Abstract
Epithelial cell movements, such as those that occur during cell intercalation, largely contribute to the formation of epithelial structures during the morphogenesis of multicellular organisms. As the architecture of epithelial tissues relies on strong adhesion between cells at adherens junctions (AJs), the intercalation or rearrangements of epithelial cells might be controlled by modulating the adhesion dynamics of the AJs by internal or external forces. In this review, we describe recent progress in understanding cell rearrangements during epithelial tube remodelling and discuss several models that might account for the developmental control of the spatial dynamics of AJs.
Keywords: adherens junctions, Drosophila, E-cadherin, epithelia, intercalation, tubulogenesis
Introduction
During development and homeostasis, epithelial tissues are remodelled extensively to account for their structural needs. In some cases, tissue morphogenesis is dependent on changes in either cell shape or orientated cell division. In other cases, morphogenesis is accompanied by cells exchanging their neighbours through ‘intercalation'. Different types of intercalation (such as radial intercalation and axial intercalation) have been described and are at the heart of the restructuring of early embryos before and during gastrulation (Keller, 2002). The importance of cell intercalation to epithelial remodelling in development in general and to morphogenesis in particular was recognized nearly two decades ago (for a review, see Fristrom (1988)). Nevertheless, the molecular scenarios that underlie the astonishing capacity of epithelial cells to remodel while remaining tightly attached to each other through adherens junctions (AJs), and the molecular mechanisms that control the extent of remodelling, are only now being unveiled in a few well-studied cases (Gumbiner, 2005). Here, we briefly discuss emerging cellular mechanisms and the molecules involved in the remodelling of epithelial tubes through cell intercalation, a process that has been recently described in the Drosophila tracheal system by a combination of live imaging, genetic and cellular assays. Whether similar mechanisms remodel epithelial tubes in other tissues and/or organisms remains to be investigated, but parallels to cell intercalation in more simple situations are apparent.
Epithelial remodelling in tubulogenesis
During the past few years, several laboratories have tried to resolve how the ordered formation of distinct tracheal tubes is orchestrated during embryonic development in Drosophila. It has been known for quite some time that starting from epithelial invaginations called ‘tracheal placodes', tracheal cells migrate towards localized sources of the secreted ligand Branchless/Fgf (Bnl/Fgf; Sutherland et al, 1996) by forming dynamic filopodial and lamellopodial extensions from their basal side (Ribeiro et al, 2002). The developmentally controlled expression of Bnl/Fgf in the Drosophila embryo prefigures the direction of branch formation and, in a first phase, bud-like epithelial outgrowths are formed, extending away from the sac that is present at the onset of the process (reviewed in Affolter et al, 2003; Ghabrial et al, 2003; Uv et al, 2003). Tracheal cells keep their adhesive contacts throughout the morphogenetic process; the apical side of the tracheal cells faces the luminal side and is later covered by cuticle structures. As tracheal cells do not divide after invagination, the morphological transformations that accompany outgrowth and elongation of branches are brought about by cell-shape changes and cell intercalation.
The tracheal network formed through branching morphogenesis consists of tubes with different cellular compositions (Samakovlis et al, 1996; Uv et al, 2003). Large tubes consist of several cells wrapped around the lumen, with more than one cell contributing their apical side to the luminal circumference. Cells in such tubes are held together by intercellular AJs, established at a subapical position between neighbouring cells (Fig 1A,B). Finer tubes consist of single cells wrapped around the lumen. Most of the AJs in such tubes represent autocellular AJs: they seal single cells along the lumen. To maintain this chain-like alignment of tube cells, ring-shaped intercellular AJs connect adjacent cells (Fig 1C). The finest tubes at the periphery of the tracheal system are seamless, intracellular tubes that lack AJ complexes along the lumen (Manning & Krasnow, 1993; Samakovlis et al, 1996).
Figure 1.
