Abstract
Guanine nucleotide exchange factors (GEFs) regulate the activity of small GTP-binding proteins in a variety of biological processes. We have identified a gain-of-function mutation in the Caenorhabditis elegans GEF ect-2, the homologue of the mammalian ect2 proto-oncogene that has an essential role during cytokinesis. Here, we report that, in addition to its known function during mitosis, ECT-2 promotes the specification of the primary vulval cell fate by activating RAS/mitogen-activated protein kinase (MAPK) signalling before the end of the S-phase. Epistasis analysis indicates that ECT-2 crosstalks to the canonical RAS/MAPK cascade upstream of the RAS GEF SOS-1 by means of a RHO-1 signalling pathway. Our results raise the possibility that the transforming activity of the mammalian ect-2 oncogene could be due to hyperactivation of the RAS/MAPK pathway.
Keywords: Caenorhabditis elegans, signal transduction, development, RAS, guanine nucleotide exchange factor
Introduction
The RHO family of small guanine triphosphate hydrolases includes the RhoA, RhoB, RAC and CDC42 subfamilies of small GTPases that control various cellular processes, such as cell migration, adhesion, axonal guidance, gene expression, cell-cycle progression and cytokinesis (reviewed by Malliri & Collard, 2003; Burridge & Wennerberg, 2004). In most of these cases, RHO proteins transduce signals from transmembrane receptors to proteins controlling actin dynamics. The activity of RHO proteins is tightly regulated. They cycle between an inactive GDP-bound and an active GTP-bound form. GTPase-activating proteins (GAPs) accelerate the intrinsic GTPase activity of RHO to inactivate RHO signalling, and guanine dissociation inhibitors block the release of GDP to keep the RHO protein in the inactive conformation. Conversely, guanine nucleotide exchange factors (GEFs) catalyse the release of GDP and thereby facilitate GTP binding and RHO activation (reviewed by Schmidt & Hall, 2002). The activity of the GEFs is regulated by signals from various growth factor receptors at the plasma membrane. Aberrant RHO signalling in humans has been implicated in tumour formation and in other diseases (Malliri & Collard, 2003).
The mammalian ECT2 protein and its Drosophila orthologue Pebble are members of the Dbl family of GEFs, and they activate a RHO-mediated signalling pathway during cytokinesis (Prokopenko et al, 1999; Saito et al, 2003). ECT2 is necessary to form an actomyosin contractile ring that separates the plasma membranes of the two daughter cells that are forming. Amino-terminally truncated ECT2 and many other Dbl family GEFs show oncogenic transforming activity in cultured cells (Miki et al, 1993; Schmidt & Hall, 2002). However, it has remained unclear as to which signalling pathways the transforming activity of the Dbl family GEFs involves.
We show that the Caenorhabditis elegans homologue of the mammalian ect2 proto-oncogene positively regulates the RAS/mitogen-activated protein kinase (MAPK) signalling pathway during vulval development (reviewed by Sternberg & Han, 1998). In the C. elegans hermaphrodite, the anchor cell (AC) in the somatic gonad secretes the epidermal growth factor (EGF) LIN-3 to activate the EGF receptor (EGFR)/RAS/MAPK pathway in the vulval precursor cells (VPCs) and to specify the vulval cell fates. In response to the inductive AC signal, the VPC closest to the AC, P6.p, adopts the primary (1°) vulval fate. As a consequence of adopting the 1° fate, P6.p produces a lateral signal that activates the LIN-12 NOTCH pathway in the neighbouring VPCs P5.p and P7.p (Sternberg & Horvitz, 1989). The lateral LIN-12 NOTCH signal prevents P5.p and P7.p from adopting the 1° fate and instructs the secondary (2°) fate (Ambros, 1999; Berset et al, 2001). The three remaining VPCs, P3.p, P4.p and P8.p, that receive lower amounts of inductive signal (Dutt et al, 2004) and no lateral signal adopt the tertiary (3°) non-vulval cell fate. We have isolated a gain-of-function mutation in C. elegans ect-2 (previously named let-21) in a genetic screen for new regulators of the EGFR/RAS/MAPK pathway. Genetic epistasis analysis indicates that ECT-2 activates a RHO-1 signalling pathway that crosstalks to the RAS/MAPK cascade upstream of the RAS GEF SOS-1 (Chang et al, 2000).
