Abstract
Myelination in Schwann cells is governed by several transcription factors, including the POU proteins Oct6 and Brn2, the high mobility group protein Sox10 and the zinc-finger protein Krox20. How the function of these factors is integrated in the control of myelination has not been established. Previously, we identified an enhancer element controlling Krox20 expression throughout myelination in Schwann cells. In this paper, cell culture experiments were combined with transgenesis to identify transcription factors acting directly upstream of Krox20. The results show that during the promyelin–myelin transition, Krox20 expression is directly activated by Oct6 and Brn2 acting on this enhancer. In addition, the enhancer-dependent synergism between these POU proteins and Sox10 suggests that Krox20 expression requires this combination of factors. These results resolve previous controversy concerning the mechanism of action of Oct6 and Brn2 during myelination and provide an explanation for myelin deficiencies in Waardenberg–Hirschsprung disease patients whereby Sox10 mutations could lead to a loss of Krox20 expression.
Keywords: Krox20/Egr2, Oct6/Tst1/SCIP/Pou3f1/Oft-6, Brn2/N-Oct-3/Pou3f2/Oft-7, myelination, peripheral nervous system, transcriptional regulation
Introduction
The myelin sheath, which serves to increase nerve conduction velocities, is deposited around axons by specialized cells in the central and peripheral nervous systems of higher vertebrates. In the peripheral nervous system, myelin is synthesized by Schwann cells and, so far, several transcription factors participating in the onset of myelination have been described. A pivotal factor is the zinc-finger transcription factor gene Krox20 (Egr2), the mutation of which in the mouse blocks Schwann cells at the promyelinating stage (Topilko et al, 1994). Consistently, Krox20/Egr2 mutations have also been identified in patients suffering from peripheral neuropathies (Warner et al, 1998). Taken together with cell culture experiments showing that myelin genes are induced by Krox20 (Nagarajan et al, 2001), these data suggest that Krox20 has characteristics of a master regulator of myelination. Previously, our studies into the regulation of Krox20 identified a transcriptional enhancer, designated the myelinating Schwann cell element (MSE), which is under the control of the POU domain transcription factor Oct6 (Ghislain et al, 2002).
Oct6 is transiently expressed in Schwann cells, peaking at the promyelinating stage (Jaegle et al, 2003). The analysis of Oct6 loss-of-function alleles indicated that mutant Schwann cells show a transient delay in myelination (Jaegle et al, 1996). More recently, the related POU gene, Brn2, expressed in Schwann cells in a manner similar to Oct6, was shown to compensate for the Oct6 mutation, the combined loss of both Oct6 and Brn2 provoking a more severe delay in myelination (Jaegle et al, 2003). Although a role for Oct6 and Brn2 in promoting myelination is established, their mechanism of action remains controversial, as no data providing a satisfactory molecular explanation have been put forward.
The SRY-related high-mobility group (HMG) domain protein Sox10 is expressed throughout Schwann cell development (Kuhlbrodt et al, 1998). However, the early loss of Schwann cells in Sox10 mutant mice precluded the analysis of a possible function in myelin formation (Britsch et al, 2001). Nevertheless, Waardenberg–Hirschsprung disease patients carrying dominant Sox10 mutations often show peripheral neuropathies characterized by normal Schwann cell numbers in the absence of myelin (Inoue et al, 2002), which suggests that Sox10 is involved in myelination. Indeed, the capacity of Sox10 to directly regulate myelin genes in Schwann cells provides a possible basis for this phenotype (Peirano et al, 2000). The paradigm of Sox–Oct cooperation in gene regulation (Kamachi et al, 2000) could provide a further explanation. However, whereas cell culture transfection studies have shown that Sox10 can cooperate with Oct6 (Kuhlbrodt et al, 1998), this activity has not been linked to Schwann cell myelination.
In this paper, transgenic and cell culture experiments were combined to show that the related POU proteins Oct6 and Brn2 activate transcription of Krox20 by directly binding to the MSE. In addition, Sox10 was found to synergize with these POU proteins on this element. Overall, these data suggest a molecular model that integrates the principal myelin transcription factors, in which Oct6 and Brn2 cooperate with Sox10 in driving Krox20 expression and thereby controlling myelination in Schwann cells.