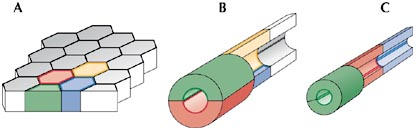
Cellular architecture of epithelial tubes. (A) All tubes of the tracheal system originate from a flat epithelial sheet. By invagination, this flat epithelium is transformed into an epithelial sac from which epithelial tubes form through cell migration, cell intercalation and cell shape changes. (B) Initially, cells in larger tubes retain their intercellular adherens junctions (AJs) that connect them to their neighbours. In such cases, several cells make up the circumference of the lumen. (C) In certain branches, cells intercalate and eventually transform most of their intercellular AJs into autocellular AJs. In these cases, single cells make up the circumference of the lumen.
Recent studies that take advantage of methods allowing the in vivo imaging of individual cell outlines in real time during branch elongation have started to provide significant insight into how cell intercalations transform the epithelial sheet into tubes with differing cellular architectures. In particular, these studies have shown how larger tubes with intercellular AJs are remodelled into finer tubes with mostly autocellular AJs through cell intercalation, a process that must be accompanied by a dramatic rearrangement of the AJ complexes.
Tube remodelling occurs through distinct steps
Several distinct steps involved in the process of tube elongation have been identified by following the rearrangement of AJs during tracheal remodelling in vivo with the help of an α-catenin–GFP (green fluorescent protein) fusion, which specifically labels the AJ complexes in Drosophila (Fig 2; Oda & Tsukita, 1999; Jazwinska et al, 2003; Ribeiro et al, 2004). Starting from a bud-like extension, tracheal cells first seem to align in a pairwise fashion along the tube, with two cells making up the luminal circumference in a cross section, a process we refer to as ‘pairing' (step 1). Pairing seems to precede intercalation, and such an arrangement is conceptually crucial as it allows cells to ‘squeeze' in-between their neighbours. After pairing, cells start to intercalate. The intercalation process is accompanied by extensive AJ remodelling, and we have subdivided this phase into three distinct steps. At the onset, one of the two neighbouring cells starts to reach around the lumen (step 2), a process that ultimately leads to the formation of the first autocellular contact and the formation of the first autocellular AJ, as a cell touches its own membrane extension at the opposite side of the tube circumference. For intercalation to proceed properly, both of the paired cells eventually reach around the lumen but they do so at opposite ends, one cell on the proximal side and its neighbour on the distal side along the axis of elongation. Once the initial autocellular AJ contact has been established, cells seem to ‘zip up' or extend their autocellular junctions by continuously replacing intercellular AJ complexes with autocellular AJs in a process referred to as zipping (step 3). During this zipping, cells transform most of their AJs into autocellular AJs, and only small, ring-like intercellular AJs remain intact and connect to neighbouring cells. To ensure that this contact remains intact and holds cells together in a head-to-tail arrangement, the zipping process must be stopped before it completes and leads to a total loss of intercellular AJs (termination, step 4).
Figure 2.
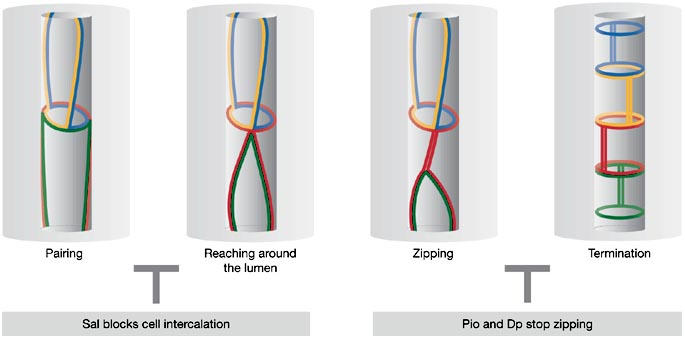
Steps of adherens junction remodelling during cell intercalation and tube elongation. At the onset of intercalation, cells are paired (pairing). On pairing, one cell reaches around the lumen with its apical surface leading to the formation of the first autocellular adherens junction (AJ) contact (reaching around the lumen). Starting from this initial autocellular contact, conversion of intercellular AJs into autocellular AJs proceeds through a process that resembles a zipping mechanism (zipping). The zipping process is stopped by proteins (Piopio (Pio) and Dumpy (Dp)) that are deposited in the tracheal lumen (termination). The entire intercalation process can be blocked by Spalt (Sal).
