Abstract
Activation of the superoxide-producing phagocyte NADPH oxidase, crucial in host defense, requires the cytosolic proteins p67phox and p47phox. They translocate to the membrane upon cell stimulation and activate flavocytochrome b558, the membrane-integrated catalytic core of this enzyme system. The activators p67phox and p47phox form a ternary complex together with p40phox, an adaptor protein with unknown function, comprising the PX/PB2, SH3 and PC motif- containing domains: p40phox associates with p67phox via binding of the p40phox PC motif to the p67phox PB1 domain, while p47phox directly interacts with p67phox but not with p40phox. Here we show that p40phox enhances membrane translocation of p67phox and p47phox in stimulated cells, which leads to facilitated production of superoxide. The enhancement cannot be elicited by a mutant p40phox carrying the D289A substitution in PC or a p67phox with the K355A substitution in PB1, each being defective in binding to its respective partner. Thus p40phox participates in activation of the phagocyte oxidase by regulating membrane recruitment of p67phox and p47phox via the PB1–PC interaction with p67phox.
Keywords: NADPH oxidase/p40phox/p67phox/PB1 domain/PC motif
Introduction
Professional phagocytes, including neutrophils and macrophages, play a crucial role in host defense against microbial infection. During phagocytosis of invading microorganisms or upon cell stimulation with soluble agents, phagocytes produce superoxide (O2–), a precursor of microbicidal oxidants, by catalysis of an activated NADPH oxidase (Sumimoto et al., 1997; Babior, 1999; Clark, 1999; Nauseef, 1999). The soluble stimulants include phorbol myristate acetate (PMA) and chemoattractants, the latter of which bind to their own receptors to activate the oxidase via the trimeric GTPase Gi (Bokoch, 1995). The significance of the oxidase in host defense is exemplified by recurrent and life-threatening infections that occur in patients with chronic granulomatous disease (CGD), whose phagocytes are defective in the superoxide-producing activity (Roos et al., 1996).
The redox core of the phagocyte NADPH oxidase is a membrane-spanning flavocytochrome b558, comprising the two subunits gp91phox and p22phox. The cytochrome is catalytically inactive in resting cells but acquires the capacity to transfer electrons from NADPH to molecular oxygen upon cell stimulation, leading to superoxide production (Roos et al., 1996; Babior, 1999). The activation of cytochrome b558 requires stimulus-induced membrane translocation of cytosolic proteins, namely the small GTPases Rac and two SH3 domain-harboring oxidase factors p47phox and p67phox (Sumimoto et al., 1997; Babior, 1999; Clark, 1999; Nauseef, 1999). In CGD neutrophils lacking cytochrome b558, neither p47phox nor p67phox can be recruited to the membrane upon cell stimulation (Heyworth et al., 1991). In p47phox-deficient phagocytes, membrane targeting of p67phox does not occur, whereas p47phox is independently targeted in p67phox-defective cells (Heyworth et al., 1991; Dusi et al., 1996). Thus p47phox appears to directly interact with cytochrome b558 and recruits p67phox to the membrane. Indeed the SH3 domains of p47phox bind to a proline-rich region (PRR) of p22phox, the small subunit of cytochrome b558 (Leto et al., 1994; Sumimoto et al., 1994). Although the SH3 domains are normally masked via an intramolecular interaction, the conformation of p47phox becomes changed upon cell stimulation to render the domains in a state accessible to p22phox, which leads to the activation of the NADPH oxidase (Sumimoto et al., 1994; Ago et al., 1999). The P156Q substitution in the p22phox PRR, a mutation that has occurred in a case of CGD (Dinauer et al., 1991), results in not only impaired interaction between p22phox and p47phox in vitro (Sumimoto et al., 1994), but also defective translocation of p47phox to the membrane in vivo (Leusen et al., 1994). It is also known that p67phox constitutively associates with p47phox via a tail-to-tail interaction: the C-terminal SH3 domain of p67phox directly binds to a PRR in the C-terminal region of p47phox (Leusen et al., 1994; Ito et al., 1996). On the other hand, membrane translocation of Rac is independent of the presence of cytochrome b558, p47phox and p67phox: even in CGD neutrophils lacking one of these proteins, the GTPase is normally recruited to the membrane (Heyworth et al., 1994).
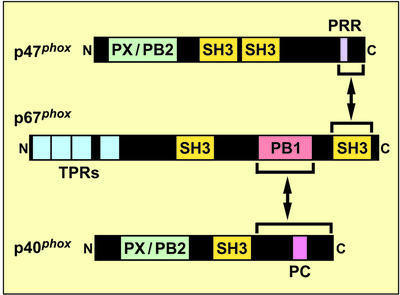
Another SH3 domain-containing oxidase factor, p40phox, has been identified as a protein that constitutively associates with p67phox in the cytosol of resting phagocytes (Someya et al., 1993; Wientjes et al., 1993; Tsunawaki et al., 1994). It comprises three portions: a PX (Phox) domain [also known as PB2 (Phox and Bem 2) domain] at the N-terminus, an SH3 domain in the middle and the C-terminal region containing a PC (Phox and Cdc) motif (Ponting, 1996; Sumimoto et al., 1997; Mizuki et al., 1998; Nakamura et al., 1998; Hiroaki et al., 2001) (Figure 1). The PC motif is present in a variety of signaling proteins such as the yeast polarity protein Cdc24p, the protein kinase MEK5 and Zip, a protein that interacts with the ζ isoform of protein kinase C, and serves as a target of PB1 (Phox and Bem 1) domain, thereby mediating modular protein–protein interactions (Ito et al., 2001; Terasawa et al., 2001). The protein p67phox contains a PB1 domain between the two SH3 domains, which recognizes the PC motif of p40phox and plays an essential role in interaction between p67phox and p40phox (Ito et al., 2001). Thus, in a ternary oxidase complex of resting phagocytes, p67phox is thought to tether p47phox to p40phox: the C-terminal SH3 domain binds to the PRR of p47phox and the PB1 domain directly interacts with the PC motif of p40phox (Figure 1).
Fig. 1. Protein–protein interactions in a complex of the phagocyte NADPH oxidase factors p67phox, p47phox and p40phox. The NADPH oxidase proteins p67phox, p47phox and p40phox form a complex in resting phagocytes. The three proteins harbor multiple modular domains: p67phox comprises a domain containing four units of TPR (tetratricopeptide repeat), the first SH3 domain, PB1 domain and the second SH3 domain; p47phox contains PX or PB2 domain, two SH3 domains and the C-terminal PRR; and p40phox harbors PX/PB2 and SH3 domains, followed by PC motif. In the oxidase complex, p67phox directly binds to p47phox via a tail-to-tail interaction between the C-terminal SH3 of p67phox and the PRR of p47phox; and p67phox also directly binds to p40phox via a novel modular interaction between the PB1 domain of p67phox and the PC motif-containing region of p40phox. Thus p67phox tethers p47phox to p40phox.
