Summary
Workshop on Notch Signalling in Development and Cancer
Keywords: Notch signalling, development, cancer, haematopoiesis, differentiation
As the white smoke cleared on one bank of the Tiber, a conclave of a different sort began on the opposite side of the river. Such was the setting of this EMBO workshop, which was the first large international meeting exclusively devoted to Notch signalling. Appropriately, given the timing and location of the meeting, the keynote lecturer S. Artavamis-Tsakonas (Charlestown, MA, USA) was nicknamed the Notch ‘Pope' by one anonymous student. This meeting brought together a diverse group of researchers whose interests span invertebrate and vertebrate development, structural biology, biochemistry, cell signalling, immunology and tumour biology. Despite the diversity, a common language emerged during the meeting, which allowed Notch signalling to be addressed from several vantage points. In this report, after a brief introduction to the Notch signalling pathway, we summarize the presentations that covered different aspects of Notch signalling, including the pathway itself, development, haematopoiesis and cancer.

The EMBO Workshop on Notch Signalling in Development and Cancer was held between 22 and 25 April 2005 in Rome, Italy, and was organized by I. Screpanti, B. Osborne, L. Miele, S. Krishna and U. Lendahl.
Notch signalling occurs as a result of cell-to-cell contact through the interactions of Notch receptors and their DSL (Delta and Serrate for Drososphila and LAG-2 for Caenorhabditis elegans) ligands. Mammals have four Notch receptors (Notch1–Notch4), and five DSL ligands (Jagged1 and Jagged2 (homologues of Serrate) and Delta-like 1 (Dll1), Dll3 and Dll4 (homologues of Delta); Fig 1). Ligand–receptor engagement results in two successive proteolytic cleavages in Notch. The first cleavage occurs extracellularly, close to the transmembrane (TM) domain and is mediated by the metalloprotease tumour necrosis factor-α-converting enzyme (TACE). The extracellular domain that is cleaved is then ‘transendocytosed' by the ligand-expressing neighbouring cell. The second cleavage occurs in the TM domain and is mediated by a multiprotein complex that consists of presenilins, nicastrin, APH1 and PEN2 proteins and has γ-secretase activity (Fortini, 2002). This final cleavage allows the translocation of the cytoplasmic domain of the Notch receptor (NICD) into the nucleus, where it binds to its downstream transcription factor CSL (CBF1 in humans, Suppressor of Hairless in Drosophila and LAG in C. elegans; also known as RBP-J in the mouse) and thus activates transcription (Fig 2).
Figure 1.
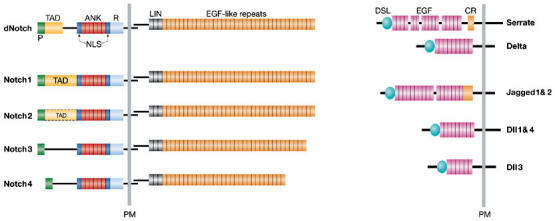
Notch receptors and their ligands. Drosophila contains one Notch receptor (dNotch) that is bound by two transmembrane DSL-ligands (Delta and Serrate). Mammalians possess four Notch receptors (Notch1–4) and five ligands (Jagged1 and 2, which are homologous to Serrate, and Delta-like (Dll) 1, 3 and 4, which are homologous to Delta). Notch receptors are expressed on the cell surface as heterodimeric proteins. Their extracellular portion contains 29–36 epidermal growth factor (EGF)-like repeats that are associated with ligand binding, followed by three cysteine-rich LIN repeats that prevent ligand-independent signalling, and a heterodimerization domain. The intracellular portion of the receptor harbours two protein interaction domains, the RAM domain (R) and six ankyrin repeats (ANK), two nuclear localizations signals (NLS) and a transactivation domain (TAD, which has not yet been defined for Notch3 and 4), and a PEST (P) sequence. Notch ligands are also expressed as membrane-bound proteins. They all contain an amino-terminal DSL domain (Delta, Serrate and Lag2) followed by EGF-like repeats. Ligands of the Serrate family also harbour a cysteine-rich (CR) domain downstream of the EGF-like repeats. PM, plasma membrane.
