Abstract
During homologous recombination (HR), a heteroduplex DNA is formed as a consequence of strand invasion. When the two homologous strands differ in sequence, a mismatch is generated. Earlier studies showed that mismatched heteroduplex often triggers abortion of recombination and that a pivotal component of this pathway is the mismatch repair Msh2 protein. In this study, we analysed the roles of AtMSH2 in suppression of recombination in Arabidopsis. We report that AtMSH2 has a broad range of anti-recombination effects: it suppresses recombination between divergent direct repeats in somatic cells or between homologues from different ecotypes during meiosis. This is the first example of a plant gene that affects HR as a function of sequence divergence and that has an anti-recombination meiotic effect. We discuss the implications of these results for plant improvement by gene transfer across species.
Keywords: mismatch repair, genome stability, somatic recombination, meiotic recombination, sequence divergence
Introduction
Mismatch repair (MMR) systems have an important role in promoting genetic stability by repairing DNA replication errors (Kornberg & Bake, 1992; Modrich & Lahue, 1996), inhibiting recombination between divergent DNA sequences (Petit et al, 1991), maintaining barriers against massive genetic flow (Rayssiguier et al, 1989), preventing productive meiosis in interspecies hybrids (Hunter et al, 1996) and participating in responses to DNA damage (Stojic et al, 2004).
The best-characterized MMR pathway is the Escherichia coli methyl-directed MutHLS system that is involved in mismatch recognition and removal (Modrich & Lahue, 1996). MutS is an ATPase involved in mismatch recognition; MutL, another ATPase, couples mismatch recognition by MutS to downstream processing steps, and MutH is a methylation-sensitive endonuclease (Aravind et al, 1999). Both eubacteria and eukaryotes express highly conserved MutS and MutL proteins. In eukaryotes, there are at least six MutS and five MutL homologues of the bacterial proteins (Modrich & Lahue, 1996; Flores-Rozas & Kolodner, 1998).
In Arabidopsis, several MutS homologues have been isolated. Four MutS homologues have been identified on the basis of their sequence conservation and through inspection of the Arabidopsis genomic sequence: AtMSH2, AtMSH3, AtMSH6 and AtMSH7 (Culligan & Hays, 1997; Eisen, 1998; Ade et al, 1999). AtMSH1 was cloned through map-based cloning of a chlorophyll variegated mutant (Abdelnoor et al, 2003). AtMSH4 was identified and cloned on the basis of sequence similarity with the human, mouse and yeast Msh4 amino-acid sequences (Higgins et al, 2004). Biochemical studies also point to conservation of protein functions (Culligan & Hays, 2000; Ade et al, 2001). The AtMsh2 protein was shown to form heterodimers with AtMsh3 and AtMsh6 in vitro, and the resulting complexes have mismatch recognition specificities similar to those shown by the corresponding yeast and mammalian complexes (Culligan & Hays, 2000). In addition, AtMsh2 forms heterodimers with AtMsh7, and this complex has a mismatch recognition spectrum distinct from that of the other two AtMsh2-containing complexes (Wu et al, 2003).
The plant MSH-like genes are involved in a broad range of functions, all of which are related to genome stability. AtMsh1 is involved in maintaining the stability of the mitochondrial genome (Abdelnoor et al, 2003). AtMSH4 was shown to be expressed in floral tissues, and a mutant in this gene is defective in chromosome synapsis during meiosis, giving rise to a partially sterile phenotype (Higgins et al, 2004). The best-characterized plant gene so far is AtMSH2. Functional studies on the Atmsh2 mutant in Arabidopsis showed microsatellite instability, indicating a direct involvement of AtMSH2 in the repair of replication errors (Leonard et al, 2003; Depeiges et al, 2005). Moreover, the Atmsh2 mutant shows a mutator effect when propagated for a number of generations, thus highlighting its importance in the control of genome stability (Hoffman et al, 2004).
In a previous study, we have shown that a minor sequence divergence can cause a drop-off in the rates of somatic recombination between direct repeats that differ by only 1 out of 618 identical base pairs (Opperman et al, 2004). Similarly, sequence divergence was shown to repress homologous recombination (HR) between inverted repeats (Li et al, 2004). The implication of AtMSH2 or of any other plant MMR gene in such repression has not yet been tested.