Genetic control of cell intercalation
Using this four-step model as a conceptual framework, many questions need to be answered to better understand how cell intercalation is controlled during tube formation. Where do the forces that trigger cell intercalation come from? Which molecules are implicated in exerting or regulating the different steps of intercalation? Why do certain branches undergo intercalation whereas others do not?
During the past decade, many genes involved in tracheal development have been identified. Several interesting observations were made when the functions of these gene products were re-examined with regard to intercalation. It was shown that the intercalation process can be blocked by expression of the zinc-finger transcription factor Spalt (Sal) in groups of tracheal cells (Ribeiro et al, 2004). In fact, the Drosophila embryo makes use of Sal to inhibit cell intercalation in the dorsal trunk; as a result of this inhibition, the dorsal trunk remains a large, multicellular tube consisting entirely of cells sharing intercellular AJs with neighbouring cells (Kuhnlein & Schuh, 1996; Ribeiro et al, 2004). The expression of sal is induced by Wnt signalling in dorsal cells of the tracheal sac (Chihara & Hayashi, 2000; Llimargas, 2000). In a few of the dorsal-most cells that ultimately form a neighbouring, finer branch—the dorsal branch—sal transcription is turned off by the transcription factors Knirps and Knirps-related, which are themselves induced by Decapentaplegic (Dpp) signalling (Chen et al, 1998). Therefore, the Wnt and Dpp signalling systems generate two distinct groups of tracheal cells. One group lacks Sal and undergoes intercalation by proceeding with steps 2–4 (that is, reaching around the lumen, zipping and termination; Fig 2), which generates an elongated tube with autocellular AJs. The other group of cells in the dorsal trunk cannot initiate the intercalation process owing to the presence of Sal and thus remains in a multicellular arrangement (Ribeiro et al, 2004). Given the important role of the Sal transcription factor in the developmental control of intercalation, the identification of the genes that are regulated by Sal will enable us to better understand, at the molecular level, how their products interfere with cell intercalation and AJ remodelling.
Little is known about the molecules that are involved in cell pairing (step 1), in cells reaching around the lumen (step 2) or in the zipping process (step 3). However, it seems likely that the regulation of adhesion is crucial for these steps to occur properly. Although many proteins are involved in the establishment and maintenance of epithelial cell–cell contacts, E-cadherin is probably the most important protein for strong intercellular adhesion. E-cadherin is a single-pass transmembrane protein, in which the extracellular domain forms homophilic transdimers between adjacent cell membranes. The cytoplasmic domain interacts with p120 and β-catenin and with Hakai, an E3-ubiquitin ligase (Fujita et al, 2002). β-Catenin binds to α-catenin, which in turn associates with actin filaments. The anchoring of cadherin–catenin complexes to the actin cytoskeleton contributes to the strong adhesiveness of cadherin-based cell–cell contacts, and must presumably be modulated during cell rearrangements. Regulation could occur at many levels, for example by the control of E-cadherin turnover or recycling, by the modulation of the interaction of E-cadherin with the cytoskeleton, or by the regulation of adhesive strength through inside-out signalling (reviewed in Bryant & Stow, 2004; D'Souza-Schorey, 2005; Gumbiner, 2005). Promising candidates for analysis with regard to the intercalation process are: Src, which has been shown to induce the dissociation of epithelial cells; Hakai, an E3-ubiquitin ligase targeting E-cadherin (Fujita et al, 2002); and Arf6, a GTPase that mediates the internalization of E-cadherin (Paterson et al, 2003). Candidates implicated in the regulation of the interaction of E-cadherin with the actin cytoskeleton are, for example, the small GTPases Rho, Rac and Cdc42 (reviewed in Fukata & Kaibuchi, 2001) and the Ras family member Rap1 (Hogan et al, 2004; Price et al, 2004). In addition, various components of the AJ complex itself, including α-catenin, β-catenin and p120, might have specific functions in the regulation of intercalation.