Results
An activating mutation in ect-2 causes excess induction
To identify novel regulators of the EGFR/RAS/MAPK signalling pathway in C. elegans, we screened for mutations that cause excess vulval induction and a multivulva (Muv) phenotype in a sensitized gap-1(0) background. gap-1 encodes a GTPase-activating protein that inhibits LET-60 RAS signalling, but gap-1(0) single mutants develop a wild-type vulva (Fig 2A; Table 1, row 5; Hajnal et al, 1997). Out of 30,000 EMS mutagenized haploid genomes, we isolated 27 mutants that show a synthetic Muv phenotype and define at least four complementation groups. The zh8 mutation causes a penetrant Muv phenotype in the gap-1(0) background but no obvious vulval phenotype as a single mutant (Table 1, rows 3,7). The gene was mapped to the right arm of chromosome II to an 86 kbp interval containing 15 candidate genes (supplementary figure online; for details on cloning, see the supplementary information online). As we observed a weak Muv phenotype in zh8/+; gap-1(0) animals (Table 1, row 6), we speculated that zh8 might represent a weak gain-of-function (gf) allele. We therefore tested whether RNA interference against one of the 15 candidate genes might suppress the Muv phenotype of zh8; gap-1(0) double mutants. RNA interference against the open reading frame T19E10.1, which encodes the ect-2 gene (previously named let-21; Omachi & Lambie, personal communication), efficiently suppresses the zh8 Muv phenotype. In another sensitized background created by the elevated expression of the MAPK under control of the heat-shock promoter along with D-mek-2 (hs∷mpk-1; Lackner & Kim, 1998), the semi-dominant nature of ect-2(zh8) was more apparent (Table 1, rows 8–10). Moreover, ect-2(zh8) is a semi-dominant suppressor of the vulvaless (Vul) phenotype caused by a reduction-of-function mutation in the lin-3 egf gene (Table 1, rows 11–13).
Figure 2.
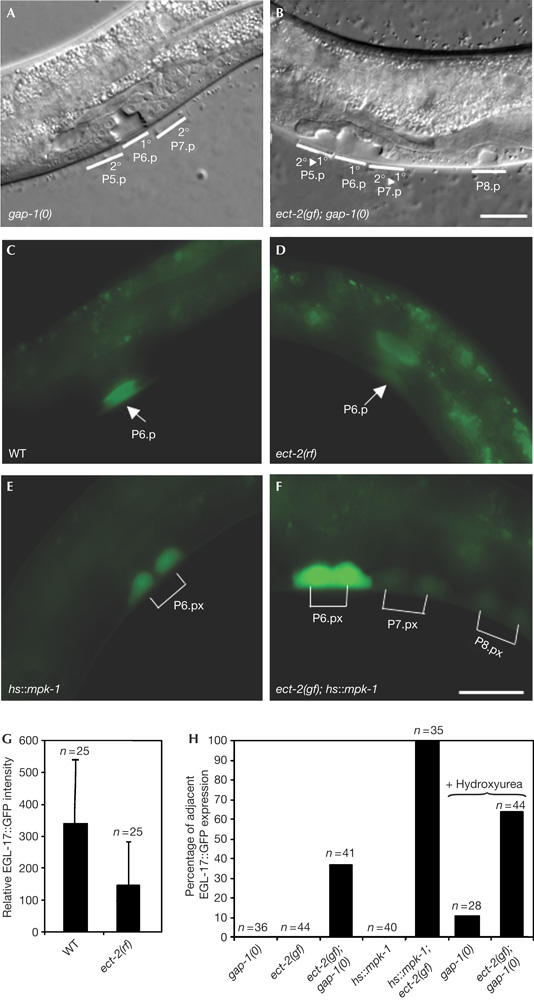
Vulval morphology and 1° cell fate marker expression in ect-2 mutants. (A) Wild-type vulval morphology in a gap-1(0) L4 larva. (B) An ect-2(zh8gf); gap-1(0) double mutant showing a multivulva (Muv) phenotype. Note the detachment and invagination of the P5.p and P7.p descendants, which is characteristic for a 2° to 1° cell fate transformation, and the ectopic differentiation of the P8.p descendants. (C) Expression of the 1° cell fate marker EGL-17∷GFP (green fluorescent protein) in a wild-type (WT) and (D) an ect-2(rf) L3 larva (Pn.p stage). (E) EGL-17∷GFP expression in an [hs∷mpk-1] and (F) an [hs∷mpk-1]; ect-2(zh8gf) L3 larva at the Pn.px stage. Note the ectopic EGL-17∷GFP expression in the P7.p and P8.p descendants, in (F). (G) Relative EGL-17∷GFP fluorescence intensity in P6.p of wild-type and ect-2(rf) L3 larvae (see Methods). (H) Percentage of animals at the Pn.px stage showing EGL-17∷GFP expression in P5.p and/or P7.p descendants in addition to the P6.p descendants. Where indicated, animals were treated with 40 mM hydroxyurea, as described (Ambros, 1999), and EGL-17∷GFP expression in the undivided Pn.p cells was scored 5 h later. Scale bars, 10 μm (B,F).
Table 1.