The role of p40phox in the phagocyte NADPH oxidase activation has remained largely elusive, although it probably resides in a complex of the oxidase as described above. The oxidase can be activated in a cell-free system reconstituted with cytochrome b558, cytosolic proteins (p47phox, p67phox and Rac in the GTP-bound form) and anionic amphiphiles such as arachidonate and sodium dodecyl sulfate (Bromberg and Pick, 1985; Hata et al., 1998). It is well known that the cell-free activation does not require p40phox. The effect of the addition of p40phox to the cell-free system, however, seems controversial: Cross (2000) has shown that p40phox only slightly elevates the oxidase activity under cell-free conditions, while other investigators have reported that p40phox represses the oxidase activation at high concentrations (Sathyamoorthy et al., 1997). Although the cell-free system has greatly contributed to understanding of the molecular mechanism for activation of the NADPH oxidase, results obtained using this system sometimes seem inconsistent with those using intact cells. For instance, p67phox lacking the C-terminal SH3 domain is incapable of activating the NADPH oxidase when it is expressed in cells, while the mutant p67phox almost fully activates the oxidase under cell-free conditions (de Mendez et al., 1994).
Here we describe the role of p40phox in activation of the phagocyte NADPH oxidase at the cellular level. This protein enhances membrane translocation of the cytosolic activators p67phox and p47phox, and subsequent activation of the NADPH oxidase by 2- to 3-fold, when cells are stimulated with PMA. The enhancements are totally dependent on the binding of p40phox to p67phox, which is mediated via the PB1–PC interaction. Intriguingly, in cells treated with a Gi-activating peptide instead of PMA, p40phox serves as a much more efficient activator of the oxidase, thereby underscoring its importance in a physiological context that cannot be reproduced under cell-free conditions.
Results
Expression of p40phox leads to enhanced production of superoxide in PMA-stimulated cells
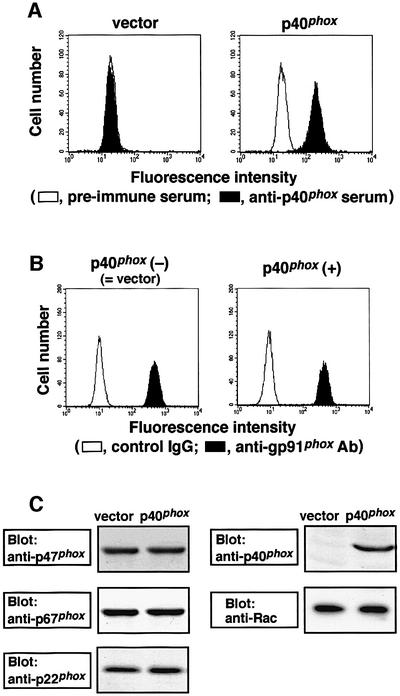
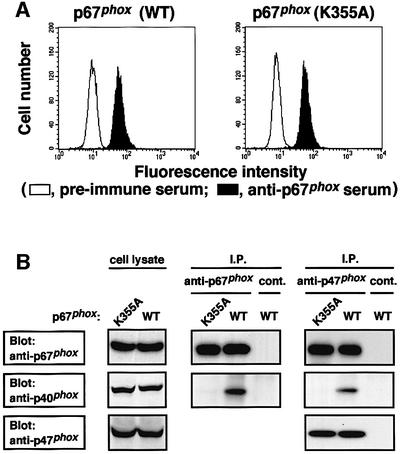
To investigate the role of p40phox in activation of the phagocyte NADPH oxidase, we transfected the plasmid vector pREP4 encoding the full-length cDNA of p40phox or vector alone to K562 cells that express functional cytochrome b558, p47phox and p67phox (Ago et al., 1999; Koga et al., 1999), and cloned seven independent transformants, each expressing p40phox at the same level (for details see Materials and methods). The transformants stably express p40phox as estimated by flow cytometric analysis (Figure 2A). The p40phox-expressing cells harbored the same amounts of functional gp91phox as the p40phox-deficient cells, containing the vector pREP4 that did not encode this protein (Figure 2B). In addition, analyses by immunoblot (Figure 2C) and flow cytometry (data not shown) revealed that no difference existed between these cells in expression of the proteins p22phox, p47phox, p67phox and the small GTPase Rac.
Fig. 2. Stable expression of p40phox in K562 cells transfected with pREP4 encoding the full-length of this protein. (A) Expression of p40phox in the K562 cells. Parent K562 cells stably expressing gp91phox, p67phox and p47phox were transfected with pREP4 encoding the full-length p40phox (p40phox) or vector alone (vector). The cells were stained with anti-p40phox serum (filled histogram) or pre-immune serum (open histogram) and analyzed by flow cytometry. For details see Materials and methods. (B) Expression of functional gp91phox. The cells were stained with the monoclonal antibody 7D5 to detect gp91phox in functional cytochrome b558 (filled histogram) or control IgG (open histogram) and analyzed by flow cytometry. (C) Expression of p47phox, p67phox, p40phox and p22phox. The lysates of the K562 cells were analyzed by immunoblot with an anti-p47phox, an anti-p67phox, an anti-p40phox or an anti-p22phox antibody, as described in Materials and methods.
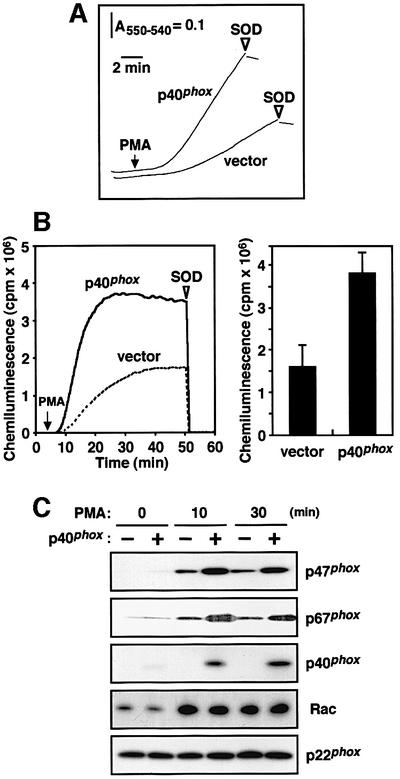
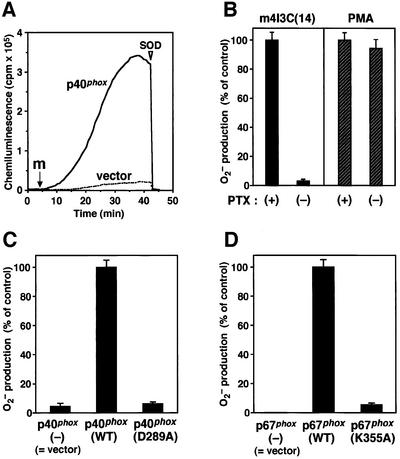
When the K562 cells expressing p40phox were stimulated with PMA, they produced a large amount of superoxide, which was estimated by superoxide dismutase (SOD)-inhibitable cytochrome c reduction (Figure 3A). We also tested four other clones (data not shown) and found that the cells exhibited essentially the same superoxide-producing activity of 4.2 ± 0.14 nmol/min per 106 cells (n = 5). On the other hand, the activity of the cells without p40phox was 1.8 ± 0.12 nmol/min per 106 cells (n = 5 independent clones) (Figure 3A; data not shown). Using another method, SOD-inhibitable chemiluminescence, we also tested the effect of p40phox in activation of the phagocyte NADPH oxidase (Figure 3B). In response to PMA, seven independent clones of the p40phox-expressing K562 cells all produced 2- to 3-fold more superoxide than the cells lacking p40phox (Figure 3B). Thus expression of p40phox leads to enhanced production of superoxide in PMA-stimulated cells, raising the possibility that p40phox facilitates the assembly of the phagocyte oxidase factors.