Interestingly, mutations in the genes that encode some of these molecules cause tracheal defects. Mutations in the Drosophila homologue of the nucleoside diphosphate kinase NM23H, which is recruited by Arf6 to facilitate the internalization of E-cadherin (Palacios et al, 2002), results in defects in all tracheal branches (Dammai et al, 2003). Also, double mutants inactivating both Drosophila Src genes show tracheal defects (Takahashi et al, 2005). In addition, the small GTPase Rac1 has been shown to influence cell rearrangements during tracheal development (Chihara et al, 2003). These phenotypes have not been analysed in detail with regard to cell rearrangements, and so the defects cannot yet be related to different steps of intercalation. Furthermore, Rac and Src affect diverse cellular functions, making it difficult to assign direct and indirect roles in the adhesive state of tracheal cells.
The identification of two other mutations, however, has provided significant insight into the last step of the intercalation process, the termination of the conversion of intercellular AJs into autocellular AJs (Jazwinska et al, 2003). Two genes, piopio (pio) and dumpy (dp) were shown to be required for the integrity of the fine, autocellular AJ-containing branches; in the absence of either pio or dp, all fine tubes are transformed into epithelial cysts that are disconnected from the multicellular tubes in the embryo. No obvious defects are observed in the multicellular as well as in the intracellular, seamless tubes in these two mutants. Both pio and dp encode zona pellucida (ZP)-domain-containing proteins that are produced by all tracheal cells and are secreted apically into the luminal space. As it has been shown that several ZP domains trigger the formation of extracellular filaments (Wassarman et al, 2004), it was proposed that Pio and Dp might form rigid heteromeric filaments that are deposited in the lumen; such filaments could provide a physical barrier and hinder the complete zipping of the AJs of tracheal cells. Live imaging of the disruption process in the mutant conditions indeed supports such a model (Jazwinska et al, 2003).
What about the role of other proteins that link neighbouring tracheal cells, such as septate junction and desmosomal proteins, in cell intercalation during tube elongation? Several proteins localizing to the lateral membrane of tracheal cells have been identified in the past few years and mutations in many of them result in an irregular tube size (Bauer et al, 2005; Wu & Beitel, 2004). Whether these molecules influence the intercalation process remains to be shown.
A model for AJ shortening during cell intercalation
As outlined above, the transformation of an epithelial sheet into a network of distinct tracheal tubes requires extensive AJ remodelling, in particular during cell intercalation. Nevertheless, AJ contacts between neighbouring cells need to be maintained throughout the rearrangement procedure to keep cells attached to each other and to form an interconnected, tubular network. Relatively little is known about how epithelial cells physically exchange their neighbours in a coordinated manner, and which molecules are involved in and control this process.
Two recent studies, however, have started to shed some light on these issues (Bertet et al, 2004; Zallen & Wieschaus, 2004). During Drosophila gastrulation, cell intercalation is used to elongate the body axis. It has now been shown that this intercalation depends on the differential localization and action of at least two molecules, Myosin II and Bazooka. Myosin II localizes in the vicinity of AJs orientated along the dorsal–ventral axis, whereas Bazooka is excluded from these junctions but is present on the AJs orientated along the anterior–posterior axis. It turns out that Myosin II and its activation by Rho kinase is required to destabilize and shorten the AJs orientated along the dorsal–ventral axis; by contrast, Bazooka might stabilize the other AJs. On an abstract level, the AJ remodelling events in the epidermis of the early fly embryo have been proposed to resemble the AJ rearrangements during cell intercalation in the tracheal system described above (compare the upper parts of Fig 3A–C with the corresponding tubes shown below; Lecuit, 2005). It has been hypothesized that similar mechanisms drive these two intercalation processes.
Figure 3.
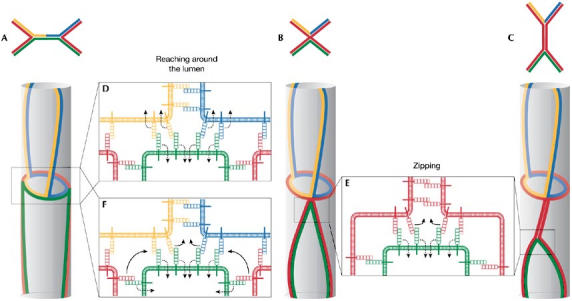
Speculative models for adherens junction remodelling. (A–C) Stages of adherens junction (AJ) remodelling during germband extension (as proposed in Bertet et al, 2004; note that A–C are drawn with anterior at the top). The AJs are coloured to correspond to the tracheal AJs. (D) Analogous to this model, ‘reaching around the lumen' might be mediated by the shrinking of a common AJ between neighbouring cells. (E) By contrast, the ‘zipping' process might rely on the exchange of cadherin interactions between different cells. (F) A similar mechanism could also account for ‘reaching around the lumen'.
