Abstract
How does the biological clock tick?
The lives of all organisms are restricted. After a more or less prolonged phase of ageing, every living thing dies. We all accept this inevitable fate as 'biologically' normal, but this fatalistic attitude stems largely from our experience with artificial objects. These are subject to natural wear and tear during their use, eventually break and become unusable—'dead' in the biological sense. But the wear and tear and loss of function of technical objects and the ageing and death of a living organism are fundamentally different processes. Artificial objects are 'static' closed systems. They usually consist of the same basic material that becomes 'older' over time. Their 'ageing' follows the laws of thermodynamics. And even if we can replace defective parts, such as in a broken car, the object as a whole is slowly worn out until it breaks. Although the same law holds true for a living organism, ageing and death are not inexorable in the same way. An organism is an open, dynamic system through which material continuously flows. The destruction of old and formation of new material are in a permanent dynamic equilibrium. Within about seven years, a human replaces roughly 90% of the material of which he or she is built. This continuous exchange of substance is comparable with a spring, which more or less maintains its form and function, but in which the water molecules are always different.
In principle, it is not necessary that a living organism should age and die as long as it retains the ability to repair and renew
Ageing and death should therefore not be seen as inevitable, particularly as biological systems have many mechanisms to repair damage and replace defective cells. In principle, it is not necessary that a living organism should age and die as long as it retains the ability to repair and renew. Nevertheless, ageing followed by death is a basic characteristic of life, as nature regularly needs to replace existing organisms with new ones. Because of variations in their genetic material due to mutations or recombination, these new individuals have different characteristics, which in the course of their lives are tested for improved adaptation to existing environmental conditions. Immortality would disturb this system of mutation and adaptation as it depends on the availability of room for new and improved life forms. Thus, death is the basic precondition for the frictionless and rapid development of new species that can successfully adapt to changing environmental conditions. This is an evolutionary principle.
... death has a programmatic character in many, if not all, living organisms
Death of an organism is therefore not left only to ecological factors such as disease, accident or predation. To ensure the exchange of existing organisms with new variants, death is an inherent property from the first moment of development. Lifespan and death are obviously programmed, a hypothesis known as genetically programmed ageing. This theory is not particularly controversial among scientists, even though they often use wear-and-tear arguments.
The programme theory does not necessarily explain ageing as a slow loss of body functions—in fact, there are many organisms that die at the zenith of their physiological abilities. A wide range of plant species, for example, die shortly after flowering, and there are thousands of animal species, among them insects, worms and fish, in which death occurs immediately after reproduction or even soon after successful copulation. One of the most dramatic examples is the male Argiope spider, which dies shortly after copulation by a programmed stop of the heartbeat and is then eaten by the female. The programme theory is further supported by mutant variations of Drosophila and rodents that produce long-lived progeny (Martin & Loeb, 2004; Trifunovic et al, 2004), as well as by human genetic defects such as Werner's syndrome and other forms of progeria (accelerated ageing). Apoptosis—the programmed and intrinsically released death of cells—is also known as a characteristic and absolutely necessary phenomenon of normal growth and development (Höffeler, 2004; Brenner & Kroemer, 2000). These examples clearly show that death has a programmatic character in many, if not all, living organisms.
The programme thesis is further supported by observations that every organism has a physiological lifespan that is highly characteristic for its species (Prinzinger, 1996). There are large variations in lifespan between different species, but within a species the potential lifespan is relatively constant. For example, the maximum duration of human life has hardly changed over thousands of years. Although more and more people reach an advanced age as a result of better medical care and nutrition, the characteristic upper limit for most people remains the fourscore (80) years mentioned in the Bible. In 2002, German women lived an average of 82.0 years (in 1881, it was 38.5) and men 75.5 years (1881, 35.6; Statistisches Bundesamt Deutschland, www.destatis.de). The difference in lifespan between males and females is also a general characteristic of all cultures.
