Abstract
Mutations in the L1 gene induce a spectrum of human neurological disorders due to abnormal development of several brain structures and fiber tracts. Among its binding partners, L1 immunoglobulin superfamily adhesion molecule (Ig CAM) associates with neuropilin-1 (NP-1) to form a semaphorin3A (Sema3A) receptor and soluble L1 converts Sema3A-induced axonal repulsion into attraction. Using L1 constructs containing missense pathological mutations, we show here that this reversion is initiated by a specific trans binding of L1 to NP-1, but not to L1 or other Ig CAMs, and leads to activation of the NO/cGMP pathway. We identified the L1–NP-1-binding site in a restricted sequence of L1 Ig domain 1, as a peptide derived from this region could reverse Sema3A repulsive effects. A pathological L1 missense mutation located in this sequence specifically disrupts both L1–NP-1 complex formation and Sema3A reversion, suggesting that the cross-talk between L1 and Sema3A might participate in human brain development.
Keywords: axon guidance/MASA syndrome/neuropilin/receptor complex/semaphorin
Introduction
Axon path finding is regulated by a number of chemotropic and cell contact guidance cues that act as attractants or repellents for developing neuronal projections. At choice points along the pathways, a complex series of events may take place to ensure appropriate decisions made by the growth cone (Cook et al., 1998). In particular, this has been documented for commissural projections where a highly dynamic mechanism of up- and down-regulation of axonal responses to guidance cues occurs in order sequentially to orientate the axons towards, across or away from the midline. These events were found to involve cell adhesion molecules of the immunoglobulin (Ig) superfamily as well as secreted factors of the netrin, slit and semaphorin families (Stoeckli and Landmesser, 1998). In rodents, loss of function of the Ig superfamily cell adhesion molecule (Ig CAM) L1 has implicated this protein in axonal guidance at the pyramidal decussation (Cohen et al., 1998). This particular stage in corticospinal pathfinding has been characterized by two major events: axon growth across the midline followed by a switch from a ventral to a dorsal trajectory in the spinal cord. L1 is likely to be important for each of these steps as it may be required as a cell contact cue for crossing the midline (Cohen et al., 1998). In addition, L1 has also been shown in vitro to be required for axons to respond to a ventrally secreted semaphorin, Sema3A, suggesting that this signal regulates the dorsalization of the pathway (Castellani et al., 2000).
Recent studies suggest that semaphorins signal through multi-molecular receptor complexes, containing neuropilin-1 (NP-1) and/or neuropilin-2 (NP-2) as specific ligand-binding subunits (Raper, 2000), and plexins as signal transducers (Tamagnone and Comoglio, 2000). L1 has also been shown to associate with NP-1 and is required as part of the Sema3A receptor complex for axon guidance responses (Castellani et al., 2000). A functional link between L1 and NP-1/Sema3A signaling is supported by the finding that a soluble L1Fc chimera switches Sema3A-induced chemorepulsion to attraction (Castellani et al., 2000). Previously it has been suggested that growth cones can switch between attraction and repulsion via a modulation of internal cyclic nucleotide levels (Song et al., 1998). How binding of soluble L1 on the cell surface can induce such a switch is largely unclear. L1 displays a highly complex pattern of homophilic and heterophilic interactions. In addition to L1 and NP-1, as components of the Sema3A receptor complex, axons express a number of other binding partners that potentially could be the receptor for L1Fc in the Sema3A reversion. These include other Ig CAMs such as F3/contactin and TAG-1/axonin-1, α3β5 integrins and several extracellular matrix proteins, such as laminin and some chondroitin sulfate proteoglycans (Brümmendorf and Rathjen, 1996). Furthermore, L1 expression on the growth cone is controlled in part by internalization, which is a critical point of L1-mediated adhesion and signaling (Kamigushi and Lemmon, 2000; Schaefer et al., 2002).
A clear requirement for L1 in axon development is reflected by the variety of neurological abnormalities associated with L1 mutations in the human disorder referred to as X-linked hydrocephalus, MASA syndrome or SPG type 1 (hydrocephalus, enlarged ventricles, corpus callosum agenesis, corticospinal tract hypoplasia; Rosenthal et al., 1992; Jouet et al., 1994; Vits et al., 1994). Mutations in L1 that give rise to these disorders include many that would truncate the mature protein and eliminate cell surface expression. More subtle missense mutations within L1 have been shown to have multiple effects on L1 function, disrupting intracellular protein trafficking or ligand binding, or both (De Angelis et al., 1999). The possibility that these mutations affect axonal responses to Sema3A has not been examined hitherto.
In the present study, we have dissected the molecular mechanisms underlying the switch of axonal responses to Sema3A by soluble L1, using L1Fcs that contain pathological missense mutations. We demonstrate that binding of L1Fc to NP-1 but not to L1 or to two other Ig CAMs expressed on growth cones is crucial for L1Fc to activate the conversion process. Binding and co-immunoprecipitation studies implicate the region containing mutation L120V in Ig domain 1 of L1 in both trans and cis L1–NP-1 complex formation. Moreover, a peptide composed of the FASNKL120 amino acid sequence was sufficient to reverse Sema3A-mediated repulsion. L1–NP-1 trans interaction activates nitric oxide (NO) synthase and cGMP guanylyl cyclase. This study therefore identifies molecular mechanisms involved in switching Sema3A repulsion to attraction. It also provides a new explanation for the effects of human pathological mutations in the X-linked L1 gene.
Results
Mutation L120V in Ig domain 1 of L1Fc abrogates the switch of Sema3A repulsion to attraction
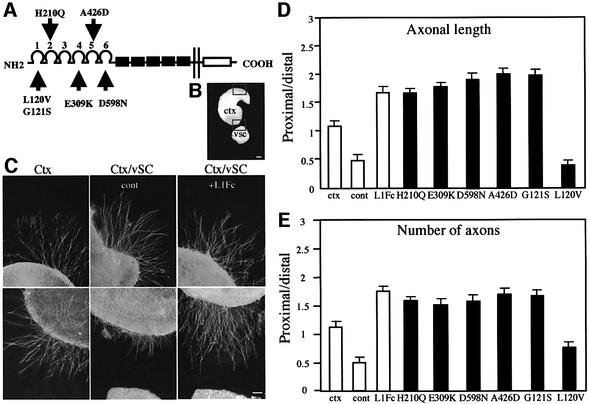
We previously developed an assay system using explants of ventral spinal cord co-cultured with cortical axons in order to determine the factors involved in axonal repulsion and attraction (Castellani et al., 2000). Using this system, we have shown that Sema3A secreted from the ventral spinal cord repels cortical axons. In the presence of soluble wild-type L1Fc chimeric protein, however, repulsion is switched to attraction (Castellani et al., 2000; Figure 1C). In the present study, we aimed to identify the L1 interactions underlying the switching process. As Ig domains have been implicated in binding of Ig CAMs to many of their ligands (Brümmendorf and Rathjen, 1996), we hypothesized that they would also be required for L1Fc-mediated switching. Six L1Fc chimeras containing pathological missense mutations affecting specific Ig domains (De Angelis et al., 1999; Figure 1A) were assessed for their ability to reverse Sema3A repulsion to attraction.