In this study, we tested the role of AtMSH2 in two different types of HR: somatic and meiotic. The Atmsh2-1 mutant we used is similar to that used in earlier studies (Alonso et al, 2003; Leonard et al, 2003; Hoffman et al, 2004). The somatic assay of recombination between divergent repeats has been described previously (Opperman et al, 2004). The effect of AtMSH2 on the rates of meiotic recombination was determined using a novel seed-based assay in which linked markers (green fluorescent protein (GFP) or red fluorescent protein (RFP)) become separated following crossover (Melamed-Bessudo et al, 2005). We show that AtMSH2 has a broad range of anti-recombination effects. We compare the roles of MSH2 in different kingdoms and the implication of our results for plant improvement by broad gene transfer.
Results
Somatic recombination between divergent repeats
To explore the role of AtMSH2 on HR frequency in the presence of sequence divergence, we tested HR in the background of the Atmsh2-1 mutant (Leonard et al, 2003; Hoffman et al, 2004) using the previously described assay (Opperman et al, 2004). The assay construct contains two overlapping parts of the β-glucuronidase (uidA) gene (GUS gene), namely the U repeat, as shown for the GU-NPT-US construct in Fig 1. The U repeat is the segment in which mismatches were inserted. HR between the two direct repeats (U sequences) leads to the formation of an intact GUS gene and results in a blue sector on histochemical staining with the enzyme substrate 5-bromo-4-chloro-3-indolylglucuronide (X-gluc). We used a construct with two identical repeats (A0) and a construct with ten mismatches between the repeats (A10), as described previously (Opperman et al, 2004).
Figure 1.

Assay for recombination between divergent repeats. A schematic representation of the assay construct is shown, before (top) and after (bottom) recombination. The GU-NPT-US construct (top) contains two overlapping halves of the β-glucuronidase GUS gene, GU and US, with the U (=618 bp) overlapping region and the NPTII gene in-between the repeats. Mutations are introduced in the U part of the GU half. Homologous recombination (HR) between the U direct repeats gives rise to an active GUS reporter gene (bottom). A blue sector is obtained following X-gluc histological staining in the cell in which HR occurred and in its daughters. RB and LB represent the right and left borders of the pMLBART binary vector.
The Atmsh2-1 mutant, in the background of ecotype Columbia, was previously shown (Leonard et al, 2003) to be a true knockout in the AtMSH2 gene (At3g18524). This was confirmed in this study by reverse transcription (RT)–PCR (data not shown). The Atmsh2-1 homozygote and the wild-type plants were transformed with the assay constructs (Fig 1). About 40 transformants from each of the four combinations studied (2 genotypes × 2 mismatch categories) were grown, representing a broad range of position effects in the genome. The number of spots per seedling was determined in 120–150 individuals of each combination and served as an estimate of the recombination frequency (supplementary information online). In the absence of mismatches (A0), the Atmsh2-1 mutant and wild type showed a statistically similar frequency of recombination (Fig 2). In the presence of mismatches, there was a significant drop-off in recombination frequency in the wild type, of the same magnitude as that previously reported (Opperman et al, 2004). However, in the Atmsh2-1 background, this drop-off was not observed. Conversely, there was an increase in recombination frequency, although this increase was not statistically significant.
Figure 2.

Recombination frequency between identical and divergent sequences in wild-type or mutant background. The recombination frequency is expressed as the average number of blue spots per seedling. It was determined for the recombination between identical (A0) and divergent repeats (A10) in wild-type and Atmsh2-1 backgrounds. Black circles represent Atmsh2-1; black squares represent wild type. Statistical analysis between the different groups was carried out with the Wilcoxon's test for non-parametric variables. n.s. indicates that the difference in the distribution of spots between the groups was not statistically significant (P(χ2)>0.05). ** and *** indicate that the difference between the groups was statistically significant with P(χ2)<0.05 or P(χ2)<0.01, respectively.