An ect-2 gain-of-function mutation activates the epidermal growth factor receptor/RAS/mitogen-activated protein kinase pathway upstream of SOS-1
| Row | Genotype | Vulval induction* | Vulval phenotype‡ | n |
|---|---|---|---|---|
| 1 | Wild type | 3.0 | WT | Many |
| 2 | Wild type, gonad ablated | 0.0 | 100% Vul | 22 |
| 3 | ect-2(zh8gf) | 3.0 | WT | 44 |
| 4 | ect-2(zh8gf), gonad ablated | 0.6 | 93% Vul | 27 |
| 5 | gap-1(0) | 3.0 | WT | 36 |
| 6 | ect-2(zh8gf)/+; gap-1(0) | 3.02 | 5% Muv | 65 |
| 7 | ect-2(zh8gf); gap-1(0) | 4.6 | 76% Muv | 17 |
| 8 | [hs∷mpk-1] | 3.0 | WT | 40 |
| 9 | ect-2(zh8gf)/+; [hs∷mpk-1] | 3.4 | 25% Muv | 57 |
| 10 | ect-2(zh8gf); [hs∷mpk-1] | 5.6 | 94% Muv | 35 |
| 11 | lin-3(rf) | 0.9 | 95% Vul | 20 |
| 12 | ect-2(zh8gf)/+; lin-3(rf) | 1.8 | 87% Vul | 47 |
| 13 | ect-2(zh8gf); lin-3(rf) | 2.9 | 22% Vul | 23 |
| 14 | sem-5(rf) | 1.5 | 76% Vul | 21 |
| 15 | ect-2(zh8gf); sem-5(rf) | 2.7 | 13% Vul | 30 |
| 16 | sos-1(0); let-60(gf) | 2.5 | 32% Vul | 31 |
| 17 | ect-2(zh8gf); sos-1(0); let-60(gf) | 2.7 | 26% Vul, 2% Muv | 40 |
| 18 | let-60(rf) | 0.0 | 100% Vul | 33 |
| 19 | ect-2(zh8gf); let-60(rf) | 0.2 | 94% Vul | 33 |
| 20 | mpk-1(0) | 0.0 | 100% Vul | 25 |
| 21 | ect-2(zh8gf); mpk-1(0) | 0.0 | 100% Vul | 93 |
*Average number of vulval precursor cells per animal that have adopted a 1° or 2° vulval fate scored under Nomarski optics.
‡Percent Vul, animals with fewer than three induced VPCs; percent Muv, animals with more than three induced VPCs. Alleles used: ect-2(zh8gf) II, cis-linked with unc-4(e120), mpk-1(ga117) III, cis-linked with unc-32(e189), lin-3(e1417) IV, cis-linked with unc-5(e51), let-60(n1876) IV, cis-linked with unc-22(s7), gaIs36[HS-mpk-1(+), EFα-D-mek(+), unc-30(+)] V, cis-linked with him-5(e1490), sos-1(s1031), cis-linked with unc-46(e177), sem-5(n2019) X, gap-1(ga133) X.
Sequence analysis of the ect-2 locus in zh8gf mutants identified a C-to-T transition in exon 6 at position +998 relative to the ATG start codon (supplementary figure online). ect-2 encodes a protein of 924 amino acids containing two breast cancer gene carboxy-terminal (BRCT) domains followed by a Dbl homology (DH) and a Plekstrin homology (PH) domain (Fig 1A). The zh8gf mutation substitutes the conserved Glu residue 225 in the second BRCT domain with a Lys. ect-2 is the closest C. elegans homologue of Drosophila pebble (Prokopenko et al, 1999) and the mammalian ect2 proto-oncogene (Miki et al, 1993). C. elegans ECT-2 shows 23% overall sequence identity and 53% similarity with human ECT2. Pebble and ECT2 are members of the Dbl family of GEFs for the Rho family of small GTPases (Schmidt & Hall, 2002). The residues that are necessary for the catalytic activity of DH domains in the α-helices α1 or α9 are all conserved in C. elegans ECT-2, which indicates that the protein could function as a GEF (Fig 1B, asterisks; Liu et al, 1998).
Figure 1.
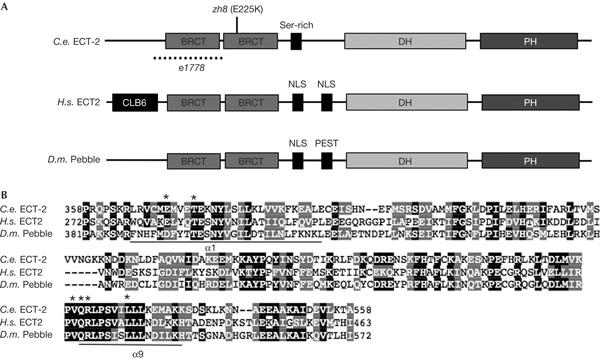
Molecular analysis of ect-2. (A) Domain structure of the ECT-2 protein compared with human ECT2 and Drosophila Pebble. The amino-acid change induced by zh8 and the location of the n1778 in-frame deletion are shown. PH, Plekstrin homology. (B) Sequence alignment of the Dbl homology (DH) domain of C. elegans (C.e.) ECT-2, human (H.s.) ECT2 and Drosophila (D.m.) Pebble. The asterisks indicate the conserved residues in the two α-helices (α1 or α9) that are necessary for the nucleotide exchange factor activity (Liu et al, 1998).