Fig. 1. Co-cultures of cortical slices and ventral spinal cord explants in the presence of mutated L1Fc chimeras. (A) L1Fc chimeras with individual missense mutations affecting distinct Ig domains selected for the study. (B) Microphotograph of the co-culture system of cortical slice and ventral spinal cord explant. Scale bar: 250 µm. (C) The microphotographs illustrate that axons extending from cortical slices cultured in the absence of ventral spinal cord exhibit a symmetrical outgrowth (Ctx) in contrast to the reduced growth of axons towards the source of Sema3A, when cortical slices are co-cultured with ventral spinal cord explants (Ctx/vSC). Axonal growth is increased by L1Fc application in the culture medium (Ctx/vSC + L1Fc). Scale bar: 100 µm. (D and E) Histograms showing the proximal/distal values obtained in the co-cultures when the mutated chimeras were applied. H210Q, E309K, D598N, A426D and G121S mutant L1Fc chimeras were able to reverse Sema3A repulsion to attraction. The values obtained for axonal length (D) and number of axons (E) were comparable with the value obtained in the cultures treated with L1Fc (P < 0.001 compared with control; NS when compared with L1Fc). In the culture with the chimera carrying the L120V mutation, axon outgrowth from the proximal side was reduced as observed in the control (NS compared with control; P < 0.001 when compared with L1Fc). Thus this mutation prevented the reversion process from occurring.
For each mutant, soluble Fc chimeric protein was produced in mammalian cells and purified by protein A–Sepharose affinity chromatography. The purified L1Fc chimeras were subjected to SDS–PAGE and immunoblotted with anti-Fc antibodies to verify their integrity. In the co-culture assay, axons extending from the cortical slice co-cultured with a spinal cord explant grew preferentially away from the co-cultured spinal cord explant (Figure 1C, middle panel). Axons elongating from cortical slices cultured alone exhibited a radial growth (Figure 1C, left panel). Axonal length and number of axons from the proximal and distal regions were analyzed and the proximal/distal ratio calculated. This ratio is ∼1 when cortical slices are cultured alone (Figure 1D and E, ctx). This ratio was strikingly reduced when cortical slices were co-cultured with spinal cord explants (Figure 1D and E, cont). This effect was reversed by application of L1Fc, as the proximal/distal value increased to 1.75 (Figure 1C, right panel; and D and E, L1Fc). In this co-culture assay, five out of six L1Fc proteins containing a single point mutation in Ig domains 1, 2, 3, 5 or 6 (G121S, H210Q, E309K, A426D and D598N, respectively) were found to efficiently induce switching of Sema3A repulsion to attraction. As indicated by the proximal/distal value, the outgrowth directed towards the Sema3A source was strongly increased (Figure 1D and E, total of 170 co-cultures in three separate experiments, statistical difference when compared with control with P < 0.001 for each chimera; not significantly different from the co-cultures with wild-type L1Fc). In contrast, one missense mutation, L120V in Ig domain 1, abrogated the reversal of Sema3A repulsion to attraction. When the L120V chimeric Fc protein was added to the culture medium, axons continued to be repelled from the spinal cord explant. This resulted in a proximal/distal value that was comparable with the control (Figure 1D and E, statistical difference of P < 0.001 compared with L1Fc). Thus, at least part of Ig domain 1 is required for L1Fc to switch the axonal response.
We aimed to identify the receptor on the cortical axons that binds L1Fc and initiates the reversal of Sema3A repulsion to attraction. L1 and/or NP-1 could fulfill this function, as they are both components of the Sema3A receptor complex and both interact in trans with L1 (Castellani et al., 2000). Plexin-A1 may also mediate the reversion of Sema3A as it associates with NP-1 in the Sema3A receptor. Other known L1 ligands, including Ig CAMs F3/contactin and TAG-1/axonin, are also candidate receptors for L1Fc. Previous work has indicated that the mutations described above can selectively affect either homophilic binding or heterophilic binding to F3 or axonin-1 (De Angelis et al., 1999). Two of these muta tions, G121S and A426D, reduce homophilic binding and heterophilic binding to F3, axonin-1 and the human homo log of axonin-1, TAX-1. H210Q and E309K affect homo philic or heterophilic binding, respectively. D598N has a very modest effect on homophilic binding but significantly reduces heterophilic binding to F3 and axonin-1, though not to TAX-1. L120V does not affect homophilic or heterophilic binding. Therefore, we were able to compare the ability of the mutated chimeras to reverse the Sema3A repulsion with their binding capabilities.
IgCAMs including L1 are not axonal receptors for L1Fc in the reversion of Sema3A repulsion
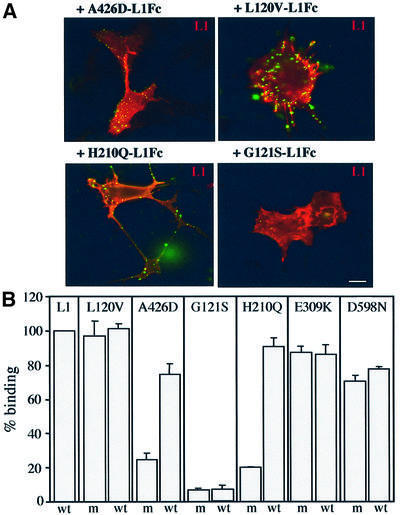
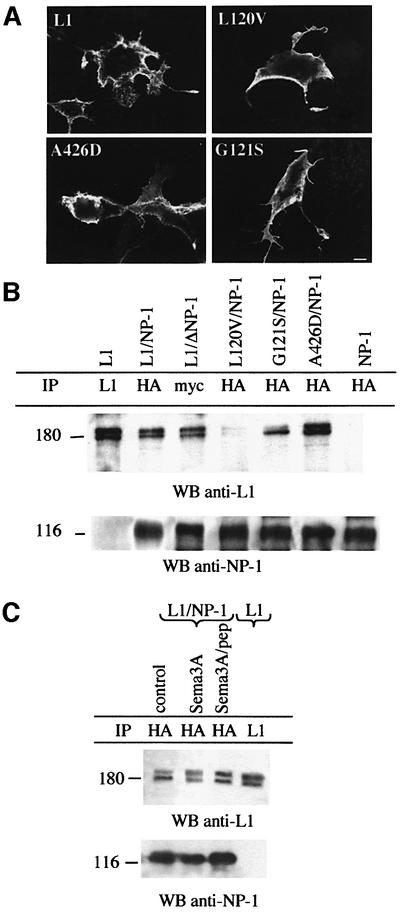