Results
Oct6 and Brn2 activate Krox20 by binding to the MSE
In previous studies, a 1.3 kb cis-regulatory element located at +35 kb relative to the start site of transcription of the mouse Krox20 gene was identified (Ghislain et al, 2002). This element, designated the MSE, directs Krox20 expression throughout myelination in Schwann cells. To facilitate the search for transcription factor-binding sites in the enhancer, orthologous sequences from human and chicken genomes were identified (Fig 1).
Figure 1.

Alignment of human and chick sequences orthologous to the mouse myelinating Schwann cell element (MSE) shows several conserved transcription factor-binding sites. The nucleotide sequence of the mouse 1.3 kb MSE is shown (Ghislain et al, 2002). Nucleotide numbering corresponds to the mouse sequence. Restriction sites used in deletion studies are indicated. Conserved residues identified in the human and chick genomes are aligned to the mouse sequence and indicated as dashes. Oct6 footprints are indicated (I–IV; see Fig 2C). In these regions, conserved candidate binding sites for Oct6 and Brn2 are double underlined. These sites are based on the Brn2 binding site matrix (lower left; n can be either 0, 2 or 3 nucleotides) and Oct6 and Brn2 show similar binding characteristics (Li et al, 1993). The AT to GC substitutions introduced to inactivate these sites are shown. Conserved candidate Sox10-binding sites are shown in red with arrows. These sites are based on the Sox9 binding site matrix (lower right; Mertin et al, 1999), and Sox9 and Sox10 have similar DNA-binding characteristics (Peirano et al, 2000). Binding site matrices were generated using the WebLogo program (Crooks et al, 2004). Stack height reflects conservation and symbol height indicates frequency of residue. C, chick; H, human; M, mouse.
Previously, we showed that Oct6 acts upstream of the MSE to control Krox20 expression during Schwann cell myelination (Ghislain et al, 2002). Here, the possibility that Krox20 transactivation is mediated by the direct binding of Oct6 to sequences in the MSE was tested. Co-transfection of an Oct6 expression vector and the mouse 1.3 kb MSE fused to a minimal promoter/lacZ reporter led to a significant increase in reporter gene activity in both the glioblastoma cell line U138 and HeLa cells when compared with controls (Fig 2A). To identify regions responding to Oct6 in the MSE, a series of 5′ deletions were generated. A significant decrease in activity was observed on deletion of the PstI–BanII region, with a further weak but reproducible contribution of the Psp1406I–PstI region in HeLa cells (Fig 2A). These results indicate that essential cis-regulatory information for Oct6 transactivation is located between Psp1406I and BanII. Direct binding of Oct6 to this region was tested in a bandshift assay. The addition of bacterial extracts containing the Oct6 protein produced several specific complexes that were absent in the control extracts (Fig 2B; data not shown). The Oct6-binding sites in this region were then delimited in DNase I footprinting assays. Two subregions, Psp1406I–PstI and PstI–BanII, were assayed, showing four footprints with extracts containing Oct6 that were absent with control extracts (Fig 2C, I–IV; data not shown). Sequence comparisons between human, mouse and chick confirmed the presence of several conserved sequences similar to the Oct6 consensus binding site in these footprints (Li et al, 1993; Fig 1). AT to GC substitutions were then introduced to specifically eliminate the putative Oct6-binding sites (Fig 1), resulting in the elimination of the Oct6-binding activity of the Psp1406I–BanII fragment (Fig 2B).
Figure 2.

