation maintaining neuronal survival by NT3. Our new results are in agreement with the latter studies and show that, in the inner ear, NT3 signals exclusively through TrkC in vivo. Our results strongly argue that there is a high degree of specificity for NT3 receptor engagement in vivo and show that TrkB and TrkC receptors are not functionally redundant, even when coexpressed in individual neurons of the cochleovestibular system.
Methods
Animals. In this study, we used offspring from the BDNF+/− (Ernfors et al, 1994), BDNF+/NT3 (Agerman et al, 2003), TrkB+/− (Klein et al, 1993) and TrkC+/− (Klein et al, 1994) mice. The offspring were genotyped with PCR.
Tissue preparation. For immunohistochemical analysis and neuronal quantification, embryos were obtained from overnight mating and the morning of the vaginal plug was considered as E0. Tissues from E18 mice were immersionfixed in 4% paraformaldehyde overnight, equilibrated in 10% sucrose followed by 30% sucrose, and frozen. The tissue was sectioned in a cryostat at a thickness of 14 μm.
Quantification of neuronal numbers. Sections were stained with cresyl violet. Neuronal numbers were established by counting neurons with a clear nucleus and nucleoli in every third section in the ganglia. The total number of cells in a ganglion was calculated as described before (Coggeshall, 1992). One-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test was used for statistical analyses. A P-value of <0.05 was considered to be significant.
Immunohistochemistry. Sections were stained with mouse anti-βIII tubulin (1:2,000; Promega, Southampton, UK), and rabbit anti-p75NTR (1:200; Promega). Photomicrographs (Fig 3A–E; supplementary Fig 1A–E online) were obtained on a confocal microscope (Zeiss LSM510).
Semiquantification of innervation. Frames were captured from anti-βIII-tubulin-immunostained sections at × 20. The total area of the utricular sensory epithelium and that of the subepithelial layer were quantified separately, and the area covered with signal above threshold was computed using ImageJ. Wild type was set to 1 and the other genotypes were normalized against this value.
Quantitative real-time PCR. Total RNA from the inner ears of E18 mice was extracted using the Absolutely RNA™ Nanoprep kit (Stratagene, La Jolla, CA, USA). Primers amplifying the extracellular domain (all) or the intracellular tyrosine kinase domain (FL) of either TrkB or TrkC and primers for the HPRT (positive control) were designed in different exons to avoid amplification of eventual DNA contamination. PCR products were verified by sequencing and used for standard curves in the real-time PCR. The samples were run on a Perkin-Elmer ABI Prism 5700.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary Information
Acknowledgments
This research was supported by the Swedish Medical Research Council, the Swedish Cancer Society, Hedlunds Foundation, the EU Quality of Life program (QLG3-CT-2000-01343) and the Swedish Foundation for Strategic Research (CEDB grant). K.A. was supported by a fellowship from the Swedish National Network for Neuroscience.
References
- Agerman K, Baudet C, Fundin B, Willson C, Ernfors P (2000) Attenuation of a caspase-3 dependent cell death in NT4- and p75-deficient embryonic sensory neurons. Mol Cell Neurosci 16: 258–268 [DOI] [PubMed] [Google Scholar]
- Agerman K, Hjerling-Leffler J, Blanchard MP, Scarfone E, Canlon B, Nosrat C, Ernfors P (2003) BDNF gene replacement reveals multiple mechanisms for establishing neurotrophin specificity during sensory nervous system development. Development 130: 1479–1491 [DOI] [PubMed] [Google Scholar]
- Barbacid M (1994) The Trk family of neurotrophin receptors. J Neurobiol 25: 1386–1403 [DOI] [PubMed] [Google Scholar]
- Coggeshall RE (1992) A consideration of neural counting methods. Trends Neurosci 15: 9–13 [DOI] [PubMed] [Google Scholar]
- Coppola V, Kucera J, Palko ME, Martinez-De Velasco J, Lyons WE, Fritzsch B, Tessarollo L (2001) Dissection of NT3 functions in vivo by gene replacement strategy. Development 128: 4315–4327 [DOI] [PubMed] [Google Scholar]
- Davies AM, Minichiello L, Klein R (1995) Developmental changes in NT3 signalling via TrkA and TrkB in embryonic neurons. EMBO J 14: 4482–4489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElShamy WM, Ernfors P (1996) Requirement of neurotrophin-3 for the survival of proliferating trigeminal ganglion progenitor cells. Development 122: 2405–2414 [DOI] [PubMed] [Google Scholar]
- Ernfors P (2001) Local and target derived actions of neurotrophins during peripheral nervous system development. Cell Mol Life Sci 58: 1036–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernfors P, Merlio J-P, Persson H (1992) Cells expressing mRNA for neurotrophins and their receptors during embryonic rat development. Eur J Neurosci 4: 1140–1158 [DOI] [PubMed] [Google Scholar]
- Ernfors P, Lee KF, Jaenisch R (1994) Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature 368: 147–150 [DOI] [PubMed] [Google Scholar]
- Ernfors P, Van De Water T, Loring J, Jaenisch R (1995) Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron 14: 1153–1164 [DOI] [PubMed] [Google Scholar]
- Farinas I, Jones KR, Backus C, Wang XY, Reichardt LF (1994) Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature 369: 658–661 [DOI] [PubMed] [Google Scholar]
- Farinas I, Wilkinson GA, Backus C, Reichardt LF, Patapoutian A (1998) Characterization of neurotrophin and Trk receptor functions in developing sensory ganglia: direct NT-3 activation of TrkB neurons in vivo. Neuron 21: 325–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF (2001) Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24: 677–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EJ, Wilkinson GA, Farinas I, Backus C, Zang K, Wong SL, Reichardt LF (1999) Expression of Trk receptors in the developing mouse trigeminal ganglion: in vivo evidence for NT-3 activation of TrkA and TrkB in addition to TrkC. Development 126: 2191–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE (2001) NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development 128: 417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL, Barbacid M (1993) Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell 75: 113–122 [PubMed] [Google Scholar]
- Klein R, Silossantiago I, Smeyne RJ, Lira SA, Brambilla R, Bryant S, Zhang L, Snider WD, Barbacid M (1994) Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature 368: 249–251 [DOI] [PubMed] [Google Scholar]
- Kuruvilla R, Zwifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, Ginty DD (2004) A neurotrophin singaling cascade coordinates sympathetic neuron development through differential control of trkA trafficking and retrograde signaling. Cell 118: 243–255 [DOI] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Palgi J, Lehtonen E, Arumae U, Saarma M (1992) Brain-derived neurotrophic factor and neurotrophin 3 mRNAs in the peripheral target fields of developing inner ear ganglia. Proc Natl Acad Sci USA 89: 9915–9919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmang T, Minichiello L, Vazquez E, San Jose I, Giraldez F, Kelin R, Represa J (1995) Developing inner ear sensory neurons require TrkB and TrkC receptors for innervation of their peripheral targets. Development 121: 3381–3391 [DOI] [PubMed] [Google Scholar]
- Tessarollo L, Tsoulfas P, Donovan MJ, Palko ME, Blair-Flynn J, Hempstead BL, Parada LF (1997) Targeted deletion of all isoforms of the trkC gene suggests the use of alternate receptors by its ligand neurotrophin-3 in neuronal development and implicates trkC in normal cardiogenesis. Proc Natl Acad Sci USA 94: 14776–14781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessarollo L, Coppola V, Fritzsch B (2004) NT-3 replacement with brain-derived neurotrophic factor redirects vestibular nerve fibers to the cochlea. J Neurosci 24: 2575–2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Silossantiago I, Molliver DC, Nishimura M, Phillips H, Barbacid M, Snider WD (1996) Synchronous onset of NGF and trkA survival dependence in development of dorsal root ganglia. J Neurosci 16: 4662–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Moshnyakov M, Palgi J, Arumae U, Saarma M (1993) Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hear Res 65: 69–78 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