Meiotic recombination
The role of AtMSH2 in meiotic recombination was determined using tester line Le5-11/22 (Melamed-Bessudo et al, 2005) that is in the Landsberg background. This line harbours two seed markers, RFP and GFP, driven by a seed-specific Napine promoter, linked in cis, ∼6 cM apart. This tester enables one to estimate meiotic recombination rates by counting seeds expressing both parental markers (red and green) or recombinant seeds expressing only one marker (red or green). To test meiotic recombination in the mutant background, the marker genes were introgressed in Atmsh2-1, as shown in Fig 3. The tester is in the Landsberg background, whereas the mutant is in the Columbia background. Therefore, in this plant material, crossover occurred between two non-identical homologues. Recombination in the mutant Atmsh2-1 background was compared with a control in the AtMSH2 background. In both mutant and control, recombination rates were tested in the same genetic background, namely the BC1F2 generation (see Methods; Fig 3).
Figure 3.

Scheme for introgression of the green fluorescent protein and red fluorescent protein meiotic markers in the Atmsh2-1 background. (A) The tester line with the green fluorescent protein (GFP) and red fluorescent protein (RFP) markers, in the Landsberg background, is on the left side in the top row. The RFP and GFP markers are marked by a red and green square, respectively. The AtMSH2 locus is indicated by a + sign on a different chromosomal pair. On the right side is the Atmsh2-1 mutant in the Columbia background. The mutated Atmsh2 locus is shown by a − sign. As a control, the same scheme of crosses was carried out, using the wild-type AtMSH2 (Columbia background) for the first cross (F1) as well as for the backcross (BC1). (B) Crossover rates are determined by counting seeds of the parental types, seen as red and green (r&g) or non-fluorescent (n) versus seeds of the recombinant types seen as red only (r) or green only (g). This analysis was carried out in the self-pollinated seeds from plants that were heterozygote for the tester markers and homozygote for Atmsh2-1 (or homozygote for AtMSH2 in the control), namely in the BC1F2 generation.
In each genotype, we tested 4–6 plants and an average of 500 seeds per plant (Table 1). Atmsh2-1 showed a significant 40% percent increase in crossover rates between the markers (8.8 versus 6.3 cM) compared with the AtMSH2 wild type (Table 1). Note that each of the markers segregated in a mendelian 3:1 ratio, and the number of ‘green-only' was similar to the number of ‘red-only' seeds, indicating that there was no silencing of the marker genes.
Table 1.
Recombination rates between the green fluorescent protein and red fluorescent protein markers in tester line Le5-11/22 in the background of Atmsh2-1 compared with wild type
|
Genotype |
Total seeds |
Seed phenotype* |
Percentage of recombination |
Standard error |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Total red | Total green | Red only | Green only | Red and green | Non-glowing | ||||
| Atmsh2-1 | 550 | 401 | 402 | 25 | 26 | 376 | 123 | ||
| 508 | 374 | 380 | 17 | 23 | 357 | 111 | |||
| 505 | 374 | 371 | 23 | 20 | 351 | 111 | |||
| 537 | 388 | 387 | 22 | 21 | 366 | 128 | |||
| Total | 2,100 | 1,537 | 1,540 | 87 | 90 | 1,450 | 473 | 8.81 | ±0.68 |
| Percentage of total | 73.2‡ | 73.3‡ | |||||||
| AtMSH2 | 543 | 404 | 406 | 10 | 12 | 394 | 127 | ||
| 456 | 319 | 325 | 11 | 17 | 308 | 120 | |||
| 493 | 336 | 328 | 22 | 14 | 314 | 143 | |||
| 517 | 384 | 379 | 24 | 19 | 360 | 114 | |||
| 532 | 392 | 394 | 14 | 16 | 378 | 124 | |||
| 546 | 407 | 411 | 12 | 16 | 395 | 123 | |||
| Total | 3,087 | 2,242 | 2,243 | 93 | 94 | 2,149 | 751 | 6.31 | ±0.56 |
| Percentage of total | 72.6† | 72.7† | |||||||
*Seed phenotypes were determined in the BC1F2 generation for both Atmsh2-1 and AtMSH2 plants, as described in Methods and in Fig 3.
†These ratios do not differ from a mendelian 3 (coloured):4 (total) segregation: P(χ2>0.13).