Identification of Oct6-binding sites in the myelinating Schwann cell element. (A) 5′ deletions of the 1.3 kb myelinating Schwann cell element (MSE) fused to a minimal β-globin promoter/lacZ reporter were transfected into U138 (left) and HeLa (right) cells, with 300 or 6 ng/well, respectively, of the expression vector, empty (−) or carrying the Oct6 coding sequence (+). The data show the mean β-galactosidase activity of two independent, normalized experiments carried out in duplicate. Values for transfections with the empty promoter/reporter and expression plasmids were set to one. Data from all other transfections are presented as the fold induction over this level. Error bars represent the standard error. (B) The wild-type MSE subfragment, Psp1406I–BanII (left), or a mutant version containing the AT to GC substitutions indicated in Fig 1 (right) were used as probes in bandshift experiments with increasing amounts of Oct6-containing bacterial extracts. As a control, both probes were combined without the bacterial extract (far right). To identify specific complexes, unlabelled competitor oligonucleotides corresponding to a high-affinity Oct6-binding site (wt) or a mutant version unable to bind to Oct6 (mt) were included in the binding reaction at a 200-fold molar excess. Specific complexes are indicated with brackets. FP, free probe. (C) The upper (left) and the upper (centre) and lower (right) strands of the Psp1406I–PstI and the PstI–BanII fragments, respectively, were analysed for Oct6 binding in DNase I footprinting assays using extracts from Oct6-expressing bacteria. Nucleotide numbering corresponds to the mouse 1.3 kb MSE sequence (Fig 1). The positions of the Oct6 footprints are indicated (I–IV).
Having defined several Oct6-binding sites in the MSE, the role of these sites in the activity of the MSE by Oct6 was tested both in vitro and in vivo. In addition, as the related POU protein Brn2 has been shown to compensate for the myelination defect in Oct6 mutant nerves (Jaegle et al, 2003), the possible involvement of these sites in transactivation by Brn2 was also investigated. Mutations in Oct6-binding sites II–IV or I–IV were introduced into the full-length MSE fused to a minimal promoter/lacZ reporter (Fig 3A). Whereas strong stimulation of reporter activity was detected with the wild-type MSE following co-transfection of the Oct6 expression vector in both HeLa and U138 cells, both mutant MSE constructs showed behaviour similar to the enhancerless control construct (Fig 3B). Similar results were obtained following co-transfection of the Brn2 expression vector (Fig 3B). The POU factors Brn5 and Oct1 were inactive on the wild-type MSE (data not shown), which suggested that MSE-dependent reporter activity is specific to Oct6 and Brn2. The effects of the Oct6 binding site mutations were then analysed by mouse transgenesis. Whereas the wild-type MSE led to 54% of lines expressing a high level of β-galactosidase activity in sciatic nerve Schwann cells after birth (Ghislain et al, 2002; Fig 3A,C), mutation of the Oct6-binding sites II–IV and I–IV reduced this frequency to 22% and virtually eliminated this activity, respectively (Fig 3A,D; data not shown), disclosing important roles of Oct6-binding sites II–IV and of site I in the activity of the MSE during myelination. In the mature nerve, although the wild-type element led to 30% of high-expressing lines (Fig 3A,E), such levels of expression were never observed with either of the mutant constructs (Fig 3A,F; data not shown).
Figure 3.
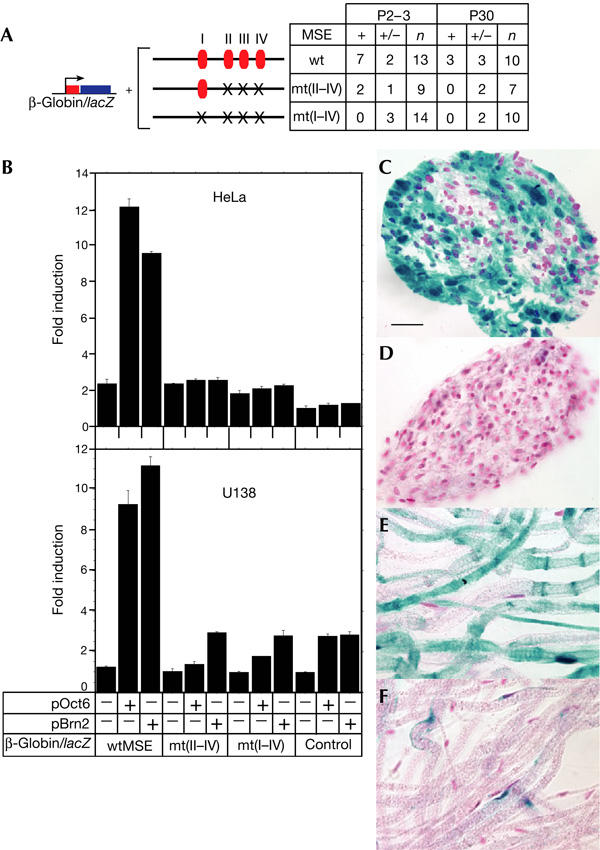