Four mutations (E309K, D598N, A426D and G121S) that were found to alter L1Fc binding to the Ig CAMs F3 and axonin-1 switched Sema3A from repulsion to attraction to the same extent as wild-type L1Fc. Thus, Ig CAMs F3 and TAG-1 (the rodent homolog of axonin-1) are unlikely to be axonal receptors for L1Fc in the reversion process. Mutations A426D, H210Q and G121S were found previously to reduce homophilic binding when individual mutant L1Fc proteins were bound to themselves (De Angelis et al., 1999). In the present study, however, mutated L1Fc proteins were required to interact with wild-type L1 expressed on growth cones. To exclude formally a role for homophilic binding of L1 in the initiation of the reversion process, it was therefore necessary to examine whether homophilic binding of the mutated L1Fc chimeras to wild-type L1 was also disrupted. Mutated L1Fcs were therefore incubated with COS7 cells transfected with full-length wild-type L1 cDNA, and binding was assessed by immunofluorescence. In parallel, homophilic binding of mutant–mutant and mutant–wild-type L1Fcs was compared in a two-color fluorescence-activated cell sorting (FACS) aggregation assay, allowing a quantitative assessment to be made (see Materials and methods). Interest ingly, the immunofluorescence studies showed that the A426D and H210Q L1Fc chimeras bound to wild-type L1 (Figure 2A). Similarly, in the two-color FACS assay, homophilic binding was restored to 74.4 and 90.5% of wild-type levels for A426D and H210Q, respectively (Figure 2B). In contrast, the mutation G121S almost abolished homophilic binding to wild-type L1 in both assays (Figure 2A and B). This mutation, however, still promoted the L1Fc-induced switching of Sema3A repulsion to attraction in the co-culture assay. Thus, the ability of L1Fc to switch Sema3A repulsion is not dependent on homophilic binding. Consequently, the trans interaction of L1Fc with L1 in the receptor complex is not required for the change in growth cone responsiveness to Sema3A. In support of this conclusion, we also observed that the L120V mutated L1Fc retained 100% homophilic binding capability but failed to reverse the Sema3A effect (Figure 2A and B).
Fig. 2. Homophilic binding of mutant L1 chimeras to wild-type L1. (A) Binding of mutated L1Fc protein to wild-type L1 expressed in COS cells and detected by immunofluorescence analysis. Cell surface L1 was detected using Texas Red-conjugated secondary antibodies, and mutant L1Fc chimeras using FITC-conjugated secondary antibodies. A426D, H210Q and L120V mutated chimeras bound to wild-type L1 whereas G121S did not. Scale bar: 5 µm. (B) Homophilic binding in wild-type–mutant and mutant–mutant combinations is compared by FACS analysis in the two-color aggregation assay. The histogram represents the percentage of mixed aggregates formed at the 30 min time point, with values expressed as percentage wild-type binding; error bars represent the SEM of three independent experiments. Wild-type L1-Fc is 100%. For mutations A426D and H210Q, increased mutant–wild-type compared with mutant–mutant binding was observed, whereas binding of G121S remained extremely low for both combinations. Wt, wild-type; m, mutant.
NP-1 is the axonal receptor for L1Fc in the switch of Sema3A repulsion
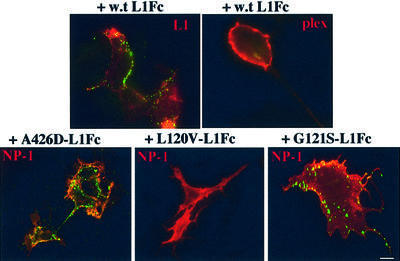
To determine whether soluble L1Fc binds plexin-A1, COS7 cells were transiently transfected with a vector encoding plexin-A1 fused to a VSV tag (Rohm et al., 2000) and incubated with wild-type L1Fc protein. As a positive control, chimeric protein was also incubated with COS7 cells transiently transfected with full-length wild-type L1. Immunodetection of L1Fc with anti-Fc antibodies showed clear binding of the chimera on L1-expressing cells but no binding to plexin-A1-expressing cells (Figure 3). Therefore, it is very unlikely that plexin-A1 is the axonal binding partner of L1Fc in the switch of Sema3A repulsion to attraction, although we cannot exclude it formally as other components of the complex which might influence plexin-A1 conformation and therefore its binding abilities are not present in our heterologous cell model.
Fig. 3. Binding assay with L1Fcs on plexin-A1- and NP-1 expressing cells. Cell surface expression of L1, plexin-A1 and NP-1 was detected using Texas Red-conjugated secondary antibodies. Mutant L1Fc chimeras were visualized using FITC-conjugated secondary antibodies. Wild-type L1Fc bound to L1- but not plexin-A1-expressing cells. A426D and G121S L1Fc chimeras bound to NP-1-expressing cells, whereas the L120V chimera did not. Scale bar: 5 µm.
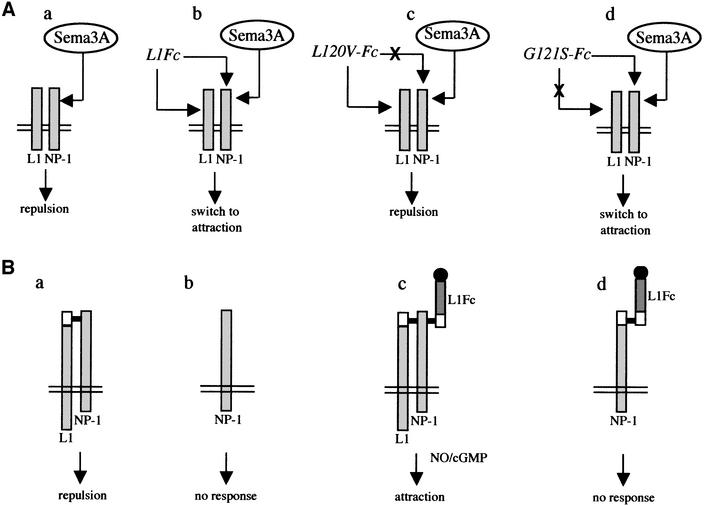
Next, we examined the ability of the mutant L1Fc chimeras to bind NP-1. COS7 cells were transfected with a vector encoding the extracellular and transmembrane domains of NP-1 fused to a myc tag (ΔNP-1; Renzi et al., 1999), and incubated with the mutant L1Fc chimeras. Binding was detected by immunofluorescence. The assays showed that mutant chimeras containing G121S, H210Q, E309K, D598N and A426D bound NP-1-expressing cells. In contrast, L120V-L1Fc did not bind NP-1. Binding of L120V, G121S and A426D L1Fcs to NP-1 is illustrated in Figure 3. As L120V-L1Fc does not interact with NP-1 in binding assays nor switches Sema3A repulsion to attraction in the co-culture experiments, this strongly suggests that NP-1 is the axonal receptor for L1Fc-mediated switching (see model in Figure 7A).
