Abstract
The novel Drosophila mutant löchrig (loe) shows progressive neurodegeneration and neuronal cell death, in addition to a low level of cholesterol ester. loe affects a specific isoform of the γ-subunit of AMP-activated protein kinase (AMPK), a negative regulator of hydroxymethylglutaryl (HMG)-CoA reductase and chol esterol synthesis in vertebrates. Although Drosophila cannot synthesize cholesterol de novo, the regulatory role of fly AMPK on HMG-CoA reductase is conserved. The loe phenotype is modified by the level of HMG-CoA reductase and suppressed by the inhibition of this enzyme by statin, which has been used for the treatment of Alzheimer patients. In addition, the degenerative phenotype of loe is enhanced by a mutation in amyloid precursor protein-like (APPL), the fly homolog of the human amyloid precursor protein involved in Alzheimer’s disease. Western analysis revealed that the loe mutation reduces APPL processing, whereas overexpression of Loe increases it. These results describe a novel function of AMPK in neurodegeneration and APPL/APP processing which could be mediated through HMG-CoA reductase and cholesterol ester.
Keywords: amyloid precursor protein-like/cholesterol/Drosophila/neurodegeneration
Introduction
Cholesterol metabolism has been investigated for a long time in peripheral cells, yet relatively little is known about it in brain cells. This is all the more surprising as the brain is the organ richest in cholesterol (Dietschy and Turley, 2001). Most cells in the body take up the required amount of cholesterol via the LDL or VLDL (low- and very low-density lipoprotein) receptor pathway (Fisher et al., 1999; Simons et al., 2001). After uptake, the lipoproteins are degraded and the cholesterol released within the cell where it can be either used as free cholesterol or stored in the form of cholesterol ester (Poirier, 1994; Weisgraber and Mahley, 1996). This transport mechanism is highly conserved in vertebrates and invertebrates (Fisher et al., 1999). In addition, vertebrate cells can produce cholesterol by de novo synthesis in the endoplasmic reticulum (Simons et al., 2001). Due to the blood–brain barrier, brain cells are unable to receive their supply of lipoproteins from the plasma and it has been suggested that only very little is supplied by uptake (Kabara, 1973). At least oligodendrocytes seem to meet their demand for cholesterol by de novo synthesis (Morell and Jurevics, 1996). Nevertheless, the cerebrospinal fluid contains special lipoproteins, the apolipoproteins apoE and apoAI (Roheim et al., 1979; Ladu et al., 2000), and most probably these brain lipoproteins are not involved in the transport of cholesterol to and from the brain but in the redistribution of cholesterol within the brain (Mahley, 1988).
Cholesterol regulates the physical properties of the cell membrane, and its level is therefore tightly controlled. Recent work has shown that cholesterol plays a role in membrane compartmentalization and in the formation of lipid rafts (Simons and Ikonen, 1997). This important function might be the reason for the connection between cholesterol and neurodegeneration. Studies have shown that the cholesterol level influences the production of the pathogenic Aβ peptide, which is produced from the amyloid precursor protein (APP) by cleavage through β- and γ-secretase (Refolo et al., 2000; DeStrooper and Annaert, 2000). It has been suggested that Aβ processing occurs within rafts, whereas the non-amyloidogenic α-processing occurs outside (Lee et al., 1998; Simons et al., 1998; Kojro et al., 2001). Cholesterol synthesis in neurons is regulated by hydroxymethylglutaryl-CoA (HMG-CoA) reductase, which again has been connected to Alzheimer’s disease. Inhibition of this enzyme by statins not only reduces cholesterol synthesis but also inhibits β-secretase cleavage of APP (Frears et al., 1999). In addition, clinical studies indicate that patients treated with statins have a decreased prevalence of Alzheimer’s disease (Wolozin et al., 2000). HMG-CoA reductase activity is negatively regulated via phosphorylation through the AMP-activated protein kinase (AMPK), a heterotrimeric complex, consisting of the catalytic α-subunit and β- and γ-subunits, found in all eukaryotes (Hardie et al., 1998; Kemp et al., 1999).
Here we show that the Drosophila mutant löchrig (loe) disrupts a specific isoform of the AMPK γ-subunit, which leads to a low level of cholesterol ester together with a strong neurodegenerative phenotype. loe interacts genetically with HMG-CoA reductase and influences processing of the β-amyloid protein precursor-like (APPL) gene. Although the regulation and most downstream targets of HMG-CoA reductase are conserved, this enzyme is not involved in cholesterol synthesis in insects, because they cannot synthesize cholesterol de novo (Gertler et al., 1988). Our mutant now shows that HMG-CoA reductase and its regulator AMPK are also involved in neurodegeneration in insects. The low level of cholesterol ester suggests that the mediator could be cholesterol ester rather than cholesterol, which might be important in the context of Alzheimer’s disease because the level of cholesterol ester has been directly correlated with Aβ production in cell culture experiments (Puglielli et al., 2001).
Results
Progressive degeneration and necrosis of neurons in loe
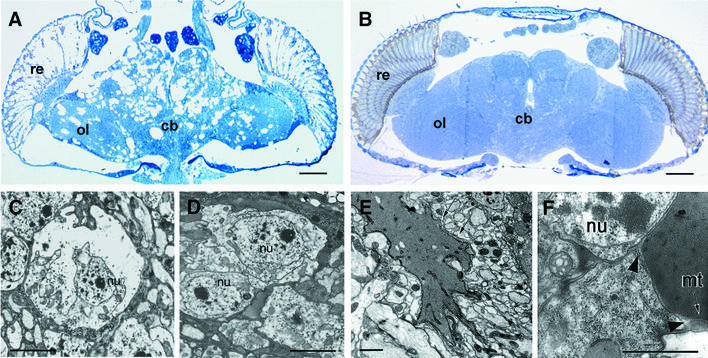
loe was isolated from a collection of P-element insertion lines from Deak et al. (1997). About 800 lines which have a shortened adult life span were aged and screened histologically for signs of neurodegeneration. Two of these lines showed severe vacuolization of the central nervous system (CNS) which increased with aging, and one of them was named löchrig (the German word for full of holes). The vacuolar pathology is most prominent around the central complex and in the central parts of the brain, while the optic lobes are less affected (Figure 1A). Previous developmental studies suggested that the vacuolization and degeneration in loe are confined to differentiated, probably synaptically active neurons, whereas neuroblasts and developing neurons are unaffected (Tschäpe et al., 2002).
Fig. 1. Necrotic cell death, accumulation of fatty acids and neurodegeneration in loe. (A and B) Horizontal plastic sections of the brain stained with toluidine blue. (A) A 10-day-old loe fly shows massive degeneration compared with wild-type (B). (C–F) Electron microscopy on adult heads. (C) Dying neurons, in this case a monopolar cell of the optic system, in 7-day-old loe flies show the characteristic swelling and lysis of necrotic cell death, while the nucleus (nu) remains intact. (D) Wild-type monopolar cells. (E) EM brain sections from 7-day-old loe flies reveal fatty inclusions (arrows). (F) The unsaturated fatty acids seem to originate from within the cell because they are surrounded by residual cell cytoplasm (arrowheads), including a mitochondrion (mt). re, retina; cb, central part of the brain; ol, optic lobes. Bars: (A) and (B) 50 µm; (C–F) 2 µm.
