Abstract
Neurofibromatosis type 1 (NF1) is a common tumour predisposition syndrome associated with numerous clinical complications. Mutations in the tumour suppressor gene NF1 are responsible for disease pathogenesis. This gene encodes the 320 kDa protein neurofibromin, the only clearly defined function of which is to act as a Ras-specific GTPase-activating protein (RasGAP). Here we report the structural discovery of a novel module in neurofibromin, composed of a Sec14p homologous segment and a previously undetected pleckstrin homology (PH)-like domain of potentially novel function. We show phospholipid binding by this bipartite module and identify residues that are involved in this activity; we also show that the PH-like domain is not sufficient for lipid binding. The unique architecture of the domain interface points to a model of how the PH-like domain may regulate binding of a ligand by the Sec14 module.
Keywords: neurofibromin, PH domain, phospholipid, Sec14, tumour suppressor
Introduction
Neurofibromatosis type 1 (also known as von Recklinghausen neurofibromatosis or NF1) is a common autosomal dominant genetic disease affecting 1 in 3,500 births, with 50% of the cases representing spontaneous alterations (Riccardi, 1992). Hallmark symptoms include pigment anomalies (café au lait macules), skeletal deformations, optic gliomas, learning disabilities and nervous tumours called neurofibromas. Mutations of the tumour suppressor gene NF1 are responsible for disease pathogenesis, with 90% of the alterations being nonsense codons (Upadhyaya et al, 1994). The NF1 gene encodes a 320 kDa protein, termed neurofibromin, which is a Ras-specific GTPase-activating protein (RasGAP). Its GAP activity is associated with a central portion of the protein, termed GAP-related domain (GRD) (Cichowski & Jacks, 2001).
Although several interacting proteins have been reported (Izawa et al, 1996; Tokuo et al, 2001; Feng et al, 2004), including tubulin (Bollag et al, 1993) and heparan sulphate proteoglycans (Hsueh et al, 2001), the RasGAP activity remains the only well-characterized biochemical function. Observations according to which the expression of a protein kinase A (PKA) transgene could rescue NF1−/− phenotypes in Drosophila melanogaster suggested a role of neurofibromin in regulating cyclic AMP controlled signalling pathways (The et al, 1997), although the responsible mechanisms have remained elusive.
We aim to identify neurofibromin functions by comparing results of structural analyses of the protein with proteins of known structures (www.rcsb.org). Structural similarities or bound ligands would be a source of experimental ideas to investigate biochemical/functional properties. Applying this approach to the carboxy-terminal half of neurofibromin, we have identified and crystallized a novel segment distinct from the GAP-related domain (Bonneau et al, 2004). We present here its crystal structure, shown as a novel bipartite module composed of a Sec14p homologous domain (Aravind et al, 1999) and an unexpected pleckstrin homology (PH)-like portion. We also show phospholipid binding and identify residues involved in this activity by using site-directed mutagenesis and phospholipid-binding assays. Our structural model suggests that large conformational changes would be required to support ligand binding in the Sec14p portion and that the PH-like domain may regulate this process.
Results and Discussion
Overall structure
The refined crystal structure (see Methods; supplementary Table 1 online) includes a non-crystallographic dimer of the neurofibromin fragment comprising residues 1,560–1,816 of human neurofibromin, two Triton X-100 molecules, two pyrophosphate (PPi) ions and 113 solvent molecules. The structural model uncovers the architecture of the neurofibromin-Sec14 homology domain (NF1-Sec, residues 1,560–1,698) as a lipid-binding cage (Sha et al, 1998) and shows the presence of an unexpected PH-like (Lemmon, 2004) domain (NF1-PH, residues 1,715–1,816) that has not been detected previously. Both domains are connected by a partly helical linker peptide (residues 1,699–1,714) forming a bipartite module with intriguing interdomain contacts (NF1-Sec-PH; Fig 1A,B). A structure-based sequence alignment including known Sec14- and PH-like domains is shown in Fig 2. Two monomers of the asymmetric unit interact through their PH portions and bind two PPi ions, partially involved in crystal contacts.
Figure 1.