Fig. 7. Models for L1–NP-1 interaction and modulation of the Sema3A signal. (A) The diagrams represent L1–L1 and L1–NP-1 trans interactions and the effects of missense mutations on the modulation of Sema3A signaling. (a) cis interaction of L1 with NP-1 triggers the repulsive Sema3A signal. (b) Soluble L1 (L1Fc) binds both L1 and NP-1 on the growth cone and switches Sema3A repulsion to attraction. (c) Soluble L1 containing the L120V mutation binds L1 but no longer NP-1, and fails to reverse Sema3A repulsion. (d) G121S, a mutation that destroys homophilic binding, does not affect L1 binding to NP-1 or alter the ability of the chimera to induce the switch. This shows that interaction of soluble L1 with NP-1 but not with L1 is crucial for the reversion process. (B) A model for the interaction of L1 with NP-1 in the Sema3A receptor complex. (a) Association of L1 with the Sema3A receptor complex activates a chemorepulsive signal. (b) In the absence of L1 (L1-deficient mice), the receptor complex is no longer functional and the repulsive signaling is blocked. (c) Simultaneous cis and trans interactions of L1 with NP-1 occur on the growth cone. Activation of the nNOS/cGMP pathway is involved in the switch from repulsive to attractive responses. (d) L1–NP-1 complex formation in the cell membrane is also necessary for triggering Sema3A attractive responses since wild-type L1Fc cannot switch Sema3A repulsion to attraction in the absence of neuronal L1 (L1-deficient mice) even though it binds NP-1.
A six amino acid peptide mimics the L1Fc-reversing bioactivity
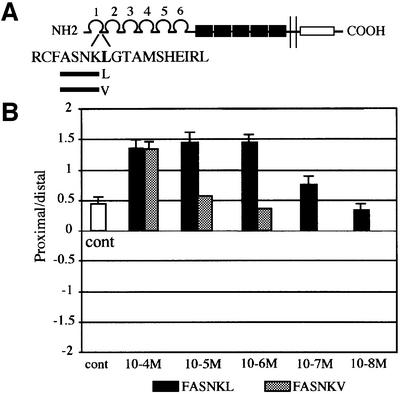
We postulated that only a restricted sequence within Ig domain 1 of L1 is involved in the interaction with NP-1. G121S, a mutation that is likely to disrupt Ig domain 1 conformation (Bateman et al., 1996), modified neither NP-1 binding nor the switching process, whereas the neighboring mutation L120V, affecting a surface residue, abrogated both. According to this hypothesis, isolated peptides should mimic L1Fc in reversing Sema3A repulsion to attraction. Since the G121S mutation did not abrogate L1Fc switching, this amino acid should be dispensable for peptidic bioactivity. Based on these criteria, a six amino acid peptide, FASNKL120, with free N- and C-termini (Schafer-N, Copenhagen DK), was designed and tested in the co-culture assay (Figure 4A and B). It successfully converted Sema3A-induced chemorepulsion into attraction at 10–6 M concentration (Figure 4B). Furthermore, mimicking the human mutation by replacing the C-terminal Leu120 by valine in the peptide induced a 100-fold decrease in its converting activity. A residual effect was only observed at 10–4 M (Figure 4B). Thus, the six amino acid peptide contains the L1 sequence that binds to NP-1, and Leu120 is a critical residue for the interaction. FASNKL120 also prevented at similar concentrations, the Sema3A-collapsing effect on dorsal root ganglion growth cones (data not shown).
Fig. 4. Switching Sema3A repulsion by a mimetic peptide of L1. (A) Part of the amino acid sequence of the L1 Ig domain 1. Peptides of six amino acids containing either the native leucine or the mutant valine 120 residue were synthesized. (B) Analysis of axon guidance effects in the co-culture assay for five different concentrations of the peptides. As indicated by the proximal/distal values, outgrowth from the proximal side was increased by application of the peptide with C-terminal leucine but not with C-terminal valine at 10–5 and 10–6 M (for each concentration, P < 0.001 compared with control for the peptide with C-terminal leucine and NS for the peptide with C-terminal valine). Thus, at these concentrations, the peptide with C-terminal leucine switched Sema3A repulsion to attraction, whereas the C-terminal valine did not. The control bar represents the proximal/distal value obtained in the absence of peptide treatment.
The pathological mutation L120V disrupts the L1–NP-1 receptor complex for Sema3A
We showed previously that on the cell membrane L1 and NP-1 associate in a common molecular complex that serves as a receptor for Sema3A (Castellani et al., 2000). We investigated the effects of L1 mutations on this complex formation. First, we determined whether L1 and NP-1 extracellular domains are required for the cis interaction. COS7 cells were co-transfected with vectors encoding NP-1 lacking its cytoplasmic domain (ΔNP-1; Renzi et al., 1999) and full-length wild-type L1. The cells were lysed and proteins immunoprecipitated by protein A–Sepharose coupled to rabbit anti-mouse and mouse anti-myc antibodies. Immunoprecipitated proteins were identified by western blot analysis using anti-L1 and anti-NP-1 antibodies. Co-immunoprecipitation of both ΔNP-1 and L1 indicated that cis association of the extracellular domains is required for the formation of the L1–NP-1 complex (Figure 5B, lane 3). Secondly, we expressed the mutated L1 proteins in COS7 cells and verified their cell surface expression by immunofluorescence (Figure 5A). Thirdly, COS7 cells were co-transfected with vectors encoding full-length hemagglutinin (HA)-tagged NP-1 and wild-type or mutant L1. Cells were lysed and immunoprecipitated with protein A–Sepharose coated with anti-HA antibodies. Recovered proteins were subjected to SDS–PAGE, transferred and immunoblotted with anti-L1 and anti-HA antibodies. The results show that L1 containing missense mutations A426D and G121S successfully immunoprecipitated NP-1, whereas L1 containing L120V did not (Figure 5, lanes 4–6). Therefore, both trans and cis interactions of L1 with NP-1 depend on the same binding site within the six amino acid sequence of Ig domain 1 described above.
Fig. 5. Effects of pathological L1 mutations on the formation of L1–NP-1 receptor complex. (A) Expression plasmids encoding full-length L1 containing missense mutations were transfected into COS7 cells, and cell surface expression was detected by immunofluorescence analysis. As illustrated, mutant L1 constructs were expressed on the cell surface. Scale bar: 5 µm. (B) Co-immunoprecipitation experiments. Cells transfected with NP-1 lacking its cytoplasmic domain (ΔNP-1) and full-length L1 were lysed and proteins were precipitated with anti-myc antibodies (see Materials and methods). Immunoblots were probed with anti-L1 and anti-NP-1 antibodies. Both L1 and NP-1 were detected in the precipitates, indicating that the cis interaction involves the extracellular domains of each protein. Western blot analysis of cell lysate immunoprecipitated with anti-HA antibodies is shown for double transfectants expressing mutant L1 constructs (L120V, G121S or A426D) and full-length HA-NP-1. L1 proteins with either the G121S or the A426D mutation co-immunoprecipitated with NP-1, whereas L120V-L1 did not. (C) Sema3A or Sema3A/peptide treatment did not prevent L1/NP-1 complex formation.
