Abstract
The antimicrobial defence of Drosophila relies on cellular and humoral processes, of which the inducible synthesis of antimicrobial peptides has attracted interest in recent years. Another potential line of defence is the activation, by a proteolytic cascade, of phenoloxidase, which leads to the production of quinones and melanin. However, in spite of several publications on this subject, the contribution of phenoloxidase activation to resistance to infections has not been established under appropriate in vivo conditions. Here, we have isolated the first Drosophila mutant for a prophenoloxidase-activating enzyme (PAE1). In contrast to wild-type flies, PAE1 mutants fail to activate phenoloxidase in the haemolymph following microbial challenge. Surprisingly, we find that these mutants are as resistant to infections as wild-type flies, in the total absence of circulating phenoloxidase activity. This raises the question with regard to the precise function of phenoloxidase activation in defence, if any.
Keywords: Drosophila, innate immunity, PAE, prophenoloxidase, serine protease
Introduction
Besides the well-documented induction of antimicrobial peptides (Hoffmann, 2003; Brennan & Anderson, 2004; Leclerc & Reichhart, 2004), a second potential defence mechanism in insects (and in most invertebrates) is the activation of phenoloxidase (PO; Cerenius & Soderhall, 2004). In Drosophila, the crystal cells, a specific class of haemocytes, synthesize PO as an inactive prophenoloxidase (proPO) precursor. Biochemical data derived from large insects, such as Manduca sexta, led to the present model of PO activation, in which the recognition of microorganisms triggers a proPO-activating enzyme (PAE) proteolytic cascade culminating in the liberation of active PO. The activated PO then catalyses the oxidation of tyrosine-derived phenols to quinones. Quinones are believed to be directly toxic to microorganisms and also to polymerize non-enzymatically to form insoluble melanin. Melanin deposition is observed at all infection sites, where it possibly contributes to wound healing and control of microorganism growth.
In spite of several publications on this subject in various invertebrates (for reviews, see Sugumaran, 2002; Cerenius & Soderhall, 2004; Christensen et al, 2005), the precise contribution of PO activation to survival to microbial infections has not been genetically investigated. To address this problem, we decided to generate mutant flies that are unable to activate PO, targeting the PAE upstream regulators. In Drosophila, except for a proteolytic activity purified from total pupae extracts (Chosa et al, 1997), no PAE has been characterized so far. However, we have recently identified a serine protease inhibitor, Serpin27A, involved in the control of PO activation. Loss-of-function mutations in this serpin result in constitutive PO activation and an excessive melanization at the site of injury, indicating that at least one serine protease is involved in PO activation in Drosophila (De Gregorio et al, 2002a; Ligoxygakis et al, 2002b). Here, we present a new mutation that renders the flies unable to activate circulating PO. Surprisingly, we observed that, in these mutant flies, the survival to infections is not affected.
Results and Discussion
Detection of proPO cleavage in the haemolymph
The haemolymph of uninfected flies is devoid of any detectable PO activity, and injection of a mix of Gram-negative and Gram-positive bacteria induces a marked level of activity in 3 h, as reported earlier (Ligoxygakis et al, 2002b). Drosophila and Anopheles proPOs share a high sequence conservation, and antibodies raised against Anopheles PPO2 protein (Muller et al, 1999) have been successfully used to detect Drosophila proPO in crystal cells, by immunolocalization (Duvic et al, 2002). Assuming that the induction of PO activity results from the cleavage of proPO in the haemolymph of the flies (Chosa et al, 1997), we used an anti-Anopheles PPO2 serum on western blot. We detected a single band of about 75 kDa in the absence of infection, and a second band of about 72 kDa following microbial challenge (Fig 1), which correlates with the calculated molecular weight for Drosophila proPO and PO, respectively. As expected, we could not detect any band in the haemolymph of the Black cell (Bc) mutant, which is devoid of circulating PO activity (Rizki et al, 1980; Fig 2A).
Figure 1.

Anti-Anopheles gambiae PPO2 antibodies detect prophenoloxidase (proPO) cleavage in Drosophila. Immunoblotting experiments detect a single band of about 75 kDa in control (c) wild-type Drosophila (WT) haemolymph and a faster migrating band 4 h after infection (i) with a mixture of Gram-positive (Micrococcus luteus) and Gram-negative (Escherichia coli) bacteria (the same infection procedure is used in all figures unless otherwise stated). After immune challenge, the low-molecular-weight band is absent in Dif but not in kenny (key) mutants. The high-molecular-weight band corresponds to the proPO (PPO, double arrowhead) and the lower band to phenoloxidase (PO, single arrowhead). The same symbols are used in all figures. Note that, depending on the experiment, some proPO is still present after microbial challenge (see wild type in Fig 2A for example).