Figure 2.

Notch signalling. Notch receptors are synthesized as single precursor proteins that are cleaved in the Golgi by a Furin-like convertase during their transport to the cell surface where they are expressed as heterodimers. Fringe glycosyltransferases modify EGF-like repeats by adding N-acetylglucosamine within the Golgi. Notch signalling is initiated after ligand-receptor interaction, which induces two sequential proteolytic cleavages. The first cleavage within the extracellular domain is mediated by the metalloprotease TACE (tumor necrosis factor α-converting enzyme). The cleaved extracellular subunit of the receptor is ‘trans-endocytosed' by the neighbouring ligand-expressing cell. This process seems to be controlled by Neuralized and/or Mindbomb E3 ubiqutin ligases. The second cleavage occurs within the transmembrane domain and is mediated by the γ-secretase activity of the multi-protein complex of presenilins (PS), which includes Nicastrin, APH-1 and PEN-2. The liberated intracellular domain of Notch (NICD) translocates into the nucleus and binds to the transcription factor CSL (CBF1 in humans, Supressor of Hairless in Drosophila and LAG in C. elegans). This interaction leads to transcriptional activation by displacement of corepressors (CoR) and simultaneous recruitment of coactivators (CoA), including mastermind-like proteins (MAML1). Receptors modified by Fringe glycosyltransferases cannot mediate signalling via Jagged ligands, whereas Delta-mediated Notch signalling is still possible.
Notch signalling
The endocytosis of Notch and its ligands has emerged recently as a key mechanism in the regulation of Notch signalling (Le Borgne et al, 2005a). Several talks at this meeting gave a flavour of the fruitful convergence of genetic approaches in the fly with biochemical approaches in mammalian cells for the study of membrane transport in Notch signalling. With respect to the ligand, F. Schweisguth (Paris, France) presented recently published data on the role of the Mind bomb (D-mib) and Neuralized (Neur) E3 ubiquitin ligases in Drosophila. Genetic analysis revealed that the two genes encoding these ligases are required for distinct subsets of Notch signalling events during Drosophila development. In addition, they are involved in the regulation of the endocytosis of DSL ligands, which is essential for Notch receptor activation (Le Borgne et al, 2005b). In particular, D-mib is required for Serrate (Ser) signalling at the dorsal–ventral boundary of the wing. During wing development, Ser acts in trans to activate Notch and also inhibits Notch when present at high concentrations in the same cell. S. Bray (Cambridge, UK) reported data from a structure–function analysis of the intracellular tail of Ser, which identified a region that is essential for cis inhibition. This analysis indicated further that cis inhibition occurs through interactions at the cell surface and that the activating and inhibitory activities of Ser have different sensitivities to the glycosyltransferase Fringe.
With regard to receptors, C. Brou (Paris, France) has used biochemical and cell-culture assays to study the processing of a constitutively active Notch derivative, NotchDE, which mimics the structure of the TACE-processed product. These analyses showed that monoubiquitylation and clathrin-dependent endocytosis of NotchDE is required for γ-secretase processing. This suggests that monoubiquitylation and endocytosis of Notch are a prerequisite for its presenilin-dependent cleavage (Gupta-Rossi et al, 2004). Data suggesting that similar events are also necessary for the γ-secretase cleavage of Delta 1 and the amyloid precursor protein (APP) were also presented. A different view was offered by E. Hansson (Stockholm, Sweden) who presented work on the assembly of a functional γ-secretase complex. These data support a model in which nicastrin and APH1 initially form a subcomplex in the endoplasmic reticulum, and presenilin and PEN2 are subsequently recruited to generate a functional γ-secretase complex. This complex has been shown to interact with Notch early in the secretory pathway as well as on the cell surface (Hansson et al, 2005).
