Abstract
A prominent response during the Drosophila host defence is the induction of proteolytic cascades, some of which lead to localized melanization of pathogen surfaces, while others activate one of the major players in the systemic antimicrobial response, the Toll pathway. Despite the fact that gain-of-function mutations in the Toll receptor gene result in melanization, a clear link between Toll activation and the melanization reaction has not been firmly established. Here, we present evidence for the coordination of hemolymph-borne melanization with activation of the Toll pathway in the Drosophila host defence. The melanization reaction requires Toll pathway activation and depends on the removal of the Drosophila serine protease inhibitor Serpin27A. Flies deficient for this serpin exhibit spontaneous melanization in larvae and adults. Microbial challenge induces its removal from the hemolymph through Toll-dependent transcription of an acute phase immune reaction component.
Keywords: Drosophila/innate immunity/melanization/serine protease cascade/serpin
Introduction
Insects are particularly resistant to invading microorganisms. In response to microbial infection, they mount a multitude of defence reactions that include synthesis of potent antimicrobial peptides by the fat body (systemic antimicrobial response), phagocytosis by macrophage-like blood cells and activation of protease cascades leading to localized melanization and coagulation (reviewed in Hoffmann and Reichhart, 2002). Genetic studies have shown that antimicrobial responses against fungi and Gram-positive bacteria are regulated by the transmembrane receptor protein Toll, and several additional genes of the dorsoventral regulatory cassette of Drosophila (Lemaitre et al., 1996), whereas resistance to Gram-negative bacteria depends on the imd gene (for immune deficiency). This gene has since been shown to act upstream of a regulatory pathway independent of Toll (reviewed in Hoffmann and Reichhart, 2002). Gain-of-function mutations in the Toll receptor gene result in spontaneous melanization (Gerttula et al., 1988; Lemaitre et al., 1995; Govind 1996; Qui et al., 1998). It has been hypothesized for some time that a link exists between the challenge-dependent proteolytic cascades leading to melanization and the systemic Toll-mediated antimicrobial response (Lemaitre et al., 1995; Govind, 1996). However, the molecular and genetic basis of this putative link has remained elusive so far.
The infection-dependent melanization reaction requires the activation of phenoloxidase (PO), which is an oxidoreductase that catalyzes the conversion of phenols to quinones. The quinones may be directly toxic to bacteria, fungi and eucaryotic parasites, and can also polymerize to form melanotic capsules that surround parasites (Pye, 1974; Nappi and Vass, 1993; Nappi et al., 1995; for a review, see Ashida and Brey, 1997). PO is present as an inactive precursor prophenoloxydase (PPO) that must be proteolytically activated by a serine protease (for a review, see Ashida and Brey, 1997). This protease, which is the terminal component of a proteolytic cascade, has been identified in several insect species and has been called prophenoloxydase-activating enzyme (PPAE) (Ashida and Brey, 1995; Chosa et al., 1997; Jiang et al., 1998). Biochemical studies in crustaceans by Söderhäll and co-workers (for a review, see Söderhäll and Cerenius, 1998) and in the silkworm Bombyx mori by Ashida and collaborators (for a review, see Ashida and Brey, 1997) have established that this protease cascade can be initiated upon recognition of foreign material. In arthropods, the presence of serine protease inhibitors regulates this process by preventing excessive activation (Ashida and Sasaki, 1994; Jiang and Kanost, 1997; Park et al., 2000; for a review, see Jiang and Kanost, 2000).
In this study, we show that the blood serine protease inhibitor Spn27A controls melanization in Drosophila. Absence of this serpin in the hemolymph leads to a high rate of spontaneous melanization both in larvae and in adults and a constitutively elevated PO activity. In wild-type adult flies, we find that Spn27A is depleted from the hemolymph during the melanization process. Moreover, this depletion is infection-dependent and controlled by the Toll pathway. In Toll pathway mutants, serpin depletion and PO activation are blocked. Elimination of the serpin during melanization is most probably dependent on a protease. Infection-dependent secretion of this protease or of the factor that triggers its activation in the hemolymph is in need of NF-κB-mediated transcription and requires de novo protein synthesis. These results present the first genetic link between melanization and Toll pathway activation.