Fig. 3. p40phox-enhanced superoxide production and membrane translocation of p47phox and p67phox upon cell stimulation with PMA. (A) Superoxide production by the K562 cells with stable expression of p40phox or without p40phox was measured as reduction of cytochrome c. The cells (5.0 × 105 cells/ml) were stimulated with PMA (200 ng/ml) (arrow), and the superoxide-producing activity was measured by determining the rate of ferricytochrome c reduction at 550 to 540 nm. The reduction was stopped by addition of superoxide dismutase (SOD) (50 µg/ml) (arrowhead). (B) Superoxide production by the K562 cells with p40phox or without p40phox was measured as change of chemiluminescence. The K562 cells (5.0 × 103 cells) were stimulated with PMA (200 ng/ml), and the chemiluminescence change was continuously monitored with DIOGENES. SOD (50 µg/ml) was added where indicated (arrowhead). For details see Materials and methods. (C) Membrane translocation of p47phox, p67phox, p40phox and Rac upon PMA stimulation. The K562 cells with or without p40phox (1.0 × 107 cells) were incubated with PMA (200 ng/ml) for the indicated time. After the incubation, the cells were sonicated and ultracentrifuged as described in Materials and methods. The membrane fractions were analyzed by immunoblot with the antibody against p47phox, p67phox, p40phox, Rac or p22phox. The data are representative of results from five independent experiments.
p40phox facilitates membrane translocation of p47phox and p67phox but not of Rac upon cell stimulation
It is well established that stimulus-elicited translocation of p47phox and p67phox from the cytosol to the membrane is required for activation of the phagocyte NADPH oxidase (Roos et al., 1996; Clark, 1999; Nauseef, 1999). Since p40phox is constitutively associated with p67phox and enhances the oxidase activation as shown above (Figure 3A and B), it seems likely that p40phox affects the membrane recruitment of p47phox and p67phox. To test this possibility, we fractionated the membrane of the K562 cells with or without p40phox and estimated the amounts of p47phox and p67phox. When the p40phox-lacking cells were stimulated with PMA, both p47phox and p67phox translocated to the membrane in a time-dependent manner (Figure 3C). In the cells expressing p40phox, this protein as well as p47phox and p67phox was targeted upon cell stimulation to the membrane (Figure 3C). The amounts of p47phox and p67phox at the membrane were increased by 2- to 3-fold, compared with those of the p40phox-deficient cells (Figure 3C). On the other hand, p40phox did not affect PMA-elicited membrane targeting of Rac (Figure 3C). Thus the enhancement of the oxidase activation by p40phox is likely to be due to the p40phox-induced facilitation of membrane translocation of p47phox and p67phox.
p40phox carrying the D289A substitution is incapable of binding to p67phox and fails to enhance superoxide production and membrane translocation of p47phox and p67phox
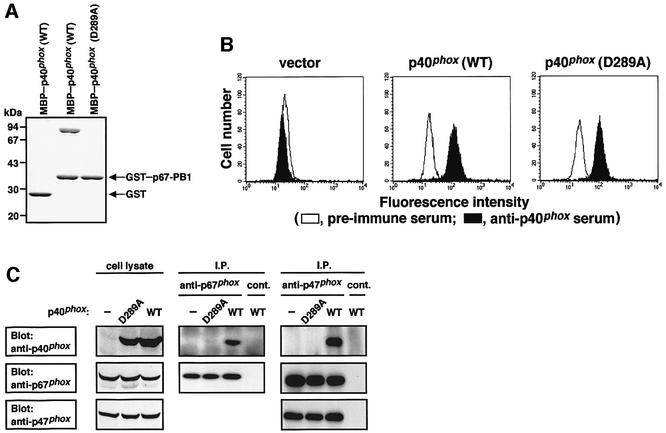
We next tested whether the interaction of p40phox with p67phox participates in the p40phox-enhanced translocation of p47phox and p67phox. As we have previously shown, this interaction is mediated via a direct binding of the p40phox PC motif to the p67phox PB1 domain (Nakamura et al., 1998; Ito et al., 2001). Consistent with previous observations, the maltose-binding protein (MBP)-tagged full-length protein of p40phox, MBP–p40phox (WT), directly interacted with the p67phox PB1 domain as a glutathione S-transferase (GST) fusion (GST–p67-PB1) as analyzed by a pull-down assay (Figure 4A). To confirm the role for the PC motif of p40phox, we purified a mutant p40phox carrying the amino acid substitution of Ala for Asp289, an invariant residue among PC motifs (Nakamura et al., 1998); the corresponding residue in the PC motif of the yeast polarity protein Cdc24p plays an essential role in its binding to the PB1 domain of Bem1p (Ito et al., 2001). As shown in Figure 4A, the D289A substitution resulted in a complete loss of the interaction of p40phox with p67phox.
Fig. 4. Effect of the D289A substitution in the PC motif of p40phox on its interaction with p67phox both in vivo and in vitro. (A) Direct interaction of p40phox with p67phox. GST-tagged PB1 domain of p67phox (GST–p67-PB1) or GST alone was incubated with MBP–p40phox (WT) or MBP–p40phox (D289A) and proteins were pulled down with glutathione–Sepharose-4B. The precipitated proteins were subjected to SDS–PAGE, followed by staining with CBB, as described, see Materials and methods. Positions for marker proteins are indicated in kDa. (B) Expression of p40phox (D289A) in K562 cells. K562 cells stably expressing gp91phox, p67phox and p47phox were transfected with pREP4 encoding p40phox with the D289A substitution, p40phox (D289A). The K562 cells were stained with anti-p40phox serum (filled histogram) or pre-immune serum (open histogram) and analyzed by flow cytometry. (C) Interaction of p40phox with p67phox in the K562 cells. The cell lysates of the K562 cells were analyzed by immunoprecipitation with an anti-p67phox or control IgG (cont.) (left panel), or an anti-p47phox antibody or control IgG (cont.) (right panel) followed by immunoblot with the indicated antibody, as described under Materials and methods.
To examine the effect of the D289A substitution in the PC motif at the cellular level, we prepared seven clones of the K562 cells stably expressing p40phox (D289A). The amount of p40phox as well as those of other oxidase proteins including gp91phox, p22 phox, p47phox and p67phox in the p40phox (D289A)-expressing cells was identical to that in the cells expressing the wild-type p40phox (WT) (Figure 4B and C; data not shown). An immunoprecipitation assay demonstrated that p67phox binds to p40phox (WT) but not to p40phox (D289A) in vivo (Figure 4C), indicating that the binding of p40phox to p67phox is mediated solely via the PC motif even at the cellular level. Although p47phox was involved in a complex with p40phox and p67phox (Figure 4C), it should be noted that the interaction between p47phox and p67phox occurs in a manner independent of the presence of p40phox (Figure 4C).
When cells were stimulated with PMA, p40phox (D289A) was incapable of enhancing the activation of the NADPH oxidase: the amount of superoxide produced by the cells expressing p40phox (D289A) was similar to that by the cells without p40phox (Figure 5A). The amounts of p67phox and p47phox in the membrane of the p40phox (D289A)-expressing cells were half-to-one-third of those of the p40phox (WT)-expressing ones. In addition, p40phox (D289A) did not translocate to the membrane in response to PMA (Figure 5B), suggesting that its localization is totally dependent on the interaction with p67phox. The present observations thus indicate that membrane translocation of p47phox and p67phox is facilitated by the binding of p40phox to p67phox, which leads to enhanced activation of the NADPH oxidase.