Notch endocytosis is thought to be regulated negatively by Numb, a key Notch regulator that acts as a cell-fate determinant during asymmetrical cell division (Betschinger, 2004). U. Lendahl (Stockholm, Sweden) reported data confirming that Numb antagonizes Notch signalling. Specifically, Numb and Numb-like inhibit Notch signalling when expressed at low levels in mouse myoblast C2C12 cells. However, high levels of Notch activation in C2C12 cells were found, unexpectedly, to regulate negatively the amount of Numb and Numb-like proteins in a proteasome-dependent manner. The PEST domain of Numb-like is required for this Notch-mediated degradation, suggesting a model in which Numb and Notch have reciprocal antagonistic activities.
Cell-fate specification of the peripheral nervous system in Drosophila is regulated through asymmetrical cell division. Numb and α-adaptin segregate together into one of two daughter cells during asymmetrical cell division, which creates different levels of Notch signalling in the two cells. J. Knoblich (Vienna, Austria) showed that the asymmetrical localization of Numb and α-adaptin is regulated by a protein complex that contains atypical protein kinase C (aPKC) and its substrate Lethal(2) giant larvae (Lgl). Knoblich also reported new data revealing that at least one other endocytic protein, Rab11, localizes differently between the two daughter cells. However, differences in its intracellular localization seem to be independent of aPKC and Lgl. This suggests that another pathway also establishes asymmetry during cell division (Emery et al, 2005).
Notch and development
The list of developmental processes that require Notch signalling is increasing to such an extent that it is difficult to name a tissue or a developing organ that does not depend on Notch function at one stage or another. Developmental aspects of Notch function were discussed by several participants at the meeting. T. Gridley (Bar Harbor, ME, USA) presented work on the function of Notch signalling during differentiation and patterning of the inner ear. The mammalian auditory sensory epithelium, known as the organ of Corti or cochlea, contains sensory hair cells and non-sensory support cells arranged in a highly patterned mosaic. Notch-mediated lateral inhibition is the proposed mechanism for patterning this mosaicism between sensory hair cells and supporting cells. The principle of lateral inhibition is that cells in the process of adopting one particular cell fate express Notch ligands on their surface, which activate Notch receptors on neighbouring cells and thereby prevent them from adopting the same cell fate. Gridley reported that the ligands Dll1 and Jagged2 synergistically regulate sensory-hair-cell differentiation in the inner ear. Supernumerary hair cells in the cochlea of Dll1/Jagged2 double mutants arise due to the inability of mutant cells to deliver a lateral inhibitory signal. In addition, supporting cells in the cochlea show a prolonged cell division cycle, indicating that the Notch pathway has a dual role in regulating cellular differentiation and patterning in the cochlea through lateral inhibition and the control of cellular proliferation (Kiernan et al, 2005).
Vertebrate somitogenesis is another process that requires Notch function. Somites are clusters of mesodermal cells that give rise to segmental body structures such as vertebrae, spinal nerves, striated muscles and blood vessels during embryonic development. R. Kopan (St Louis, MI, USA) presented recently published data on presenilin activity during mouse somitogenesis (Huppert et al, 2005). He showed that activated Notch (NICD) is produced cyclically during somite formation and established that somitogenesis requires less NICD than any other tissue in early-stage mouse embryos. In contrast to mice that lack all of the presenilin alleles and that have no somites, the formation of anterior somites proceeds normally in Notch1:Notch2-deficient embryos. As nicastrin-deficient embryos (which lack γ-secretase activity) have anterior somites, presenilin might have a γ-secretase-independent role in somitogenesis.
Notch signalling is also crucially involved in cardiac development (Grego-Bessa et al, 2004) as reported by J. Luis de la Pompa (Madrid, Spain). During this process, NICD can be detected in the endocardium, which is a specialized endothelium crucial for cardiac valve and trabeculae formation. Using Notch-deficient mouse mutants as well as Notch gain- and loss-of-function experiments in zebrafish, de la Pompa showed that in pre-valve territory, Notch promotes the epithelial–mesenchyme transition (EMT) that will form the valve primordium through the transcriptional induction of the transcription factor Snail (a well-known inducer of the EMT). In the ventricles, Notch mediates the inductive and proliferative signals between the endocardium and myocardium that promote trabecullar formation. Thus, Notch establishes a field of competence of endocardial cells, which enables them to respond to developmental cues.