Figure 2.
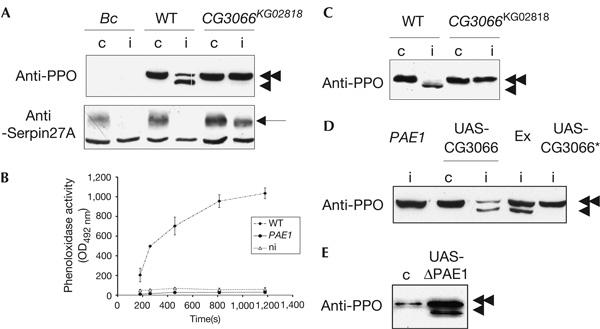
CG3066 encodes a prophenoloxidase-activating enzyme. (A) Prophenoloxidase (proPO), which is absent from the haemolymph of Black cell (Bc) flies, is cleaved after immune challenge in wild-type (WT) flies but not in flies homozygous for the CG3066KG02818 insertion (CG3066KG02818). The blot, incubated with anti-proPO antibodies (anti-PPO), was stripped and re-probed with anti-Serpin27A antibodies (anti-Serpin27A, arrow, the lowest band is unspecific; Ligoxygakis et al, 2002b). Serpin27A is depleted after immune challenge in the haemolymph of WT and Bc mutant flies, but not in the haemolymph of flies homozygous for CG3066KG02818. (B) Phenoloxidase activity is detected (as absorbance at an optical density of 492 nm (OD492 nm) after conversion of L-3,4-dihydroxyphenylalanine) in the haemolymph of immune-challenged WT flies, but not in the haemolymph of CG3066KG02818 immune-challenged flies (PAE1) or WT non-challenged flies (ni). (C) proPO fails to be cleaved when CG3066KG02818 homozygous flies are infected (i) with the yeast Candida albicans, as is the case after bacterial infection. (D) Immunoblotting experiments with anti-proPO antibodies on the haemolymph of control (c) or infected (i) flies show that the proPO cleavage, which is absent in CG3066KG02818 (PAE1) mutant flies, is restored when the KG02818 insertion is excised (Ex) or when a WT copy of the CG3066 gene (UAS-CG3066), but not a version mutated in the catalytic domain of the protein (UAS-CG3066*), is expressed in the CG3066KG02818 background. (E) In the absence of any challenge (c), the expression of an activated form of the CG3066-encoded protein (UAS-ΔPAE1) leads to the constitutive cleavage of proPO.
We had previously shown that PO activation in the haemolymph was dependent on the Toll pathway but did not require genes of the immune deficiency (IMD) pathway (Ligoxygakis et al, 2002b). Using the anti-proPO antibodies, we could confirm that, after microbial challenge, the lowest band is absent from the haemolymph of Toll pathway mutants (Dif; Fig 1) but still present in the IMD pathway mutant kenny (key; Fig 1), although reduced in intensity. Therefore, in all conditions tested, the appearance on western blots of the 72 kDa band correlates with the detection of PO activity (see supplementary Fig 1 online for analysis of antibody specificity). This validates the use of the anti-Anopheles PPO2 antibodies as a tool to detect activation of PO in the Drosophila haemolymph.
CG3066 encodes a PAE
Biochemical studies on several invertebrates have shown that the PAE serine proteases contain a regulatory clip domain that has to be cleaved for activation of the enzyme (reviewed by Cerenius & Soderhall, 2004). A total of 24 genes encoding clip domain-containing serine proteases are present in the Drosophila genome. Among these, ten have been classified as potential PAEs on the basis of sequence similarity with the PAEs purified from several insect species (Ross et al, 2003).
The serine protease Easter is one of these candidate PAEs. Together with Snake and Gastrulation defective, Easter forms the proteolytic cascade that activates, under the control of Serpin27A, the Toll receptor during the establishment of the dorso-ventral axis in the Drosophila early embryo (Anderson, 1998; Hashimoto et al, 2003; Ligoxygakis et al, 2003). However, in easter, snake or gastrulation defective mutant flies, proPO cleavage is similar to that in wild-type flies (supplementary Fig 2 online), indicating that the embryonic proteolytic cascade is not used for proPO activation in adult Drosophila.