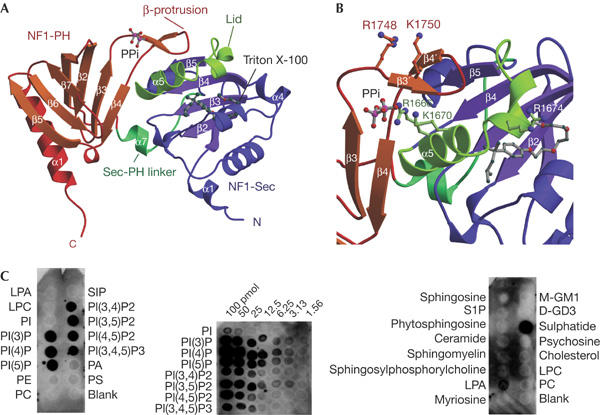
Structure and phospholipid binding of the NF1-Sec-PH module. (A) Ribbon representation of the NF1-Sec-PH module of the human neurofibromin, clearly showing the two domain architecture. (B) Close-up view of the domain interface with selected side chains of basic residues included in ball and stick. The β-protrusion of NF1-PH seems to stabilize a closed conformation by contact with the lid–helix of NF1-Sec. (C) Overlay assays with NF1-Sec-PH and membranes spotted with 100 pmol phospholipids (left panel), indicating binding selecting for phosphorylated phosphatidylinositol (PI). Overlay assays (PIP-array™) spotted with increasing amounts of phosphorylated PI (middle panel), suggesting preference for monophosphorylated species. Overlay assays spotted with 100 pmol of various lipid compounds (Sphingo-Strips™, right panel) show that sulphatide is the preferred binder in this assay, with minor signal derived from lysophosphatidic acid (LPA).
Figure 2.
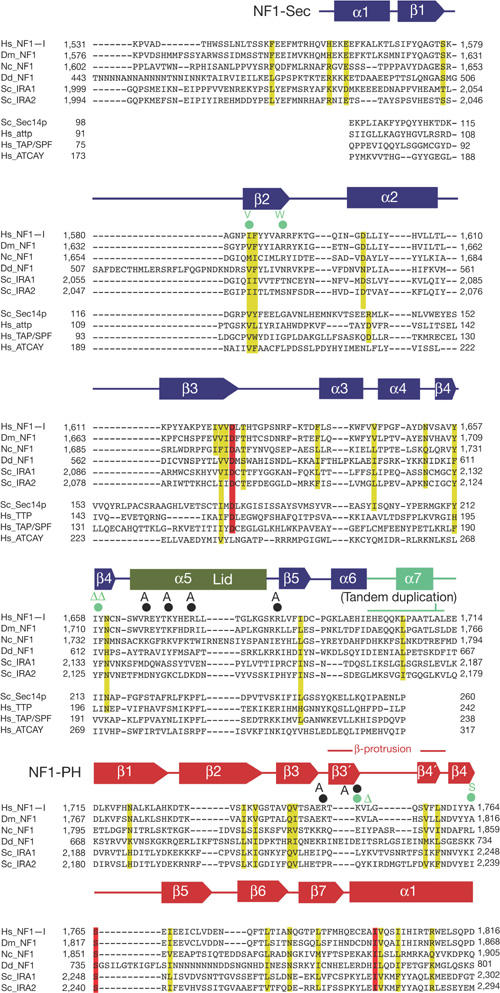
Structure-based sequence alignment of the NF1-Sec-PH region showing similarities with other neurofibromin orthologues as well as with disease-related Sec14p-like proteins (NF1-Sec only). Sequence identity is highlighted in red and similarity in yellow. Structure-based mutations are indicated with black circles; disease-related mutations are indicated with green-filled circles (see Fig 3A). The resulting mutation is specified in bold writing. Sequence source codes (Uniprot): Hs_NF1-I, Homo sapiens neurofibromin (P21359-2); Dm_NF1, D. melanogaster neurofibromin (O01397); Nc_NF1, Neurospora crassa NF1 homologue (Q8WZX6); Dd_NF1, Dictyostelium discoideum NF1 homologue (Q8MNG1); Sc_IRA1, Saccharomyces cerevisiae IRA1 (P18963); Sc_IRA2: S. cerevisiae IRA2 (P19158); Sc_Sec14p: S. cerevisiae Sec14p (P24280); Hs_attp: Homo sapiens α-tocopherol-transfer protein (P49638); Hs_TAP/SPF: H. sapiens supernatant protein factor (SPF) (O76054); Hs_ATCAY: H. sapiens caytaxin (Q86WG3). The tandem duplication detected in Noonan's syndrome patients and its insertion position is indicated; the duplicated span corresponds to the linker region between the pleckstrin homology (PH) and Sec domains.