The dramatic increase in average life expectancy from 1881 to 2002 is not due to an increase in potential lifespan, but in ecological lifespan, which includes mortality by diseases, accidents, starvation, succumbing to predators and so on. It is the mean attainable age that a member of a population can reach under normal ecological conditions. Potential or physiological lifespan, however, excludes these causes and characterizes the maximum age that an organism can attain before 'natural' factors terminate life. In other words, the typical limits for physiological—but not ecological—lifespan are genetically fixed for the two sexes in humans. This is valid for man in nearly all cultures and for all races, but also for animals, as far as is known. Additionally, as shown below, physiological lifetime—and even the different phases of life, such as embryogeny, juvenile stage and adulthood—are strongly correlated with body mass in all organisms (Prinzinger, 1990).
It is therefore essential to search for the underlying genetic cause that determines lifespan. An obvious candidate is body mass, which has an allometric and genetically determined relationship between size and function (Calder, 1984; Peters, 1983); the best known association is that a larger body size is highly correlated with increased longevity (Fig 1A). In most animals, chronological lifespan (A), measured in days or years, shows a strong correlation with body mass (M) according to the general equation:
Figure 1.
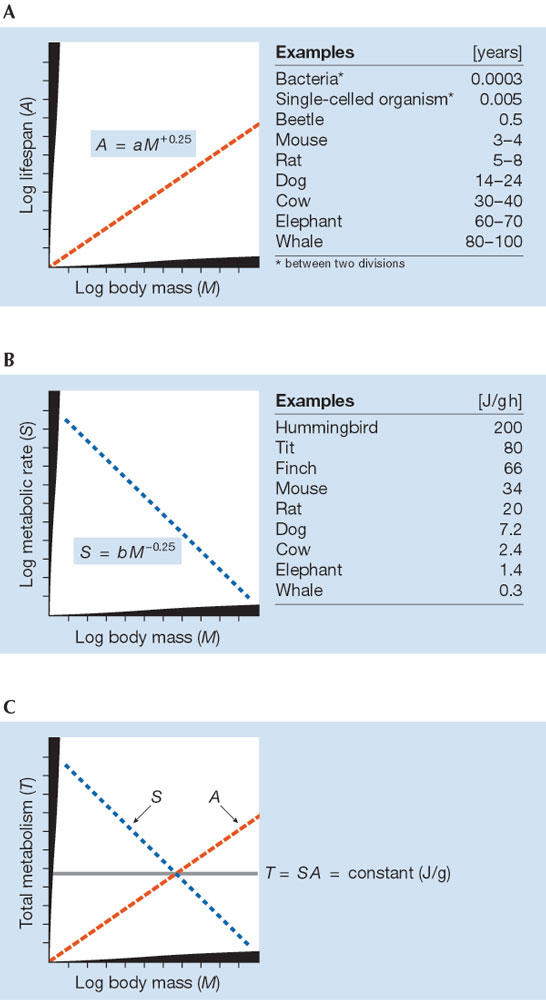
Schematic view of allometry (log–log scale) of (A) lifespan, (B) metabolic rate and (C) total lifetime energy consumption.
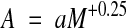 |
1 |
That is, the physical or chronological lifetime of most animals consistently varies with the fourth root of body mass. Only the coefficient a shows a marked difference between taxa, whereas the exponent is almost constant (overall range, 0.23–0.27). This correlation is valid not only for adults, but also for other life stages; for instance, the chronological durations of embryogeny, ontogeny and the adult phase show identical mass correlations in birds (Fig 2; Prinzinger, 1979, 1990). We also find that a nearly identical exponent applies to many other biological times (Fig 3). It seems clear that this allometry has very high significance in terms of physiological lifespan.
Figure 2.
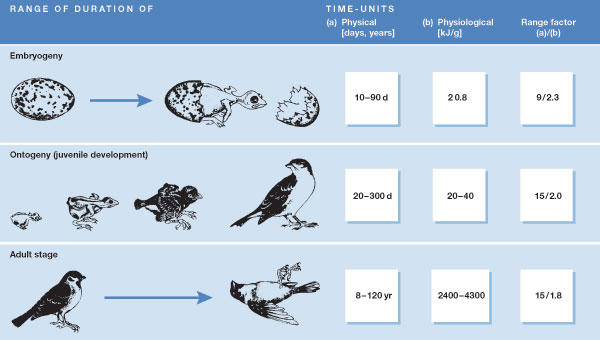
Duration of the three life stages in birds expressed in different time units.