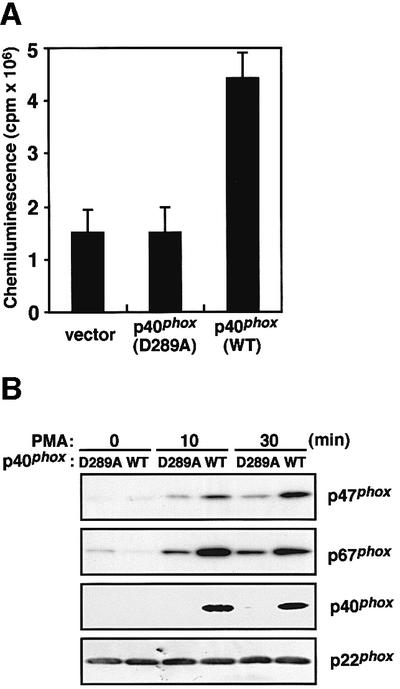
Fig. 5. Effect of the D289A substitution in the PC motif of p40phox on superoxide production and membrane translocation of p47phox and p67phox upon PMA stimulation. (A) Superoxide production by the K562 cells with p40phox (WT) or p40phox (D289A) was measured as change of chemiluminescence. The K562 cells (5.0 × 103 cells) were stimulated with PMA (200 ng/ml) and the chemiluminescence change was measured. Each histogram indicates the average from five independent experiments, with bars representing SD. (B) Membrane translocation of p47phox, p67phox and p40phox upon PMA stimulation. The K562 cells stably expressing p40phox (WT) or p40phox (D289A) (1.0 × 107 cells) were stimulated with PMA (200 ng/ml) for the indicated time and the amounts of p47phox, p67phox and p40phox in the membrane fractions were analyzed by immunoblot as described under Materials and methods.
p67phox carrying the K355A substitution is incapable of interacting with p40phox, and fails to enhance superoxide production and membrane translocation of p47phox and p67phox
To further study the importance of the interaction between p40phox and p67phox, we used a mutant p67phox with the substitution of Ala for Lys355 (Figure 6A), a conserved residue among PB1 domains (Ito et al., 2001; Terasawa et al., 2001). In an in vitro pull-down assay, GST–p67-PB1 (K355A) was incapable of interacting with MBP–p40phox, while the wild-type PB1 domain fully bound to p40phox (data not shown). Similarly, p67phox (K355A) could not associate with p40phox in vivo (Figure 6B). On the other hand, both p67phox (WT) and p67phox (K355A) were co-immunoprecipitated with p47phox (Figure 6B), indicating that p40phox binds to p47phox indirectly but via p67phox (see Figure 1).
Fig. 6. Effect of the K355A substitution in the PB1 domain of p67phox on its interaction with p40phox. (A) Expression of p67phox in K562 cells. K562 cells stably expressing gp91phox, p47phox and p40phox were transfected with pREP4 encoding p67phox (WT) or p67phox (K355A). The K562 cells were stained with anti-p67phox serum (filled histogram) or pre-immune serum (open histogram), and analyzed by flow cytometry. (B) In vivo interaction of p67phox with p40phox in the K562 cells. The cell lysates of the K562 cells were analyzed by immunoprecipitation with the anti-p67phox or control IgG (cont.) (left panel), or the anti-p47phox antibody or control IgG (cont.) (right panel) followed by immunoblot (Blot) with the indicated antibody.
We next investigated the effect of the K355A substitution in the p67phox PB1 domain on superoxide production and membrane translocation of p47phox and p67phox. As shown in Figure 7A, the amounts of p47phox and p67phox in the membranes of the p67phox (K355A)-expressing cells were half-to-one-third of those of the p67phox (WT)-expressing cells. In the cells expressing p67phox (K355A), p40phox failed to translocate to the membranes. As shown in Figure 7B, the amount of superoxide produced by the cells expressing p67phox (K355A) was half-to-one-third of that by those expressing p67phox (WT). In response to PMA, the cells with p67phox (K355A) produced the same low amount of superoxide irrespective of the presence of p40phox (Figure 7B). These observations further supported the idea that the p40phox–p67phox interaction enhances the membrane translocation of p47phox and p67phox, thereby facilitating the assembly of the active oxidase complex.
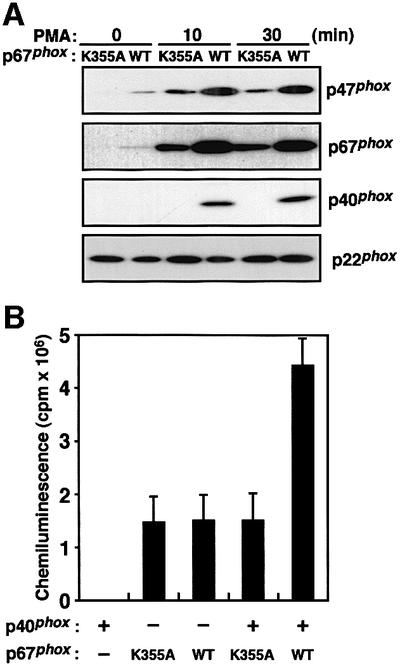
Fig. 7. Effect of the K355A substitution in the PB1 domain on membrane translocation of p47phox and p67phox and superoxide production upon PMA stimulation. (A) Membrane translocation of p47phox, p67phox and p40phox upon PMA stimulation. The K562 cells stably expressing p67phox (WT) or p67phox (K355A) (1.0 × 107 cells) were stimulated with PMA (200 ng/ml) for the indicated time and the amounts of p47phox, p67phox and p40phox in the membrane fractions were analyzed by immunoblot. (B) Superoxide production by the K562 cells with p67phox (WT) or p67phox (K355A) was measured as change of chemiluminescence. The K562 cells (5.0 × 103 cells) were stimulated with PMA (200 ng/ml) and the chemiluminescence change was measured. The chemiluminescence change by K562 cells without the expression of p40phox and with p67phox (WT) or p67phox (K355A) was also measured. Each histogram indicates the average from five independent experiments, with bars representing SD.
p40phox greatly enhances superoxide production in cells stimulated with the muscarinic receptor peptide m4I3C(14), an agent that can activate the trimeric GTPase Gi
It is well documented that, in neutrophils, chemoattractants such as N-formyl peptides induce superoxide production via the trimeric GTPase Gi (Bokoch, 1995). The chemotactic receptor, however, is not expressed in K562 cells. To further clarify the role of p40phox in the oxidase activation, we used the muscarinic receptor peptide m4I3C(14) as a stimulant.
In response to m4I3C(14), K562 cells expressing p40phox produced superoxide (Figure 8A). The superoxide production, albeit 10-fold less than that by PMA-stimulated cells, is likely to be catalyzed by the phagocyte NADPH oxidase, since the peptide did not act on parental K562 cells that lack oxidase proteins (data not shown) or cells expressing all oxidase factors except p67phox (see later). Furthermore, we did detect, although qualitatively, the peptide-induced membrane translocation of the cytosolic oxidase proteins (data not shown). The treatment of the K562 cells with pertussis toxin (PTX) resulted in a loss of the m4I3C(14)-induced superoxide production, while the toxin did not affect that induced by PMA (Figure 8B). The peptide thus activates the oxidase via Gi.