To assess whether dying cells undergo apoptotic or necrotic cell death, we performed TUNEL stainings (Gavrieli et al., 1992) and electron microscopic (EM) studies. The observed swelling and lysis of cell bodies, while the nucleus stays intact, are characteristic features of necrotic cell death (Figure 1C), which is supported by the negative TUNEL staining on head cryosections (data not shown). The EM sections confirmed that the dying cells are neurons because glial cells appeared morphologically normal.
In addition, the electron microscopic analysis revealed the accumulation of a substance (Figure 1E and F), which presumably is unsaturated fatty acids due to the stabilization by osmium in the fixative (Ruthmann, 1966). The accumulations are sometimes still embedded in the cell cytoplasm (Figure 1F).
loe encodes a subunit of the AMP-dependent protein kinase complex
To verify that the mutation is caused by the insertion of the P-element, we remobilized the P-element (O’Kane, 1998) to restore the wild-type phenotype. We established two lines which showed a reversion of the vacuolization phenotype in paraffin head sections (data not shown) and a precise excision of the P-element. These confirm the mutagenic effect of the P-element, which consequently was used to isolate neighboring genes via plasmid rescue (O’Kane, 1998).
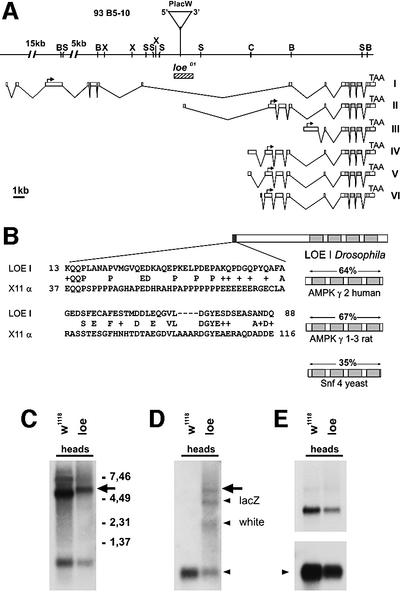
We isolated ∼20 kb of genomic DNA adjacent to the P-element insertion site. Within this region, we found homology to a cDNA fragment from the Berkley Sequencing Project and to genomic clones from the Drosophila Genome Project. Various other cDNAs were isolated by their homology to either of these clones. Their further characterization revealed that they represent at least six alternatively spliced transcripts for the Drosophila γ-subunit of AMPK (Figure 2A). The different mRNAs encode at least three different protein isoforms, all sharing the same C-terminus while varying in their N-terminal part. The C-terminus includes the so-called CBS (cystathionine-β-synthase) domains which are highly conserved between yeast, mammals (Hardie et al., 1998) and Drosophila (Figure 2B). Interestingly, a region in the unique N-terminus of the LoeI isoform shows homology to the X11α protein which can bind to the APP protein (Borg et al., 1998); LoeI and X11α are 28% identical and 41% similar over a stretch of 80 amino acids (Figure 2B). The P-element is inserted in the seventh intron of this transcript and 38 bp upstream of the transcription start site of LoeII (Figure 2A), suggesting that one or two transcripts are affected by the insertion (all other transcripts are >10 kb downstream of the insertion site and therefore most probably are not affected by the P-element). We created a small deletion of 1.3 kb around the insertion site, removing exon 1 of the LoeII transcript (Figure 2A, loeD1), and these flies do not show a degeneration phenotype. This indicates that LoeII is not required for CNS integrity.
Fig. 2. Analysis of the loe gene. (A) Genomic region adjacent to the P-element (PlacW). The exon–intron structures of the LoeI to LoeVI transcripts are shown underneath. Start codons are indicated by arrows. The deletion loeD1 is indicated by a striped bar. B, BamHI; S, SstI; X, XbaI; C, ClaI. (B) The homology to other AMPK γ-subunits is restricted to the C-terminus (only LoeI shown), including the cystathionine-β-synthase domains (light gray). The identity is given above. The N-terminal fragment of LoeI (dark gray) shows homology to the rat X11α protein. (C–E) mRNA expression of loe. (C) Three transcripts are detected with a probe derived from exons 1–3 of LoeI in the heads of w1118 flies. Analysis of these transcripts reveals a larger fusion transcript for the strongly expressed 4.7 kb form (arrow in C) in the loe mutant, which is also recognized by a P-element-specific probe (D) (lacZ and white are transcripts encoded by the P-element, arrowheads). (E) The expression of LoeII is unaltered. w1118 was used as control because this line provides the same genetic background as the mutant. rp49 was used as loading control.
The mutation is due to an aberrant LoeI transcript
Northern blot analysis of adult head mRNA fractions further supported the conclusion that the mutation is due to an effect on the LoeI transcript. A probe comprised of exons 1–3 from LoeI detected three transcripts (Figure 2C), with a size of 7.6, 4.7 and 0.7 kb in w1118 flies. Comparing transcripts in head homogenates from w1118 and loe mutant flies revealed a change of the 4.7 kb LoeI transcript only, increasing it in size to ∼5.5 kb in the mutant (Figure 2C, arrow). The hybridization of this aberrant transcript with a P-element-specific probe proves that it is due to splicing parts of the P-element to the LoeI transcript (Figure 2D). As expected, other transcripts, including LoeII, are unaltered in mutant flies (Figure 2E).
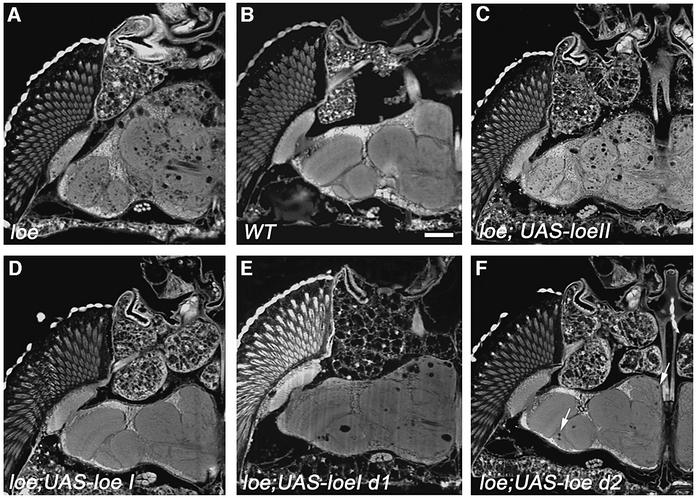
To confirm the specific role of LoeI, we expressed the LoeI and LoeII cDNAs in different cell types using the UAS/Gal4 system (Brand and Perrimon, 1993). Lines carrying P-element vectors with either the LoeI or LoeII cDNA under the control of the Gal4-binding sequence (UAS) were crossed with various Gal4 lines to induce expression of Loe in different cell types. A rescue of the loe phenotype could only be achieved by using the neuron- specific elav-Gal4 line (Luo et al., 1994) in combination with UAS-LoeI (Figure 3D). Expression in glia using loco-Gal4 did not rescue the phenotype (data not shown) nor did expression of LoeII in neurons (Figure 3C). This finally proves that the mutation is caused by a disruption of the LoeI transcript. In addition, these experiments reveal a requirement for this transcript in neurons, because glial expression cannot rescue the phenotype.