Results
A candidate serpin for the control of PO activation in Drosophila
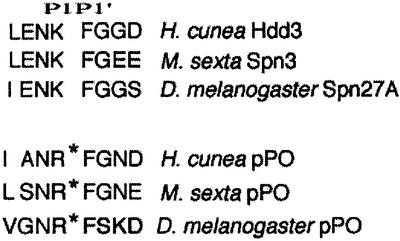
Serpins belong to a family of proteins, which preferentially inhibit serine proteinases (Silverman et al., 2001). Each serpin interacts with its target enzyme via an exposed reactive centre loop. This loop places the reactive centre in an accessible position for interaction with its target, allowing the formation of a stable complex between enzyme and inhibitor. The amino acid sequence and conformation of the loop largely dominate the selectivity of inhibition. More specifically, inhibition relies primarily on the residues P1–P1′ of the reactive centre, which provide a cleavage site for the target protease. Recently, an immune inducible serpin whose P1–P1′ reactive site sequence is reminiscent of the activation cleavage site in insect PPOs was cloned from the fall webworm Hyphantria cunea (Park et al., 2000). A Manduca sexta serpin with similar P1–P1′ sequence was subsequently shown to inhibit PPAEs in vitro (Zhu, 2001). We have now identified a gene encoding a homologous protein, designated Spn27A (CG11331), in the Drosophila genome by BLAST search. As illustrated in Figure 1, the three serpins have P1–P1′ reactive sites that resemble the P1–P1′ cleavage sites of their respective PPOs, suggesting that the PPAE of each species could be the target of these serpins. It is relevant to note here that also the P4 (hydrophobic) and P2 (Asn) residues are strikingly similar among these PPOs and the corresponding serpins.
Fig. 1. Spn27A is a candidate for a serine protease inhibitor involved in hemolymph-borne melanization. The top three alignments show the resemblance between the P1–P1′ amino acids of the reactive site loops in three insect serpins. The serpins of Manduca and Hyphantria have been biochemically shown to interact with PPAEs. The bottom three sequences show the cleavage sites of the respective PPOs for each species. Our working hypothesis was that the homology between these cleavage sites and the P1–P1′ sequence of the serpins could imply the same proteases.
Spn27A-deficient larvae and adults exhibit spontaneous melanization
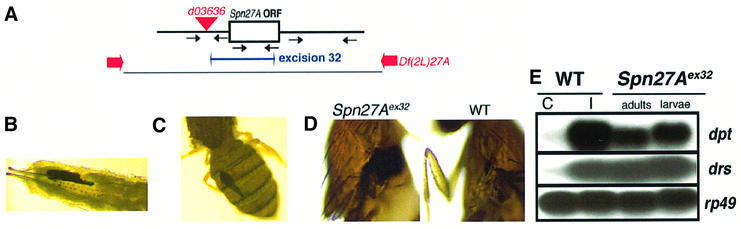
We carried out a genetic analysis to investigate the role of Spn27A in vivo. For this, we mobilized an XP element inserted 200 bp upstream of the translational start site of the gene. We obtained an imprecise excision, which removed along with the XP, a DNA fragment of 1.4 kb in total, comprising 1.2 kb of the serpin open reading frame (Figure 2A; see also Materials and methods). This mutant (Spn27Aex32) is a protein null (see below). To eliminate possible contributions from other mutations in the genetic background of the excision allele, we combined this mutation with a chromosomal deletion (Bloomington Stock Centre), Df(2L)6374, which uncovers the region to which Spn27A maps. Larvae and adults homozygous or hemizygous for the Spn27Aex32 allele showed constitutive melanization (40% for larvae; 35% for adults; Figure 2B and C; Table I). Melanization was particularly conspicuous around internal organs such as gut and fat body, but was never associated with barrier epithelia of the body wall. Statistics of repeated Cyo-GFP/Spn27Aex32 self crosses or Cyo-GFP/Spn27Aex32 with Cyo-GFP/Df(2L)6374 crosses revealed a high rate of lethality for Spn27Aex32 homozygous or hemizygous progeny (Table I). Most of the homozygous or hemizygous larvae (40%) that developed spontaneous melanization died in mid-pupal stages (Table I; see also Materials and methods). To demonstrate that the above observations were due to the absence of the corresponding serpin, we re-introduced a wild-type copy of Spn27A into the mutant background using the UAS/GAL4 system (Brand and Perrimon, 1993). The UAS-Spn27A rescue construct was driven by daGAL4. Addition of the UAS-Spn27A transgene in an Spn27Aex32 genetic background suppressed lethality, as the homozygous progeny showed the expected Mendelian ratios (Table I). Moreover, in the presence of the UAS-Spn27A transgene, spontaneous melanization in these mutants was suppressed (Table I). We conducted survival experiments with Spn27Aex32 homozygous flies infected with various classes of microorganisms (see Materials and methods). Survival of these mutants following bacterial challenge with Gram-negative or Gram-positive bacteria or fungal infection was comparable to wild-type flies (data not shown). Nevertheless, we observed that the Spn27Aex32 mutants were particularly sensitive in terms of their melanization reaction to injury-coupled infection as this treatment resulted in the appearance of extended melanization around the wound (Figure 2D, left panel), in stark contrast to wild-type flies (Figure 2D, right panel). A clean injury with a sterile needle did not produce such an effect (data not shown). Finally, we analyzed expression of the antimicrobial peptide genes drosomycin and diptericin. In the absence of any immune challenge, we observed a constitutive expression of both peptide genes in serpin-deficient flies. However, this expression was restricted to those individuals that developed spontaneous melanization (Figure 2E; data not shown).