Thus, an activating mutation in the RHO GEF ect-2 causes excess vulval induction when combined with mutations that sensitize the EGFR/RAS/MAPK pathway.
Overexpression of ECT-2 causes hyperinduction
The Muv phenotype caused by the zh8gf mutation could be due to either an increase in normal ECT-2 function or a newly acquired (neomorphic) activity of ECT-2 that is created through the zh8gf mutation. The mutant ECT-2(zh8gf) protein might recognize a substrate that the wild-type protein does not accept. To distinguish between these two possibilities, we tested whether overexpression of wild-type ECT-2 is sufficient to cause excess vulval differentiation. For this purpose, transgenic lines carrying multiple copies of a genomic DNA fragment spanning the ect-2 locus were introduced into a wild-type and gap-1(0) background (supplementary figure online). All three independent lines that were tested showed a Muv phenotype in a gap-1(0) background, and two transgenic lines even showed a Muv phenotype in a gap-1(+) background (Table 2). Thus, increasing the dosage of wild-type ECT-2 causes excess vulval induction, which indicates that the zh8gf Muv phenotype is due to an increase in normal ECT-2 activity.
Table 2.
Overexpression of wild-type ect-2 causes excess vulval induction
| Genotype | Array | P3.p | P4.p | P5.p | P6.p | P7.p | P8.p* | Induction‡ | n |
|---|---|---|---|---|---|---|---|---|---|
| Wild type | None | 0 | 0 | 100 | 100 | 100 | 0 | 3.0 | Many |
| ect-2(gf); gap-1(0) | None | 38 | 59 | 100 | 100 | 100 | 62 | 4.6 | 17 |
| Wild type | zhEx44.1[ect-2(+)] | 9 | 19 | 100 | 100 | 100 | 9 | 3.4 | 43 |
| gap-1(0) | zhEx44.1[ect-2(+)] | 10 | 17 | 100 | 100 | 100 | 15 | 3.4 | 41 |
| Wild type | zhEx44.2[ect-2(+)] | 0 | 5 | 100 | 100 | 100 | 0 | 3.1 | 22 |
| gap-1(0) | zhEx44.2[ect-2(+)] | 7 | 27 | 100 | 100 | 100 | 13 | 3.5 | 15 |
| Wild type | zhEx44.3[ect-2(+)] | 0 | 0 | 100 | 100 | 100 | 0 | 3.0 | 21 |
| gap-1(0) | zhEx44.3[ect-2(+)] | 10 | 24 | 100 | 100 | 100 | 20 | 3.5 | 51 |
*Percent VPCs adopting 1° or 2° induced fates.
‡Average number of VPCs per animal that have adopted a 1° or 2° vulval fate scored under Nomarski optics. Alleles used: ect-2(zh8gf) II, gap-1(ga133) X, zhEx44.1 through zhEx44.3 are three independent lines.
ECT-2 promotes primary cell fate specification
Drosophila Pebble and mammalian ECT2 have an essential role in cytokinesis during the M-phase (Prokopenko et al, 1999; Saito et al, 2003). However, the specification of the 1° vulval cell fate in C. elegans occurs during the G1-phase of the VPC cell cycle (Ambros, 1999). We therefore tested whether ECT-2 controls cell fate specification before the M-phase, using the egl-17∷gfp reporter transgene as a molecular marker for the 1° fate (Burdine et al, 1998). For this purpose, we examined the ect-2(e1778) reduction-of-function mutation, which is caused by an in-frame deletion that removes the first BRCT domain but leaves the remaining open reading frame intact (Fig 1B). This allele has been identified in a screen for mutations that cause meiotic arrest, and shows the same germline phenotype as a null allele but only a weak P-cell migration defect (Omachi & Lambie, personal communication and our own observation; also, see below).
In wild-type L3 larvae, EGL-17∷GFP (green fluorescent protein) is expressed in P6.p and its 1° descendants (Burdine et al, 1998). In ect-2(e1778rf) larvae, we found two- to threefold lower levels of EGL-17∷GFP in P6.p when compared with the wild-type controls (Fig 2C,D,G). Conversely, in a gap-1(0) or an [hs∷mpk-1] sensitized background, ect-2(zh8gf) causes ectopic EGL-17∷GFP expression in other VPCs besides P6.p (Fig 2E,F,H). This ectopic expression of the 1° fate marker is accompanied by morphological changes of the vulva that are characteristic of a 2° to 1° cell fate transformation in P5.p and P7.p descendants (Fig 2A,B; Berset et al, 2001).