O. Basak (Freiburg, Germany) is studying CSL-dependent Notch1 function in the developing central nervous system (CNS) of the mouse. He presented data on the generation of a reporter mouse that expresses the green fluorescent protein (GFP) specifically in the nervous system under the control of a promoter of an endogenous Notch1-target gene. This mouse enables the identification and isolation of multipotent neural stem cells (NSCs) from the developing nervous system. Another interesting reporter mouse was presented by M. Vooijs (Utrecht, The Netherlands), which he developed while working in the Kopan laboratory. This reporter mouse marks the lineage of cells once they have received a Notch1-specific signal. Additional work on the developing nervous system was reported by D. Yabe (Kyoto, Japan) who presented work on the Cre–loxP-mediated inactivation of the Msx2-interacting nuclear target (Mint) gene. Mint competes with NICD for binding to CSL and thereby negatively regulates Notch signalling. Conditional inactivation of Mint in the CNS results in increased neuronal progenitors and reduced post-mitotic neurons. This suggests that Mint deficiency leads to delayed neurogenesis in the developing brain, which is in striking contrast to the premature neuronal differentiation seen in mice that lack Notch activity. L. Bally-Cuif (Munich, Germany) reported on the role of E(Spl) factors in the definition and maintenance of neuronal progenitor pools at the zebrafish midbrain–hindbrain boundary (Ninkovic et al, 2005). During development, neuronal differentiation is actively inhibited by the two E(Spl) factors Her5 and Him/Her11. Although it is commonly believed that E(Spl) genes are target genes of Notch, it is interesting to note that this process appears to be independent of Notch signalling and might involve the direct downregulation of expression of proneural and cell-cycle inhibitor genes.
In addition to regulating developmental processes during embryogenesis, Notch has also been reported to have important roles in self-renewing systems of adult animals. Most of the self-renewing organs can be subdivided into three compartments: stem cells, transient amplifying cells and terminally differentiated cells. The interplay and correct regulation of these three compartments is essential for homeostasis and self-renewal of such tissues. G.-P. Dotto (Lausanne, Switzerland) reported on the integration of Notch and calcineurin/nuclear factor of activated T cells (NFAT) signalling during keratinocyte growth and differentiation control (Mammucari et al, 2005). Both the Notch and calcineurin/NFAT pathways are implicated in the control of keratinocyte differentiation. Induction of the p21 (also known as p21/WAF1/Cip1) gene by Notch1 activation in differentiating keratinocytes is associated with direct targeting of the CSL protein to the p21 promoter. In addition, Dotto showed that Notch1 activation also functions through a second calcineurin-dependent mechanism that acts on the p21 TATA box proximal region. Increased calcineurin/NFAT activity by Notch signalling involves downregulation of calcipressin (an endogenous calcineurin inhibitor) through a HES1 (homologue of Drosophila Hairy/Enhancer-of-Split, a downstream target of Notch signalling)-dependent mechanism. Besides control of the p21 gene, calcineurin contributes significantly to the transcriptional response of keratinocytes to Notch1 activation, both in vitro and in vivo. In addition, deletion of the calcineurin B1 gene in the skin results in a cyclic alopecia phenotype, which is associated with altered expression of the Notch-responsive genes that are involved in hair-follicle structure and/or adhesion to the surrounding mesenchyme.
The intestine is another self-renewing system that has a unique topology consisting of finger-like protrusions, known as villi, and invaginations into the submucosa, known as the crypts of Lieberkühn. Stem cells and proliferating transiently amplifying cells are located in the crypts, whereas terminally differentiated cells are mostly found in villi with the exception of paneth cells, which reside at the bottom of the crypts. F. Radtke (Lausanne, Switzerland) reported that inducible abrogation of Notch signalling in the intestine results in the conversion of undifferentiated proliferative crypt cells into post-mitotic mucus-secreting goblet cells (van Es et al, 2005). Gain-of-function studies for Notch in the intestine were reported by Artavanis-Tsakonas, who showed that forced Notch signalling in the intestine results in undifferentiated cells only (Fre et al, 2005). These complementary loss- and gain-of-function studies suggest that Notch signalling is a gatekeeper of the progenitor/stem-cell compartment of the gut.