NF1-Sec, a lipid-binding module
NF1-Sec shows the characteristic α/β-fold of Sec14p-like core domains (Sha et al, 1998; Stocker et al, 2002; Meier et al, 2003), with a central β-sheet forming the bottom of a hydrophobic cavity, which is closed by surrounding helices and contains a Triton X-100 molecule (Fig 1A,B; supplementary material 1 and supplementary Fig 1 online), presumably incorporated during protein purification.
Sec14p has originally been identified as an essential component of the protein secretory pathway in yeast (Kearns et al, 1997), catalysing the exchange of phosphatidyl choline (PC) for phosphatidylinositol (PI) in lipid membranes (Cleves et al, 1991). In the meantime, it has become clear that the Sec14 core domain is present in numerous proteins including regulators of signalling proteins such as RhoGAPs/GEFs and in phosphotyrosine phosphatases (Aravind et al, 1999). Although their specific functions are not well established, they have been implicated in the regulation of subcellular localization of their host proteins (Kostenko et al, 2004).
Structural comparison with the core domains of known Sec14p homologous proteins (Sha et al, 1998; Stocker et al, 2002; Meier et al, 2003) suggests that NF1-Sec adopts a closed conformation, which is mediated by a mostly helical peptide segment (lid–helix, residues 1,663–1,682), covering the potential ligand entry site (Figs 1A,B, 4A).
Figure 4.
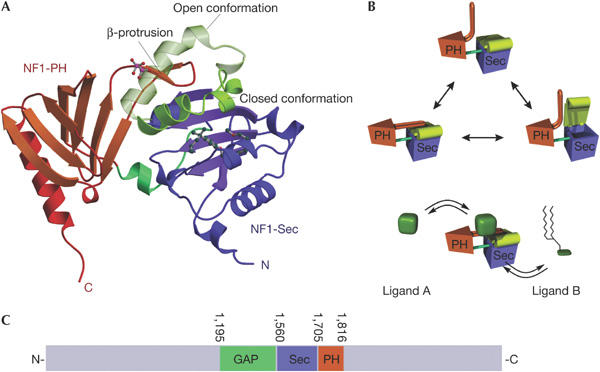
Proposed functional mechanism of the NF1-SEC-PH module. (A) Ribbon diagram of NF1-Sec-PH showing superimposed open (derived from Sec14p; Sha et al, 1998) and closed conformation (this structure). It is clearly visible that the open conformation would clash with the β-protrusion, making us postulate conformational rearrangements in this region to allow movement of the lid–helix region. (B) Hypothetical mechanism of how conformational changes in the NF1-PH domain may regulate access to the NF1-Sec lipid-binding cage. The putative regulatory ligand A could be a phospholipid, but the possibility of other types of molecule including proteins has to be considered (see text). On interaction with ligand A, conformational changes involving the β-protrusion of the NF1-PH domain and the lid–helix of the NF1-Sec module may control binding of ligand B, which may be a membrane lipid binding inside the NF1-Sec. (C) Structurally validated domain scheme of neurofibromin with domain boundaries indicated.
NF1-PH, a previously undetected PH-like domain
NF1-PH shows a β-sandwich fold flanked by a characteristic C-terminal α-helix, which is typical for PH-like domains (Lemmon, 2004), but has not been detected previously in neurofibromin. As in other PH modules, an elongated loop is visible between the canonical strands β3 and β4. In neurofibromin, this loop forms a small β-protrusion, consisting of two short antiparallel β-strands (β3′,β4′), which folds on top of the lid–helix (α5) of NF1-Sec (see below).