Fig. 2. Absence of Spn27A results in spontaneous melanization. (A) Schematic representation of our genetic approach to generate a null mutant for Spn27A. An XP element inserted ∼200 bp upstream of the translational start site of Spn27A was mobilized. Spn27A is coded by a single exon of 1.6 kb. We obtain an imprecise excision (excision 32) of the XPd03636 element comprising 1.2 kb of the serpin’s open reading frame (see also Materials and methods). As illustrated in Figure 3A (lane Spn27Aex32), this mutant is a protein null. Excisions were verified by PCR. Black arrows represent the position of the different primers. Larvae (B) and adults (C) deficient for Spn27A develop a spontaneous melanized reaction. Moreover, adults deficient for Spn27A exhibit an over-reaction following injury-coupled infection (D, left panel). This is not the case for wild-type flies where melanization is restricted around the wounding site (D, right panel). Finally, melanized individuals have an infection-independent activation of the host defence as exemplified by the expression of the two most representative antimicrobial peptide genes drosomycin (drs) and diptericin (dpt) (E). rp49 was used as a loading control.
Table I. Crosses and progeny of flies used.
| Progeny | Crosses |
|
|---|---|---|
| CyO-GFP/Df(2L)6374 × CyO-GFP/Spn27Aex32 | CyO-GFP/Df(2L)6374; MKRS/daGAL4 × CyO-GFP/Spn27Aex32; MKRS/UAS-Spn27A | |
| Spn27A–/+ | 145 | 130 |
| Spn27A– | 31 (melanized: 12) | 22 (melanized: 10) |
| Spn27A–; UAS-Spn27A | – | 31 (melanized: 0) |
A protein null mutant for Spn27A (Spn27Aex32) was obtained (Figure 3A, lane Spn27Aex32). When CyO-GFP/Spn27Aex32 flies were crossed to CyO-GFP/Df(2L)6374 flies, the resulting number of the homozygous progeny Spn27Aex32/Df(2L)6374 (Spn27A–) was significantly lower than expected (expected ratio, 2CyO:1non-CyO; obtained ratio, 5CyO:1non-CyO). This indicated a death rate that was confirmed by counting (in early pupariation) the non-GFP versus the GFP pupal cases, which gave the expected mendelian ratio (data not shown). Introducing a wild-type copy of the Spn27A gene (CyO-GFP/Df(2L)6374; MKRS/daGAL4 crossed with CyO-GFP/Spn27Aex32; MKRS/UAS-Spn27A) resulted in the expected ratio for the homozygous progeny (sum of non-CyO and non-Cyo; non-MKRS flies versus CyO flies).
Spn27A is depleted from the hemolymph in response to infection
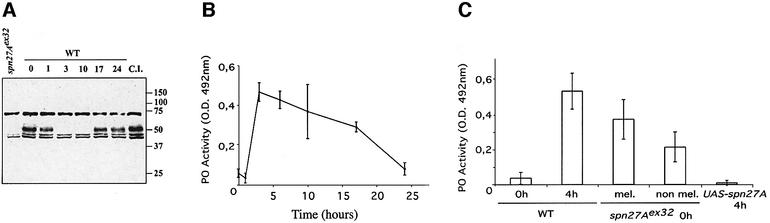
The genetic data presented above suggest a role of Spn27A in blood-borne melanization. We developed an antibody against the C-terminal part of the protein (see Materials and methods). With this antibody, we detected a marked protein band of ∼50 kDa in the hemolymph of wild-type adults. This molecular weight corresponds to that of the amino acid sequence deduced of the Spn27A gene. This 50 kDa band is absent from the hemolymph of the Spn27Aex32 mutant flies (Figure 3A). We further noted that the band corresponding to Spn27A disappeared from the hemolymph 3 h after bacterial challenge and reappeared around 17 h post challenge (Figure 3A). We interpret this disappearance as resulting from the interaction of the serpin with its cognate protease, followed by removal or degradation of the complex (see also below). Moreover, we observed that this disappearance is infection dependent since injury with a sterile needle did not have a similar effect (Figure 3A).