Oct6-binding sites are essential for the in vivo activity of the myelinating Schwann cell element. (A) Schematic representation of the wild-type (wt) 1.3 kb myelinating Schwann cell element (MSE) and mutant MSE carrying mutations in the Oct6-binding sites II–IV (mt(II–IV); Fig 1) and I–IV (mt(I–IV); Fig 1) fused to a minimal β-globin promoter/lacZ reporter. Results of transgenic experiments are shown. n, the number of transgene positive mice analysed for β-galactosidase activity in the sciatic nerve at postnatal day (P) 2–3 or 30; +, strongly β-galactosidase-positive sciatic nerves with levels similar to those shown in (C,E); +/−, weakly β-galactosidase-positive sciatic nerves with levels similar to those shown in (D,F). (B) The wild-type 1.3 kb MSE and mutant constructs were transfected into HeLa (upper) and U138 (lower), with either 6 or 75 ng/well, respectively, of the expression vector, empty (−) or carrying the murine Oct6 or Brn2 coding sequence (+). Control, promoter/reporter plasmid without MSE. Presentation of transfection data is described in the legend of Fig 2. (C–F) β-Galactosidase-positive sciatic nerves from P2–3 (C,D; transverse section) and P30 (E,F; teased nerve) mice carrying the wild-type 1.3 kb MSE (C,E, (+) in A) or mt(I–IV) mutant construct (D,F, (+/−) in A). Scale bar, 25 μm.
Overall, these results strongly suggest that during myelination, Krox20 expression is specifically controlled by the direct binding of Oct6 and Brn2 to the MSE. Interestingly, although both Oct6 and Brn2 are only transiently expressed after birth (Jaegle et al, 2003), their binding sites are still required for MSE-dependent reporter expression in the mature myelinating cells, suggesting the involvement of other POU genes.
Synergism between Sox10 and POU factors
As the HMG protein Sox10 is expressed throughout Schwann cell development and Sox10 has been shown to cooperate with Oct6 (Kuhlbrodt et al, 1998), we suggested that Krox20 expression depends on a cooperation between these factors acting on the MSE. Indeed, interspecies sequence comparisons identified several conserved putative Sox10-binding sites in the MSE (Fig 1). In the transactivation assay using the wild-type 1.3 kb MSE, although both Oct6 and Sox10 moderately activated reporter gene expression, combining these factors led to a strong synergistic activation in both U138 and HeLa cells (Fig 4A,B). Interestingly, although the responsiveness of 5′ deletions of the MSE to Sox10 alone correlated with the presence of putative Sox10-binding sites, the synergism between Sox10 and Oct6 required a core region, StuI to BanII, containing the demonstrated Oct6-binding sites and most of the putative Sox10-binding sites (Fig 4B). As Brn2 acts similar to Oct6, its activity on the wild-type MSE was also tested. Similar to Oct6, Brn2 strongly synergized with Sox10 in HeLa cells (Fig 4C). Interestingly, neither Oct6 nor Brn2 cooperated with Sox2, although this factor has been shown to synergize with other POU proteins (Fig 4C; Kamachi et al, 2000). Finally, given the synergism between Oct6/Brn2 and Sox10 on the wild-type MSE, we tested the responsiveness of the MSE Oct6 binding site mutants. Consistent with their loss of activity in transgenic experiments, both mutant constructs II–IV and I–IV, although they largely retained their responsiveness to Sox10 alone, were not cooperatively activated by Oct6 or Brn2 with Sox10 (Fig 4C). The addition of Oct6 or Brn2 actually led to a repression of the Sox10 inducing activity, which may be due to Sox10 sequestering.
Figure 4.

Myelinating Schwann cell element (MSE)-dependent enhancer activity involves synergism between Sox10 and the POU proteins Oct6 and Brn2. The wild-type and mutant 1.3 kb MSE constructs (Fig 3A) and 5′ deletion constructs (Fig 2A) fused to a minimal β-globin promoter/lacZ reporter were co-transfected with the expression vector, empty (−) or carrying the murine Oct6 or Brn2 coding sequence (++, 6 ng/well; +, 0.6 ng/well) and/or Sox10 or Sox2 coding sequence (+, 60 ng/well), as indicated, into U138 (A) and HeLa (B,C) cells. Control, promoter/reporter plasmid without MSE. Presentation of transfection data is described in the legend of Fig 2.