Notch and haematopoiesis
The haematopoietic compartment is probably the best-studied self-renewing system. A. Robert-Moreno (from A. Bigas' laboratory, Madrid, Spain) showed that Notch signalling is involved in the early events of embryonic haematopoietic determination in the aorta endothelium of the aorta gonade mesonephros (AGM) region by controlling GATA2 expression (Robert-Moreno et al, 2005). Furthermore, the few cells that line the aorta endothelium in the AGM region at embryonic days 9.5–10.5 simultaneously express Notch1 and GATA2; Notch1 associates physically with the GATA2 promoter in wild-type embryos but not in CSL mutants.
The regulatory mechanism that underlies stem-cell maintenance during adult haematopoiesis is an important question under investigation. Whether Notch has a physiological role during this process is unresolved at present. However, it seems clear that certain molecules of the Notch signalling pathway, such as the Notch ligands, can be used as tools for the in vitro expansion of either mouse or human haematopoietic progenitors. In this context, I. Bernstein (Seattle, WA, USA) reported on studies using an engineered form of the Notch ligand Delta1ext-IgG in cultures with murine bone marrow Lin−Sca1+c-Kit+ (LSK) cells, which include long-term haematopoietic stem-cell precursors (Dallas et al, 2005). A multi-log increase in the number of Sca1+c-Kit+ precursors with short-term lymphoid- and myeloid-repopulating ability was observed in such co-culture systems. This increase in precursor number was associated with the sustained expression of stem-cell-associated genes, including GATA2, Tal1/SCL, Tie2, Nestin, Tcf3, c-mpl and the Polycomb group genes Bmi1 and Phc1. Furthermore, RNA interference (RNAi) studies showed that Bmi1 is required for Notch-induced self-renewal. To address whether quantitative aspects of ligand-induced activation of Notch signalling might influence lineage choices, LSK cells were cultured with increasing densities of Delta1ext-IgG. The results show that relatively higher densities of Delta1ext-IgG inhibited myeloid differentiation, enhanced the generation of LSK cells and promoted early T-cell differentiation. By contrast, lower densities of Delta1ext-IgG enhanced the generation of LSK cells and unexpectedly promoted the generation of early B-cell precursors, thereby indicating density-dependent effects of Delta1 on fate decisions. Ongoing studies suggest that these effects might result from the differential activation of Notch target genes. In addition, Bernstein proposed that the density of Notch ligands also influences the fate determination of human cord blood cells. Enhanced generation of CD34+ precursors that are able to repopulate the non-obese diabetic/severe combined immunodeficiency (NOD/SCID) mouse was found after their culture with lower densities of Delta1ext-IgG. Conversely, increased amounts of Delta1ext-IgG induced the apoptosis of CD34+ precursors, resulting in decreased cell numbers. Furthermore, lower densities enhanced the generation of CD34+ as well as CD14+ myeloid cells and CD7+ T-cell precursors, whereas higher densities inhibited the generation of CD14+ cells but did not affect the generation of CD7+ cells. These studies suggest that the density of Delta1 found in different anatomic regions might be important in vivo for determining cell-fate outcomes, and that the density of Delta1 might be crucial ex vivo when manipulating stem cells for therapeutic purposes.
H. Neves (Lisbon, Portugal) showed that Delta1- and Jagged1- expressing stromal cells have distinct effects on the clonogenic and differentiation capacities of committed human CD34+CD38+ cells. Jagged1 increases the number of bi-potent (CFU-GM; colony forming units-granulocyte macrophage) and uni-potent (CFU-G and CFU-M) progenitors without quantitatively affecting terminal cell differentiation, whereas Delta1 reduces the number of CFU-GM and differentiated monocytic cells. Gene expression analyses of the different CD34+CD38+ cell subpopulations that arise on contact with Jagged1 or Delta1 revealed that they have distinct transcription profiles for genes related to cell-lineage-affiliation programmes and to Notch-signalling itself.