Fig. 8. p40phox-enhanced superoxide production upon cell stimulation with the muscarinic receptor peptide m4I3C(14). (A) Superoxide production by the K562 cells with stable expression of p40phox or without p40phox. The K562 cells (5.0 × 103 cells) were stimulated with the muscarinic receptor peptide m4I3C(14) (m) (200 µM) and the chemiluminescence change was continuously monitored with DIOGENES, and SOD (50 µg/ml) was added where indicated (arrowhead). For details see Materials and methods. (B) Effect of pertussis toxin (PTX) on superoxide production upon stimulation with m4I3C(14) or PMA. The K562 cells (5.0 × 103 cells) were pretreated with PTX (18 µg/ml) and stimulated with m4I3C(14) (200 µM) or PMA (200 ng/ml) and superoxide production was measured as change of chemiluminescence. Each histogram indicates the average from five independent experiments, with bars representing SD. (C) Effect of the D289A substitution in the PC motif of p40phox on superoxide production upon stimulation with m4I3C(14). The K562 cells with p40phox (WT) or p40phox (D289A) or without p40phox (5.0 × 103 cells) were stimulated with 200 µM m4I3C(14) and superoxide production was measured as change of chemiluminescence. Each histogram represents the average from five independent experiments, with bars representing SD. (D) Effect of the K355A substitution in the PB1 domain of p67phox on superoxide production upon stimulation with m4I3C(14). The K562 cells with p67phox (WT) or p67phox (K355A) or without p67phox (5.0 × 103 cells) were stimulated with 200 µM m4I3C(14), and superoxide production was measured as change of chemiluminescence. Each histogram indicates the average from five independent experiments, with bars representing SD.
Intriguingly, in response to m4I3C(14), K562 cells expressing p40phox produced superoxide ∼20-fold as much compared with the cells lacking this adaptor protein (Figure 8C). In addition, the peptide served as a weak agonist when cells expressed p40phox (D289A) but not the wild-type p40phox (Figure 8C). Consistent with this, only a small amount of superoxide was generated in cells expressing p67phox (K355A) instead of the wild-type p67phox, while no superoxide production was detected in cells lacking p67phox (Figure 8D). Thus, in cells stimulated with the Gi activator m4I3C(14), p40phox greatly enhances activation of the phagocyte NADPH oxidase, which is dependent on its interaction with p67phox.
Discussion
Although p40phox is known as a protein that constitutively associates with the phagocyte oxidase activator p67phox, the role of p40phox in the oxidase activation has remained elusive. In the present study, we show that p40phox facilitates the activation of the phagocyte NADPH oxidase at the cellular level (Figures 3 and 8). The facilitation is thought to be caused by enhancing the stimulus-induced recruitment of p67phox and p47phox to the membrane, an essential step for the oxidase activation, without affecting the membrane translocation of the small GTPase Rac (Figure 3). We also demonstrate that both enhanced recruitment of the oxidase activators and facilitated activation of the oxidase are totally dependent on the binding of p40phox to p67phox, which is mediated via a novel modular interaction between the p40phox PC motif and the p67phox PB1 domain (Figures 5, 7 and 8).
One of the reasons why the role of p40phox has been obscure for a long time is due to the fact that p40phox is dispensable for NADPH oxidase activation under both cell-free and whole-cell conditions (de Mendez et al., 1994; Sathyamoorthy et al., 1997). In addition, it has been reported that the presence of p40phox marginally affects the oxidase activity in a cell-free activation system: this protein only slightly enhances the activation (Cross, 2000). Although studies under cell-free conditions have shed light on the understanding of the molecular mechanism for the oxidase activation, results obtained in such studies sometimes seem discrepant with those observed in a whole-cell system. For instance, p67phox binds to p47phox via the C-terminal SH3 domain of p67phox, a domain which is required for the oxidase activation in a whole-cell system (de Mendez et al., 1994) but not for the cell-free activation (de Mendez et al., 1994; Leusen et al., 1995; Hata et al., 1998). Here we show, using cells stably expressing p40phox, that p40phox enhances activation of the NADPH oxidase. When cells are stimulated with PMA, a potent activator of protein kinase C, expression of p40phox leads to a 2- to 3-fold enhancement (Figure 3). On the other hand, the oxidase activation is much more drastically (∼20-fold) facilitated by p40phox in response to the muscarinic receptor peptide m4I3C(14), that acts as an activator of Gi (Figure 8). The peptide probably functions as a more physiological stimulant, since PTX blocks the peptide-induced superoxide production but not the PMA-elicited one (Figure 8). It seems probable that, in in vivo activation of the oxidase, p40phox plays a role that is much more important than expected from the results of experiments with PMA. Alternatively, the significance of p40phox could be dependent on the types of stimulants or signaling pathways for the oxidase activation. In either case, p40phox is deeply involved in activation of the phagocyte NADPH oxidase.
Although no case has been thus far reported of CGD with the defect of p40phox, it is conceivable that its defect causes less severe forms of compromised host defense and hence has escaped the clinical screening for CGD. Alternatively, since the phagocyte oxidase is known to participate not only in host defense but also inflammation, an aberrance in inflammatory response, rather than vulnerability to infection, may be manifested in the cases with defect of p40phox. It is thus intriguing to search single nucleotide polymorphisms or mutations in the p40phox gene NCF4 showing significant association with those displaying these symptoms. However, one should note that extra-phagocytic roles for p40phox and hence its involvement in other diseases are plausible, because mouse Ncf4 gene is also expressed in T cells and neurons, with no expression of p67phox (Mizuki et al., 1998). In any case, pathophysiological roles for p40phox remain largely elusive.
In contrast with the present results, Sathyamoorthy et al. (1997) have reported that transient expression of p40phox leads to a repressed activation of the NADPH oxidase when cells are stimulated with PMA. The reason for this discrepancy is presently unknown. They have also shown that expression of the SH3 domain of p40phox by itself represses the oxidase activation more efficiently than the full-length p40phox does (Sathyamoorthy et al., 1997). Since the SH3 domain of p40phox is capable of binding to p47phox but to a much lesser extent than the C-terminal SH3 domain of p67phox (Ito et al., 1996), overexpression of the p40phox SH3 domain may compete with the SH3 domain of p67phox to prevent p67phox from interacting with p47phox, thereby inhibiting the oxidase activation.
The adaptor protein p40phox is present in a complex containing not only p67phox but also p47phox. In this complex, p40phox directly binds to p67phox via the PB1–PC interaction, while p40phox interacts with p47phox in an indirect manner: p67phox tethers p40phox to p47phox (Figure 1). If the association between p40phox and p67phox is absent, p40phox and p47phox cannot reside in the same complex (Figures 4 and 6). Thus membrane translocation of p47phox is not enhanced by p40phox under conditions where the association between p40phox and p67phox is specifically disrupted (Figures 5 and 7). On the other hand, the membrane translocation of p40phox requires p67phox. When a mutant p67phox (K355A), that cannot interact with p40phox, is expressed instead of the wild-type p67phox, p40phox is incapable of translocating to the membrane upon cell stimulation (Figure 7). This is consistent with a previous observation that, in neutrophils of a p67phox-deficient CGD patient, p40phox is not targeted to the membrane in response to cell stimuli (Dusi et al., 1996). Even in these cells, stimulus-induced translocation of p47phox can still be observed (Dusi et al., 1996). Taken together, p47phox plays an essential role in membrane recruitment of the p67phox–p47phox–p40phox complex, an event which is strongly enhanced by p40phox.