A possible molecular mechanism by which soluble L1 and its mimetic peptide could exert their modulatory effect on Sema3A signaling is that they compete with transmembrane L1 in the L1–NP-1 complex. To address this issue, we examined in immunoprecipitation experiments the L1–NP-1 complex in conditions mimicking either a repulsive (Sema3A alone) or an attractive (Sema3A combined to the peptide) environment. We observed that the presence of the peptide did not abrogate the cis interaction between L1 and NP-1 (Figure 5C), as L1 was found to co-precipitate with NP-1 in the presence of Sema3A alone or combined with the peptide, as well as in the control conditions. Thus, cis and trans L1–NP-1 interactions can occur simultaneously at the cell surface.
L1–NP-1 trans interaction activates NO synthesis and subsequently increases cGMP levels
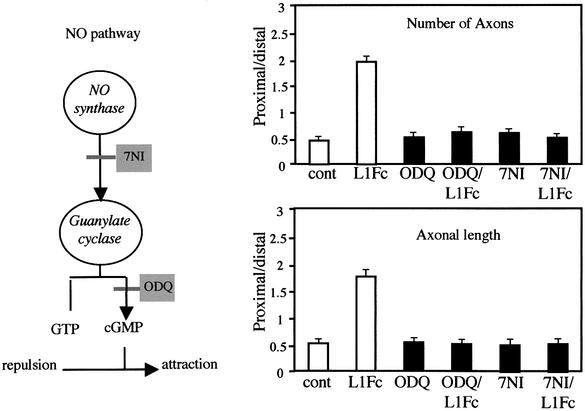
Previous studies have shown that Sema3A chemorepulsion is converted into attraction by an increase in the internal level of cGMP (Song et al., 1998). In order to explore the possibility that L1–NP-1 trans interaction activates the cGMP pathway to specify a Sema3A attractive effect, we used a pharmacological approach. 1H-(1,2,4)oxadiazolo (4,3-a)quinoxalin-1-one (ODQ) prevents cGMP synthesis by specifically blocking soluble guanylyl cyclase. ODQ was added to co-cultures of spinal cord and cortical explants either alone or in combination with L1Fc (Figure 6). Addition of ODQ alone (10–5 M) did not modify chemorepulsion compared with control, as illustrated by comparable proximal/distal values (no statistical difference between these two conditions). As expected, L1Fc alone switched repulsion to attraction (statistically significant increase in length and number of axons from the proximal region of the cortical slice, compared with control, P < 0.001; Figure 6). However, when ODQ was combined with L1Fc (3 µg/ml), reversion was prevented (Figure 6, P < 0.001 compared with L1Fc alone and not significant compared with control). This suggests that cGMP synthesis is necessary for L1Fc to reverse Sema3A repulsion. NO is a well-known activator of guanylyl cyclase (Hanafy et al., 2001). We examined whether activation of the neuronal NO synthase (nNOS) is also required for the reversion process. A specific inhibitor of NOS, 7 nitroindazole (7NI) was added to cortical slices and ventral spinal cord co-cultures. As observed with ODQ, 7NI alone (10–4 M) did not modify the chemorepulsion observed in the controls (no significant difference from the control; Figure 6), but it did block L1Fc-mediated chemoattraction since axons grew preferentially away from the spinal cord explants, as in controls (P < 0.001 compared with L1Fc alone and not significant when compared with control; Figure 6). This suggests that L1–NP-1 trans interaction triggers NO synthesis and a subsequent activation of cGMP guanylyl cyclase to induce the reversion of Sema3A repulsion to attraction.
Fig. 6. Activation of the NO/cGMP pathway in the reversion of Sema3A repulsion, pharmacological blockade of soluble guanylyl cyclase (with ODQ) and nNOS (with 7NI) in the co-culture of cortical slices and ventral spinal cord explants. As indicated by the proximal/distal values, in the presence of ODQ and 7NI alone, axons were repelled by the Sema3A source (NS when compared with control). In the presence of L1Fc alone, axons switched from repulsion to attraction for the Sema3A source (P < 0.001 when compared with control). After addition of ODQ and 7NI in combination with L1Fc, axon outgrowth from the proximal side was reduced (NS when compared with control). Thus, ODQ and 7NI prevented the L1Fc-induced switching of Sema3A from repulsion to attraction.
Discussion
We provide evidence that switching of Sema3A repulsion into attraction is initiated via a specific trans interaction of L1 with NP-1. This is followed by downstream activation of the intracellular NO/cGMP pathway. One individual missense mutation L120V located in Ig domain 1 of L1 completely prevented the switch from repulsion to attraction and disrupted both the trans and cis L1–NP-1 interactions. In contrast, other mutant L1 proteins that previously had been shown to alter homophilic and heterophilic binding to other Ig CAMs still effectively reversed Sema3A repulsion and bound NP-1 (Figure 7A). A six amino acid peptide derived from Ig domain 1 was sufficient to convert Sema3A-induced repulsion, suggesting that the L1–NP-1 binding site is restricted to the FASNKL amino acid sequence. In the light of these findings, we propose a model whereby cis and trans L1–NP-1 interaction may control the responsiveness of growth cones to Sema3A (Figure 7B).
The L1–NP-1-binding site is located in L1 Ig domain 1
The Sema3A receptor complex has been shown to contain a binding subunit (NP-1) and a signal transducer (plexin) (Tamagnone and Comoglio, 2000). Recently, we proposed that L1 is also a component of this receptor complex (Castellani et al., 2000). Previous studies have shown that several Ig domains of L1 are required for homophilic and heterophilic Ig CAM binding (Appel et al., 1993; Su et al., 1998; Haspel et al., 2000). This is highlighted by the number of pathological mutations in Ig domains that affect these interactions (De Angelis et al., 1999). In contrast, our finding that several of these mutations did not alter the L1–NP-1 interaction suggested that a restricted region of L1 might define the binding site for NP-1. This idea was confirmed by our results showing that a six amino acid peptide could mimic the integral L1Fc in reversing Sema3A repulsion to attraction. As demonstrated by the experiments using L1Fc with the mutation L120V and the peptide, modifying Leu120 to valine completely disrupted both the L1–NP-1 interaction and the reversion process. Together, these findings indicate that the binding site for NP-1 is contained in the FASNKL sequence, with the leucine being a crucial residue. Interestingly, such a sequence is also found in CHL1, a close homolog of L1 (Holm et al., 1996), and it will be worth exploring whether this Ig CAM also interacts with NP-1.