Apart from these apparent similarities, there are also profound differences between the two systems. Although the forces that control the axial extension of the germband in the Drosophila embryo are cell intrinsic and might be spatially displayed through the localization of Myosin II, the forces that drive tracheal cell intercalation most probably originate from the migration of tip cells away from the sac-like structure towards the more distant sources of Bnl/Fgf, producing a pulling force that is exerted on the attached, non-migrating cells. It is not known whether this pulling force is the only force tracheal cells experience during the intercalation process, but clearly there is no cell-autonomously driven intercalation in the absence of Bnl/Fgf signalling. Indeed, it has been shown previously that the application of external force can be sufficient to induce intercalation of epithelial cells in Xenopus explants (Beloussov et al, 2000).
Despite the apparent differences in the force-producing mechanism, we have schematically compared the remodelling of AJs during germband extension and tracheal tube morphogenesis. By analogy to germband extension, the shrinking of a common AJ between neighbouring tracheal cells might pull the two ends of an intercalating cell around the lumen (Fig 3D). In this particular scenario, the cell that pairs with the intercalating cell shrinks its common AJ with the two cells sitting above it in the tubular arrangement, leading to an AJ arrangement similar to Fig 3B. During the zipping process, however, it seems that the cell that forms autocellular AJ complexes does so at the expense of the intercellular AJ complexes it initially formed with the neighbouring cell. This process does not intuitively resemble a junctional shrinking but rather a junctional ‘zipping' involving the extracellular remodelling of junctional complexes of one cell (the exchange of the homophilic cadherin-binding partners located on neighbouring cells for cadherin molecules located on the same cell), accompanied by a relocalization/degradation of the unliganded junctional complexes of its neighbour (Fig 3E). In principle, the same mechanism could also account for the process by which cells reach around the lumen (Fig 3F). In this case, one and the same cell would shrink its junctional surface at either the distal or proximal end and, by doing so, induce both steps (‘reaching around the lumen' and ‘zipping') during the intercalation process. In molecular terms, it is thus possible that different mechanisms are at work during germband extension and tracheal cell intercalation. Other models for epithelial rearrangements, in which intercalating epithelial cells behave similarly to mesenchymal cells and form lateral protrusions in-between individual cells, have also been proposed (the ‘cortical tractor' model; Jacobson et al, 1986; Williams-Masson et al, 1998).
At present, most of these models explaining cell rearrangement in tightly adhering epithelial sheets are rather speculative. To understand better the events that occur at the AJs during cell rearrangements, it will be essential to dissect the process molecularly using forward and reverse genetic approaches. In addition, new molecular tools should be introduced into well-defined systems such as the ones described here. Pulse-chase labelling of AJ proteins during intercalation would be helpful to obtain a clearer picture of AJ dynamics and remodelling in different situations. Such methods are available in principle (Griffin et al, 1998; Keppler et al, 2003), but have not yet been well-adapted for applications in living, multicellular organisms. In addition, the dynamics of the lateral membranes should be investigated in single intercalating cells to find out whether this membrane compartment actively forms protrusive activities, or participates relatively passively in the process. Using these and other approaches should eventually provide a molecular view of epithelial remodelling, allowing both for an appreciation of an astonishing biological process and for an eventual interference with the process in certain situations, be it experimental or clinical.
Acknowledgments
We thank C. Cabernard and G. Pyrowolakis for comments on the manuscript. The authors are supported by funds from the Swiss National Science Foundation, the Kantons Basel-Stadt and Basel-Land and the Network of Excellence grant ‘Cells into Organs' from the European Commission.