To identify the Drosophila PAEs, we screened the databases for transposon insertions in the vicinity of candidate proteases and found that the P element KG02818 could potentially inactivate CG3066, as it is inserted in the second exon of the gene. In flies homozygous for the CG3066KG02818 insertion, PO cleavage could not be detected after immune challenge with a mix of Gram-positive and Gram-negative bacteria or with the yeast Candida albicans (Fig 2A,C). This is correlated with an absence of PO activity in the haemolymph (Fig 2B). We verified by two different rescue experiments that the phenotype is due to the insertion of the transposon into CG3066. In the first experiment, we precisely excised the KG02818 element from the mutant chromosome (Fig 2D, Ex), and, in the second, we expressed the CG3066 complementary DNA in the homozygous CG3066KG02818 mutant background, using the UAS-GAL4 system (Brand & Perrimon, 1993) and a female fat-body-specific yolk promoter driving Gal4 (Fig 2D, UAS-CG3066). In both cases, proPO cleavage was fully restored after immune challenge. The proteolytic function of the CG3066-encoded protein is absolutely required, as the expression of a mutated non-catalytic form of the serine protease, in which the serine of the active site was replaced by a glycine (Fig 2D, UAS-CG3066*), could not restore a normal cleavage of the proPO in the mutant background.
The activation of the melanization cascade is known to be under the control of the Toll pathway (Fig 1; Ligoxygakis et al, 2002b). The Toll pathway is activated by a proteolytic cascade, and the observed lack of proPO cleavage in the CG3066 mutant background could result from a default in the activation of this pathway. We observed that the expression of the Toll pathway target gene Drosomycin was affected neither in a CG3066KG02818 mutant background nor in flies overexpressing CG3066, using the UAS-GAL4 system (data not shown). CG3066 is thus not involved in the activation of the Toll pathway. The protein meets all the requirements for a bona fide proPO-activating enzyme, and we propose to name this protease PAE1.
proPO is activated by a proteolytic cascade in Drosophila
Biochemical studies with large insect species have established a model of PO activation, in which proPO is cleaved after the activation of a cascade of serine proteases. Under normal circumstances, this hypothetic cascade is kept inactive by Serpin27A in Drosophila. Serpin27A could potentially inhibit the end protease of the proPO activation cascade in the same way as it inhibits Easter, the end protease of the cascade that controls embryonic dorso-ventral axis formation (De Gregorio et al, 2002a; Ligoxygakis et al, 2002b, 2003; Hashimoto et al, 2003).
Serpin27A is degraded in wild-type flies shortly after immune challenge, probably through interaction with its target protease (Fig 2A; Ligoxygakis et al, 2002b), but not in PAE1 mutant flies (Fig 2A, CG3066KG02818), which means that the serpin inhibits either PAE1 itself or a downstream protease. Moreover, Spn27A;CG3066KG02818 double mutants still present spontaneous large melanization spots, with the same frequency as Spn27A simple mutants (data not shown), indicating that PAE1 is not required for the activation of proPO in the absence of Serpin27A and is therefore not its target. PAE1 does not directly cleave proPO, but activates a downstream protease ultimately responsible for the cleavage of proPO and regulated by Serpin27A. To our knowledge, this is the first genetic demonstration that PO activation requires a cascade of at least two proteolytic enzymes.
Regulation of proPO activation
As previously shown, the Toll pathway controls PO activation, probably by inducing the expression of a protease resulting in disequilibrium in the respective concentrations of serpin and proteases and the subsequent activation of the cascade (Ligoxygakis et al, 2002b). PAE1 transcription is controlled by the Toll pathway and increases as early as 1.5 h after an infection (De Gregorio et al, 2001, 2002b; Irving et al, 2001). However, overexpression of PAE1 in the absence of challenge does not induce proPO cleavage (Fig 2D), suggesting that PAE1 is unable to self-activate without immune challenge.