Discussion
Across-kingdom comparison of MSH2 genes functions
This study shows the first example of a plant gene, AtMSH2, involved in the repression of HR between divergent sequences. Repression was found for somatic recombination between direct repeats and for meiotic recombination between homologous chromosomes derived from different ecotypes. MSH2-mediated suppression of recombination between divergent repeats has been previously reported in yeast (Chen & Jinks-Robertson, 1999) and in mammalian cells (Elliott & Jasin, 2001) and thus seems to be a conserved feature across kingdoms. The role of MSH2 in meiotic recombination between homologous chromosomes is less well known. Studies on the role of the mouse MSH2 homologue in meiotic recombination have not been conclusive (Qin et al, 2002). Studies on meiotic recombination in yeast have focused on MSH2-mediated repression of meiotic recombination between repeats (Chen & Jinks-Robertson, 1999) or between homeologous chromosomes that originated from different species, for example, Saccharomyces cerevisiae versus S. paradoxus (Chambers et al, 1996). The divergence in nucleotide sequences between S. cerevisiae and S. paradoxus is 10% on average (Kellis et al, 2003). This divergence is associated with a decrease in recombination rates in the MSH2 wild-type background. In the msh2 mutant, recombination between the homeologous chromosomes was enhanced 5.5-fold (Chambers et al, 1996). In this study, we show a 40% increase in meiotic recombination rates in the absence of AtMsh2 compared with the wild-type background. This increase is lower than that reported in yeast; however, it was found for recombination between homologous, rather than homeologous, chromosomes that diverge by ∼2% at the nucleotide level in the region between the two markers in tester Le5-11/22. To our knowledge, this is the first report on MSH2-mediated repression of meiotic recombination between homologous chromosomes. We assume that the increase in meiotic recombination reported here results from the lack of suppression caused by sequence divergence between the two ecotypes, Landsberg and Columbia. Indeed, we found, in a different set of experiments (Fig 4), that when recombination between the RFP and GFP markers in tester Le5-11/22 is measured in an isogenic background (Landsberg × Landsberg), it is higher than in a non-isogenic background (Columbia × Landsberg). The same, divergence-related repression of recombination was found for a different tester (data not shown).
Figure 4.
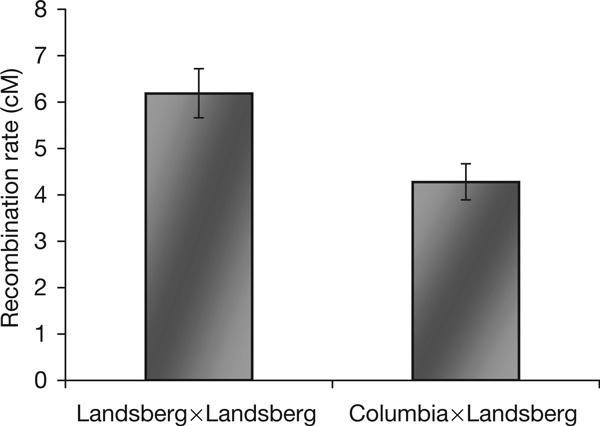
Divergence-related repression of meiotic recombination frequencies. Meiotic recombination between the red fluorescent protein and green fluorescent protein markers in tester Le5-11/22 is measured in an isogenic background (Landsberg × Landsberg) and in a non-isogenic background (Columbia × Landsberg).
The anti-recombination mechanism
There are several alternative pathways that can lead to recombination between repeats (Prado et al, 2003). How exactly it occurs in plants is not fully understood, although there is evidence for the involvement of single-strand annealing pathways (Gorbunova & Levy, 1999; Puchta, 2005). With these pathways, the mismatched heteroduplex is formed following the annealing of two single-strand DNA molecules. Heteroduplex rejection and abortion of the recombination process would thus occur after annealing, possibly, as proposed in yeast (Sugawara et al, 2004; Goldfarb & Alani, 2005), by Msh2-mediated recruiting of Sgs1p, a helicase that would unwind the annealed region. During meiotic recombination, Holliday junction formation is associated with annealing of single-stranded DNA from two homologues, forming a heteroduplex-containing Holliday junction. Statistically, there is more chance for forming a heteroduplex when recombination is initiated between homeologous chromosomes that diverge by ∼10% at the nucleotide level (for example, cerevisiae versus paradoxus) than between homologous chromosomes that diverge by ∼2%, as reported here, thus increasing the chances for recombination abortion. The mechanism whereby Msh2 mediates the rejection of heteroduplex-containing Holliday junctions is still unknown.