References
- Affolter M, Bellusci S, Itoh N, Shilo B, Thiery JP, Werb Z (2003) Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev Cell 4: 11–18 [DOI] [PubMed] [Google Scholar]
- Bauer R, Loer B, Ostrowski K, Martini J, Weimbs A, Lechner H, Hoch M (2005) Intercellular communication: the Drosophila innexin multiprotein family of gap junction proteins. Chem Biol 12: 515–526 [DOI] [PubMed] [Google Scholar]
- Beloussov LV, Louchinskaia NN, Stein AA (2000) Tension-dependent collective cell movements in the early gastrula ectoderm of Xenopus laevis embryos. Dev Genes Evol 210: 92–104 [DOI] [PubMed] [Google Scholar]
- Bertet C, Sulak L, Lecuit T (2004) Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature 429: 667–671 [DOI] [PubMed] [Google Scholar]
- Bryant DM, Stow JL (2004) The ins and outs of E-cadherin trafficking. Trends Cell Biol 14: 427–434 [DOI] [PubMed] [Google Scholar]
- Chen CK, Kuhnlein RP, Eulenberg KG, Vincent S, Affolter M, Schuh R (1998) The transcription factors KNIRPS and KNIRPS RELATED control cell migration and branch morphogenesis during Drosophila tracheal development. Development 125: 4959–4968 [DOI] [PubMed] [Google Scholar]
- Chihara T, Hayashi S (2000) Control of tracheal tubulogenesis by Wingless signaling. Development 127: 4433–4442 [DOI] [PubMed] [Google Scholar]
- Chihara T, Kato K, Taniguchi M, Ng J, Hayashi S (2003) Rac promotes epithelial cell rearrangement during tracheal tubulogenesis in Drosophila. Development 130: 1419–1428 [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C (2005) Disassembling adherens junctions: breaking up is hard to do. Trends Cell Biol 15: 19–26 [DOI] [PubMed] [Google Scholar]
- Dammai V, Adryan B, Lavenburg KR, Hsu T (2003) Drosophila awd, the homolog of human nm23, regulates FGF receptor levels and functions synergistically with shi/dynamin during tracheal development. Genes Dev 17: 2812–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fristrom D (1988) The cellular basis of epithelial morphogenesis. A review. Tissue Cell 20: 645–690 [DOI] [PubMed] [Google Scholar]
- Fujita Y, Krause G, Scheffner M, Zechner D, Leddy HEM, Behrens J, Sommer T, Birchmeier W (2002) Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat Cell Biol 4: 222–231 [DOI] [PubMed] [Google Scholar]
- Fukata M, Kaibuchi K (2001) Rho-family GTPases in cadherin-mediated cell–cell adhesion. Nat Rev Mol Cell Biol 2: 887–897 [DOI] [PubMed] [Google Scholar]
- Ghabrial A, Luschnig S, Metzstein MM, Krasnow MA (2003) Branching morphogenesis of the Drosophila tracheal system. Annu Rev Cell Dev Biol 19: 623–647 [DOI] [PubMed] [Google Scholar]
- Griffin BA, Adams SR, Tsien RY (1998) Specific covalent labeling of recombinant protein molecules inside live cells. Science 281: 269–272 [DOI] [PubMed] [Google Scholar]
- Gumbiner BM (2005) Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol 6: 622–634 [DOI] [PubMed] [Google Scholar]
- Hogan C, Serpente N, Cogram P, Hosking CR, Bialucha CU, Feller SM, Braga VM, Birchmeier W, Fujita Y (2004) Rap1 regulates the formation of E-cadherin-based cell–cell contacts. Mol Cell Biol 24: 6690–6700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson AG, Oster GF, Odell GM, Cheng LY (1986) Neurulation and the cortical tractor model for epithelial folding. J Embryol Exp Morphol 96: 19–49 [PubMed] [Google Scholar]
- Jazwinska A, Ribeiro C, Affolter M (2003) Epithelial tube morphogenesis during Drosophila tracheal development requires Piopio, a luminal ZP protein. Nat Cell Biol 5: 895–901 [DOI] [PubMed] [Google Scholar]
- Keller R (2002) Shaping the vertebrate body plan by polarized embryonic cell movements. Science 298: 1950–1954 [DOI] [PubMed] [Google Scholar]