To test in which phase of the cell cycle ECT-2 promotes 1° cell fate specification, we examined the EGL-17∷GFP expression pattern in hydroxyurea-arrested ect-2(zh8gf) larvae. Treatment of L2 larvae with hydroxyurea blocks the VPC cell cycle in the S-phase, but it does not prevent 1° fate specification (Ambros, 1999). In arrested ect-2(zh8gf); gap-1(0) larvae, the frequency of ectopic EGL-17∷GFP expression is enhanced rather than reduced when compared with untreated ect-2(zh8gf); gap-1(0) larvae (Fig 2H). This slight enhancement of EGL-17∷GFP expression by hydroxyurea treatment could be due to the fact that the VPCs are exposed to the inductive LIN-3 EGF signal for a longer time than in untreated animals and thus accumulate higher levels of MAPK activity. However, it is important to note that in hydroxyurea-treated ect-2(zh8gf); gap-1(0) double mutants also, the frequency of ectopic EGL-17∷GFP expression is enhanced when compared with hydroxyurea-treated gap-1(0) single mutants (Fig 2H). Taken together, these experiments indicate that ECT-2 positively regulates 1° cell fate specification before exit from the S-phase, most probably during the G1-phase.
ECT-2 acts upstream of or at the level of SOS-1 GEF
The removal of the gonadal AC prevents the production of the inductive EGF signal and causes a completely penetrant Vul phenotype (Table 1, row 2; Kimble, 1981). However, in ect-2(zh8gf) animals that lack a gonad, partial vulval induction can be observed, which indicates that ECT-2 promotes vulval differentiation even in the absence of an inductive AC signal (Table 1, row 4).
To determine at which step ECT-2 interacts with the inductive signalling pathway, we combined ect-2(zh8gf) with mutations that reduce or eliminate the activity of the EGFR/RAS/MAPK pathway at different steps. ect-2(zh8gf) efficiently suppresses the Vul phenotype caused by rf mutations in lin-3 egf and sem-5, which encodes a GRB2 adaptor protein that transduces the signal between EGFR and RAS (Table 1, rows 11–15; Clark et al, 1992). In contrast, neither a null mutation in the RAS GEF sos-1 (Chang et al, 2000) nor in mpk-1 (Lackner & Kim, 1998) is suppressed by ect-2(zh8gf) (Table 1, rows 16,17,20, 21; to rescue embryonic lethality of sos-1(0), we carried out the epistasis test in a let-60 ras(gf) background, as described by Chang et al, 2000). Furthermore, the strong let-60 ras(e1678rf) mutation that causes a completely penetrant Vul phenotype is only slightly suppressed by ect-2(zh8gf) (Table 1, rows 18,19; Beitel et al, 1990).
We also tested whether reducing ect-2 function suppresses the Muv phenotype caused by hyperactivation of the RAS/MAPK pathway. As no VPCs are generated in ect-2 null mutants owing to an earlier requirement for ECT-2 during the ventral migration of the P cells that generate the VPCs (K. Morita and M. Han, personal communication), we examined the vulva in ect-2(e1778rf) mutants. Among the 21 ect-2(e1778rf) animals examined, we found two cases in which an abnormal vulva was formed, because one of the proximal VPC predecessors (P5, P6 or P7) had not migrated ventrally. In another three animals, all proximal Pn.p cells were present but some of them adopted a hypodermal 3° fate (that is, they divided once) instead of a 1° or 2° vulval cell fate. Considering only those cells that were present in ect-2(e1778) mutants, the average number of induced vulval cells per animal is 2.7 (Table 3, row 2). Thus, the Vul phenotype of ect-2(e1778) animals is partly caused by defects in vulval fate specification or execution. We then examined the effect of reducing ect-2 function on transgenic worms overexpressing LIN-3 EGF under the control of its own promoter [lin-3(+)] and on animals carrying a gain-of-function mutation in let-60 ras (Table 3, rows 3,5). Although both alleles cause a Muv phenotype of similar strength, ect-2(e1778rf) partially suppresses [lin-3(+)] but has no effect on the let-60 ras(gf) Muv phenotype (Table 3, rows 4,6,7). We conclude that ECT-2 positively regulates the EGFR/RAS/MAPK pathway at the level of or upstream of SOS-1 GEF.
Table 3.
ect-2 acts upstream of or in parallel with let-60 ras
| Row | Genotype | Vulval induction* | Vulval phenotype‡ | n |
|---|---|---|---|---|
| 1 | Wild type | 3.0 | WT | Many |
| 2 | ect-2(rf) | 2.7 | 19% Vul | 21 |
| 3 | zhEx68[lin-3(+)] | 4.4 | 86% Muv | 28 |
| 4 | ect-2(rf); zhEx68[lin-3(+)] | 3.6 | 44% Muv | 27 |
| 5 | let-60(gf) | 3.8 | 64% Muv | 30 |
| 6 | ect-2(rf)/+; let-60(gf) | 3.8 | 73% Muv | 26 |
| 7 | ect-2(rf); let-60(gf) | 3.9 | 81% Muv | 26 |
*Average number of VPCs per animal that have adopted a 1° or 2° vulval fate scored under Nomarski optics.
‡Percent Vul, animals with fewer than three induced VPCs; percent Muv, animals with more than three induced VPCs. Alleles used: ect-2(e1778) II, cis-linked with unc-4(e120) and balanced with mIn1, let-60(n1046gf) IV, zhEx68[lin-3(+), sur-5∷gfp].