We designed an activated form of PAE1 (ΔPAE1), with the same strategy as that used to construct an activated form of Easter lacking the amino-terminal regulatory Clip domain (Chasan et al, 1992; Ligoxygakis et al, 2003). When expressed under the control of the fat-body-specific yolk-Gal4 driver, it resulted in a constitutive proPO cleavage in the haemolymph (Fig 2E, UAS-ΔPAE1). The expression of an activated PAE1 is therefore able to turn on the downstream target protease and override its inhibition by Serpin27A. During an infection, the protease cascade has to be activated, probably by the recognition of microorganisms. Shortly after a challenge, a dark melanized spot is usually observed at the site of the wound. In Spn27A mutants, this spot is considerably enlarged, presumably because Serpin27A is not able to restrict anymore proPO activation to the site of injury (De Gregorio et al, 2002a; Ligoxygakis et al, 2002b). Flies overexpressing the full-length PAE1 protein present no constitutive proPO cleavage (Fig 2D), but show the same phenotype of excessive melanization around the infection site after pricking (supplementary Fig 3 online). Hence, proPO activation seems to be controlled by both the Toll pathway-driven expression of a protease that is usually present in limited amounts and by the activation signal resulting from the recognition of microorganisms. Peptidoglycan recognition protein-LE is a good candidate for a receptor delivering such a signal, as it activates PO in larval haemolymph when overexpressed (Takehana et al, 2002), and is required for PO activation after Escherichia coli infection (Takehana et al, 2004). When the activated form of PAE1 was driven by daughterless-Gal4, no or very few adult flies emerged, probably because of the toxic effect of the ectopic protease activity. This larval lethality associated with large melanization spots is similar to the lethality observed for Spn27A mutant larvae. Therefore, we can assume that a strict double control is required to prevent inappropriate activation of PO that is deleterious to the flies.
Absence of susceptibility to infections
As the PAE1 mutant is unable to activate PO in the haemolymph after an infection, it is a good tool to test the potential function of PO activity in defence against infections. We analysed the susceptibility of PAE1 mutants to infections with different microorganisms: natural infection with the entomopathogenic fungus Beauveria bassiana and challenge with a needle contaminated with the yeast C. albicans, the Gram-negative bacteria Agrobacterium tumefaciens and E. coli or the Gram-positive bacteria Enterococcus faecalis and Staphylococcus aureus. Surprisingly, the PAE1 mutant flies survived as wild-type flies after all infections that we could test (Fig 3; data not shown). Accordingly, the number of E. coli bacteria still alive inside the body cavity 20 h after infection was similar in PAE1 mutant flies and wild-type flies and almost 500 times lesser than in IMD pathway mutant flies (supplementary Fig 4 online). This correlates well with recent results showing that Drosophila PO has no major killing effect on bacteria (Bidla et al, 2005).
Figure 3.

Prophenoloxidase activation mutants survive similar to wild-type flies to infection. Representative survival curve of white (w, wild-type control) and relish (rel, immune deficiency pathway mutant), spaetzle (spz, Toll pathway mutant), PAE1 and Black cell (Bc) mutant flies after infection with Gram-positive bacteria (Enterococcus faecalis (A)), Gram-negative bacteria (Agrobacterium tumefaciens (B)) or after natural infection with the fungus Beauveria bassiana (C).
These results clearly indicate that PO activity in the haemolymph of adult flies is dispensable for survival to infections, raising the question of its role, if any, in immune defence in Drosophila. A possible role for PO was suggested by the analysis of the Bc mutant phenotype. This mutation is characterized by the absence of PO in the haemolymph (Rizki et al, 1980; Lanot et al, 2001). The gene corresponding to the Bc mutation has not yet been isolated, but it probably affects a gene required for secretion of proPO into the haemolymph. Bc mutant flies are slightly susceptible to all infections, with a survival rate lower than wild-type flies but significantly higher than that of Toll or IMD pathway mutant flies (Fig 3; De Gregorio et al, 2002a). This indicates that the Bc mutation affects a gene or function that has a weak but general role in resistance to infections. However, this phenotype is independent of the lack of PO secretion. Our results on the PAE1 mutant indicate that survival of adult flies to microbial infections does not require circulating PO activity.