Fig. 3. LoeI expression rescues the phenotype. Paraffin sections from 14-day-old flies reveal the characteristic loe phenotype. (A) A control loe fly carrying only the UAS-LoeI construct without the Gal4 driver construct. (B) Wild-type. (C) Expressing LoeII in neurons with elav-Gal4 does not rescue the phenotype. (D) In contrast, expression of LoeI in neurons completely restores the wild-type phenotype in loe. (E) A construct deleting the first 738 amino acids of LoeI, while leaving the conserved C-terminus intact, shows only partial rescue ability. (F) The construct without the domain similar to X11α (deleting amino acids 1–319) reveals a better but still incomplete rescue, because some holes are forming (arrows). Bar: 50 µm.
The unique N-terminus of LoeI is required for wild-type function
To investigate the function of the specific N-terminal region of LoeI, we created N-terminally truncated LoeI transgenes. Expressing a LoeI transgene deleting amino acids 1–738, leaving the conserved C-terminus intact, in neurons, could only partially improve the degeneration phenotype (Figure 3E). This indicates the importance of the unique N-terminus for the function of the LoeI protein. A construct deleting amino acids 1–319, removing the domain similar to X11α (amino acids 13–88), could rescue more efficiently. We could, however, still detect some holes (Figure 3F). The X11α similarity domain is therefore required for wild-type function, but other functionally important domains must reside within the N-terminus of LoeI because longer deletions result in a more incomplete rescue.
Loe is involved in cholesterol homeostasis
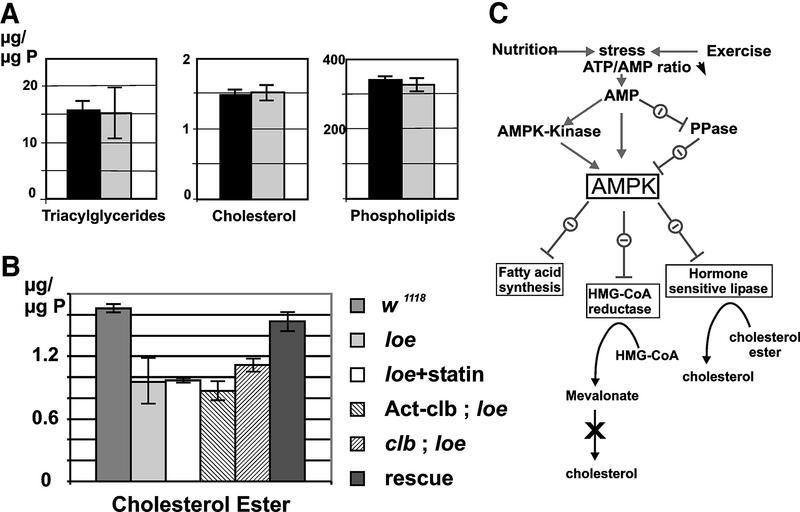
To assess whether the loe mutation influences cholesterol metabolism, a role described for AMPK (Kemp et al., 1999; Figure 4C), we measured the lipid composition of fly heads. The analysis of phospholipids, triglycerides and free cholesterol (Figure 4A) did not reveal any significant differences between 1- to 5-day-old wild-type and mutant flies. The amount of cholesterol ester, however, was reduced by ∼40% (Figure 4B). Expressing LoeI in neurons restored the wild-type level of cholesterol ester in the mutant (Figure 4B), confirming the role of Loe/AMPK in cholesterol homeostasis. The expression of LoeI restores the cholesterol ester level as well as the neurodegenerative phenotype, directly connecting cholesterol ester and neurodegeneration in the loe mutant. These results reveal an involvement of AMPK in cholesterol ester levels in the brain independent of de novo cholesterol synthesis. In peripheral tissues, vertebrate AMPK inhibits the activation of a hormone-sensitive lipase, an enzyme involved in the breakdown of cholesterol ester (Garton et al., 1989). A conserved regulatory pathway in the brain could account for the decreased amount of cholesterol ester.
Fig. 4. Cholesterol ester is reduced in loe. (A) The amount of triglycerides, phospholipids and free cholesterol is unchanged in the loe mutant (black bar) compared with w1118 (the genetic background of loe; light gray bar). (B) The level of cholesterol ester is reduced to ∼60% (mean value from nine measurements) in loe. It can be restored to wild-type levels by expressing the LoeI transcript in neurons using elav-Gal4 (light gray bar). Expression of Clb (striped bar, –45°) in loe slightly increases and one copy of mutant clb in loe slightly lowers the amount of cholesterol ester (striped bar, 45°), although not significantly. Statin treatment (white bar) does not cause a difference in cholesterol ester. The SEM is indicated. (C) AMPK is involved in several regulatory pathways, including fatty acid synthesis and the breakdown of cholesterol ester. AMPKK, AMPK kinase; PPase, protein phosphatase. Modified from Kemp et al. (1999).
A functional homology to the mammalian AMPK is supported further by the accumulation of fatty acids in the mutant, another pathway regulated by AMPK (Figure 4C).