This study clearly demonstrates that p40phox facilitates membrane targeting of the essential oxidase factors p67phox and p47phox, which requires the interaction of p40phox with p67phox. The molecular mechanism whereby p40phox functions, however, is not fully understood at present. In the N-terminal region, p40phox harbors the PX/PB2 domain (Figure 1), a module that interacts with phosphoinositides (Ago et al., 2001; Kanai et al., 2001; Ellson et al., 2001b). The PX domain of p40phox specifically binds to phosphatidylinositol-3-phosphate [PtdIns(3)P] in vitro, and the domain expressed as a fusion to green fluorescent protein localizes to early endosomes where this phosphoinositide is enriched (Ago et al., 2001; Kanai et al., 2001; Ellson et al., 2001b). It is possible that the phospholipid-binding activity of p40phox is involved in its membrane localization. In this context, it is intriguing to note a current report showing that PtdIns(3)P is enriched in phagosomes (Ellson et al., 2001a), where the NADPH oxidase is activated. It has been also reported that p40phox can interact with coronin, an actin-binding protein (Grogan et al., 1997). Interaction of p40phox with cytoskeletal elements may participate in a process of membrane translocation of this protein. In addition, stimulus-induced phosphorylation of p40phox (Bouin et al., 1998) possibly plays a role, an event which has escaped analysis in a cell-free system. These possibilities should be tested in future studies.
The PB1 domain and PC motif prevail in a variety of proteins and mediate protein–protein interactions: the PB1 domain of Bem1p, scd2, atypical protein kinase Cζ or p67phox recognizes and binds to the PC motif of Cdc24p, scd1, ZIP or p40phox, respectively (Ito et al., 2001). In the budding yeast Saccharomyces cerevisiae, the interaction between Bem1p and Cdc24p is essential for polarity establishment of cells (Ito et al., 2001; Butty et al., 2002). The present study provides another example of functional interactions mediated via these modules, i.e. the binding of p40phox to p67phox plays a significant role in activation of the phagocyte NADPH oxidase, an enzyme involved in host defense. Further studies are required to clarify the importance of other PB1–PC interactions.
Materials and methods
Plasmid constructions
cDNA fragments encoding p40phox, p47phox and p67phox were prepared as described previously (Hata et al., 1998; Nakamura et al., 1998; Ago et al., 1999) and cloned into the vectors pREP (Invitrogen), pGEX-4T1 (Amersham Pharmacia Biotech) and pMALc2 (New England Biolabs). Mutations leading to the indicated amino acid substitutions were introduced by PCR-mediated site-directed mutagenesis. All the constructs were sequenced to confirm their identities.
Expression of p40phox in K562 cells containing cytochrome b558, p47phox and p67phox
The doubly transduced K562 cells that stably express both cytochrome b558 and p67phox were prepared using the monoclonal antibody 7D5 to detect gp91phox in functional cytochrome b558 (Yamauchi et al., 2001) and anti-p67phox polyclonal antibodies raised to a C-terminal peptide of this protein (Imajoh-Ohmi et al., 1992), as previously described (Ago et al., 1999). The cells were electroporated in the presence of the full-length cDNA of p47phox in pREP9. After incubation with G418 (1.0 mg/ml), the cells were cloned by limiting dilution and checked by flow cytometry with an anti-p47phox monoclonal antibody (Transduction Laboratories). The stable transformants were further transfected with pREP4 encoding the wild-type p40phox or a mutant one carrying the D289A substitution. The transfected cells were cloned by limiting dilution in the presence of hygromycin B (100 µg/ml) and expression of p40phox was confirmed by flow cytometry with anti-p40phox polyclonal antibodies raised to an N-terminal peptide of this protein (Tsunawaki et al., 1994).
Expression of p67phox in K562 cells containing cytochrome b558, p47phox and p40phox
The doubly transduced K562 cells that stably express both functional cytochrome b558 and p47phox were prepared as previously described (Koga et al., 1999). The cells were electroporated in the presence of pREP9 (Invitrogen) encoding the full-length cDNA of p40phox or vector alone. After incubation with G418 (1.0 mg/ml), the K562 cells were cloned by limiting dilution. The stable transformants were further transfected with pREP10 encoding the wild-type p67phox or a mutant one carrying the K355A substitution. The transfected cells were cloned by limiting dilution in the presence of hygromycin B (100 µg/ml), and expression of p67phox was confirmed by flow cytometry with the anti-p67phox antibodies.
Flow cytometric analysis
Cells were washed with phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4 and 1.5 mM KH2PO4 pH 7.4), and fixed with 2.0% paraformaldehyde for 1 h at 25°C. For detection of cytosolic proteins, the fixed cells were permeabilized with PBS including 0.1% Triton X-100 and 2.0% bovine serum albumin (BSA). The cells were incubated with the indicated antibody, washed twice with 2.0% BSA in PBS, and indirectly labeled with fluorescein isothiocyanate-conjugated goat-anti mouse or rabbit IgG (BioSource International). Cells were washed twice with PBS and analyzed by FACS scan.
Activation of the phagocyte NADPH oxidase
Superoxide production was determined as SOD-inhibitable chemiluminescence, as previously described (Ago et al., 1999; Koga et al., 1999). Cells in HEPES-buffered saline (120 mM NaCl, 5 mM KCl, 5 mM glucose, 1 mM MgCl2, 0.5 mM CaCl2 and 17 mM HEPES pH 7.4) were stimulated at 37°C with PMA (200 ng/ml), and the reaction was terminated by the addition of SOD (50 µg/ml). The chemiluminescence was counted with an enhancer-containing luminol-based detection system (DIOGENES; National Diagnostics) using a luminometer (Auto Lumat LB953; EG&G Berthold).
Alternatively, the superoxide-producing activity was measured by determining the rate of SOD-inhibitable cytochrome c reduction at 550–540 nm using a dual-wavelength spectrophotometer (Hitachi 557) (Sumimoto et al., 1994).
Translocation of p47phox, p67phox and p40phox to the membrane in stimulated K562 cells
Membrane translocation of cytosolic oxidase factors were determined by the method of Leusen et al. (1994) with minor modifications. Briefly, cells were stimulated for the indicated time at 37°C with PMA (200 ng/ml). The incubation was terminated by the addition of ice cold PBS. The cells were resuspended in 3 ml of ice cold buffer A (75 mM NaCl, 170 mM sucrose, 1 mM MgCl2, 0.5 mM EGTA, 10 µM ATP, 2 mM NaN3, 5 µM GTPγS, 100 µg/ml of p-amidinophenyl methanesulfonyl fluoride hydrochloride and 20 mM HEPES pH 7.0) and lyzed by sonication. The sonicate was layered on a discontinuous sucrose gradient consisting of 4 ml of 40% (w/v) sucrose and 3 ml of 15% (w/v) sucrose with 1 mM MgCl2, 40 mM NaCl, 0.5 mM EGTA and 5 µM 5′-3-O- (thio)-triphosphate (GTPγS). After ultracentrifugation for 45 min at 100 000 g, membrane-associated proteins (1.0 µg) were analyzed by immunoblot with the indicated antibody, which were developed using ECL-plus (Amersham Pharmacia Biotech).