Overall, these data show a specific cooperation between Sox10 and the POU proteins Oct6 and Brn2 in MSE-dependent enhancer activity and suggest that Sox10 participates in myelin formation by controlling Krox20 expression.
Discussion
In this paper, transcription factors acting directly upstream of Krox20 during myelination in Schwann cells have been identified. The results indicate that Krox20 expression depends on a synergistic interaction of the POU proteins, Oct6 and Brn2, with Sox10 on sequences in the Krox20 MSE enhancer. As Krox20 directs myelination in Schwann cells, the MSE has a central role in this process by integrating the activities of Oct6, Brn2 and Sox10.
Our results establish that Oct6 activates transcription of Krox20 by binding to the MSE, providing a molecular explanation for the Oct6 mutant phenotype. However, Oct6 is required only transiently (Jaegle et al, 1996), whereas Krox20 is necessary throughout myelination (Topilko et al, 1994; L. Decker, E. Taillebourg & P.C., unpublished data). Consistent with this, Krox20 is expressed in Oct6 mutants as myelination resumes (Ghazvini et al, 2002). Previously, Brn2 was shown to act redundantly to Oct6: their combined deletion leads to a more severe block in myelination than that of Oct6 mutants alone and Brn2 can substitute for Oct6 in rescue experiments of Oct6 mutant Schwann cells (Jaegle et al, 2003). As shown in this study, the capacity of Brn2 to substitute for Oct6 and directly activate Krox20 provides a molecular explanation for the redundancy of these factors. Is Krox20 the sole major target of Oct6 and Brn2 in Schwann cells? The mutant phenotypes of Oct6 and Brn2 are similar to that of Krox20, suggesting that this may be the case. However, confirmation of this hypothesis awaits the forced expression of Krox20 in Oct6/Brn2 double-mutant nerves.
Myelination in the Oct6 and Brn2 double mutant, although severely delayed, resumes (Jaegle et al, 2003). Assuming that as myelination proceeds in this mutant, Krox20 is expressed (as in the Oct6 single mutant), then which factors are controlling its expression? An involvement of Oct6 cannot be totally excluded, as the Oct6 mutant allele used in these studies is hypomorphic (Ghazvini et al, 2002). However, as Krox20 expression is maintained in the mature myelinating nerve, in which both Oct6 and Brn2 have been downregulated (Jaegle et al, 2003), we favour the involvement of other factors. As we have shown that the Oct6 binding site MSE mutants were significantly less active in the adult compared with the wild type (Fig 3), the factors required for Krox20 adult expression are likely to belong to the POU family. Although Brn5 and the ubiquitous Oct1 are expressed in myelinating Schwann cells (Wu et al, 2001; Jaegle et al, 2003), their inactivity on the MSE, either alone or in combination with Sox10, suggests that these are not appropriate candidates (data not shown). The involvement of other POU proteins in the adult activity of the MSE therefore needs investigation.
Our data suggest that both Oct6 and Brn2 cooperate with Sox10 to activate Krox20 transcription through the MSE, identifying for the first time a natural cis-element responding synergistically to these factors. These results are consistent with previous findings identifying a synergism between Oct6 and Sox10 on the FGF4 enhancer (Kuhlbrodt et al, 1998) and extend these studies to show that Brn2 can also synergize with Sox10. Although a direct role of Sox10 in myelin gene regulation may in part be responsible for Schwann cell myelin deficiencies in Waardenberg–Hirschsprung disease patients carrying mutations in Sox10 (Inoue et al, 2002), our results provide an alternative explanation. Owing to the synergistic role of Sox10, mutations reducing its activity could lead to a marked loss of Krox20 expression, in turn blocking myelination. Although we have found numerous candidate Sox10-binding sites in the MSE (Fig 1), they do not present the particular dimeric organization characteristic of the P0 promoter (Peirano et al, 2000). This suggests that other types of dimeric site, as well as monomeric sites, are functional, at least in the context of the MSE.