Biological significance and applications
The repressing effect of sequence divergence on HR is well known in plants, as well as in other species. In plants, this repression is necessary to stabilize the repeat-rich genome, to maintain barriers between species and to prevent promiscuous recombination between homeologous chromosomes in allopolyploid species. For example, meiotic recombination between chromosomes or chromosomal segments of the edible tomato and those of its wild relatives is strongly suppressed (Rick, 1969; Chetelat et al, 2000). In hexaploid wheat, there is virtually no recombination between the homeologous chromosomes in the wild-type PH1 background (Sears, 1976). The findings reported here, that AtMSH2 is involved in anti-recombination between divergent DNA fragments in plants, are therefore relevant to the understanding of plant genome stability and evolution and to the improvement of crops by sexual transfer of genes from distant wild relatives. AtMSH2 is certainly not the only barrier for gene transfer between distant species; however, this work suggests that it has an important role. Crops mutated in MSH2, or in other MMR genes, might therefore promote distant gene transfer. It has been suggested that the PH2 locus that represses pairing between homeologous chromosomes in wheat might be an MMR gene (Dong et al, 2002).
Methods
Plant material and Agrobacterium-mediated transformation. Arabidopsis thaliana and homozygous mutant plants were grown and transformed by Agrobacterium-mediated transformation, using the dipping procedure (Clough & Bent, 1998). Plants were transformed with the A0 and the A10 constructs described previously (Opperman et al, 2004). Transformed seeds (T0) were collected, planted and selected for BASTA resistance. The selected plants (T1) were seed harvested as pools for intrachromosomal recombination experiments (30–40 plants per pool).
Intrachromosomal recombination experiments and GUS staining. Histochemical staining for GUS activity was carried out in 3-week-old plants, using the following staining protocol. Plants were grown on agar plates with 1/2 MS (Murashige and Skoog) medium (Duchefa, Haarlem, The Netherlands), 2% sucrose and 25 mg/l gluphosinate. Growth conditions were 16–18 h of light at 25–28°C. Plants were harvested and incubated for 24–48 h at 37°C in sterile staining buffer containing 1 mg/ml X-gluc substrate (Duchefa) in a final concentration of 50 mM sodium phosphate buffer (pH 7.0), 0.1% Triton X-100, 0.1% dimethylsulphoxide and 5 mM potassium ferricyanide and potassium ferrocyanide trihydrate (Sigma-Aldrich, Rehovot, Israel). Bleaching was carried out at 25°C using 70% ethanol.
DNA extraction, genotyping and reverse transcription–PCR. Total genomic DNA was extracted from 50–100 mg young leaves, as described previously (Melamed-Bessudo et al, 2005). All the plants were genotyped using PCR. Primers that were used for insertion verification and RT–PCR analysis are salk_0020708L 5′-AGCGCAATTTGGGCATGTCT-3′ and salk_0020708R 5′-CCTCCCATGTTAGGCCCTGTT-3′. Multiplex PCR reactions were carried out using these primers in combination with the LBb1 primer (5′-GCGTGGACCGCTTGCTGCAACT-3′) recommended by the Salk Institute. PCR conditions were 3 min at 95°C followed by 35 cycles of 93°C 30 s, 60°C 45 s, 72°C 120 s and a final step of 8 min at 72°C. Confirmation of the null phenotype was carried out using RT–PCR-based analysis (data not shown).
Meiotic recombination. The Atmsh2-1 mutant previously described (Leonard et al, 2003) was crossed with the previously described tester line Le5-11/22 in the Landsberg ecotype background (Melamed-Bessudo et al, 2005). The GFP and RFP markers of this tester line are located on chromosome 5, 6 cM apart. To exclude hybrid seeds that might result from self-pollination, the tester line was used as male. Seeds of the F1 generation expressing the GFP and RFP markers were sown and backcrossed with the homozygous mutant. The resulting F1 plants were backcrossed with the mutant (BC1 generation). BC1 seeds were selected for expressing both RFP and GFP markers and for being homozygote for the Atmsh2-1 mutation. The recombination rate was monitored in self-pollinated seeds of this Atmsh2-1 homozygote BC1 plants (BC1F2 generation), as described (Melamed-Bessudo et al, 2005). As a control for the effect of the mutation, the same scheme of crosses and backcrosses was carried out with wild-type (AtMSH2) Columbia plants. The recombination rate in the AtMSH2 background was also checked in BC1F2 seeds.