Our previous finding suggested that L1Fc may act independently of the Sema3A signaling pathway, activating through homophilic binding an intracellular cascade that results in increased cGMP levels and a subsequent switch from repulsion to attraction (Castellani et al., 2000; He, 2000). Although we found that L1Fc requires guanylyl cyclase activation, this is not dependent on homophilic interaction as shown by the experiments using G121S-L1Fc. We therefore propose that the switch occurs through a selective interaction with NP-1 in the receptor complex. Interestingly, cis and trans L1–NP-1 interactions were both found to use the same binding site. However, soluble and transmembrane L1 do not compete for the binding to NP-1. This is consistent with our previous finding that indicated that L1Fc did not enable L1-deficient axons to become susceptible to Sema3A-induced chemorepulsion. In addition, L1Fc was also unable to induce attraction of L1-deficient axons (Castellani et al., 2000). L1–NP-1 cis interaction is therefore a pre-requisite for the Sema3A receptor complex to transduce both repulsive and attractive signals. Thus, dimeric forms of L1 and NP-1 in the membrane might allow both trans and cis interaction to occur simultaneously, and possibly these interactions involve different region of the NP-1 protein. This might result in a conformational change leading to the activation of a pathway selective for attractive responses to Sema3A. Another possibility that deserves to be explored in the light of recent data (Thelen et al., 2002) is that the binding of soluble L1 modifies endocytosis of the complex. If it were the case, soluble L1 would modify cell surface levels of the receptor or its composition, thereby reducing or elevating the intensity of receptor signaling below or above a threshold that modifies the biological response. In addition, it is possible that endocytosed Sema3A receptors activate signal transduction pathways that differ from those activated by cell surface receptors.
The switch of Sema3A chemorepulsion to attraction requires activation of the NO/cGMP pathway
Blocking soluble guanylyl cyclase in co-culture assays using a pharmacological inhibitor prevented L1Fc from reversing Sema3A repulsion to attraction. The requirement for cGMP is consistent with previous work (Song et al., 1998) which established increased levels of cGMP as pivotal to Sema3A signaling. In the developing cortex, Sema3A has been shown to exert a dual effect, repelling axons whilst attracting dendrites (Polleux et al., 2000). Based on its asymmetric subcellular localization in the cell body, it was proposed that guanylyl cyclase was responsible for the specific attractive behavior of the dendritic growth cones to Sema3A (Polleux et al., 2000). Our findings, however, indicate that cortical axons also express the appropriate molecular machinery to display attractive responses to Sema3A. This may be due to the different stages in development at which the cortical neurons were examined in each study (postnatal neurons were used in the present work compared with embryonic cells in the Polleux study). Alternatively, changes in either the localization of the guanylyl cyclase during maturation or up- or downstream events may account for the differences observed in the response of cortical axons and dendrites.
Furthermore, our findings also suggest that NO acts as a key messenger in the reversal of Sema3A chemorepulsion to attraction. Switching of Sema3A-induced responses may therefore depend closely upon the maturation of nNOS expression. In the mouse cerebral cortex, nNOS immunoreactivity is present from late embryonic stages, peaking during the first postnatal week, and then declining to adult levels (Oermann et al., 1999). NO is thought to regulate the refinement of immature synaptic connections (Wu et al., 1994) and some forms of adult synaptic plasticity (reviewed by Kiss and Vizi, 2001). Our observations suggest a novel function of NO in patterning of neuronal projections by modulating axonal responses to guidance cues, particularly in postnatally developing cortical tracts.
Modulation of Sema3A signaling and axonal pathfinding
It is likely that modification of Sema3A-mediated responses by soluble L1 also occurs in vivo. The dynamic changes within the Sema3A receptor complex may occur as a result of contact between the growth cone and either membrane-bound L1 or L1 released from the cell surface. In support, several studies reported proteolytic cleavage of L1 by plasmin(ogen) and by ADAM10, a member of the disintegrin metalloproteinase family (Nayeem et al., 1999; Gutwein et al., 2000; Mechtersheimer et al., 2001). Furthermore, membrane-proximal cleavage of L1 is detected in the postnatal brain and might be required for L1-dependent migratory processes (Mechtersheimer et al., 2001). With respect to axon guidance, our data allow us to propose that soluble L1 may act in at least two non-exclusive ways. On the one hand, soluble L1 can induce the conversion of Sema3A-induced responses from repulsion to attraction in neighboring growth cones. On the other hand, shedding of the L1 ectodomain may disrupt the Sema3A receptor complex, thereby desensitizing the growth cone to Sema3A. This is consistent with recent findings that revealed metalloprotease activity as the determining factor in axon responsiveness to other types of guidance cues (Galko et al., 2000; Hattori et al., 2000). Switched responses to a guidance cue of the Slit family recently have been shown to be operative in directing the migration of muscle pioneers (Kramer et al., 2001). It is therefore tempting to speculate that switching Sema3A responsiveness is important during the formation of neuronal projections.
Sema3A signaling and the human disease associated with L1 mutations
Interestingly, our data may elucidate molecular events that could contribute to the human disease pathology. Several theories have been proposed to explain the pathological defects associated with the L120V mutation. The first proposal is that this mutation generates a nucleotide sequence with a potential donor splicing site (De Angelis et al., 1999). Secondly, it has been suggested that the L120V mutation affects extracellular matrix interactions as it was able to disrupt L1 binding to the chondroitin sulfate proteoglycan neurocan in in vitro assays (Oleszewski et al., 2000). Our data would favor a third possibility whereby some aspects of the human pathology may be the result of a defective L1–NP-1 interaction affecting Sema3A signal transduction, thus leading to axon guidance defects during the development of cortical fiber tracts.
Materials and methods
Co-culture experiments
Blocks of cortex were dissected from P0 (postnatal day 0) to P2 mice and cut into 250 µm thick slices with a McIlwain tissue chopper. The ventral part of the cervical spinal cord was dissected and cut into 250 µm thick slices. Co-culture of cortical slices and ventral spinal cord explants was performed in a three-dimensional plasma clot, as described previously (Castellani et al., 2000). Cortical slices were oriented in the plasma clot as indicated in Figure 1B to give axons equivalent chances to be deflected away or towards the spinal cord explant. Purified wild-type or mutant L1Fc chimeras were added to the culture medium (3 µg/ml). Inhibitors of guanylyl cyclase (ODQ, 10–5 M; Sigma) and NOS (7NI, 10–4 M; Sigma) were added to the culture medium, either alone or with wild-type L1Fc (3 µg/ml). After 1 or 2 days in vitro (DIV), the cultures were fixed with 4% paraformaldehyde (PFA) and analyzed by phase contrast microscopy (Axiovert 35-M, Zeiss). To quantify the guidance effects, a proximal and a distal region, each of them covering a 600 µm piece of cortical tissue, was determined for each culture, as described previously (Castellani et al., 2000). All fibers extending from these two regions were counted, and the length of the three longest fibers was measured, using computer analysis software (Visiolab 2000; Biocom). The ratio between axon outgrowth from the proximal and the distal region was calculated. A ratio of 1 indicates a lack of guidance activity in the culture as axons grow similarly from proximal and distal sides. Any significant changes in this ratio would reflect a guidance effect. Statistical analysis of the data was performed with ANOVA. Immunofluorescence labeling of axons was performed using the mouse anti-phosphorylated neurofilament antibody SMI31 (Sternberger and Meyer Inc.), and Texas Red-conjugated secondary anti-mouse antibody.
Binding assays
The mutant L1Fc chimeras were produced and purified as described previously (De Angelis et al., 1999). Briefly, COS-7 cells were transiently transfected with 10 µg of DNA per 150 mm culture dish, the soluble Fc chimeric protein was allowed to accumulate in the medium for 6 days and was recovered and purified by protein A–Sepharose affinity chromatography. In the binding experiments, L1Fcs were cross-linked to anti-human Fc antibodies (50 µg/ml, Jackson Immunoresearch) for 1 h at 37°C. Detection of binding to NP-1 required concentrations of L1Fcs 10-fold higher than L1. Antibody-conjugated L1Fc protein was then incubated with monolayers of COS7 cells transiently transfected with expression vectors encoding full-length human L1 or myc-tagged NP-1 where the cytoplasmic domain is deleted (gift of J.Raper; Renzi et al., 1999). Following incubation, the cells were fixed with 4% PFA and processed for double immunofluorescence detection. NP-1 was detected using a mouse anti-c-myc antibody (clone 9E10, Sigma) and L1 using a rat monoclonal anti-L1 antibody. Secondary antibody anti-c-myc (Texas Red-conjugated anti-mouse antibodies) was used to detect NP-1, anti-L1 (Texas Red-conjugated anti-rat antibodies) to detect L1, and anti-Fc [fluorescein isothiocyanate (FITC)-conjugated anti-rabbit antibodies] to detect bound L1Fc chimeras. After secondary antibody incubation, the cells were washed with phosphate-buffered saline (PBS) and mounted in Mowiol (Calbiochem).
Two-color aggregation assay
A two-color aggregation assay was developed, modified from the homophilic binding assay described by De Angelis et al. (1999). For the two-color assay, red and green fluorescent microsphere beads (0.6 µm, Duke Scientific Corps.) were coated with anti-human IgG antibody (Fc specific; Sigma, I-2136). A 2.5 µg aliquot of wild-type (red beads) or mutant (green beads) L1Fc proteins was conjugated to 10 µl of antibody-coated beads by incubating for 2 h at 3°C. Excess unbound protein was removed by washing with PBS/5% fetal calf serum (FCS). For each mutant, a 1:1 mixture of wild-type L1- and mutant L1-coated beads was prepared, disaggregated to produce a single bead suspension and allowed to form mixed aggregates at 37°C with samples removed in duplicate over a 30 min time course. Samples were diluted 1:5000 into ice-cold PBS, and the proportion of two-color aggregates was assessed by FACS (Becton Dickenson FACSort). Ten thousand particles were counted per sample and categorized as red only (WT–L1:WT–L1), green only (mutant L1:mutant L1) or mixed aggregates. Every mutant protein was assayed at least three separate times and standardized to a wild-type control conducted in parallel.
Co-immunoprecipitation experiments
COS cells were transfected with various expression vectors coding for full-length HA-tagged NP-1, myc-tagged cyt-deleted NP-1, wild-type L1 and L1 mutant constructs (G121S, A426D and L120V) using a lipofection transfection method (lipofectamine). After 2 DIV, cells were lysed with immunoprecipitation buffer (25 mM HEPES, 5 mM EDTA, 1 mM MgCl2, 2 mM PMSF, 10% glycerol and 1% Triton X-100 pH 7). In some experiments, cells were incubated for 1 h with Sema3A alone or combined with the FASNKL peptide. The lysate was incubated at 4°C for 30 min, centrifuged for 5 min and pre-cleared with protein A–Sepharose for 2 h at 4°C. Samples were immunoprecipitated with mouse anti-HA (3 µl; clone 12CA5; Roche), rabbit anti-L1 antibodies (3 µl), mouse anti-myc antibodies (3 µl; 9E10; Sigma) coupled to rabbit anti-mouse antibodies on protein A pre-formed complexes for 16 h at 4°C. The beads were washed, and the precipitates analyzed by immunoblot using anti-HA (1:1000) and anti-L1 (1:1000) antibodies.
Acknowledgments
Acknowledgements
We thank M.Schachner, A.Chédotal, A.W.Püschel and J.A.Raper for constructs, and F.Rathjen for L1 antibodies. We are grateful for funding from the Centre National de la Recherche Scientifique (CNRS), Ministère de la Recherche (MRT) (Action Concertée Incitative Molécules et Cibles Thérapeutiques), European Union (EU) programme Quality of Life (Key Action QLRT 1999-02187) for V.C. and G.R., and from the Wellcome Trust for S.K.