In conclusion, the pivotal role of Oct6, Brn2, Sox10 and Krox20 in Schwann cell myelination and the importance of both Krox20 and Sox10 in peripheral neuropathies emphasize the importance of this study. By showing a direct link between these factors, our work provides a first insight into the hierarchy of transcription factors controlling myelination in Schwann cells and a better understanding of the molecular mechanisms underlying myelin disorders in humans.
Methods
Cell culture transfection and transgenic mice. U138 and HeLa cell lines were maintained in DMEM supplemented with 10% FCS. Transfection and assay techniques have been described previously (Ghislain et al, 2003). The murine Oct6, Brn2 and Brn5 expression vectors (Jaegle et al, 2003) were gifts from D. Meijer. The murine Sox10 expression vector was obtained from RZPD (clone ID: IRAVp968C0325D). The murine Sox2 (Yuan et al, 1995) and human Oct1 (Tanaka & Herr, 1990) expression vectors were gifts from L. Dailey and W. Herr, respectively. Transgenesis, identification of transgenic mice by PCR, 5-bromo-4-chloro-3-indolyl-β-D-galactoside staining of sciatic nerves and sectioning were carried out, as described previously (Ghislain et al, 2002).
Sequence analyses and isolation of the chick myelinating Schwann cell element. Sequence analyses were carried out, as described previously (Ghislain et al, 2003). The GenBank accession numbers for the human and mouse contigs containing sequences shown in Fig 1 are AC067751 and AC068424, respectively. Chick sequences containing homology to these sequences have been submitted to GenBank (AY519467). This sequence was isolated from a chicken genomic BAC clone carrying the Krox20 gene (Giudicelli et al, 2001) by low-stringency hybridization, using the mouse 1.3 kb MSE as a probe.
DNase I footprinting, bandshift assays and mutagenesis. The pET11a (Novagen, La Jolla, CA, USA) bacterial expression plasmid containing the full-length murine Oct6 coding sequence was a gift from D. Meijer. Protein extraction from Oct6-expressing and control bacteria, DNase I footprinting and bandshift experiments were carried out, as described previously (Ghislain et al, 2003). The mutant PstI–BanII restriction fragment of the mouse MSE carrying the AT to GC substitutions (Fig 1, sites II–IV) was synthesized chemically. The AT to GC substitutions in the Psp1406I–PstI fragment (Fig 1, site I) were introduced using the Exsite™ PCR-based site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). All mutated subfragments were verified by sequencing. MSE mutants were generated by replacing the PstI–BanII fragment of either the wild-type or the mutant 1.3 kb MSE carrying the mutation in site I with the mutant version to generate MSE mutants in sites I–IV and II–IV, respectively. The wild-type and mutant 1.3 kb MSE were cloned into pBGZ40 (Ghislain et al, 2003).
Acknowledgments
We thank P. Gilardi-Hebenstreit and A. Baron-Van Evercooren for critical reading of the manuscript. J.G. was supported by postdoctoral fellowships from the European Union (TMR), the Association Française contre les Myopathies (AFM), the Institut de France and the Association pour la Recherche sur la Sclérose en Plaque. This work was supported by grants from the Institut National de la Santé et de la Recherche Médicale, the Ministére de l'Éducation Nationale de la Recherche et de la Technologie, the European Community, the Association pour la Recherche sur le Cancer, and the AFM.