Statistical analysis. The mitotic recombination data were analysed using the Wilcoxon's non-parametric test. With this test, the number of blue sectors of the respective mutant and the wild type are converted to rank order data. The ranks from each group are compared. The meiotic recombination data were analysed by two-way ANOVA. All statistical analyses were carried out using the JMP program 5.0.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400577-s1.pdf).
Supplementary Material
Supplementary data
Acknowledgments
We thank R. Opperman for the intrachromosomal assay and the rest of the members of our lab for streaming discussions. This work was carried with the support of the Israeli Science Foundation. A.A.L. holds the Gilbert de Botton chair of Plant Science.
References
- Abdelnoor RV, Yule R, Elo A, Christensen AC, Meyer-Gauen G, Mackenzie SA (2003) Substoichiometric shifting in the plant mitochondrial genome is influenced by a gene homologous to MutS. Proc Natl Acad Sci USA 100: 5968–5973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ade J, Belzile F, Philippe H, Doutriaux MP (1999) Four mismatch repair paralogues coexist in Arabidopsis thaliana: AtMSH2, AtMSH3, AtMSH6-1 and AtMSH6-2. Mol Gen Genet 262: 239–249 [DOI] [PubMed] [Google Scholar]
- Ade J, Haffani Y, Beizile FJ (2001) Functional analysis of the Arabidopsis thaliana mismatch repair gene MSH2. Genome 44: 651–657 [PubMed] [Google Scholar]
- Alonso JM et al. (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Aravind L, Walker DR, Koonin EV (1999) Conserved domains in DNA repair proteins and evolution of repair systems. Nucleic Acids Res 27: 1223–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers SR, Hunter N, Louis EJ, Borts RH (1996) The mismatch repair system reduces meiotic homeologous recombination and stimulates recombination-dependent chromosome loss. Mol Cell Biol 16: 6110–6120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Jinks-Robertson S (1999) The role of the mismatch repair machinery in regulating mitotic and meiotic recombination between diverged sequences in yeast. Genetics 151: 1299–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetelat RT, Meglic V, Cisneros P (2000) A genetic map of tomato based on BC(1) Lycopersicon esculentum × Solanum lycopersicoides reveals overall synteny but suppressed recombination between these homeologous genomes. Genetics 154: 857–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Culligan KM, Hays JB (1997) DNA mismatch repair in plants. An Arabidopsis thaliana gene that predicts a protein belonging to the MSH2 subfamily of eukaryotic MutS homologs. Plant Physiol 115: 833–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culligan KM, Hays JB (2000) Arabidopsis MutS homologs—AtMSH2, AtMSH3, AtMSH6, and a novel AtMSH7—form three distinct protein heterodimers with different specificities for mismatched DNA. Plant Cell 12: 991–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depeiges A, Farget S, Degroote F, Picard G (2005) A new transgene assay to study microsatellite instability in wild-type and mismatch-repair defective plant progenies. Plant Science 168: 939–947 [Google Scholar]
- Dong C, Whitford R, Langridge P (2002) A DNA mismatch repair gene links to the Ph2 locus in wheat. Genome 45: 116–124 [DOI] [PubMed] [Google Scholar]
- Eisen JA (1998) A phylogenomic study of the MutS family of proteins. Nucleic Acids Res 26: 4291–4300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott B, Jasin M (2001) Repair of double-strand breaks by homologous recombination in mismatch repair-defective mammalian cells. Mol Cell Biol 21: 2671–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Rozas H, Kolodner RD (1998) The Saccharomyces cerevisiae MLH3 gene functions in MSH3-dependent suppression of frameshift mutations. Proc Natl Acad Sci USA 95: 12404–12409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfarb T, Alani E (2005) Distinct roles for the Saccharomyces cerevisiae mismatch repair proteins in heteroduplex rejection, mismatch repair and nonhomologous tail removal. Genetics 169: 563–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbunova VV, Levy AA (1999) How plants make ends meet: DNA double-strand break repair. Trends Plant Sci 4: 263–269 [DOI] [PubMed] [Google Scholar]