An in vitro binding assay using purified recombinant proteins
The PB1 domain of p67phox (amino acids 335–427) and the protein with the K355A substitution were expressed as GST fusion proteins in Escherichia coli and purified by glutathione–Sepharose-4B (Amersham Pharmacia Biotech) (Ago et al., 1999; Koga et al., 1999). MBP-tagged full-length p40phox and the protein with the D289A substitution were also expressed and purified by amylose resin (New England BioLab). For in vitro pull-down binding assays, a pair of a GST fusion (250 µg) and an MBP-tagged protein (500 µg) were incubated for 30 min in 1 ml PBS containing 0.5% Triton X-100. Proteins were precipitated with glutathione–Sepharose-4B and eluted with 5 mM glutathione. The eluates were subjected to SDS–PAGE and stained with Coomassie Brilliant Blue (CBB).
An in vivo binding assay
Cells were lyzed with 1 ml of a lysis buffer (1% Triton X-100, 100 mM NaCl, 5 mM MgCl2, 1 mM EDTA and 40 mM HEPES pH 7.4). The lysate was precipitated with the anti-p47phox or anti-p67phox antibody in the presence of protein G–Sepharose (Amersham Pharmacia Biotech). After washing with the lysis buffer, precipitated proteins were analyzed by immunoblotting.
Preparation of the muscarinic receptor peptide m4I3C(14)
m4I3C(14) is a 14-residue peptide fragment corresponding to the C-terminal portion of the third intracellular loop and the N-terminal portion of the sixth transmembrane helix of human m4 muscarinic acetylcholine receptor (Bonner et al., 1987) (residues 393–406: RERKVTRTIFAILL). This peptide, like mastoparan (Ross and Higashijima, 1994), activates trimeric Gi reconstituted in phospholipid vesicles in a similar manner as the parental m4 muscarinic acetylcholine receptor (K.Wakamatsu, in preparation) yet does not show any cell-damaging activity as observed for mastoparan (Nakajima et al., 2000). The peptide was synthesized by a standard fluoren-9-ylmethoxycarbonyl-based solid-phase method. The purity and the identity of the synthesized peptide were confirmed by analytical HPLC and time-of-flight mass spectroscopy.
Acknowledgments
Acknowledgements
We are grateful to Professor Michio Nakamura (Nagasaki University) for the monoclonal antibody 7D5 and to Dr Shinobu Imajoh-Ohmi (University of Tokyo) for the anti-p67phox antibody. This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan, ONO Medical Research Foundation, CREST JST (Japan Science and Technology) and the BIRD project of JST Corporation.
References
- Ago T., Nunoi,H., Ito,T. and Sumimoto,H. (1999) Mechanism for phosphorylation-induced activation of the phagocyte NADPH oxidase protein p47phox. J. Biol. Chem., 274, 33644–33653. [DOI] [PubMed] [Google Scholar]
- Ago T., Takeya,R., Hiroaki,H., Kuribayashi,F., Ito,T., Kohda,D. and Sumimoto,H. (2001) The PX domain as a novel phosphoinositide-binding module. Biochem. Biophys. Res. Commun., 287, 733–738. [DOI] [PubMed] [Google Scholar]
- Babior B.M. (1999) NADPH oxidase: an update. Blood, 93, 1464–1476. [PubMed] [Google Scholar]
- Bokoch G.M. (1995) Chemoattractant signaling and leukocyte activation. Blood, 86, 1649–1660. [PubMed] [Google Scholar]
- Bonner T.I., Buckley,N.J., Young,A.C. and Brann,M.R. (1987) Identification of a family of muscarinic acetylcholine receptor genes. Science, 237, 527–532. [DOI] [PubMed] [Google Scholar]
- Bouin A.-P., Grandvaux,N., Vignais,P.V. and Fuchs,A. (1998) p40phox is phosphorylated on threonine 154 and serine 315 during activation of the phagocyte NADPH oxidase: implication of a protein kinase C-type kinase in the phosphorylation process. J. Biol. Chem., 273, 30097–30103. [DOI] [PubMed] [Google Scholar]
- Bromberg Y. and Pick,E. (1985) Activation of NADPH-dependent superoxide production in a cell-free system by sodium dodecyl sulfate. J. Biol. Chem., 260, 13539–13545. [PubMed] [Google Scholar]
- Butty A.-C., Perrinjaquet,N., Petit,A., Jaquenoud,M., Segall,J.E., Hofmann,K., Zwahlen,C. and Peter,M. (2002) A positive feedback loop stabilizes the guanine-nucleotide exchange factor Cdc24 at sites of polarization. EMBO J., 21, 1565–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R.A. (1999) Activation of the neutrophil respiratory burst oxidase. J. Infect. Dis., 2, S309–S317. [DOI] [PubMed] [Google Scholar]
- Cross A.R. (2000) p40phox participates in the activation of NADPH oxidase by increasing the affinity of p47phox for flavocytochrome b558. Biochem. J., 349, 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Mendez I., Garrett,M.C., Adams,A.G. and Leto,T.L. (1994) Role of p67-phox SH3 domains in assembly of the NADPH oxidase system. J. Biol. Chem., 269, 16326–16332. [PubMed] [Google Scholar]
- Dinauer M.C., Pierce,E.A., Erickson,R.W., Muhlebach,T.J., Messner,H., Orkin,S.H., Seger,R.A. and Curnutte,J.T. (1991) Point mutation in the cytoplasmic domain of the neutrophil p22-phox cytochrome b subunit is associated with a nonfunctional NADPH oxidase and chronic granulomatous disease. Proc. Natl Acad. Sci. USA, 88, 11231–11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusi S., Donini,M. and Rossi,F. (1996) Mechanisms of NADPH oxidase activation: translocation of p40phox, Rac1 and Rac2 from the cytosol to the membranes in human neutrophils lacking p47phox or p67phox. Biochem. J., 314, 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellson C.D., Anderson,K.E., Morgan,G., Chilvers,E.R., Lipp,P., Stephens,L.R. and Hawkins,P.T. (2001a) Phosphatidylinositol 3-phosphate is generated in phagosomal membranes. Curr. Biol., 11, 1631–1635. [DOI] [PubMed] [Google Scholar]
- Ellson C.D. et al. (2001b) PtdIns(3)P regulates the neutrophil oxidase complex by binding to the PX domain of p40phox. Nat. Cell Biol., 3, 679–682. [DOI] [PubMed] [Google Scholar]
- Grogan A., Reeves,E., Keep,N., Wientjes,F., Totty,N.F., Burlingame,A.L., Hsuan,J.J. and Segal,A.W. (1997) Cytosolic phox proteins interact with and regulate the assembly of coronin in neutrophils. J. Cell Sci., 110, 3071–3081. [DOI] [PubMed] [Google Scholar]
- Hata K., Ito,T., Takeshige,K. and Sumimoto,H. (1998) Anionic amphiphile-independent activation of the phagocyte NADPH oxidase in cell-free system by p47phox and p67phox, both in C terminally truncated forms. Implication for regulatory Src homology 3 domain-mediated interactions. J. Biol. Chem., 273, 4232–4236. [DOI] [PubMed] [Google Scholar]