N. Carlesso (Boston, MA, USA) reported on the molecular mechanisms by which Notch1 signalling can inhibit the differentiation of human bone-marrow progenitors. She showed that Notch1 activation directly modulates cell-cycle progression by promoting the transcription of SKP2—the F-box subunit of the ubiquitin-ligase complex SCFSKP2, which targets proteins for degradation (Sarmento et al, 2005). Upregulation of SKP2 by Notch results in enhanced proteasome-mediated degradation of the cyclin-dependent kinase inhibitors (CDKIs) p27Kip1 and p21Cip1 and causes premature S-phase entry. Accordingly, silencing of SKP2 by RNAi in G1 stabilized p27Kip1 and p21Cip1 and abolished Notch effects on G1–S progression. This process is independent of cell proliferation or cellular transformation, but does alter the relative time that cells spend in G1—the temporal window when differentiation decisions are made—and cell differentiation. Carlesso suggested that the Notch/SKP2/p27Kip1 pathway represents a new molecular mechanism by which Notch signalling fine-tunes the proliferation–differentiation balance during the physiological response to stress. Together with additional genetic and epigenetic alterations, the ability of Notch to modulate a key cell-cycle regulator such as SKP2 might prove to be crucial for the acquisition of a malignant phenotype.
T-cell development is a process in which Notch influences many events during the development of a single lineage. W. Pear (Philadelphia, PA, USA) described the use of a dominant-negative Mastermind allele to inhibit Notch signalling. This truncated form of Mastermind-like 1 (DN MAML1) binds to the CSL:NICD complex in the nucleus but is unable to recruit transcriptional co-activators, thus creating a complex that is incapable of signalling. This construct should prevent transcriptional activation by Notch but should not affect the CSL transcriptional repression complex. Pear presented data showing that DN MAML1 inhibits signalling by all four Notch receptors. When DN MAML1 was expressed in haematopoietic stem cells, the earliest steps of T-cell commitment were completely blocked. This is a stage of development when the cell is known as the early T-lineage progenitor (ETP), and is the most robust T-cell progenitor in the adult. This suggests that Notch signalling, presumably Notch1, is required to generate ETPs (Sambandam et al, 2005). Whether this occurs in the thymus and whether this cell is a multipotent T-cell:B-cell progenitor were questions addressed by C. Bleul (Freiburg, Germany). He used a mouse that had a GFP knock-in to the CCR9 locus and OP9 Delta cultures to show that T-cell commitment probably occurs in the thymus from a progenitor that can give rise to T and B cells at the clonal level (Benz & Bleul, 2005). After commitment to the T-cell lineage, Notch is also required for further differentiation of immature thymocytes. J. Zuniga-Pflucker (Toronto, Canada) showed that Notch signalling is required for both proliferation and differentiation at the stage of development known as β-selection, during which an immature T-cell receptor is expressed at the cell surface. Using OP9 cultures, Zuniga-Pflucker showed that cells lacking Notch signalling that are poised to undergo β-selection do not express phosphorylated AKT (a serine-threonine kinase, also known as protein kinase B). He also showed that ectopic expression of myristoylated AKT in these cells rescues both Notch-dependent proliferation and differentiation (Ciofani & Zuniga-Pflucker, 2005). How Notch influences AKT is not known. Many functions for Notch have also been described in peripheral T cells. T-helper cells are classified as either Th1 or Th2 according to their ability to secrete interferon-γ or interleukin-4, respectively. These two subclasses arise from a common precursor. B. Osborne (Amherst, MA, USA) presented data showing that Notch signalling is required for Th1 but not Th2 differentiation (Minter et al, 2005). By contrast, Pear presented data showing that Notch signalling is required for Th2 but not Th1 differentiation. These opposing results could reflect the modes of Notch inhibition used and suggest that additional work needs to be done to clarify the role of Notch in T-helper-cell development.