- Keppler A, Gendreizig S, Gronemeyer T, Pick H, Vogel H, Johnsson K (2003) A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat Biotechnol 21: 86–89 [DOI] [PubMed] [Google Scholar]
- Kuhnlein RP, Schuh R (1996) Dual function of the region-specific homeotic gene spalt during Drosophila tracheal system development. Development 122: 2215–2223 [DOI] [PubMed] [Google Scholar]
- Lecuit T (2005) Adhesion remodeling underlying tissue morphogenesis. Trends Cell Biol 15: 34–42 [DOI] [PubMed] [Google Scholar]
- Llimargas M (2000) Wingless and its signalling pathway have common and separable functions during tracheal development. Development 127: 4407–4417 [DOI] [PubMed] [Google Scholar]
- Manning G, Krasnow MA (1993) Development of the Drosophila tracheal system. In Bate M, Martinez-Arias A (eds), The Development of Drosophila melanogaster, pp 609–685. Cold Spring Harbor, NY, USA: Cold Spring Harbor Laboratory Press [Google Scholar]
- Oda H, Tsukita S (1999) Dynamic features of adherens junctions during Drosophila embryonic epithelial morphogenesis revealed by a Dα-catenin–GFP fusion protein. Dev Genes Evol 209: 218–225 [DOI] [PubMed] [Google Scholar]
- Palacios F, Schweitzer JK, Boshans RL, D'Souza-Schorey C (2002) ARF6–GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat Cell Biol 4: 929–936 [DOI] [PubMed] [Google Scholar]
- Paterson AD, Parton RG, Ferguson C, Stow JL, Yap AS (2003) Characterization of E-cadherin endocytosis in isolated MCF-7 and Chinese hamster ovary cells: the initial fate of unbound e-cadherin. J Biol Chem 278: 21050–21057 [DOI] [PubMed] [Google Scholar]
- Price LS, Hajdo-Milasinovic A, Zhao J, Zwartkruis FJT, Collard JG, Bos JL (2004) Rap1 regulates E-cadherin-mediated cell–cell adhesion. J Biol Chem 279: 35127–35132 [DOI] [PubMed] [Google Scholar]
- Ribeiro C, Ebner A, Affolter M (2002) In vivo imaging reveals different cellular functions for FGF and Dpp signaling in tracheal branching morphogenesis. Dev Cell 2: 677–683 [DOI] [PubMed] [Google Scholar]
- Ribeiro C, Neumann M, Affolter M (2004) Genetic control of cell intercalation during tracheal morphogenesis in Drosophila. Curr Biol 14: 2197–2207 [DOI] [PubMed] [Google Scholar]
- Samakovlis C, Hacohen N, Manning G, Sutherland DC, Guillemin K, Krasnow MA (1996) Development of the Drosophila tracheal system occurs by a series of morphologically distinct but genetically coupled branching events. Development 122: 1395–1407 [DOI] [PubMed] [Google Scholar]
- Sutherland D, Samakovlis C, Krasnow MA (1996) branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell 87: 1091–1101 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Takahashi F, Ui-Tei K, Kojima T, Saigo K (2005) Requirements of genetic interactions between Src42A, armadillo and shotgun, a gene encoding E-cadherin, for normal development in Drosophila. Development 132: 2547–2559 [DOI] [PubMed] [Google Scholar]
- Uv A, Cantera R, Samakovlis C (2003) Drosophila tracheal morphogenesis: intricate cellular solutions to basic plumbing problems. Trends Cell Biol 13: 301–309 [DOI] [PubMed] [Google Scholar]
- Wassarman PM, Jovine L, Litscher ES (2004) Mouse zona pellucida genes and glycoproteins. Cytogenet Genome Res 105: 228–234 [DOI] [PubMed] [Google Scholar]
- Williams-Masson EM, Heid PJ, Lavin CA, Hardin J (1998) The cellular mechanism of epithelial rearrangement during morphogenesis of the Caenorhabditis elegans dorsal hypodermis. Dev Biol 204: 263–276 [DOI] [PubMed] [Google Scholar]
- Wu VM, Beitel GJ (2004) A junctional problem of apical proportions: epithelial tube-size control by septate junctions in the Drosophila tracheal system. Curr Opin Cell Biol 16: 493–499 [DOI] [PubMed] [Google Scholar]
- Zallen JA, Wieschaus E (2004) Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell 6: 343–355 [DOI] [PubMed] [Google Scholar]