PH domains have originally been discovered as PI binding domains that were implicated in membrane recruitment processes (Haslam et al, 1993; Mayer et al, 1993). Meanwhile, research has shown the now PH superfold (Lemmon, 2004) as an interaction domain in numerous signalling proteins, which has accommodated several different functions (e.g., binding of specific peptides or proteins) on a common scaffold, primarily by changes in loop regions connecting the β-strands. The mere structural identification of a PH-like domain in neurofibromin, similar to that in TFIIH (Gervais et al, 2004) or BEACH proteins (Jogl et al, 2002), may point to a novel function of PH modules, which we believe involves regulation of the neighbouring NF1-Sec domain.
A β-protrusion of NF1-PH blocks ligand access to NF1-Sec
The domain interface involves the linker peptide that contacts residues derived from β3PH as well as from the C-terminal tips of β2Sec, β3Sec and β4Sec. As a most remarkable feature in NF1-PH, the β-hairpin protrusion inserted between strands β3 and β4 (see above) interacts extensively with the lid–helix region of the NF1-Sec portion (Fig 1A,B), suggesting its functional importance (see below), presumably in controlling access of ligands to the interior of the lipid binding cavity of the NF1-Sec. The interface architecture reveals an inter-domain cleft (Figs 1A,B, 3A) that has been predicted to be a functional site of the PH domain (P. Aloy, unpublished) and is in the immediate vicinity of the lid–helix of NF1-Sec and the β-protrusion from NF1-PH, mentioned above.
Figure 3.
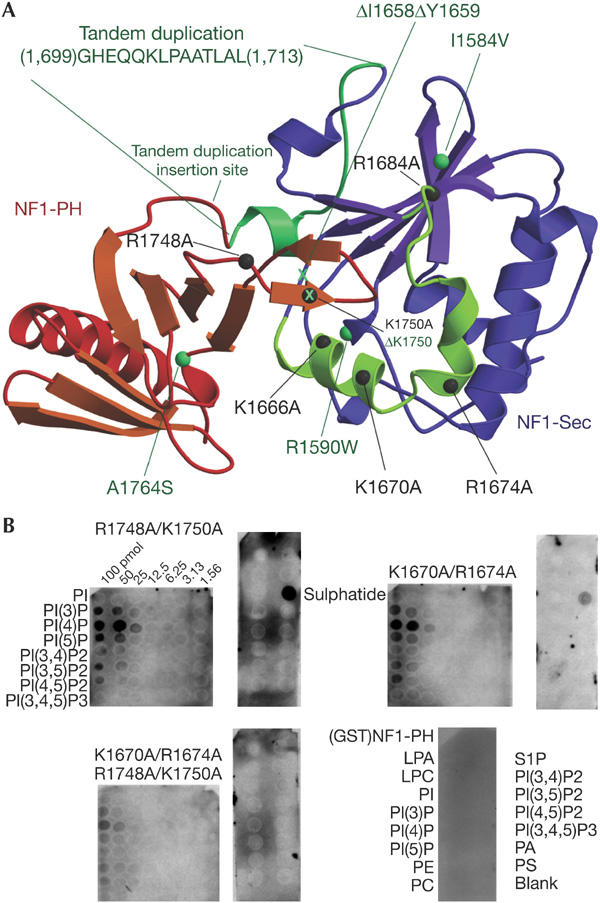
Biochemical analysis of phospholipid binding to NF1-Sec-PH by site-directed mutagenesis along with phospholipid-overlay assays. (A) Top view of the NF1-Sec-PH model with positions of mutated residues indicated with dark grey spheres. Positions of missense mutations identified in NF1 patients are indicated by green spheres and deletions by a green X. The region of the tandem duplication in the domain linker is also indicated. (B) Overlay assays (PIP-arrays™ and Sphingo-Strips™) performed with various mutants and the GST-fused NF1-PH (bottom right panel; see text).