Notch and cancer
The failure to regulate Notch signalling precisely during T-cell development can lead to T-cell leukaemia. In fact, mammalian Notch was first described in a rare subset of T-cell tumours that are characterized by a translocation between the T-cell receptor and Notch1. J. Aster (Boston, MA, USA) described recently published data showing that Notch1 mutations are the most common mutation in T-cell leukaemia (Weng et al, 2004). So far, they have identified Notch-activating mutations in approximately 50% of T-cell leukaemias, all of which occur in Notch1. The mutations cause either ligand-independent signalling and/or prolong Notch1 half-life in the nucleus. Many of these gain-of-function alleles are dependent on γ-secretase cleavage and signalling, and the growth of leukaemic cell lines can be abolished by treatment with γ-secretase inhibitors. A clinical trial using γ-secretase inhibitors to treat patients with T-cell leukaemia is now in progress. In addition to the human disease, B. Kee (Chicago, IL, USA) showed that activating Notch mutations occur frequently in various murine T-cell leukaemias.
Progress was also described on the potential role of Notch signalling in other tumours. S. Krishna (Bangalore, India) presented data on the induction and role of phosphatidylinositol 3-kinase (PI3K) in Notch signalling in human papillomavirus (HPV)-derived cancers. Jagged1, in contrast to Delta1, is preferentially upregulated in human cervical tumours. The expression of Jagged1, but not Delta1, sustained in vivo tumours that were induced by HPV16 oncogenes in HaCaT cells. Furthermore, Jagged1 expression correlated with the rapid induction of PI3K-mediated EMT in both HaCaT cells and a human cervical tumour-derived cell line, which suggests that Delta1, Serrate/Jagged and Lag2 have ligand-specific roles. The Notch–PI3K oncogenic functions seem to be independent of CSL activation and, instead, rely on Deltex1, an alternative Notch effector.
C. Eberhart (Baltimore, MD, USA) discussed the dysregulation of the Notch pathway and its role in the pathogenesis of embryonal brain tumours. Notch1 inhibits whereas Notch2 promotes the proliferation of medullablastoma cells in culture (Fan et al, 2004). Despite these opposing activities of Notch1 and Notch2, global Notch-pathway blockade in medulloblastoma cultures using γ-secretase inhibitors resulted in a cell mass decrease of more than 50%. This effect was rescued by expression of the constitutively active NICD2. Tumour cells treated with γ-secretase inhibitors either exited the cell cycle and differentiated, or underwent programmed cell death, raising the possibility that therapies targeting the Notch pathway might be beneficial for patients suffering from medullablastoma.
As exemplified by the presentations described above, this exhilarating meeting was a huge success. As the participants dispersed to the four corners of the globe, it was clear that some issues of the Notch ‘gospel' have now been clarified whereas others await further investigation. Much was shared and learned, and many of the important challenges in this field have now been defined.

Acknowledgments
We thank our colleagues for sharing information and allowing their work to be described. We apologize to the participants whose work could not be cited due to space limitations.
References
- Benz C, Bleul CC (2005) A multipotent precursor in the thymus maps to the branching point of the T versus B lineage decision. J Exp Med 202: 21–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betschinger J, Knoblicj JA (2004) Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr Biol 14: R674–R685 [DOI] [PubMed] [Google Scholar]
- Ciofani M, Zuniga-Pflucker JC (2005) Notch promotes survival of pre-T cells at the β-selection checkpoint by regulating cellular metabolism. Nat Immunol 6: 881–888 [DOI] [PubMed] [Google Scholar]
- Dallas MH, Varnum-Finney B, Delaney C, Kato K, Bernstein ID (2005) Density of the Notch ligand Delta1 determines generation of B and T cell precursors from hematopoietic stem cells. J Exp Med 201: 1361–1366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery G, Hutterer A, Berdnik D, Mayer B, Wirtz-Peitz F, Gaitan MG, Knoblich JA (2005) Asymmetric Rab 11 endosomes regulate Delta recycling and specify cell fate in the Drosophila nervous system. Cell 122: 763–773 [DOI] [PubMed] [Google Scholar]