Methods
Drosophila strains. OregonR flies were used as wild-type controls. Other stocks have been described previously: Dif1 (Rutschmann et al, 2000b), key1 (Rutschmann et al, 2000a), Bc (Rizki et al, 1980), daGAL4 (Ligoxygakis et al, 2002a) and ea1, ea2, snk073, snk233 and gd7 (Ligoxygakis et al, 2003). The transposon insertion P{SUPor-P}CG3066KG02818 stock was obtained from the Bloomington Stock Center. We observed a strong susceptibility to B. bassiana infections in the original CG3066KG02818 insertion that is not rescued by the excision of KG02818 and that we could easily separate, using meiotic recombination, from the P-element insertion responsible for the defective proPO cleavage. Clone SD07170 from the BDGP EST project matches the CG3066 gene. For the inactive version of the protease, the catalytic serine (Ser 341) was replaced by a glycine using PCR-directed mutagenesis. To obtain an activated form of the protease, we used the strategy described by Chasan et al (1992) for Easter: the PCR-amplified proteolytic domain (starting Val 137) with cysteine 264 replaced by serine was linked to the Easter signal sequence. The resulting constructs were cloned into pUAST (Brand & Perrimon, 1993).
Microbial strains and survival experiments. We used the following microbial organisms: E. coli (1106), Micrococcus luteus (CIP A270), E. faecalis (a kind gift from H. Monteil), S. aureus, A. tumefaciens, C. albicans (a pathogenic strain isolated in patient no. 3 by Pr. M. Koenig, CHU Strasbourg-Hautepierre) and B. bassiana (80.2 strain).
Survival experiments were carried out as described previously (Rutschmann et al, 2000b; Ligoxygakis et al, 2002a). Each experiment was repeated at least three times.
Sample preparation and analysis. Infections, haemolymph collection, sample preparation, western blot analysis and PO activity assay were as described by Ligoxygakis et al (2002b). Western blots were incubated with rabbit anti-glutathione S-transferase–Serpin27A antibodies (Ligoxygakis et al, 2002b) and rabbit anti-Anopheles gambiae PPO2 antibodies (a generous gift from H.M. Müller) overnight at 4°C at a dilution of 1/5,000 and 1/10,000, respectively.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400592-s1.pdf).
Supplementary Material
Supplementary information
Acknowledgments
We are grateful to A. Meunier, R. Walther and S. Ozkan for technical help. We thank H.-M. Müller for the anti-PPO2 antibodies and D. Ferrandon and M. Meister for a critical reading of the manuscript. This work was supported financially by the Centre National de la Recherche Scientifique and a National Institutes of Health Program Grant PO1 AI44220.
References
- Anderson KV (1998) Pinning down positional information: dorsal–ventral polarity in the Drosophila embryo. Cell 95: 439–442 [DOI] [PubMed] [Google Scholar]
- Bidla G, Lindgren M, Theopold U, Dushay MS (2005) Hemolymph coagulation and phenoloxidase in Drosophila larvae. Dev Comp Immunol 29: 669–679 [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415 [DOI] [PubMed] [Google Scholar]
- Brennan CA, Anderson KV (2004) Drosophila: the genetics of innate immune recognition and response. Annu Rev Immunol 22: 457–483 [DOI] [PubMed] [Google Scholar]
- Cerenius L, Soderhall K (2004) The prophenoloxidase-activating system in invertebrates. Immunol Rev 198: 116–126 [DOI] [PubMed] [Google Scholar]
- Chasan R, Jin Y, Anderson KV (1992) Activation of the easter zymogen is regulated by five other genes to define dorsal–ventral polarity in the Drosophila embryo. Development 115: 607–616 [DOI] [PubMed] [Google Scholar]
- Chosa N, Fukumitsu T, Fujimoto K, Ohnishi E (1997) Activation of prophenoloxidase A1 by an activating enzyme in Drosophila melanogaster. Insect Biochem Mol Biol 27: 61–68 [DOI] [PubMed] [Google Scholar]
- Christensen BM, Li J, Chen CC, Nappi AJ (2005) Melanization immune responses in mosquito vectors. Trends Parasitol 21: 192–199 [DOI] [PubMed] [Google Scholar]