ECT-2 regulates the RAS pathway through RHO-1
The results presented so far raised the possibility that ECT-2 regulates vulval induction by activating a RHO-1 signalling pathway, which feeds into the RAS/MAPK cascade. To test this model, we expressed a dominant-negative form of rho-1 under control of the heat-shock-inducible promoter (Spencer et al, 2001). This approach allowed us to selectively block RHO-1 signalling at a time point after the P cells had migrated to the ventral midline and the VPCs had been generated. Three independent hs∷rho-1(dn) transgenic lines were crossed to let-21(gf); gap-1(0) animals and tested for rescue of the Muv phenotype (Table 4). All three transgenes efficiently suppressed the let-21(gf); gap-1(0) Muv phenotype, causing distal VPCs to adopt the 3° cell fate when expression was induced by a single heat shock during the L2 stage (Table 4). In two lines, we even observed a weak Vul phenotype because proximal VPCs occasionally adopted the 3° rather than a 1° or 2° fate (Table 4, rows 4,6). Non-transgenic siblings that were subjected to the same heat shock as controls showed a slight reduction of the Muv phenotype, which was probably due to the heat shock (Table 4, rows 1,3,5).
Table 4.
Expression of dominant-negative rho-1 suppresses the ect-2(gf) Muv phenotype
| Row | Genotype | Transgene | Vulval induction* | Vulval phenotype‡ | n |
|---|---|---|---|---|---|
| 1 | ect-2(gf); gap-1(0) | None | 3.9 | 55% Muv | 22 |
| 2 | ect-2(gf); gap-1(0) | zhEx167.1[hs∷rho-1(dn)] | 3.0 | 4% Muv | 26 |
| 3 | ect-2(gf); gap-1(0) | None | 3.8 | 50% Muv | 20 |
| 4 | ect-2(gf); gap-1(0) | zhEx167.2[hs∷rho-1(dn)] | 2.8 | 5% Muv, 15% Vul | 19 |
| 5 | ect-2(gf); gap-1(0) | None | 4.2 | 77% Muv | 26 |
| 6 | ect-2(gf); gap-1(0) | zhEx167.3[hs∷rho-1(dn)] | 2.9 | 9% Muv, 6% Vul | 32 |
To induce rho-1(dn) expression, synchronized populations of L2 larvae were heat shocked once for 30 min at 33°C and scored as L4s.
*Average number of VPCs per animal that have adopted a 1° or 2° vulval fate scored under Nomarski optics. In all cases shown, the uninduced VPCs adopted a 3° cell fate (that is, they divided once).
‡Percent Vul, animals with fewer than three induced VPCs; percent Muv, animals with more than three induced VPCs. Alleles used: ect-2(zh8) II, cis-linked with unc-4(e120), gap-1(ga133) X, zhEx167.1[hs∷rho-1(dn), sur-5∷gfp] through zhEx167.3 are independent lines.
Thus, ECT-2 regulates the RAS/MAPK pathway by activating RHO-1 signalling.
Discussion
We have isolated a gain-of-function mutation in the C. elegans ect-2 gene, which encodes the homologue of the mammalian GEF ECT2 (Miki et al, 1993). Like most other Dbl family GEFs, mammalian ECT2 shows transforming activity when truncated at the N terminus, and its transforming potential has been correlated with hyperactivation of the GEF activity (Saito et al, 2003). It was therefore proposed that the N-terminal region of ECT2 inhibits the GEF activity of the DH domain. The second BRCT domain may interact with the catalytic centre of the DH domain and thereby prevent substrate binding (Fig 3A; Kim et al, 2005). On activation, for example by phosphorylation of the DH domain, the protein assumes an open conformation that allows it to bind to its substrates (Schmidt & Hall, 2002). The ect-2(zh8gf) mutation exchanges a conserved, negatively charged Glu with a positively charged Lys residue in the second BRCT domain, which may cause ECT-2 to remain in an open, constitutively active conformation and thus have a similar effect as the N-terminal truncations found in the oncogenic variants of mammalian ECT2 (Fig 3A).
Figure 3.