NF1-Sec-PH binds phospholipids
The proximity of the NF1-Sec-PH portion to the GRD domain together with the requirement of membrane recruitment of neurofibromin to allow interaction with Ras prompted us to test phospholipid binding of the protein. Indeed, NF1-Sec-PH binds to membrane-immobilized phospholipids (PIP-Strips™) containing phosphorylated PI head groups (Fig 1C). Array experiments (PIP-Array™) show some preference for the monophosphorylated head groups, with the trisphosphorylated head group giving consistently lower binding signal. The position of the phosphate on the inositol ring seemed to have only a mild effect on the binding signal, suggesting plasticity of the binding site (Fig 1C). Our results are consistent with co-migration of NF1-Sec-PH and a fluorescently labelled phospholipid (phosphatidylinositol (4,5)-bis phosphate (PIP(4,5))) in native gels (data not shown). Overlay assays with lipids different from PI derivatives (SphingoStrips™) indicated that out of 11 compounds, NF1-Sec-PH bound only to the galactolipid sulphatide (Fig 1C). This suggests the presence of a phosphate- or sulfate-modified ring structure, found in sugars or inositol derivatives, as a determinant of binding specificity.
In trying to dissect the individual roles of the two domains for phospholipid binding, we produced glutathione-S-transferase (GST)-fused NF1-PH, but could not overexpress NF1-Sec with satisfactory solubility. GST-fused NF1-PH did not show any binding signal in PIP-strips™ (Fig 3B), suggesting that this domain alone is not sufficient for the phospholipid binding activity.
To find out about the structural determinants of the phospholipid binding, we engineered site-directed mutations of basic residues in the extended domain interface region (Fig 3A). Although most of the single missense mutations only mildly interfered with phospholipid binding (data not shown), three mutants involving two (R1666/K1670, K1670/R1674, R1748/K1750) or four basic residues (K1670A/R1674/R1748/K1750) seemed to bind more weakly (Fig 3B). In particular, the fourfold mutation gave a consistently low binding signal. Interestingly, we did not observe significant changes in the lipid specificity pattern of the various mutants, raising the possibility that binding of the phosphate moieties may be mediated equally efficiently by more than single basic functions. In conclusion, the investigated protein region seems to represent a platform for phospholipids binding where the function of one basic residue can be taken over by another positively charged amino acid.
NF1 mutations point out the domain interface
Several alterations have been reported in the NF1-Sec-PH portion in NF1 patients (Fig 3A; Tassabehji et al, 1993; Upadhyaya et al, 1997; Fahsold et al, 2000; Han et al, 2001). In the context of our NF1-Sec-PH construct, none of the missense mutations showed a significant effect in the phospholipid overlay assays. The single deletion mutant ΔK1750 shows a reduced binding signal, suggesting that removal of the residue distorts the head group binding platform more severely than by its mere mutation to alanine. Strikingly, ΔK1750 maps to the tip of the β-protrusion interacting with the lid–helix, which we suggest to be functionally relevant. Of particular interest is the 14-residue insertion, which has been reported in an NF1 patient with Noonan's syndrome (Tassabehji et al, 1993). This insertion essentially duplicates the linker peptide between NF1-Sec and NF1-PH, suggesting significant effects on the interdomain communication.
The role of the Sec-PH module in the presumed lipid-mediated regulation of RasGAP activity (Tsai et al, 1989; Bollag & McCormick, 1991; Golubic et al, 1998) remains elusive, particularly when considering that lipid inhibition of RasGAP activity has been mapped to the GAP domain of neurofibromin and p120GAP (Golubic et al, 1998).