- De Gregorio E, Spellman PT, Rubin GM, Lemaitre B (2001) Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc Natl Acad Sci USA 98: 12590–12595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio E, Han SJ, Lee WJ, Baek MJ, Osaki T, Kawabata S, Lee BL, Iwanaga S, Lemaitre B, Brey PT (2002a) An immune-responsive Serpin regulates the melanization cascade in Drosophila. Dev Cell 3: 581–592 [DOI] [PubMed] [Google Scholar]
- De Gregorio E, Spellman PT, Tzou P, Rubin GM, Lemaitre B (2002b) The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J 21: 2568–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvic B, Hoffmann JA, Meister M, Royet J (2002) Notch signaling controls lineage specification during Drosophila larval hematopoiesis. Curr Biol 12: 1923–1927 [DOI] [PubMed] [Google Scholar]
- Hashimoto C, Kim DR, Weiss LA, Miller JW, Morisato D (2003) Spatial regulation of developmental signaling by a serpin. Dev Cell 5: 945–950 [DOI] [PubMed] [Google Scholar]
- Hoffmann JA (2003) The immune response of Drosophila. Nature 426: 33–38 [DOI] [PubMed] [Google Scholar]
- Irving P, Troxler L, Heuer TS, Belvin M, Kopczynski C, Reichhart JM, Hoffmann JA, Hetru C (2001) A genome-wide analysis of immune responses in Drosophila. Proc Natl Acad Sci USA 98: 15119–15124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanot R, Zachary D, Holder F, Meister M (2001) Postembryonic hematopoiesis in Drosophila. Dev Biol 230: 243–257 [DOI] [PubMed] [Google Scholar]
- Leclerc V, Reichhart JM (2004) The immune response of Drosophila melanogaster. Immunol Rev 198: 59–71 [DOI] [PubMed] [Google Scholar]
- Ligoxygakis P, Pelte N, Hoffmann JA, Reichhart JM (2002a) Activation of Drosophila Toll during fungal infection by a blood serine protease. Science 297: 114–116 [DOI] [PubMed] [Google Scholar]
- Ligoxygakis P, Pelte N, Ji C, Leclerc V, Duvic B, Belvin M, Jiang H, Hoffmann JA, Reichhart JM (2002b) A serpin mutant links Toll activation to melanization in the host defence of Drosophila. EMBO J 21: 6330–6337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ligoxygakis P, Roth S, Reichhart JM (2003) A serpin regulates dorsal–ventral axis formation in the Drosophila embryo. Curr Biol 13: 2097–2102 [DOI] [PubMed] [Google Scholar]
- Muller HM, Dimopoulos G, Blass C, Kafatos FC (1999) A hemocyte-like cell line established from the malaria vector Anopheles gambiae expresses six prophenoloxidase genes. J Biol Chem 274: 11727–11735 [DOI] [PubMed] [Google Scholar]
- Rizki TM, Rizki RM, Grell EH (1980) A mutant affecting crystal cells in Drosophila melanogaster. Roux Arch Dev Biol 188: 91–99 [DOI] [PubMed] [Google Scholar]
- Ross J, Jiang H, Kanost MR, Wang Y (2003) Serine proteases and their homologs in the Drosophila melanogaster genome: an initial analysis of sequence conservation and phylogenetic relationships. Gene 304: 117–131 [DOI] [PubMed] [Google Scholar]
- Rutschmann S, Jung A, Zhou R, Silverman N, Hoffmann JA, Ferrandon D (2000a) Role of Drosophila IKKγ in a Toll-independent antibacterial immune response. Nat Immunol 1: 342–347 [DOI] [PubMed] [Google Scholar]
- Rutschmann S, Jung AC, Hetru C, Reichhart JM, Hoffmann JA, Ferrandon D (2000b) The Rel protein DIF mediates the antifungal but not the antibacterial host defense in Drosophila. Immunity 12: 569–580 [DOI] [PubMed] [Google Scholar]
- Sugumaran M (2002) Comparative biochemistry of eumelanogenesis and the protective roles of phenoloxidase and melanin in insects. Pigment Cell Res 15: 2–9 [DOI] [PubMed] [Google Scholar]
- Takehana A, Katsuyama T, Yano T, Oshima Y, Takada H, Aigaki T, Kurata S (2002) Overexpression of a pattern-recognition receptor, peptidoglycan-recognition protein-LE, activates imd/relish-mediated antibacterial defense and the prophenoloxidase cascade in Drosophila larvae. Proc Natl Acad Sci USA 99: 13705–13710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehana A, Yano T, Mita S, Kotani A, Oshima Y, Kurata S (2004) Peptidoglycan recognition protein (PGRP)-LE and PGRP-LC act synergistically in Drosophila immunity. EMBO J 23: 4690–4700 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information