- Higgins JD, Armstrong SJ, Franklin FC, Jones GH (2004) The Arabidopsis MutS homolog AtMSH4 functions at an early step in recombination: evidence for two classes of recombination in Arabidopsis. Genes Dev 18: 2557–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman PD, Leonard JM, Lindberg GE, Bollmann SR, Hays JB (2004) Rapid accumulation of mutations during seed-to-seed propagation of mismatch-repair-defective Arabidopsis. Genes Dev 18: 2676–2685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter N, Chambers SR, Louis EJ, Borts RH (1996) The mismatch repair system contributes to meiotic sterility in an interspecific yeast hybrid. EMBO J 15: 1726–1733, abs.html [PMC free article] [PubMed] [Google Scholar]
- Kellis M, Patterson N, Endrizzi M, Birren B, Lander ES (2003) Sequencing and comparison of yeast species to identify genes and regulatory elements. Nature 423: 241–254 [DOI] [PubMed] [Google Scholar]
- Kornberg A, Bake TA (1992) DNA Replication. New York, USA: WH Freeman [Google Scholar]
- Leonard JM, Bollmann SR, Hays JB (2003) Reduction of stability of Arabidopsis genomic and transgenic DNA-repeat sequences (microsatellites) by inactivation of AtMSH2 mismatch-repair function. Plant Physiol 133: 328–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Santerre-Ayotte S, Boivin EB, Jean M, Belzile F (2004) A novel reporter for intrachromosomal homoeologous recombination in Arabidopsis thaliana. Plant J 40: 1007–1015 [DOI] [PubMed] [Google Scholar]
- Melamed-Bessudo C, Yehuda E, Stuitje AR, Levy AA (2005) A new seed-based assay for meiotic recombination in Arabidopsis thaliana. Plant J 43: 458–466 [DOI] [PubMed] [Google Scholar]
- Modrich P, Lahue R (1996) Mismatch repair in replication fidelity, genetic recombination, and cancer biology. Annu Rev Biochem 65: 101–133 [DOI] [PubMed] [Google Scholar]
- Opperman R, Emmanuel E, Levy AA (2004) The effect of sequence divergence on recombination between direct repeats in Arabidopsis. Genetics 168: 2207–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit MA, Dimpfl J, Radman M, Echols H (1991) Control of large chromosomal duplications in Escherichia coli by the mismatch repair system. Genetics 129: 327–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado F, Cortes-Ledesma F, Huertas P, Aguilera A (2003) Mitotic recombination in Saccharomyces cerevisiae. Curr Genet 42: 185–198 [DOI] [PubMed] [Google Scholar]
- Puchta H (2005) The repair of double-strand breaks in plants: mechanisms and consequences for genome evolution. J Exp Bot 56: 1–14 [DOI] [PubMed] [Google Scholar]
- Qin J, Baker S, Te Riele H, Liskay RM, Arnheim N (2002) Evidence for the lack of mismatch-repair directed antirecombination during mouse meiosis. J Hered 93: 201–205 [DOI] [PubMed] [Google Scholar]
- Rayssiguier C, Thaler DS, Radman M (1989) The barrier to recombination between Escherichia coli and Salmonella typhimurium is disrupted in mismatch-repair mutants. Nature 342: 396–401 [DOI] [PubMed] [Google Scholar]
- Rick CM (1969) Controlled introgression of chromosomes of Solanum pennellii into Lycopersicon esculentum: segregation and recombination. Genetics 62: 753–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears ER (1976) Genetic control of chromosome pairing in wheat. Annu Rev Genet 10: 31–51 [DOI] [PubMed] [Google Scholar]
- Stojic L, Brun R, Jiricny J (2004) Mismatch repair and DNA damage signalling. DNA Repair (Amst) 3: 1091–1101 [DOI] [PubMed] [Google Scholar]
- Sugawara N, Goldfarb T, Studamire B, Alani E, Haber JE (2004) Heteroduplex rejection during single-strand annealing requires Sgs1 helicase and mismatch repair proteins Msh2 and Msh6 but not Pms1. Proc Natl Acad Sci USA 101: 9315–9320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SY, Culligan K, Lamers M, Hays J (2003) Dissimilar mispair-recognition spectra of Arabidopsis DNA-mismatch-repair proteins MSH2*MSH6 (MutSalpha) and MSH2*MSH7 (MutSgamma). Nucleic Acids Res 31: 6027–6034 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data