A potential mechanism for the control of ligand binding
As outlined above, we found NF1-Sec in a closed conformation, with a β-protrusion derived from the C-terminal NF1-PH strongly interacting with the lid–helix, thereby stabilizing this state (Fig 1A,B). In fact, transition of NF1-Sec to the open and presumably ligand-accepting conformation would require considerable conformational changes in the β-protrusion (Fig 4A). Fig 4B shows a hypothetical model for how structural changes could allow ligand binding to NF1-Sec, of which the natural ligand is yet to be identified. Although we could not show such conformational changes on phospholipid binding, we have to consider that the detected lipid-binding activity may not represent the only functional property of this module. In this respect, we would like to emphasize that PH domains represent a family of typical protein interaction modules (Blomberg et al, 1999; Lemmon, 2004), suggesting the existence of a binding partner that could control the proposed conformational change (Fig 4B, ligand A). Compelling evidence emerges from two-hybrid studies, screening for proteins binding to the multifunctional Twenty-five suppressor 1 (Tfs1p) in Saccharomyces cerevisiae (Chautard et al, 2004), a 20 kDa phosphatidylethanolamine-binding protein, which has been implicated in the regulation of the PKA pathway (Caesar & Blomberg, 2004). Interestingly, this pathway has been shown to be involved in rescuing NF1−/− phenotypes in D. melanogaster (The et al, 1997) by overexpression of a PKA transgene. Tfs1p interacts with the yeast IRA2 protein in a region corresponding to NF1-Sec-PH and inhibits GAP activity. In addition, deletion of the portion corresponding to our NF1-PH-like domain abolished the interaction (Chautard et al, 2004). We are at present investigating whether human or fly homologues of Tfs1p are able to interact with NF1-Sec-PH. Among these homologues is the Raf kinase inhibitory protein, a putative cancer metastasis suppressor protein (Keller et al, 2005), providing a link to cell-growth-regulating signalling pathways.
Concluding remarks
With our study, we have extended the known domain scheme of neurofibromin to a structurally validated portion that now accounts for 20% of the full-length protein (Fig 4C). We have shown an unexpected bipartite module with phospholipid binding activity that we could map to the interface region between the two domains. The discovery of the PH-like domain as a potential and likely interaction module increases the chances to discover new interaction partners in two-hybrid screens or pull-down experiments to obtain deeper insight into neurofibromin functions. Finally, the presence of a RasGAP domain followed by a Sec14-PH module (Fig 4C) may define a novel class of RasGAPs that is involved in similar cellular functions or regulated by similar mechanisms like the human neurofibromin. In this context, it is noteworthy that a putative Sec14-PH portion of an NF1-like protein from Neurospora crassa (∼21% residue identity) has similar phospholipid binding properties (F.B., unpublished). Whether other proteins of this domain organization follow similar functional mechanisms will have to await further studies.
Methods
Protein purification, site-directed mutagenesis and antibody purification are described in supplementary material 3 online.
Crystallization and structure determination. A segment of the human NF1 gene coding for the residues 1,545–1,816 was expressed, purified and crystallized as described (Bonneau et al, 2004). The structure was determined by the MIRAS method and refined (supplementary Table 1 online) to a resolution of 2.3 Å with Rwork/Rfree=22.5%/27.2%, as described in supplementary material 2 online. Coordinates have been deposited with the Protein Data Bank (www.rcsb.org) accession code 2D4Q.
Membrane overlay assays. For membrane overlay assays, Phosphoinositide-Strips™ (PIP-Strips), PIP-Arrays™ and SphingoStrips™ (Echelon Biosciences Inc., Salt Lake City, UT, USA) were used as described in supplementary material 4 online. Except for the isolated PH portion, binding detection used rabbit antibodies raised against the recombinant NF1-Sec-PH protein.
Supplementary information is available at EMBO reports online (http://www.nature.com/embor/journal/vaop/ncurrent/extref/7400602-s1.pdf).
Supplementary Material
Supplementary Material
Acknowledgments
We thank F. Wieland and his group for advice in lipid biochemistry, experimental support and discussions and M. Brunner for discussion, U. Karst for help with protein purification and crystallization of the deletion mutant, the staff at the European Synchrotron Radiation Facility beam line for technical support, B. Simon for help with NMR experiments, and M. Knop, E. Izaurralde, N. Ratner, A. Bernards and K. Shannon for comments on the manuscript. This work was supported by a grant of the US Department of Defense (DAMD17-00-1-0539).