loe interacts with columbus, the fly homolog of HMG-CoA reductase
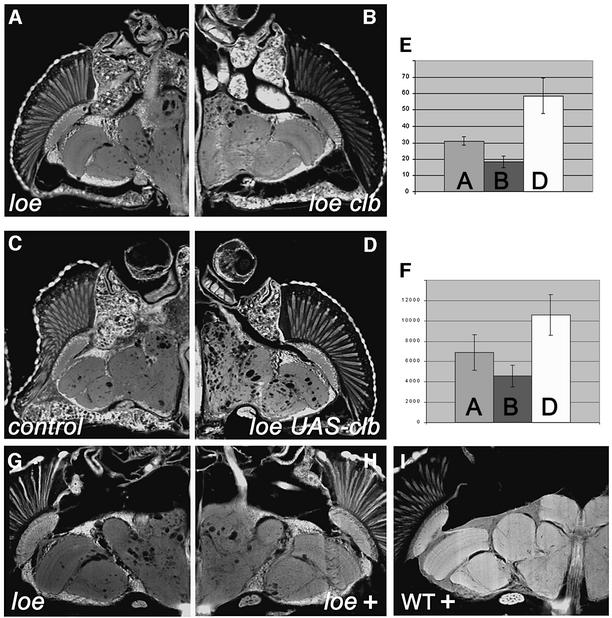
AMPK negatively regulates HMG-CoA reductase, a key enzyme in cholesterol synthesis in vertebrates. In Drosophila, this protein is encoded by the columbus (clb) gene (Van Doren et al., 1998). To assess whether loe interacts with the clb mutation, we created flies homozygous for loe and heterozygous for two strong, embryonic lethal alleles of clb (which both had the same effect). clb/+; loe showed a weak but significant suppression of vacuolization (Figure 5B) compared with loe mutant flies (Figure 5A). To confirm an interaction, we used lines expressing Clb in the loe background. In contrast to the clb mutation, Clb overexpression enhanced the phenotype (Figure 5D). Control flies, containing only the UAS-Clb construct but no neuronal promoter construct (Figure 5C), did not differ from the original loe mutants. The interaction was quantified by counting holes in the different genotypes (Figure 5E) and measuring their total volume (Figure 5F). The enhancement by Clb overexpression and suppression by the clb/+ mutant suggests that HMG-CoA reductase is negatively regulated by AMPK as in other organisms. In addition, we investigated an influence on the cholesterol ester level of loe. Overexpression of Clb slightly reduced, and introduction of one mutant copy of clb slightly increased, the cholesterol ester level in loe (Figure 4B); however, the differences are not significant. Nevertheless, they are in agreement with the results on the neurodegenerative phenotype because the clb mutation suppresses and additional Clb enhances the phenotype. Interestingly, the function of HMG-CoA reductase in cholesterol synthesis is not conserved because insects cannot synthesize cholesterol de novo. However, many other downstream genes and regulatory feedback mechanisms are conserved (Gertler et al., 1988), and one of them might connect HMG-CoA reductase and cholesterol ester.
Fig. 5. Interaction of loe with clb. (A) 7-day-old loe flies reveal the characteristic vacuolization, which is reduced in flies additionally heterozygous for a mutation in the HMG-CoA reductase homolog clb (loe clb11.54/loe, B) of the same age. (C) Control loe mutant flies (7 days) carrying only the UAS-Clb construct, without a promoter construct, are not distinguishable from the original loe strain. (D) In contrast, flies expressing additional clb in neurons (Appl-Gal; UAS-clb; loe 7 days) show an enhancement of the neurodegenerative phenotype. To quantify the enhancement, we counted the holes in these flies. (E) The number of holes is reduced to ∼59% in loe clb11.54/loe flies (dark gray bar) and almost doubled (1.9-fold) in flies expressing clb in neurons (white bar). (F) The size of the forming holes is not changed significantly because the total volume of holes (in µm3) is reduced in loe clb11.54/loe to 66% (dark gray bar), whereas it is increased to 150% in Appl-Gal; UAS-clb; loe flies (white bar). loe, light gray bar. The SD is indicated. (G) A loe fly kept for 8 days on glucose. (H) Adding mevinacor (1 ng/ml) to the glucose solution reduces the formation of holes. (I) Feeding mevinacor to wild-type flies shows no effect.
Treatment with statin suppresses the loe phenotype
As mentioned in the Introduction, HMG-CoA reductase can be inhibited pharmacologically by a class of drugs called statins, which have also been shown to decrease the prevalence of Alzheimer’s disease (Wolozin et al., 2000). To assess whether treatment with statins influences the neurodegeneration in loe, we compared flies fed on glucose with or without the drug lovastatin. Flies kept on lovastatin showed a suppression of the vacuolization (Figure 5H) compared with control animals (Figure 5G). Treatment of wild-type flies with lovastatin revealed no adverse effects (Figure 5I). These results show that the progressive neurodegeneration in loe can be slowed successfully by treatment with statins. We also tested the level of cholesterol ester in loe flies treated with statins, but could not find a significant difference (Figure 4B).
Loe interacts with amyloid precursor protein-like
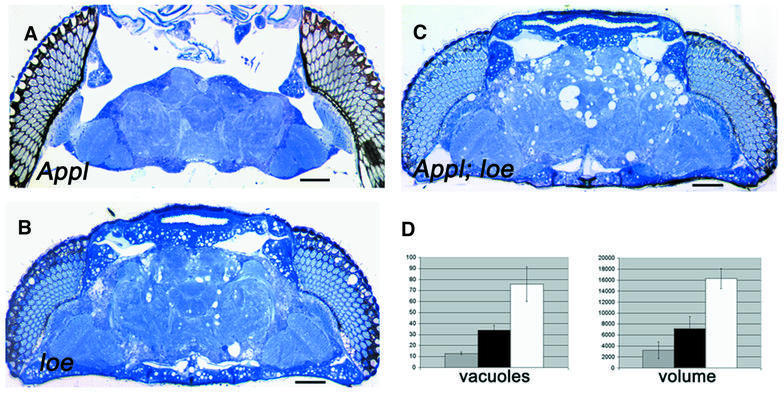
Cholesterol homeostasis has been implicated in the processing of Aβ from APP, as has statin treatment, which can dramatically decrease Aβ production (Fassbender et al., 2001). Therefore, we investigated whether loe influences APPL, the fly homolog of human APP (Rosen et al., 1989). Appld mutants, which carry a deletion in the Appl gene (Torroja et al., 1996), do not reveal any signs of neurodegeneration (Figure 6A). However, crossing Appld with loe flies shows an enhancement of the loe vacuolization (Figure 6C and D). The effect is weaker in loe flies carrying one copy of Appld (loe/loe; Appld/+) compared with homozygous double mutants (loe/loe; Appld/Appld) and can be detected in the central brain as well as the optic system (Figure 6D).
Fig. 6. Interaction between loe and Appl. (A) A 4-day-old Appld mutant reveals no vacuolization, whereas a loe fly at the same age displays many holes (B). (C) In a 4-day-old Appld; loe double mutant, this phenotype is enhanced and more and larger holes are formed. Bar: 50 µm. (D) To quantify the enhancement, we counted the holes in 7-day-old loe flies (gray bar) and flies which also carry, besides loe, a heterozygous (black bar) or homozygous (white bar) Appl mutation. The number of holes is approximately doubled in heterozygous Appld/+ flies and >4-fold increased in homozygous Appld/Appld mutants. Whereas the number increases, the size of the forming holes does not increase significantly because the total volume of holes (in µm3) also increases by two or four, respectively. The SD is indicated.