- Fan X, Mikolaenko I, Elhassan I, Ni X, Wang Y, Ball D, Brat DJ, Perry A, Eberhart CG (2004) Notch1 and Notch2 have opposite effects on embryonal brain tumor growth. Cancer Res 64: 7787–7793 [DOI] [PubMed] [Google Scholar]
- Fortini ME (2002) γ-Secretase-mediated proteolysis in cell-surface-receptor signalling. Nat Rev Mol Cell Biol 3: 673–684 [DOI] [PubMed] [Google Scholar]
- Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S (2005) Notch signals control the fate of immature progenitor cells in the intestine. Nature 435: 964–968 [DOI] [PubMed] [Google Scholar]
- Grego-Bessa J, Diez J, Timmerman L, de la Pompa JL (2004) Notch and epithelial–mesenchyme transition in development and tumor progression: another turn of the screw. Cell Cycle 3: 718–721 [PubMed] [Google Scholar]
- Gupta-Rossi N, Six E, LeBail O, Logeat F, Chastagner P, Olry A, Israel A, Brou C (2004) Monoubiquitination and endocytosis direct γ-secretase cleavage of activated Notch receptor. J Cell Biol 166: 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson EM, Stromberg K, Bergstedt S, Yu G, Naslund J, Lundkvist J, Lendahl U (2005) Aph-1 interacts at the cell surface with proteins in the active γ-secretase complex and membrane-tethered Notch. J Neurochem 92: 1010–1020 [DOI] [PubMed] [Google Scholar]
- Huppert SS, Ilagan MX, De Strooper B, Kopan R (2005) Analysis of Notch function in presomitic mesoderm suggests a γ-secretase-independent role for presenilins in somite differentiation. Dev Cell 8: 677–688 [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Cordes R, Kopan R, Gossler A, Gridley T (2005) The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development 132: 4353–4362 [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Bardin A, Schweisguth F (2005a) The roles of receptor and ligand endocytosis in regulating Notch signalling. Development 132: 1751–1762 [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Remaud S, Hamel S, Schweisguth F (2005b) Two distinct E3 ubiquitin ligases have complementary functions in the regulation of delta and serrate signalling in Drosophila. PLoS Biol 3: e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammucari C, di Vignano AT, Sharov AA, Neilson J, Havrda MC, Roop DR, Botchkarev VA, Crabtree GR, Dotto GP (2005) Integration of Notch 1 and calcineurin/NFAT signalling pathways in keratinocyte growth and differentiation control. Dev Cell 8: 665–676 [DOI] [PubMed] [Google Scholar]
- Minter LM et al. (2005) Inhibitors of γ-secretase block in vivo and in vitro T helper type 1 polarization by preventing Notch upregulation of Tbx21. Nat Immunol 6: 680–688 [PubMed] [Google Scholar]
- Ninkovic J, Tallafuss A, Leucht C, Topczewski J, Tannhauser B, Solnica-Krezel L, Bally-Cuif L (2005) Inhibition of neurogenesis at the zebrafish midbrain–hindbrain boundary by the combined and dose-dependent activity of a new hairy/E(spl) gene pair. Development 132: 75–88 [DOI] [PubMed] [Google Scholar]
- Robert-Moreno A, Espinosa L, de la Pompa JL, Bigas A (2005) RBPjκ-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells. Development 132: 1117–1126 [DOI] [PubMed] [Google Scholar]
- Sambandam A, Maillard I, Zediak VP, Xu L, Gerstein RM, Aster JC, Pear WS, Bhandoola A (2005) Notch signalling controls the generation and differentiation of early T lineage progenitors. Nat Immunol 6: 663–670 [DOI] [PubMed] [Google Scholar]
- Sarmento LM, Huang H, Limon A, Gordon W, Fernandes J, Tavares MJ, Miele L, Cardoso AA, Classon M, Carlesso N (2005) Notch1 modulates timing of G1–S progression by inducing SKP2 transcription and p27 Kip1 degradation. J Exp Med 202: 157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es JH et al. (2005) Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435: 959–963 [DOI] [PubMed] [Google Scholar]
- Weng AP, Ferrando AA, Lee W, Morris JPt, Silverman LB, Sanchez-Irizarry C, Blacklow SC, Look AT, Aster JC (2004) Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 306: 269–271 [DOI] [PubMed] [Google Scholar]