- Heyworth P.G., Curnutte,J.T., Nauseef,W.M., Volpp,B.D., Pearson,D.W., Rosen,H. and Clark,R.A. (1991) Neutrophil nicotinamide adenine dinucleotide phosphate oxidase assembly. Translocation of p47-phox and p67-phox requires interaction between p47-phox and cytochrome b558. J. Clin. Invest., 87, 352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth P.G., Bohl,B.P., Bokoch,G.M. and Curnutte,J.T. (1994) Rac translocates independently of the neutrophil NADPH oxidase components p47phox and p67phox: evidence for its interaction with flavocytochrome b558. J. Biol. Chem., 269, 30749–30752. [PubMed] [Google Scholar]
- Hiroaki H., Ago,T., Ito,T., Sumimoto,H. and Kohda,D. (2001) Solution structure of the PX domain, a target of the SH3 domain. Nat. Struct. Biol., 6, 526–530. [DOI] [PubMed] [Google Scholar]
- Imajoh-Ohmi S., Tokita,K., Ochiai,H., Nakamura,M. and Kanegasaki,S. (1992) Topology of cytochrome b558 in neutrophil membrane analyzed by anti-peptide antibodies and proteolysis. J. Biol. Chem., 267, 180–184. [PubMed] [Google Scholar]
- Ito T., Nakamura,R., Sumimoto,H., Takeshige,K. and Sakaki,Y. (1996) An SH3 domain-mediated interaction between the phagocyte NADPH oxidase factors p40phox and p47phox. FEBS Lett., 385, 229–232. [DOI] [PubMed] [Google Scholar]
- Ito T., Matsui,Y., Ago,T., Ota,K. and Sumimoto,H. (2001) Novel modular domain PB1 recognizes PC motif to mediate functional protein–protein interactions. EMBO J., 20, 3938–3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai F., Liu,H., Field,S.J., Akbary,H., Matsuo,T., Brown,G.E., Cantley,L.C. and Yaffe,M.B. (2001) The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat. Cell Biol., 3, 675–678. [DOI] [PubMed] [Google Scholar]
- Koga H., Terasawa,H., Nunoi,H., Takeshige,K., Inagaki,F. and Sumimoto,H. (1999) Tetratricopeptide repeat (TPR) motifs of p67phox participate in interaction with the small GTPase Rac and activation of the phagocyte NADPH oxidase. J. Biol. Chem., 274, 25051–25060. [DOI] [PubMed] [Google Scholar]
- Leto T.L., Adams,A.G. and de Mendez,I. (1994) Assembly of the phagocyte NADPH oxidase: binding of Src homology 3 domains to proline-rich targets. Proc. Natl Acad. Sci. USA, 91, 10650–10654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leusen J.H., Bolscher,B.G., Hilarius,P.M., Weening,R.S., Kaulfersch,W., Segal,R.A., Roos,D. and Verhoeven,A.J. (1994) 156Pro→Gln substitution in the light chain of cytochrome b558 of the human NADPH oxidase (p22-phox) leads to defective translocation of the cytosolic proteins p47-phox and p67-phox. J. Exp. Med., 180, 2329–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leusen J.H., Fluiter,K., Hilarius,P.M., Roos,D., Verhoeven,A.J. and Bolscher,B.G. (1995) Interactions between the cytosolic components p47phox and p67phox of the human neutrophil NADPH oxidase that are not required for activation in the cell-free system. J. Biol. Chem., 270, 11216–11221. [DOI] [PubMed] [Google Scholar]
- Mizuki K. et al. (1998) Functional modules and expression of mouse p40phox and p67phox SH3-domain-containing proteins involved in the phagocyte NADPH oxidase complex. Eur. J. Biochem., 251, 573–582. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Wakamatsu,K. and Mukai,T. (2000) Mastoparan as a G protein activator. In Rochat,H. and Martin-Eauclaire,H.M.F. (eds), Animal Toxins, Principles and Applications. Birkhauser, Basel, Switzerland, pp. 116–126.
- Nakamura R., Sumimoto,H., Mizuki,K., Hata,K., Ago,T., Kitajima,S., Takeshige,K., Sakaki,Y. and Ito,T. (1998) The PC motif: a novel and evolutionarily conserved sequence involved in interaction between p40phox and p67phox, SH3 domain-containing cytosolic factors of the phagocyte NADPH oxidase. Eur. J. Biochem., 251, 583–589. [DOI] [PubMed] [Google Scholar]
- Nauseef W.M. (1999) The NADPH-dependent oxidase of phagocytes. Proc. Assoc. Am. Physicians, 111, 373–382. [DOI] [PubMed] [Google Scholar]
- Ponting C.P. (1996) Novel domains in NADPH oxidase subunits, sorting nexins, and PtdIns 3-kinases: binding partners of SH3 domains? Protein Sci., 5, 2353–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos D. et al. (1996) Mutations in the X-linked and autosomal recessive forms of chronic granulomatous disease. Blood, 87, 1663–1681. [PubMed] [Google Scholar]
- Ross E.M. and Higashijima,T. (1994) Regulation of G-protein activation by mastoparans and other cationic peptides. Methods Enzymol., 237, 26–37. [DOI] [PubMed] [Google Scholar]
- Sathyamoorthy M., de Mendez,I., Adams,A.G. and Leto,T.L. (1997) p40phox down-regulates NADPH oxidase activity through interactions with its SH3 domain. J. Biol. Chem., 272, 9141–9146. [DOI] [PubMed] [Google Scholar]
- Someya A., Nagaoka,I. and Yamashita,T. (1993) Purification of the 260 kDa cytosolic complex involved in the superoxide production of guinea pig neutrophils. FEBS Lett., 330, 215–218. [DOI] [PubMed] [Google Scholar]
- Sumimoto H., Kage,Y., Nunoi,H., Sasaki,H., Nose,T., Fukumaki,Y., Ohno,M., Minakami,S. and Takeshige,K. (1994) Role of Src homology 3 domains in assembly and activation of the phagocyte NADPH oxidase. Proc. Natl Acad. Sci. USA, 91, 5345–5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumimoto H., Ito,T., Hata,K., Mizuki,K., Nakamura,R., Kage,Y., Nakamura,M., Sakaki,Y. and Takeshige,K. (1997) Membrane transclocation of cytosolic factors in activation of the phagocyte NADPH oxidase: role of protein–protein interactions. In Hamasaki,N. and Mihara,K. (eds), Membrane Proteins: Structure, Function and Expression Control. S.Karger AG, Basel, Switzerland, pp. 235–245.
- Terasawa H., Noda,Y., Ito,T., Hatanaka,H., Ichikawa,S., Ogura,K., Sumimoto,H. and Inagaki,F. (2001) Structure and ligand recognition of the PB1 domain: a novel protein module binding to the PC motif. EMBO J., 20, 3947–3956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunawaki S., Mizunari,H., Nagata,M., Tatsuzawa,O. and Kuratsuji,T. (1994) A novel cytosolic component, p40phox, of respiratory burst oxidase associates with p67phox and is absent in patients with chronic granulomatous disease who lack p67phox. Biochem. Biophys. Res. Commun., 199, 1378–1387. [DOI] [PubMed] [Google Scholar]
- Wientjes F.B., Hsuan,J.J., Totty,N.F. and Segal,A.W. (1993) p40phox, a third cytosolic component of the activation complex of the NADPH oxidase to contain src homology 3 domains. Biochem. J., 296, 557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi A. et al. (2001) Location of the epitope for 7D5, a monoclonal antibody raised against human flavocytochrome b558, to the extracellular peptide portion of primate gp91phox. Microbiol. Immunol., 45, 249–257. [DOI] [PubMed] [Google Scholar]