References
- Aravind L, Neuwald AF, Ponting CP (1999) Sec14p-like domains in NF1 and Dbl-like proteins indicate lipid regulation of Ras and Rho signaling [letter]. Curr Biol 9: R195–R197 [DOI] [PubMed] [Google Scholar]
- Blomberg N, Baraldi E, Nilges M, Saraste M (1999) The PH superfold: a structural scaffold for multiple functions. Trends Biochem Sci 24: 441–445 [DOI] [PubMed] [Google Scholar]
- Bollag G, McCormick F (1991) Differential regulation of rasGAP and neurofibromatosis gene product activities. Nature 351: 576–579 [DOI] [PubMed] [Google Scholar]
- Bollag G, McCormick F, Clark R (1993) Characterization of full-length neurofibromin: tubulin inhibits Ras GAP activity. EMBO J 12: 1923–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau F, D'Angelo I, Welti S, Stier G, Ylanne J, Scheffzek K (2004) Expression, purification and preliminary crystallographic characterization of a novel segment from the neurofibromatosis type 1 protein. Acta Crystallogr D 60: 2364–2367 [DOI] [PubMed] [Google Scholar]
- Caesar R, Blomberg A (2004) The stress-induced Tfs1p requires NatB-mediated acetylation to inhibit carboxypeptidase Y and to regulate the protein kinase A pathway. J Biol Chem 279: 38532–38543 [DOI] [PubMed] [Google Scholar]
- Chautard H, Jacquet M, Schoentgen F, Bureaud N, Benedetti H (2004) Tfs1p, a member of the PEBP family, inhibits the Ira2p but not the Ira1p Ras GTPase-activating protein in Saccharomyces cerevisiae. Eukaryot Cell 3: 459–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichowski K, Jacks T (2001) NF1 tumor suppressor gene function: narrowing the GAP. Cell 104: 593–604 [DOI] [PubMed] [Google Scholar]
- Cleves A, McGee T, Bankaitis V (1991) Phospholipid transfer proteins: a biological debut. Trends Cell Biol 1: 30–34 [DOI] [PubMed] [Google Scholar]
- Fahsold R et al. (2000) Minor lesion mutational spectrum of the entire NF1 gene does not explain its high mutability but points to a functional domain upstream of the GAP-related domain. Am J Hum Genet 66: 790–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L, Yunoue S, Tokuo H, Ozawa T, Zhang D, Patrakitkomjorn S, Ichimura T, Saya H, Araki N (2004) PKA phosphorylation and 14-3-3 interaction regulate the function of neurofibromatosis type I tumor suppressor, neurofibromin. FEBS Lett 557: 275–282 [DOI] [PubMed] [Google Scholar]
- Gervais V, Lamour V, Jawhari A, Frindel F, Wasielewski E, Dubaele S, Egly JM, Thierry JC, Kieffer B, Poterszman A (2004) TFIIH contains a PH domain involved in DNA nucleotide excision repair. Nat Struct Mol Biol 11: 616–622 [DOI] [PubMed] [Google Scholar]
- Golubic M, Harwalkar JA, Bryant SS, Sundaram V, Jove R, Lee JH (1998) Differential regulation of neurofibromin and p120 GTPase-activating protein by nutritionally relevant fatty acids. Nutr Cancer 30: 97–107 [DOI] [PubMed] [Google Scholar]
- Han SS, Cooper DN, Upadhyaya MN (2001) Evaluation of denaturing high performance liquid chromatography (DHPLC) for the mutational analysis of the neurofibromatosis type 1 (NF1) gene. Hum Genet 109: 487–497 [DOI] [PubMed] [Google Scholar]
- Haslam RJ, Koide HB, Hemmings BA (1993) Pleckstrin domain homology. Nature 363: 309–310 [DOI] [PubMed] [Google Scholar]
- Hsueh YP, Roberts AM, Volta M, Sheng M, Roberts RG (2001) Bipartite interaction between neurofibromatosis type I protein (neurofibromin) and syndecan transmembrane heparan sulfate proteoglycans. J Neurosci 21: 3764–3770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa I, Tamaki N, Saya H (1996) Phosphorylation of neurofibromatosis type 1 gene product (neurofibromin) by cAMP-dependent protein kinase. FEBS Lett 382: 53–59 [DOI] [PubMed] [Google Scholar]