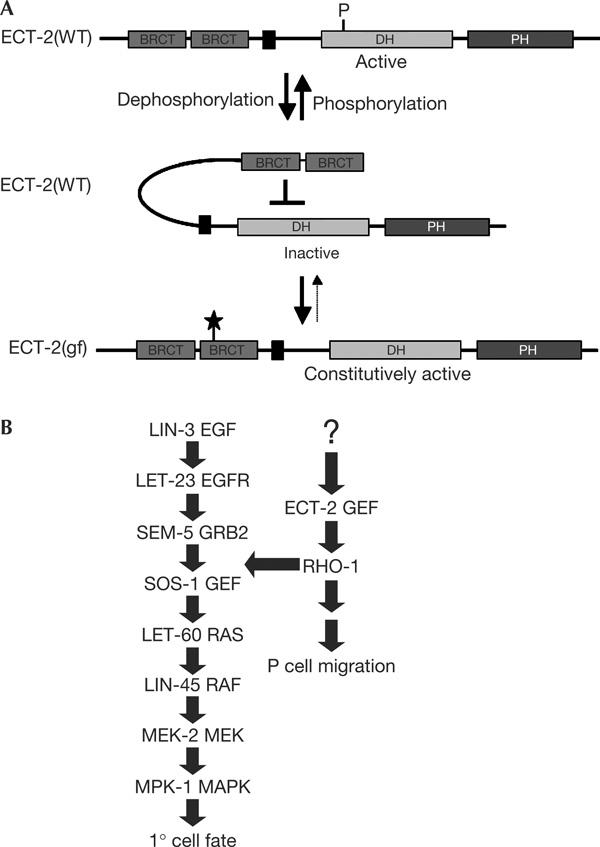
Models for ECT-2 activation and its role in activating the EGFR/RAS/MAPK pathway. (A) Model for the autoinhibitory function of the second BRCT domain and the effect of the zh8gf mutation (symbolized by a star). (B) Crosstalk between ECT-2 and the RAS/MAPK pathway. EGF, epidermal growth factor; EGFR, EGF receptor; GEF, guanine nucleotide exchange factor; MAPK, mitogen-activated protein kinase; MEK, mitogen-activated protein kinase kinase.
The Drosophila and mammalian ECT-2 homologues have an essential role in activating RHO signalling during the M-phase (Prokopenko et al, 1999; Saito et al, 2003). More recently, Drosophila Pebble was also shown to be required during interphase for mesodermal cell migrations in the embryo (Schumacher et al, 2004; Smallhorn et al, 2004). Here, we report that ECT-2 regulates 1° vulval cell fate specification, which involves a RAS/MAPK pathway and takes place during the G1-phase (Ambros, 1999). By epistasis analysis, we show that ECT-2 feeds into the canonical RAS/MAPK pathway upstream of or at the level of the SOS-1 RAS GEF. Moreover, the stimulatory effect of ECT-2(gf) on 1° vulval cell fate specification can be suppressed by a dominant-negative form of RHO-1. As we have not determined the cellular focus of ect-2 function, it is in principle possible that ECT-2 regulates vulval induction from the tissues surrounding the VPCs. However, in view of the genetic data, a cell-autonomous function of ect-2 in the VPCs seems more likely. Taken together, these results indicate that ECT-2 activates a RHO-1 signalling pathway that not only controls P-cell migration during early larval development (Spencer et al, 2001) but also regulates RAS/MAPK signalling later during vulval induction (Fig 3B).
Both positive and negative crosstalks between the RHO and RAS pathways have been observed in several cell types, but, in many cases, the molecular mechanisms connecting the two pathways are unclear (Malliri & Collard, 2003). Our results raise the intriguing possibility that the transforming activity of the mammalian Dbl family of GEFs is at least in part due to hyperactivation of the RAS/MAPK pathway. Small deletions that cause N-terminal truncations and result in the oncogenic activation of a Dbl family GEF are rather unlikely to occur spontaneously in human tumours. However, point mutations similar to the zh8gf mutation that we have isolated in C. elegans ect-2 could be more common.
Methods
General Caenorhabditis elegans methods. C. elegans strains were maintained as described (Brenner, 1974) and grown at 20°C. Wild type refers to C. elegans variety Bristol, strain N2. The induction of the VPCs was scored under Nomarski optics and the average number of 1° or 2° induced VPCs per animal was calculated as described (Dutt et al, 2004). Animals were mounted on 3% agarose slides in M9 buffer containing 20 mM NaN3. Larvae were first inspected using Nomarski optics to identify the position of the Pn.p cells or their descendants, and GFP expression was then scored under fluorescent light illumination using a Leica DM RA microscope equipped with a Hamamatsu Orca ER camera using Openlab 3.1 (Improvision, Coventry, UK) software. For quantification of the fluorescence intensity shown in Fig 2G, images of synchronized early L3 larvae (Pn.p stage) were recorded under standardized illumination and recording conditions with non-saturating exposure settings, and the average fluorescence intensity in the entire P6.p cell was determined using the Openlab 3.1 measurement tool. The same contrast settings were applied to all fluorescent images shown in Fig 2. AC ablations were carried out by removing the precursors of the somatic gonad Z1 and Z4 with a laser microbeam at the L1 stage, as described (Kimble, 1981).
For more details on alleles used, positional cloning, hydroxyurea treatment and transgenesis, see the supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplementary figure
Acknowledgments
We thank all lab members for stimulating discussions, G. Battu and M. Hengartner for comments on the manuscript, J. Ahringer for RNA interference clones and T. Schedl and the Caenorhabditis elegans Genetics Center for providing some of the strains used. This work was supported by a grant from the Swiss National Science Foundation to A.H. and by the Kanton Zürich.