Figure 3.
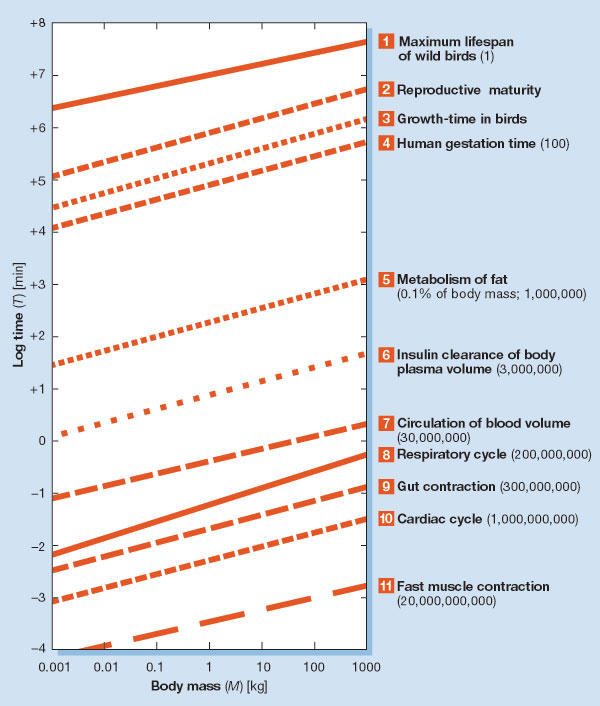
Examples for the allometry of different physiological times (log–log scale) in birds and mammals. They all show mass dependencies that are approximately proportional to M+0.25 (same slope of curves). The numbers in parentheses represent the theoretical maximum number of those cycles during one life (based on the calculation procedure of Fig 1; Prinzinger, 1996).
If lifespan is genetically determined, it is logical to assume the existence of an internal clock that in some way measures time and controls the ageing process. If there are no ecological influences, this clock eventually determines death as the last step in a fixed programme. This last step can also consist of a long succession of different processes—ageing per se. The clock itself must be able to monitor physiological rather than chronological age, which of course should be the prime determinant of lifespan.
The question, then, is what makes this clock 'tick'. There is a large number of theories on what controls ageing processes (Table 1), but none of these can easily determine whether the phenomenon is the clock itself or a subsidiary mechanism that is controlled by the clock. The latter could be quite different in different organisms, whereas the clock itself should have a fairly similar structure for all organisms.
Table 1. Theories of ageing.
| Wear-and-tear effects | After a certain amount of time, the living organism becomes 'unusable', stops working and dies. (The following theories can be summarized under this general theory.) |
| Immune function impairment | Impairment of immune function results in disease and death. Ageing may represent deterioration in immune ability. |
| Somatic mutation | Accumulated damage to cellular components results in altered cellular function. Ageing may represent accumulated cellular damage at the molecular level. |
| Free radicals | Highly reactive, oxidative free radicals damage cellular components. Ageing may represent accumulated damage from free radicals. |
| Cross-linkage of macromolecules | Abnormal chemical bonds form between cellular structures and cellular components, such as collagen, and result in altered cellular function. Ageing may represent accumulated damage in macromolecules. |
| Metabolic causes | Metabolic exhaustion causes deterioration of the organism. Ageing may represent metabolic depletion. |
| Species-specific restricted ability of cells to divide | This theory is based on the observation that normal cells in tissue culture only divide a defined number of times and then die. |
| Genetic programme theory | Lifespan concluded by death is a genetically determined characteristic. |
Furthermore, this biological clock must operate at the cellular level. This is also the underlying basis of one of the best-known theories in this field so far, which postulates that cells have a fixed maximal mitotic capability, known as the Hayflick limit (Hayflick, 1980), which is specific to every species. It is supported by the observation that the number of in vitro cell divisions in humans varies inversely with age: the older the individual, the fewer cell divisions can be achieved before cellular senescence and death. In our view, the cellular clock would therefore measure biological time in numbers of mitotic cell divisions. The mechanisms listed in Table 1 would merely be factors that eventually cause the individual's death. Nevertheless, there are many questions that cannot be explained by this theory—ageing of protozoa, for instance, or the large differences in the number of maximum mitotic divisions between various taxa. In addition, only a few species have been investigated to determine whether their cells display the Hayflick limit.
We reach a fixed physiological age as the organism works its way through a roughly constant quantity of energy until the internal clock initiates death
With respect to the question of how protozoa age, it was believed that unicellular organisms were potentially immortal—that is, they must have an unlimited capacity for cell division to have survived so long. However, recent studies suggest that the divisions might not always be equal, so that the resulting daughter cells can show symptoms of ageing and even undergo a 'natural' death. As it happens, this also applies to bacteria (Ackermann et al, 2003).
On the basis of our own work (Prinzinger & Hänssler 1980; Prinzinger, 1989, 1993, 1996) and the ideas of Rubner (1908) and Pearl (1928), we have put forward another theory about how the cellular clock measures time so as to control ageing. We now have a large amount of data on energy metabolism in man, mammals and especially in birds. Similar to lifespan, the energy turnover has a fixed mathematical relationship to overall body mass for various organisms. It, too, is an exponential relationship and is in principle the same for all species and for all phases of development (Fig 1B). Massspecific energy metabolism (S) in organisms correlates with body mass (M) according to the equation:
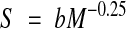 |
2 |
in which S varies with the fourth root of body mass (M−0.25). By contrast to lifespan, this relationship is inverted: the larger the organism, the lower its metabolic rate. This correlation is a fundamental physiological fact, tested on thousands of species and as in equation [1], the exponent ranges from 0.23–0.27. As found for the allometry of time, it is valid not only for adulthood but also for the embryonic and juvenile stages in birds. There is only a slight difference in the coefficient b between taxa, as was also found in the mass correlation for lifespan.
The next question is how much energy does an organism consume over its entire lifespan? Using equations [1] and [2], we can calculate the total mass-specific metabolism TM (J/g) during lifespan as the product of A and energy production S:
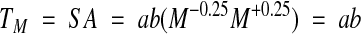 |
3 |
where TM is independent of body mass and is constant for all organisms regardless of their physical lifespan (Fig 1C) because a and b are constant for each taxa. In other words, physiological lifetime is expressed in units of energy metabolism per gram, and is almost identical within an animal taxon (Rahn, 1989). We reach a fixed physiological age as the organism works its way through a roughly constant quantity of energy until the internal clock initiates death. Of course, it is possible to find great differences in lifespan between species of different sizes and at different evolutionary levels.
The absolute amount of energy that mitochondria can generate thus may ultimately define the lifespan of the host organism
These findings are well established on the basis of extensive data from more than a hundred species. As with the metabolic rate, this relationship is evidently valid not only for birds but also for many other organisms, including humans. Some specific examples, in addition to the more general and therefore more important considerations stated above, vividly illustrate various aspects of this theory (McKay et al, 1935; Fries, 1980; Masoro, 1984; Paffenbarger et al, 1986). These relationships can be found for mammals, reptiles and other animals, and even plants (Peters, 1983; Calder, 1984), and also for many other physiological parameters (Fig 3, sidebar and Table 2). In these and other groups, only the coefficients a and b differ. Nevertheless, all show approximately equal durations of their life stages—and thus have almost identical lifespans—when the duration of their lives is measured in energy units.
Examples of the correlation between energy turnover and lifespan.
The lifespan (time to the next division) of many unicellular organisms is halved when their metabolic rate is doubled by raising the temperature of the medium.
Animals who behave 'frugally' with energy become particularly old. The sluggish crocodiles and tortoises are potential animal Methuselahs.
Parrots and birds of prey are often kept in cages. Being unable to 'experience life', they attain a high lifespan in captivity.
Among the invertebrates, the highly active octopuses only live to 4–6 years old. Equally large but immobile shellfish easily reach 20–40 years.
Animals that save energy by hibernation or lethargy, for instance bats and hedgehogs, live much longer than those who are always active. This is particularly obvious in closely related animals. Thus, white-toothed and red-toothed shrews can be differentiated by the presence or lack, respectively, of a state of lethargy for saving energy. White-toothed shrews (capable of lethargy) become much older (4–6 years) than their almost equally large red-toothed relations (2–3 years), who are not capable of lethargy.
The metabolic rate of mice can be reduced with very low food consumption (caloric restriction or hunger diet). They may live twice as long as their satiated comrades.
Male castrates (rats and men) show a marked increase in lifespan (5.3–8.1 years in rats, >14 years in humans). Their energy turnover is significantly lowered.
Females live about 10% longer than males. Metabolic rates in males are higher and roughly account for their shorter lifespan. They live 'energetically' more intensively, but not for as long.
Hyperfunction of the thyroid gland with increased metabolic rate reduces lifespan, although this is not observed for hypofunction.
Animals with high energy expenditure have shorter lifespans than less-active or slow-moving species. Sluggish turtles and mussels grow very old, whereas hectic hummingbirds and shrews are short-lived.
Deprivation of energy intake prolongs life in humans, rodents and other animals.
Caloric restriction extends lifespan by delaying ageing in numerous species (for example, Saccharomyces, Caenorhabditis and Drosophila; Wood et al, 2004).
People with a sedentary lifestyle and who have more sleep live longer than those who engage in hard physical labour.
Table 2. Factors that contribute towards increased lifespan in humans.
| Factor | Explanation |
|---|---|
| Genetics | People with longer-lived ancestors are more likely to enjoy long lives themselves. |
| Gender | Women live longer than men. |
| Race | People of taller and heavier races live longer than those of shorter or slimmer races. |
| Constitution | Leptosomal types (with slender limbs) live longer. |
| Location | Those who reside in a moderate climate or in a peaceful town or village live longer. |
| Marital status | Happily married people live longer. |
| Health | Non-smokers live longer than smokers. Moderate drinkers live longer than people with high alcohol consumption. People with reasonable nourishment live longer than those who are malnourished. |
| Financial status | People who are financially secure and have fewer money worries live longer. |
| Work | People who do mental work rather than physical labour live longer. People with a balanced work life and reduced stress live longer. |
We can, of course, find examples and arguments that contradict this theory (Lints, 1989; Enesco et al, 1990), but no theory is without exceptions. Conversely, a theory of such high universality for all living organisms can be neither proved nor disproved by examples that are based on very few or single groups of animals—including the examples provided in Table 1.
What is special about this theory of maximum metabolic scope? Along with reproduction and excitability, metabolism is the third basic systemic characteristic of organisms, and thus of life itself. But by contrast to the other two properties, metabolism is practically identical for all living creatures that live in and breathe oxygen—including many bacteria, unicellular organisms, plants and animals—as all aerobic organisms use identical metabolic pathways with the same intermediates and enzymes to convert matter into energy. There is thus no difference in principle between a unicellular organism and humans or between a bird and a tree. Such a general system would therefore be very suitable as a timer for lifespan. And as all metabolic pathways contain feedback elements, it would not be excessively complicated to imagine a physiological mechanism that measures time in terms of the energy that is used.
Practically all organisms produce energy in the mitochondria, which oxidize foodstuffs by combining them with oxygen to create ATP. These cellular power plants were probably once independent organisms that resembled bacteria, which in the course of evolution were 'incorporated' into cells as energy producers and now live in symbiosis with the 'host' cell. They divide independently and have their own hereditary substance. No matter how highly developed cells became during millions of years of evolution, the mitochondria themselves hardly changed at all. Regardless of whether they are producing energy in a simple unicellular organism or in a complex mammal, they have remained ancient in their structure as well as in their general function. And—this is especially important—even they have only a limited functionality and a limited lifespan. Regardless of their host organism, the life of which can apparently vary markedly in length as measured in physical units, the mitochondria can produce only a certain amount of energy before they cease to function. The absolute amount of energy that mitochondria can generate thus may ultimately define the lifespan of the host organism. The amount of energy that has already been produced at any time can additionally be informative about physiological development times (efforts expended) in the past, because particular syntheses always require specific quantities of energy, independent of the evolutionary state of the host. Many scientists throughout the world are now busy investigating this mitochondrial theory of ageing. It is most often mentioned in connection with damage to the mitochondrial membrane that is caused by free radicals.
...the wear and tear and loss of function of technical objects and the ageing and death of a living organism are fundamentally different processes
A further positive aspect of the theory of maximum metabolic scope is that it is highly accessible to experimental inquiry. The rate of energy metabolism would therefore be a phylogenetically old, simple and general parameter by which biological systems measure their genetically determined physiological time. It should nevertheless be reiterated: it is only a theory.

References
- Ackermann M, Stearns SC, Jenal U (2003) Senescence in a bacterium with asymmetric division. Science 300: 1920. [DOI] [PubMed] [Google Scholar]
- Brenner C, Kroemer G (2000) Mitochondria—the death signal integrators. Science 289: 1150–1151 [DOI] [PubMed] [Google Scholar]
- Calder WA (1984) Size, Function, and Life History. Cambridge, MA, USA: Harvard University Press [Google Scholar]
- Enesco HE, McTavish A, Garberi R (1990) Spontaneous activity level and life span in rotifers: lack of support for the rate of living theory. Gerontology 36: 256–261 [DOI] [PubMed] [Google Scholar]
- Fries JF (1980) Aging, natural death, and the compression of morbidity. N Engl J Med 303: 130–135 [DOI] [PubMed] [Google Scholar]
- Hayflick L (1980) The cell biology of human aging. Sci Am 242: 58–65 [DOI] [PubMed] [Google Scholar]
- Höffeler F (2004) Die Maschinerie der Apoptose. Biologie in unserer Zeit 34: 16–23 [Google Scholar]
- Lints FA (1989) The rate of living theory revisited. Gerontology 35: 36–57 [DOI] [PubMed] [Google Scholar]
- Martin GM, Loeb LA (2004) Ageing: mice and mitochondria. Nature 429: 357–359 [DOI] [PubMed] [Google Scholar]
- Masoro EJ (1984) Food restriction and the aging process. J Am Geriatric Soc 32: 296–300 [DOI] [PubMed] [Google Scholar]
- McKay CM, Crowell MF, Maynard LA (1935) The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr 10: 63–79 [PubMed] [Google Scholar]
- Paffenbarger RS Jr, Hyde RT, Wing AL, Hsieh CC (1986) Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med 314: 605–613 [DOI] [PubMed] [Google Scholar]
- Pearl R (1928) The Rate of Living. New York, NY, USA: AA Knopf [Google Scholar]
- Peters RH (1983) The Ecological Implications of Body Size. Cambridge, UK: Cambridge University Press [Google Scholar]
- Prinzinger R (1979) Life span and relative energy production in birds. J Ornithol 120: 103–105 [Google Scholar]
- Prinzinger R (1989) The energy cost of life stages in Birds. In Wieser W, Gnaiger E (eds) Energy Transformations in Cells and Organisms pp123–129. Stuttgart, Germany: Thieme [Google Scholar]
- Prinzinger R (1990) The phases of life and their physiological time in birds—an allometric view. J Ornithol 131: 47–61 [Google Scholar]
- Prinzinger R (1993) Energy metabolism—a unit for physiological time? Interdisc Sci Rev 18: 35–44 [Google Scholar]
- Prinzinger R (1996) Das Geheimnis des Alterns. Frankfurt, Germany: Campus [Google Scholar]
- Prinzinger R, Hänssler I (1980) Metabolism–weight relationship in some small nonpasserine birds. Experientia 36: 1299–1300 [Google Scholar]
- Rahn H (1989) Time, energy and body size. In Paganelli CV, Farhi LE (eds), Physiological Function in Special Environments pp203–213. New York, NY, USA: Springer [Google Scholar]
- Rubner M (1908) Das Problem der Lebensdauer und seine Beziehungen zu Wachstum und Ernährung. Munich, Germany: Oldenburg [Google Scholar]
- Trifunovic A et al. (2004) Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 429: 417–423 [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D (2004) Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 430: 686–689 [DOI] [PubMed] [Google Scholar]