- Jogl G, Shen Y, Gebauer D, Li J, Wiegmann K, Kashkar H, Kronke M, Tong L (2002) Crystal structure of the BEACH domain reveals an unusual fold and extensive association with a novel PH domain. EMBO J 21: 4785–4795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearns BG, McGee TP, Mayinger P, Gedvilaite A, Phillips SE, Kagiwada S, Bankaitis VA (1997) Essential role for diacylglycerol in protein transport from the yeast Golgi complex. Nature 387: 101–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller ET, Fu Z, Brennan M (2005) The biology of a prostate cancer metastasis suppressor protein: Raf kinase inhibitor protein. J Cell Biochem 94: 273–278 [DOI] [PubMed] [Google Scholar]
- Kostenko EV, Mahon GM, Cheng L, Whitehead IP (2004) The Sec14 homology domain regulates the cellular distribution and transforming activity of the Rho-specific guanine nucleotide exchange factor, Dbs. J Biol Chem 280: 2807–2817 [DOI] [PubMed] [Google Scholar]
- Lemmon MA (2004) Pleckstrin homology domains: not just for phosphoinositides. Biochem Soc Trans 32: 707–711 [DOI] [PubMed] [Google Scholar]
- Mayer BJ, Ren R, Clark KL, Baltimore D (1993) A putative modular domain present in diverse signaling proteins. Cell 73: 629–630 [DOI] [PubMed] [Google Scholar]
- Meier R, Tomizaki T, Schulze-Briese C, Baumann U, Stocker A (2003) The molecular basis of vitamin E retention: structure of human α-tocopherol transfer protein. J Mol Biol 331: 725–734 [DOI] [PubMed] [Google Scholar]
- Riccardi VM (1992) Neurofibromatosis: Phenotype Natural History, and Pathogenesis, 2nd edn. Baltimore and London: The Johns Hopkins University Press [Google Scholar]
- Sha B, Phillips SE, Bankaitis VA, Luo M (1998) Crystal structure of the Saccharomyces cerevisiae phosphatidylinositol-transfer protein. Nature 391: 506–510 [DOI] [PubMed] [Google Scholar]
- Stocker A, Tomizaki T, Schulze-Briese C, Baumann U (2002) Crystal structure of the human supernatant protein factor. Structure (Cambridge) 10: 1533–1540 [DOI] [PubMed] [Google Scholar]
- Tassabehji M, Strachan T, Sharland M, Colley A, Donnai D, Harris R, Thakker N (1993) Tandem duplication within a neurofibromatosis type 1 (NF1) gene exon in a family with features of Watson syndrome and Noonan syndrome. Am J Hum Genet 53: 90–95 [PMC free article] [PubMed] [Google Scholar]
- The I, Hannigan GE, Cowley GS, Reginald S, Zhong Y, Gusella JF, Hariharan IK, Bernards A (1997) Rescue of a Drosophila NF1 mutant phenotype by protein kinase A. Science 276: 791–794 [DOI] [PubMed] [Google Scholar]
- Tokuo H, Yunoue S, Feng L, Kimoto M, Tsuji H, Ono T, Saya H, Araki N (2001) Phosphorylation of neurofibromin by cAMP-dependent protein kinase is regulated via a cellular association of N(G),N(G)-dimethylarginine dimethylaminohydrolase. FEBS Lett 494: 48–53 [DOI] [PubMed] [Google Scholar]
- Tsai MH, Yu CL, Wei FS, Stacey DW (1989) The effect of GTPase activating protein upon ras is inhibited by mitogenically responsive lipids. Science 243: 522–526 [DOI] [PubMed] [Google Scholar]
- Upadhyaya M, Shaw DJ, Harper PS (1994) Molecular basis of neurofibromatosis type 1 (NF1): mutation analysis and polymorphisms in the NF1 gene. Hum Mutat 4: 83–101 [DOI] [PubMed] [Google Scholar]
- Upadhyaya M, Maynard J, Osborn M, Harper PS (1997) Six novel mutations in the neurofibromatosis type 1 (NF1) gene. Hum Mutat 10: 248–250 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