References
- Ambros V (1999) Cell cycle-dependent sequencing of cell fate decisions in Caenorhabditis elegans vulva precursor cells. Development 126: 1947–1956 [DOI] [PubMed] [Google Scholar]
- Beitel GJ, Clark SG, Horvitz HR (1990) Caenorhabditis elegans ras gene let-60 acts as a switch in the pathway of vulval induction. Nature 348: 503–509 [DOI] [PubMed] [Google Scholar]
- Berset T, Hoier EF, Battu G, Canevascini S, Hajnal A (2001) Notch inhibition of RAS signaling through MAP kinase phosphatase LIP-1 during C. elegans vulval development. Science 291: 1055–1058 [DOI] [PubMed] [Google Scholar]
- Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdine RD, Branda CS, Stern MJ (1998) EGL-17(FGF) expression coordinates the attraction of the migrating sex myoblasts with vulval induction in C. elegans. Development 125: 1083–1093 [DOI] [PubMed] [Google Scholar]
- Burridge K, Wennerberg K (2004) Rho and Rac take center stage. Cell 116: 167–179 [DOI] [PubMed] [Google Scholar]
- Chang C, Hopper NA, Sternberg PW (2000) Caenorhabditis elegans SOS-1 is necessary for multiple RAS-mediated developmental signals. EMBO J 19: 3283–3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SG, Stern MJ, Horvitz HR (1992) C. elegans cell-signalling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature 356: 340–344 [DOI] [PubMed] [Google Scholar]
- Dutt A, Canevascini S, Froehli-Hoier E, Hajnal A (2004) EGF signal propagation during C. elegans vulval development mediated by ROM-1 rhomboid. PLoS Biol 2: 1799–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajnal A, Whitfield CW, Kim SK (1997) Inhibition of Caenorhabditis elegans vulval induction by gap-1 and by let-23 receptor tyrosine kinase. Genes Dev 11: 2715–2728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Billadeau DD, Chen J (2005) The tandem BRCT domains of Ect2 are required for both negative and positive regulation of Ect2 in cytokinesis. J Biol Chem 280: 5733–5739 [DOI] [PubMed] [Google Scholar]
- Kimble J (1981) Alterations in cell lineage following laser ablation of cells in the somatic gonad of Caenorhabditis elegans. Dev Biol 87: 286–300 [DOI] [PubMed] [Google Scholar]
- Lackner MR, Kim SK (1998) Genetic analysis of the Caenorhabditis elegans MAP kinase gene mpk-1. Genetics 150: 103–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X et al. (1998) NMR structure and mutagenesis of the N-terminal Dbl homology domain of the nucleotide exchange factor Trio. Cell 95: 269–277 [DOI] [PubMed] [Google Scholar]
- Malliri A, Collard JG (2003) Role of Rho-family proteins in cell adhesion and cancer. Curr Opin Cell Biol 15: 583–589 [DOI] [PubMed] [Google Scholar]
- Miki T, Smith CL, Long JE, Eva A, Fleming TP (1993) Oncogene ect2 is related to regulators of small GTP-binding proteins. Nature 362: 462–465 [DOI] [PubMed] [Google Scholar]
- Prokopenko SN, Brumby A, O'Keefe L, Prior L, He Y, Saint R, Bellen HJ (1999) A putative exchange factor for Rho1 GTPase is required for initiation of cytokinesis in Drosophila. Genes Dev 13: 2301–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito S, Tatsumoto T, Lorenzi MV, Chedid M, Kapoor V, Sakata H, Rubin J, Miki T (2003) Rho exchange factor ECT2 is induced by growth factors and regulates cytokinesis through the N-terminal cell cycle regulator-related domains. J Cell Biochem 90: 819–836 [DOI] [PubMed] [Google Scholar]
- Schmidt A, Hall A (2002) Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev 16: 1587–1609 [DOI] [PubMed] [Google Scholar]
- Schumacher S, Gryzik T, Tannebaum S, Muller HA (2004) The RhoGEF Pebble is required for cell shape changes during cell migration triggered by the Drosophila FGF receptor Heartless. Development 131: 2631–2640 [DOI] [PubMed] [Google Scholar]
- Smallhorn M, Murray MJ, Saint R (2004) The epithelial–mesenchymal transition of the Drosophila mesoderm requires the Rho GTP exchange factor Pebble. Development 131: 2641–2651 [DOI] [PubMed] [Google Scholar]
- Spencer AG, Orita S, Malone CJ, Han M (2001) A RHO GTPase-mediated pathway is required during P cell migration in Caenorhabditis elegans. Proc Natl Acad Sci USA 98: 13132–13137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg PW, Han M (1998) Genetics of RAS signaling in C. elegans. Trends Genet 14: 466–472 [DOI] [PubMed] [Google Scholar]
- Sternberg PW, Horvitz HR (1989) The combined action of two intercellular signaling pathways specifies three cell fates during vulval induction in C. elegans. Cell 58: 679–693 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure