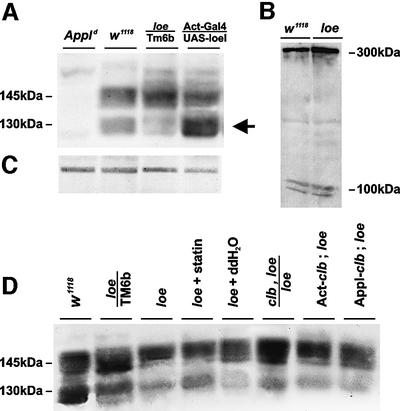
To determine whether loe might influence the APPL protein, we performed western blot analysis of brain extracts. Using an anti-APPL polyclonal antibody (Torroja et al., 1996), we detect two bands in w1118 flies, representing the genetic background used to induce the loe mutation (Figure 7A). The bands correspond to the membrane-associated 145 kDa precursor and the 130 kDa secreted form (Luo et al., 1990), which are absent in Appld. In the loe mutant, we find similar amounts of APPL precursor protein; however, the level of the processed secreted form is reduced. Conversely, we find more of the secreted form when additional LoeI is expressed in neurons. This reveals a role for loe in APPL processing or stabilization of the processed form. To assess whether this effect is specific for APPL, we investigated the processing of Notch, which is cleaved by a mechanism similar to that of APP (DeStrooper and Annaert, 2000; Nowotny et al., 2000). We could not detect any differences in the processing of Notch (Figure 7B), suggesting a specific function of loe in APPL processing, possibly mediated by the X11α similarity domain. In addition, we investigated whether Clb or statin treatment influences APPL processing in loe. Additional expression of Clb, which enhanced the neurodegenerative phenotype of loe, also enhanced the processing effect, causing a slight further reduction of APPL processing (Figure 7D). On the contrary, one copy of mutant clb or statin treatment slightly increased processing. This suggests that the neurodegenerative phenotype is correlated with the processing of APPL.
Fig. 7. Aberrant processing of APPL in loe. (A) The APPL antiserum reveals in western blots two APPL bands, which are missing in the Appld mutant. Whereas the amount of the APPL precursor protein of 145 kDa and its different post-translational modifications is similar in loe flies compared with the control line w1118, the amount of the secreted form of 135 kDa and its modifications is decreased (arrow). The opposite effect can be found in flies overexpressing loe in neurons where the amount of the secreted form is increased. (B) Western blot using an α-Notch antibody (DSHB, University of Iowa). The full-length Notch of 300 kDa and the processed species of 100 kDa (Pan and Rubin, 1997) are detected in equal amounts in wild-type and loe. (C) Load control. (D) The processing in loe is influenced by Clb because flies carrying one mutant copy of clb or treated with statin have slightly more of the processed form (lane 5 is the control for lane 4 kept under the same conditions as treated flies). Additional expression of Clb using Appl-Gal 4 or Act-Gal4 seems to decrease processing compared with loe alone. Flies were 1 day old.
Supplementary data for this paper are available at The EMBO Journal Online.
Discussion
In this report, we showed that a mutation in the AMPK γ-subunit causes progressive neurodegeneration in Drosophila. AMPK is a central component of a protein kinase cascade conserved in eukaryotes (Hardie et al., 1998; Kemp et al., 1999; Winder and Hardie, 1999) that acts as a metabolic sensor to monitor the cellular AMP and ATP levels. In cases of ATP depletion, its major function described so far is to activate energy-providing mechanisms while inactivating energy-consuming processes (Hardie et al., 1998; Kemp et al., 1999). AMPK is a heterotrimer, consisting of the catalytic α-subunit and the β- and γ-subunits which are required for stabilization of the complex and kinase activity. The activity of the complex is regulated by phosphorylation through an upstream kinase, and both phosphorylation and dephosphorylation are sensitive to AMP levels (Davies et al., 1995). For all three subunits, different isoforms have been identified that assemble into specific AMPK complexes with distinguishable tissue distribution in peripheral tissues in vertebrates (Stapleton et al., 1996; Thornton et al., 1998). Whereas most tissues predominantly express one γ isoform, the human brain expresses three different isoforms (Cheung et al., 2000). Interestingly, two of them have extended N-termini with no significant homology to each other, LoeI or any other protein (Cheung et al., 2000). The loe mutation shows, for the first time, that such a brain-specific isoform has a unique function in brain maintenance, which cannot be substituted by other isoforms. This function probably goes beyond the basic role in energy regulation because all isoforms share the C-terminus which is sufficient for a functional γ-subunit and, therefore, a functional AMPK complex. It will be interesting to discover whether one of the human isoforms is also required specifically for neuronal survival.
AMPK has a central role in cholesterol metabolism by regulating HMG-CoA reductase and hormone-sensitive lipase, which is involved in the breakdown of cholesterol ester in vertebrates (Garton et al., 1989). Although hormone-sensitive lipase has not been found in the brain, a cholesterol ester hydrolase activity is described for the brain (Gosh and Grogan, 1990); however, nothing is known about the potential regulation of this enzyme by AMPK. An inhibitory function of AMPK in the brain would lead to an overactivity of this hydrolase and, therefore, to a reduced level of cholesterol ester. A Drosophila protein with homology to hormone-sensitive lipase can be found in the Drosophila Sequencing Project, but unfortunately no mutant has been described so far. However, a deficiency deleting this enzyme was tested for genetic interactions with loe. Because this deficiency had no influence on the loe phenotype (data not shown), we assume that it is not involved in the neurodegenerative phenotype. In contrast, we could show a genetic as well as a pharmacologically induced interaction of loe with HMG-CoA reductase (clb). The interaction reveals that, as in vertebrates, AMPK acts upstream of HMG-CoA reductase. Because a mutation in clb suppresses and overexpression enhances the neurodegenerative loe phenotype, the inhibitory function of AMPK on HMG-CoA reductase seems to be conserved. Interestingly, the function of HMG-CoA reductase is not completely conserved between vertebrates and insects, because arthropods cannot synthesize cholesterol de novo. Rather, HMG-CoA reductase is involved in the production of non-sterol isoprenoids from mevalonate (Gertler et al., 1988; Duportets et al., 2000). The effect of HMG-CoA reductase on neurodegeneration cannot, therefore, be mediated through cholesterol synthesis and, as our measurements show, the cholesterol level is unaltered in loe. However, the amount of cholesterol ester is lowered in loe and adding or removing Clb has a slight influence on it, and APPL processing in loe is influenced by Clb. In this context, it is worth mentioning that statins dramatically decrease Aβ production before a reduction in cholesterol can be detected (Fassbender et al., 2001). This suggests that other members of the cholesterol pathway might regulate APP processing (Wolozin, 2001), possibly cholesterol ester.
The loe mutation reveals a connection between cholesterol ester and progressive neurodegeneration in the model system Drosophila. In vertebrates, such a link has been established by the finding that accumulation of Aβ can decrease cholesterol esterification in neurons (Koudinova et al., 1996; Liu et al., 1998). Puglielli et al. (2001) found that the level of cholesterol ester directly correlates with Aβ production, and that elevated concentrations of cholesterol ester but not free cholesterol increased the generation of Aβ. On the other hand, it has been shown that lowering the cholesterol concentration inhibits APP cleavage by secretases and interferes with the localization of APP in membrane rafts (Simons et al., 1998; Frears et al., 1999). These are membrane microdomains consisting of lipids, proteins and cholesterol, and their correct composition seems to be required for normal APP processing (DeStrooper and Annaert, 2000; Drouet et al., 2000). Our results strengthen the likelihood of a role for cholesterol ester because the loe mutant links a reduced level of cholesterol ester, leaving free cholesterol unaltered, with decreased processing of APPL.
Our results clearly reveal a function of AMPK in APPL processing. On the other hand, the Appl mutant enhances the neurodegenerative phenotype of loe. Like knock-outs of APP in mice, the Appld null mutation displays only subtle neurological deficits (Luo et al., 1992; Müller et al., 1994; Zheng et al., 1995). In the loe mutant background, however, Appl can be connected to progressive neurodegeneration, which might help to understand the function of APP proteins. Because the lack of APPL enhances the phenotype, this hints at a neuroprotective function, perhaps specifically of the soluble form, of APPL which was also suggested by cell culture studies of APP (Perez et al., 1997). In our model, neurons would be more vulnerable to the effect of the loe mutation when APPL and its soluble form are missing. The Appl mutant itself might not show degeneration because the damaging event is absent.
With the isolation of the loe mutant, we have connected AMPK, a second enzyme besides HMG-CoA reductase involved in cholesterol homeostasis, to neurodegeneration and APPL processing. This underlines the importance of the cholesterol biosynthesis pathway for the maintenance of the nervous system and for understanding of neurodegenerative diseases such as Alzheimer’s. With the Drosophila loe mutant available, we can now study the role of this pathway in neurodegeneration in an easily accessible model organism.
Materials and methods
Drosophila stocks
All stocks were maintained and raised under standard conditions. Canton S wild-type and w1118 were used as control stocks. The loco-Gal4 line was kindly provided by C.Klämbt, and the APPL-Gal4 line by L.Torroya. Act-Gal4 and elav-Gal4 were provided by the Bloomington stock center, and the P-element lines are from the Szeged stock center. The Appld mutant was kindly provided by K.White. The clb11.54 and clb26.31 alleles (both strand UAS-Clb carrying stocks) were kindly provided by R.Lehmann. Flies were raised and aged at 25°C.
Tissue sections for light and electron microscopy
Adult heads were prepared for light and electron microscopy as described in Kretzschmar et al. (1997). For light microscopy, 1 µm serial sections were cut and stained with 1% toluidine blue, 1% borax. Ultrathin Epon plastic sections were post-fixed with osmium and stained with 2% uranyl acetate, followed by Reynolds’ lead citrate (Reynolds, 1963), and stabilized for transmission EM by carbon coating. Examination was performed with a Zeiss EM10C/VR electron microscope at 40–80 kV. Paraffin mass histology was performed as described by Jäger and Fischbach (1989).
Cloning and sequencing
The cDNA clones for the various loe transcripts and genomic clones were isolated from the Drosophila Genome Project (cDNAs: SD02114, LD45665, LD28468, SD02088, GH16589, LD19285, LD41424, GH28591, LD05242, LD13337 and GH08914). The pIndy5 (kindly provided by L.Seroude) and pCaSpeR3-UAS (pUAST, Flybase) vectors were used for the pUAS-Loe constructs.
Sequencing was performed using the Thermo Sequenase fluorescent-labeled primer cycle sequencing kit from Amersham Pharmacia after subcloning cDNA fragments into pBluscript KS. Reactions were performed on a Hybaid Omn-E (MWG) thermocycler according to the instruction manual for the sequencing kit. Sequence analysis was performed with the ALFexpress sequencing system (Pharmacia) using Hydrolink Long Ranger gels (FMC Bio Products).
Northern blots
Total RNA was isolated using the Trizol method described in Goodwin et al. (1997), and poly mRNA was selected with the Promega PolyAtract system. Northern blots were performed following the protocol of Ausubel et al. (1996).
Lipid and sterol measurements
A 20 mg aliquot of heads from 1- to 5-day-old flies was homogenized mechanically and chloroform/methanol extracted as described in Folch et al. (1957). Phospholipids were separated by two-dimensional thin-layer chromatography on Silica gel 60 plates (Merck) using chloroform/methanol/25% NH3 (65:35:5 by vol.) and chloroform/acetone/methanol/acetic acid/water (50:20:10:10:5 by vol.) as solvents. Phospholipids were visualized on TLC plates by staining with iodine vapor, scraped off and quantified (Broekhuyse, 1968).
For the analysis of neutral lipids, extracts were applied to silica gel 60 plates with a sample applicator (Linomat IV; CAMAG), and chromatograms developed in an ascending manner using the solvent system light petroleum/diethyl ether/acetic acid (70:30:2 by vol.). Quantitation of sterol and sterol ester was carried out by densitometric scanning at 275 nm with ergosterol as a standard. Neutral lipids were visualized by post-chromatographic staining using a chromatogram immersion device (CAMAG). Quantification of triacylglycerols, sterol and sterol ester was carried out by densitometric scanning at 400 nm with triolein (NuCheck, Inc.). As for the phospholipid measurements, at least six measurements from three independent samples were performed for each genotype.
Western blot analysis
Fly heads were homogenized as described in Torroja et al. (1996) and loaded on 7.5% SDS–polyacrylamide gels using standard methods (Laemmli, 1970). Proteins were transferred onto nitrocellulose membranes (Towbin et al., 1979). Immunoreactions with anti-APPL (Ab952, kindly provided by K.White), diluted 1:300 and pre-adsorbed overnight against Appld embryos, were carried out according to the manufacturer’s protocol for the ECL Western Blot Detection System (Amersham). The anti-Notch supernatant (Developmental Studies Hybridoma Bank, University of Iowa) was used in a 1:100 dilution. Flies of the different genotypes were used at the same age in one set of experiments. Different ages (1 and 5 days) were tested and showed the same result.
Measurement of the vacuolar pathology
The computer set-up described in Heisenberg et al. (1995) was used to count the holes and measure their volume. Measurements were performed at two distinct levels of the brain and in two different brain areas. At least four flies of the same age and processed on one slide, using paraffin mass histology, were used for quantification.
Statin treatment
Late third instar larvae were transferred to vials containing a 5% glucose solution with or without 1 ng/ml of mevinacor. Mevinacor (MSD Sharp&Dohme GMBH) contains the cholesterol-lowering drug lovastatin.
Accession numbers
The DDBJ/EMBL/GenBank accession Nos for loe transcripts 1–7 are AY166752–AY166758.
Supplementary data
Supplementary data are available at The EMBO Journal Online.
Acknowledgments
Acknowledgements
Special thanks are due to Sinje Maruhn for her support of the injection crew, Wolfgang Drobnik for some preliminary lipid measurements, Gert Pflugfelder and Burkhard Poeck for critical reading of the manuscript, and to everyone who provided materials and fly stocks. This work was supported by fellowships to J.-A.T. from the State of Bavaria and the Schering Forschung GmbH, by a Firnberg-Fellowship to K.A. (T113) and grants to D.K. from the University of Regensburg and the Alzheimer Forschung Initiative e.V.
References
- Ausubel F.M., Brent,R., Kingston,R.E., Moore,D.D., Seidman,J.G., Smith,J.A. and Struhl,K. (1996) Current Protocols in Molecular Biology. John Wiley & Sons, New York, NY.
- Borg J.P., Yang,Y., De Taddeo-Borg,M., Margolis,B. and Turner,R.S. (1998) The X11α protein slows cellular amyloid precursor protein processing and reduced Aβ40 and Aβ42 secretion. J. Biol. Chem., 273, 14761–14766. [DOI] [PubMed] [Google Scholar]
- Brand A.H. and Perrimon,N. (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development, 118, 401–415. [DOI] [PubMed] [Google Scholar]
- Broekhuyse R.M. (1968) Phospholipids in tissues of the eye. Isolation, characterization and quantitative analysis by two-dimensional thin-layer chromatography of diacyl and vinyl-ether phospholipids. Biochim. Biophys. Acta, 260, 449–459. [DOI] [PubMed] [Google Scholar]
- Cheung P.C., Salt,I.P., Davies,S.P., Hardie,D.G. and Carling,D. (2000) Characterization of AMP-activated protein kinase γ-subunit isoforms and their role in AMP binding. Biochem. J., 346, 659–669. [PMC free article] [PubMed] [Google Scholar]
- Davies S.P., Helps,N.R., Cohen,P.T. and Hardie,D.G. (1995) 5′-AMP inhibits dephosphorylation, as well as promoting phosphorylation, of the AMP-activated protein kinase. Studies using bacterially expressed human protein phosphatase-2Cα and native bovine protein phosphatase-2AC. FEBS Lett., 377, 421. [DOI] [PubMed] [Google Scholar]
- Deak P. et al. (1997) P-element insertion alleles of essential genes on the third chromosome of Drosophila melanogaster: correlation of physical and cytogenetic maps in chromosomal region 86E–87F. Genetics, 147, 1697–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeStrooper B. and Annaert,W. (2000) Proteolytic processing and biological functions of the amyloid precursor protein. J. Cell Sci., 113, 1857–1870. [DOI] [PubMed] [Google Scholar]
- Dietschy J.M. and Turley,S.D. (2001) Cholesterol metabolism in the brain. Curr. Opin. Lipidol., 12, 105–112. [DOI] [PubMed] [Google Scholar]
- Drouet B., Pincon-Raymond,M., Chambaz,J. and Pillot,T. (2000) Molecular basis of Alzheimer’s disease. Cell. Mol. Life Sci., 57, 705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duportets L., Belles,X., Rossignol,F. and Couillaud,F. (2000) Molecular cloning and structural analysis of 3-hydroxy-3-methylglutaryl coenzyme A reductase of the moth Agrotis ipsilon. Insect Mol. Biol., 9, 385–392. [DOI] [PubMed] [Google Scholar]
- Fassbender K., Masters,C. and Beyreuther,K. (2001) Alzheimer’s disease: molecular concepts and therapeutic targets. Naturwissenschaften, 88, 261–267. [DOI] [PubMed] [Google Scholar]
- Fisher C.A, Kiss,R.S., Francis,G.A., Gao,P. and Ryan,R.O. (1999) Human apolipoprotein E N-terminal domain displacement of apolipophorin III from insect low density lipophorin creates a receptor-competent hybrid lipoprotein. Comp. Biochem. Physiol. Biochem. Mol. Biol., 122, 447–451. [DOI] [PubMed] [Google Scholar]
- Folch J., Lees,M. and Sloane-Stanley,G.H. (1957) A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem., 226, 497–509. [PubMed] [Google Scholar]
- Frears E.R., Stephens,D.J., Walters,C.E., Davies,H. and Austen,B.M. (1999) The role of cholesterol in the biosynthesis of β-amyloid. Neuroreport, 10, 1699–1705. [DOI] [PubMed] [Google Scholar]
- Garton A.J., Campell,D.G., Carling,D., Hardie,D.G., Colbran,R.J. and Yeaman,S. (1989) Phosphorylation of bovine hormone-sensitive lipase by the AMP-activated protein kinase. A possible antilipolytic mechanism. Eur. J. Biochem., 179, 249–254. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman,Y. and Ben-Sasson,S.A. (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol., 119, 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertler F.B., Chiu,C.Y., Richter-Mann,L. and Chin,D.J. (1988) Developmental and metabolic regulation of the Drosophila melanogaster 3-hydroxy-3-methylglutaryl coenzyme A reductase. Mol. Cell Biol., 8, 2713–2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin S.F., Del Vecchio,M., Velinzon,K., Hogel,C., Russell,S.R., Tully,T. and Kaiser,K. (1997) Defective learning in mutants of the Drosophila gene for a regulatory subunit of cAMP-dependent protein kinase. J. Neurosci., 17, 8817–8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosh S. and Grogan,W.M. (1990) Activation of myelin-associated cholesteryl ester hydrolase in developing rat brain. Brain Res.Dev. Brain Res., 54, 147–149. [DOI] [PubMed] [Google Scholar]
- Hardie D.G., Carling,D. and Carlson,M. (1998) The AMP-activated/SNF1 protein kinase subfamily: metabolic sensor of the eukaryotic cell. Annu. Rev. Biochem., 67, 821–855. [DOI] [PubMed] [Google Scholar]
- Heisenberg M., Heusipp,M. and Wanke,C. (1995) Structural plasticity in the Drosophila brain. J. Neurosci., 15, 1951–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäger R.J. and Fishbach,K.F. (1989) Mass histology of adult heads. In Ashburner,M., Drosophila: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 254–259.
- Kabara J.J. (1973) A critical review of brain cholesterol metabolism. Prog. Brain Res., 40, 363–382. [DOI] [PubMed] [Google Scholar]
- Kemp B.E., Mitchell,K.I., Stapleton,D., Michell,B.J., Chen,Z.-P. and Witters,L. (1999) Dealing with energy demand: the AMPK-activated protein kinase. Trends Biochem. Sci., 24, 22–25. [DOI] [PubMed] [Google Scholar]
- Kojro E., Gimpl,G., Lammich,S., Marz,W. and Fahrenholz,F. (2001) Low cholesterol stimulates the nonamyloidogenic pathway by its effect on the α-secretase ADAM 10. Proc. Natl Acad. Sci. USA, 98, 5815–5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koudinova N.V., Berezov,T.T. and Koudinov,A.R. (1996) Multiple inhibitory effects of Alzheimer’s peptide Aβ1–40 on lipid biosynthesis in cultured human HepG2 cells. FEBS Lett., 395, 204–206. [DOI] [PubMed] [Google Scholar]
- Kretzschmar D., Hasan,G., Sharma,S., Heisenberg,M. and Benzer,S. (1997) The swiss cheese mutant causes glial hyperwrapping and brain degeneration in Drosophila. J. Neurosci., 17, 7425–7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladu M.J., Reardon,C., Van Eldik,L., Fagan,A.M., Bu,G., Holtzman,D. and Getz,G.S. (2000) Lipoproteins in the central nervous system. Ann. NY Acad. Sci., 903, 167–175. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Lee S.J., Liyanage,U., Bickel,P.E., Xia,W., Lansbury,P.T.,Jr and Kosik,K.S. (1998) A detergent-insoluble membrane compartment contains Aβ in vivo. Nat. Med., 4, 730–734. [DOI] [PubMed] [Google Scholar]
- Liu Y., Peterson,D.A. and Schubert,D. (1998) Amyloid β peptide alters intracellular vesicle trafficking and cholesterol homeostasis. Proc. Natl Acad. Sci. USA, 95, 13266–13271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L.Q., Martin-Morris,L.E. and White,K. (1990) Identification, secretion and neural expression of APPL, a Drosophila protein similar to human amyloid protein precursor. J. Neurosci., 10, 3849–3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Tully,T. and White,K. (1992) Human amyloid precursor protein ameliorates behavioral deficit of flies deleted for Appl gene. Neuron, 9, 595–605. [DOI] [PubMed] [Google Scholar]
- Luo L., Liao,Y.J., Jan,L.Y. and Jan,Y.N. (1994) Distinct morphogenetic functions of similar small GTPases: Drosophila Drac1 is involved in axonal outgrowth and myoblast fusion. Genes Dev., 8, 1787–1802. [DOI] [PubMed] [Google Scholar]
- Mahley R.W. (1988) Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science, 240, 622–630. [DOI] [PubMed] [Google Scholar]
- Morell P. and Jurevics,H. (1996) Origin of cholesterol in myelin. Neurochem. Res., 21, 463–470. [DOI] [PubMed] [Google Scholar]
- Müller U., Cristina,N., Li,Z.W., Wolfer,D.P., Lipp,H.P., Rulicke,T., Brandner,S., Aguzzi,A. and Weissmann,C. (1994) Behavioral and anatomical deficits in mice homozygous for a modified β-amyloid precursor protein gene. Cell, 79, 755–765. [DOI] [PubMed] [Google Scholar]
- Nowotny P. et al. (2000) Posttranslational modification and plasma membrane localization of the Drosophila melanogaster presenilin. Mol. Cell. Neurosci., 15, 88–98. [DOI] [PubMed] [Google Scholar]
- O’Kane C.J. (1998) Enhancer traps. In Roberts,D.B. (ed.), Drosophila: A Practical Approach, 2nd edn. Oxford University Press, New York, NY, pp. 131–175.
- Pan D. and Rubin,G.M. (1997) Kuzbanian controls proteolytic processing of Notch and mediates lateral inhibition during Drosophila and vertebrate neurogenesis. Cell, 90, 271–280. [DOI] [PubMed] [Google Scholar]
- Perez R.G., Zheng,H., Van der Ploeg,L.H. and Koo,E.H. (1997) The β-amyloid precursor protein of Alzheimer’s disease enhances neuron viability and modulates neuronal polarity. J. Neurosci., 17, 9407–9414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier J. (1994) Apolipoprotein E in animal models of CNS injury and in Alzheimer’s disease. Trends Neurosci., 17, 525–530. [DOI] [PubMed] [Google Scholar]
- Puglielli L., Konopka,G., Pack-Chung,E., Ingano,L.A., Berezovska,O., Hyman,B.T., Chang,T.Y., Tanzi,R.E. and Kovacs,D.M. (2001) Acyl-coenzyme A: cholesterol acyltransferase modulates the generation of the amyloid β-peptide. Nat. Cell Biol., 3, 905–912. [DOI] [PubMed] [Google Scholar]
- Refolo L.M., Malester,B., LaFrancois,J., Bryant-Thomas,T., Wang,R., Tint,G.S., Sambamurti,K., Duff,K. and Pappolla,M.A. (2000) Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol. Dis., 7, 321–731. [DOI] [PubMed] [Google Scholar]
- Reynolds E.S. (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microskopy. J. Cell Biol., 17, 208–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roheim P.S., Carey,M., Forte,T. and Vega,G.L. (1979) Apolipoproteins in human cerebrospinal fluid. Proc. Natl Acad. Sci. USA, 76, 4646–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen D.R., Martin-Morris,L., Luo,L. and White,K.A. (1989) A Drosophila gene encoding a protein resembling the human β-amyloid precursor. Proc. Natl Acad. Sci. USA, 86, 2478–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthmann A. (1966) Methoden der Zellforschung. Franckh’sche Verlagshandlung, Stuttgart, Germany.
- Simons K. and Ikonen,E. (1997) Functional rafts in cell membranes. Nature, 387, 569–572. [DOI] [PubMed] [Google Scholar]
- Simons M., Keller,P., De Strooper,B., Beyreuther,K., Dotti,C.G. and Simons,K. (1998) Cholesterol depletion inhibits the generation of β-amyloid in hippocampal neurons. Proc. Natl Acad. Sci. USA, 95, 6460–6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M., Keller,P., Dichgans,J. and Schulz,J.B. (2001) Cholestrol and Alzheimer’s disease. Neurology, 57, 1089–1093. [DOI] [PubMed] [Google Scholar]
- Stapleton D. et al. (1996) Mammalian AMP-activated protein kinase subfamily. J. Biol. Chem., 271, 611–614. [DOI] [PubMed] [Google Scholar]
- Thornton C., Snowden,M.A. and Carling,D. (1998) Identification of a novel AMP-activated protein kinase β subunit isoform that is highly expressed in skeletal muscle. J. Biol. Chem., 273, 12443–12450. [DOI] [PubMed] [Google Scholar]
- Torroja L., Luo,L. and White,K. (1996) APPL, the Drosophila member of the APP family, exhibits differential trafficking and processing in CNS neurons. J. Neurosci., 16, 4638–4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin,T and Gordon,J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl Acad. Sci. USA, 76, 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschäpe L.-A., Cruz,A. and Kretzschmar,D. (2002) Progressive neurodegeneration in Drosophila: a model system. J. Neuronal Transm. Suppl., in press. [DOI] [PubMed] [Google Scholar]
- Van Doren M., Broihier,H.T., Moore,L.A. and Lehmann,R. (1998) HMG-CoA reductase guides migrating primordial germ cells. Nature, 396, 466–469. [DOI] [PubMed] [Google Scholar]
- Weisgraber K.H. and Mahley,R.W. (1996) Human apolipoprotein E: the Alzheimer’s disease connection. FASEB J., 10, 1485–1494. [DOI] [PubMed] [Google Scholar]
- Winder W.W. and Hardie,D.G. (1999) AMP-activated protein kinase, a metabolic master switch: possible roles in type 2 diabetes. Am. J. Physiol., 277, E1–E10. [DOI] [PubMed] [Google Scholar]
- Wolozin B. (2001) A fluid connection: cholesterol and Aβ. Proc. Natl Acad. Sci. USA, 98, 5371–5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B., Kellman,W., Ruosseau,P., Celesia,G.G. and Siegel,G. (2000) Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch. Neurol., 57, 1439–1443. [DOI] [PubMed] [Google Scholar]
- Zheng H. et al. (1995) β-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell, 81, 525–531. [DOI] [PubMed] [Google Scholar]