References
- Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossne M, Nave KA, Birchmeier C, Wegner M (2001) The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev 15: 66–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crooks GE, Hon G, Chandonia JM, Brenner SE (2004) WebLogo: A sequence logo generator. Genome Res 14: 1188–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghazvini M, Mandemakers W, Jaegle M, Piirsoo M, Driegen S, Koutsourakis M, Smit X, Grosveld F, Meijer D (2002) A cell type-specific allele of the POU gene Oct-6 reveals Schwann cell autonomous function in nerve development and regeneration. EMBO J 21: 4612–4620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghislain J, Desmarquet-Trin-Dinh C, Jaegle M, Meijer D, Charnay P, Frain M (2002) Characterisation of cis-acting sequences reveals a biphasic, axon-dependent regulation of Krox20 during Schwann cell development. Development 129: 155–166 [DOI] [PubMed] [Google Scholar]
- Ghislain J, Desmarquet-Trin-Dinh C, Gilardi-Hebenstreit P, Charnay P, Frain M (2003) Neural crest patterning: autoregulatory and crest-specific elements co-operate for Krox20 transcriptional control. Development 130: 941–953 [DOI] [PubMed] [Google Scholar]
- Giudicelli F, Taillebourg E, Charnay P, Gilardi-Hebenstreit P (2001) Krox-20 patterns the hindbrain through both cell-autonomous and non cell-autonomous mechanisms. Genes Dev 15: 567–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Shilo K, Boerkoel CF, Crowe C, Sawady J, Lupski JR, Agamanolis DP (2002) Congenital hypomyelinating neuropathy, central dysmyelination, and Waardenburg–Hirschsprung disease: phenotypes linked by SOX10 mutation. Ann Neurol 52: 836–842 [DOI] [PubMed] [Google Scholar]
- Jaegle M, Mandemakers W, Broos L, Zwart R, Karis A, Visser P, Grosveld F, Meijer D (1996) The POU factor Oct-6 and Schwann cell differentiation. Science 273: 507–510 [DOI] [PubMed] [Google Scholar]
- Jaegle M, Ghazvini M, Mandemakers W, Piirsoo M, Driegen S, Levavasseur F, Raghoenath S, Grosveld F, Meijer D (2003) The POU proteins Brn-2 and Oct-6 share important functions in Schwann cell development. Genes Dev 17: 1380–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamachi Y, Uchikawa M, Kondoh H (2000) Pairing SOX off: with partners in the regulation of embryonic development. Trends Genet 16: 182–187 [DOI] [PubMed] [Google Scholar]
- Kuhlbrodt K, Herbarth B, Sock E, Hermans-Borgmeyer I, Wegner M (1998) Sox10, a novel transcriptional modulator in glial cells. J Neurosci 18: 237–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, He X, Gerrero MR, Mok M, Aggarwal A, Rosenfeld MG (1993) Spacing and orientation of bipartite DNA-binding motifs as potential functional determinants for POU domain factors. Genes Dev 7: 2483–2496 [DOI] [PubMed] [Google Scholar]
- Mertin S, McDowall SG, Harley VR (1999) The DNA-binding specificity of SOX9 and other SOX proteins. Nucleic Acids Res 27: 1359–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan R, Svaren J, Le N, Araki T, Watson M, Milbrandt J (2001) EGR2 mutations in inherited neuropathies dominant-negatively inhibit myelin gene expression. Neuron 30: 355–368 [DOI] [PubMed] [Google Scholar]
- Peirano RI, Goerich DE, Riethmacher D, Wegner M (2000) Protein zero gene expression is regulated by the glial transcription factor Sox10. Mol Cell Biol 20: 3198–3209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Herr W (1990) Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell 60: 375–386 [DOI] [PubMed] [Google Scholar]
- Topilko P, Schneider-Maunoury S, Levi G, Baron-Van Evercooren A, Chennoufi AB, Seitanidou T, Babinet C, Charnay P (1994) Krox-20 controls myelination in the peripheral nervous system. Nature 371: 796–799 [DOI] [PubMed] [Google Scholar]
- Warner LE, Mancias P, Butler IJ, McDonald CM, Keppen L, Koob KG, Lupski JR (1998) Mutations in the early growth response 2 (EGR2) gene are associated with hereditary myelinopathies. Nat Genet 18: 382–384 [DOI] [PubMed] [Google Scholar]
- Wu R, Jurek M, Sundarababu S, Weinstein DE (2001) The POU gene Brn-5 is induced by neuregulin and is restricted to myelinating Schwann cells. Mol Cell Neurosci 17: 683–695 [DOI] [PubMed] [Google Scholar]
- Yuan H, Corbi N, Basilico C, Dailey L (1995) Developmental-specific activity of the FGF-4 enhancer requires the synergistic action of Sox2 and Oct-3. Genes Dev 9: 2635–2645 [DOI] [PubMed] [Google Scholar]