References
- Appel F., Holm,J., Conscience,J.F. and Schachner,M. (1993) Several extracellular domains of the neural cell adhesion molecule L1 are involved in neurite outgrowth and cell body adhesion. Neuroscience, 13, 4764–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman A., Jouet,M., MacFarlane,J., Du,J.S., Kenwrick,S. and Chothia,C. (1996) Outline structure of the human L1 cell adhesion molecule and the sites where mutations cause neurological disorders. EMBO J., 15, 6050–6059. [PMC free article] [PubMed] [Google Scholar]
- Brümmendorf T. and Rathjen,F.G. (1996) Structure/function relationships of axon-associated adhesion receptors of the immunoglobulin superfamily. Curr. Opin. Neurobiol., 6, 584–593. [DOI] [PubMed] [Google Scholar]
- Castellani V., Chédotal,A., Schachner,M., Faivre-Sarrailh,C. and Rougon,G. (2000) Analysis of L1-deficient mouse phenotype reveals a cross-talk between Sema3A and L1 signaling pathways in axonal guidance, Neuron, 27, 237–249. [DOI] [PubMed] [Google Scholar]
- Cohen N.R., Taylor,J.S., Scott,L., Guillery, R, Soriano,P. and Furley,A.J. (1998) Errors in corticospinal axon guidance in mice lacking the neural cell adhesion molecule L1. Curr. Biol., 8, 26–33. [DOI] [PubMed] [Google Scholar]
- Cook G., Tannahill,D. and Keynes,R. (1998) Axon guidance to and from choice points. Curr. Opin. Neurobiol., 8, 64–72. [DOI] [PubMed] [Google Scholar]
- De Angelis E., MacFarlane,J., Du,J.S., Yeo,G., Hicks,R., Rathjen,F.G., Kenwrick,S. and Brümmendorf,T. (1999) Pathological missense mutations of neural cell adhesion molecule L1 affect homophilic and heterophilic binding activities. EMBO J., 18, 4744–4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galko M.J. and Tessier-Lavigne,M. (2000) Function of an axonal chemoattractant modulated by metalloprotease activity. Science, 25, 1365–1367. [DOI] [PubMed] [Google Scholar]
- Gutwein P., Oleszewski,M., Mechtersheimer,S., Agmon-Levin,N., Krauss,K. and Altevogt,P. (2000) Role of Src kinases in the ADAM-mediated release of L1 adhesion molecule from human tumor cells. J. Biol. Chem., 275, 15490–1597. [DOI] [PubMed] [Google Scholar]
- Hanafy K., Krumenacker,J. and Murad,F. (2001) NO, nitrotyrosine and cyclic GMP in signal transduction. Med. Sci. Monit., 7, 801–819. [PubMed] [Google Scholar]
- Haspel J., Friedlander,D., Ivgy-May,N., Chickramane,S., Roonprapunt,C., Chen,S., Schachner,M. and Grumet,M. (2000) Critical and optimal Ig domains for promotion of neurite outgrowth by L1/Ng-CAM. J. Neurobiol., 42, 287–302. [PubMed] [Google Scholar]
- Hattori M., Osterfield,M. and Flanagan,J.G. (2000) Regulated cleavage of a contact-mediated axon repellent. Science, 289, 1360–1365. [DOI] [PubMed] [Google Scholar]
- He Z. (2000) Crossed wires: L1 and neuropilin interactions. Neuron, 27, 191–193. [DOI] [PubMed] [Google Scholar]
- Holm J., Hillenbrand,R., Steuber,V., Bartsch,U., Moos,M., Lubbert,H., Montag,D. and Schachner,M. (1996) Structural features of a close homologue of L1 (CHL1) in the mouse: a new member of the L1 family of neural recognition molecules. Eur. J. Neurosci., 8, 1613–1629. [DOI] [PubMed] [Google Scholar]
- Jouet M. et al. (1994) X-linked spastic paraplegia (SPG1), MASA syndrome and X-linked hydrocephalus result from mutations in the L1 gene. Nat. Genet., 7, 402–407. [DOI] [PubMed] [Google Scholar]
- Kamigushi H. and Lemmon,V. (2000) Recycling of the cell adhesion molecule L1 in axonal growth cones. J. Neurosci., 20, 3676–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss J.P. and Vizi,E.S. (2001) Nitric oxide: a novel link between synaptic and nonsynaptic transmission. Trends Neurosci., 24, 211–215. [DOI] [PubMed] [Google Scholar]
- Kramer S.G., Kidd,T., Simpson,J.H. and Goodman,C.S. (2001) Switching repulsion to attraction: changing responses to Slit during the transition in mesoderm migration. Science, 292, 737–740. [DOI] [PubMed] [Google Scholar]
- Mechtersheimer S. et al. (2001) Ectodomain shedding of L1 adhesion molecule promotes cell migration by autocrine binding to integrins. J. Cell Biol., 155, 661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayeem N., Silletti,S., Yang,X., Lemmon,V., Reisfeld,R.A., Stallcup,W. and Montgomery,A.M. (1999) A potential role for the plasmin(ogen) system in the posttranslational cleavage of the neural cell adhesion molecule L1. J. Cell Sci., 112, 4739–4749. [DOI] [PubMed] [Google Scholar]
- Oermann E., Bidmon, H, Mayer,B. and Zilles,K. (1999) Differential maturational patterns of nitric oxide synthase-I and NADPH diaphorase in functionally distinct cortical areas of the mouse cerebral cortex. Anat. Embryol., 200, 27–41. [DOI] [PubMed] [Google Scholar]
- Oleszewski M., Gutwein,P., von der Lieth,W., Rauch,U. and Altevogt,P. (2000) Characterization of the L1–neurocan-binding site. Implications for L1–L1 homophilic binding. J. Biol. Chem., 275, 34478–3485. [DOI] [PubMed] [Google Scholar]
- Polleux F., Morrow,T. and Ghosh,A. (2000) Semaphorin 3A is a chemoattractant for cortical apical dendrites. Nature, 404, 567–573. [DOI] [PubMed] [Google Scholar]
- Raper J. (2000) Semaphorins and their receptors in vertebrates and invertebrates. Curr. Opin. Neurobiol., 10, 88–94. [DOI] [PubMed] [Google Scholar]
- Renzi M.J., Feiner,L., Koppel,A. and Raper,J.A. (1999) A dominant negative receptor for specific secreted semaphorins is generated by deleting an extracellular domain from neuropilin-1. J. Neurosci., 19, 7870–7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohm B., Ottemeyer,A., Lohrum,M. and Puschel,A.W.(2000) Plexin/neuropilin complexes mediate repulsion by the axonal guidance signal semaphorin 3A. Mech. Dev., 93, 95–104. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Jouet,M. and Kenwrick,S. (1992) Aberrant splicing of neural cell adhesion molecule L1 mRNA in a family with X-linked hydrocephalus. Nat. Genet., 2, 107–112. [DOI] [PubMed] [Google Scholar]
- Schaefer A.W. et al. (2002) L1 endocytosis is controlled by a phosphorylation–dephosphorylation cycle stimulated by outside-in signaling by L1. J. Cell Biol., 157, 1223–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H., Ming,G., He,Z., Lehmann,M., McKerracher,L., Tessier-Lavigne,M. and Poo,M. (1998) Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science, 281, 1515–158. [DOI] [PubMed] [Google Scholar]
- Stoeckli E.T. and Landmesser,L.T. (1998) Axon guidance at choice points. Curr. Opin. Neurobiol., 8, 73–79. [DOI] [PubMed] [Google Scholar]
- Su X.D., Gastinel,L., Vaughn,D.E., Faye,I., Poon,P. and Bjorkman,P.J. (1998) Crystal structure of hemolin: a horseshoe shape with implications for homophilic adhesion. Science, 281, 991–995. [DOI] [PubMed] [Google Scholar]
- Tamagnone L. and Comoglio,P.M. (2000) Signalling by semaphorin receptors: cell guidance and beyond. Trends Cell Biol., 10, 377–383. [DOI] [PubMed] [Google Scholar]
- Thelen K., Kedar,V., Panicker,A., Schmid,R., Midkiff,B. and Maness,P.F. (2002) The neural cell adhesion molecule L1 potentiates integrin-dependent cell migration to extracellular matrix proteins. J. Neurosci., 22, 4918–4931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vits L. et al. (1994) MASA syndrome is due to mutations in the neural cell adhesion gene L1CAM. Nat. Genet., 7, 408–413. [DOI] [PubMed] [Google Scholar]
- Wu H., Williams,C. and McLoon,S.C. (1994) Involvement of nitric oxide in the elimination of a transient retinotectal projection in development. Science, 265, 1593–1596. [DOI] [PubMed] [Google Scholar]