Fig. 3. Depletion of Spn27A from the hemolymph correlates with increased PO activity following infection. (A) Spn27A can be detected in the hemolymph in agreement with the putative signal peptide contained in its deduced protein sequence as a band of 50 kDa. In contrast, our excision mutant (Spn27Aex32) is a protein null. In wild-type (WT) adults, Spn27A is removed from circulation 3 h following bacterial challenge and re-enters the plasma 17 h after infection. We could demonstrate that this phenomenon is infection-dependent since Spn27A is not removed following clean injury (CI) with a sterile needle. (B) PO activity in WT flies after bacterial challenge. PO activity begins to be rapidly increased following infection reaching maximum activity at 3 h post challenge. During this time, Spn27A is absent from the hemolymph [see (A)]. From 17 h post challenge [when Spn27A reappears in the hemolymph, see (A)] and on, PO activity decreases significantly reaching basal levels at 24 h post challenge. Values are from three independent experiments (see methods). (C) PO activity measurements in Spn27Aex32 mutant flies, which exhibit melanization (mel.) or do not have this phenotype (non mel.). PO activity in Spn27Aex32 mutant flies without any immune challenge is at the same levels as in WT flies 4 h following infection. Basal levels of PO activity were recorded in non-infected WT flies. All measurements following bacterial challenge were made at 4 h, a time point in which the serpin is absent from circulation [see (A)]. Overexpression of Spn27A blocks challenged-induced activation of PO. UAS-Spn27A was driven by the ubiquitously expressed daGAL4. Each bar represents three independent experiments with their respective standard deviation.
Spn27A depletion is necessary for PO activation
To determine whether the time window in which Spn27A is absent from the hemolymph correlates with increasing PO activity after infection, we examined PO activity in the hemolymph of immune-challenged wild-type flies (see Materials and methods). Our results are illustrated in Figure 3B. We observed a rapid increase in PO activity that reached its maximum at 3 h post challenge. From then on, PO activity decreased significantly following the time of serpin re-emergence in the hemolymph (17 h post challenge) and reaching basal levels 24 h following immune challenge. In parallel to each PO measurement, we examined the hemolymph of the same infected flies for the presence of the serpin. In every case, the increase in PO activity correlated with the absence of Spn27A. Conversely, reduction in PO activity coincided with Spn27A reappearance. The western blot shown in Figure 3A is representative of these series of experiments (see also Materials and methods).
In non-infected flies deficient for Spn27A, we observed the same elevated PO levels as in wild-type flies subjected to bacterial challenge (Figure 3C). This was even observed in some of the Spn27A mutant flies that did not show a spontaneous melanization phenotype (Figure 3C). Finally, overexpression of Spn27A through a UAS-Spn27A construct, using the ubiquitous driver daGA4 (Figure 3C), or in the fat body of transgenic flies using yolkGAL4 (data not shown) inhibited the increase in PO activity normally following bacterial challenge.
Spn27A depletion is dependent on Toll pathway activation
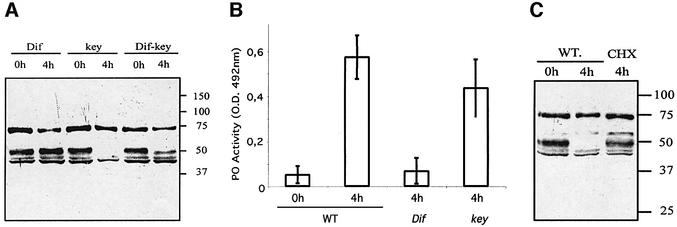
As stated above, depletion of the serpin Spn27A from the hemolymph following immune challenge is best explained by assuming that it binds to a cognate protease and that the resulting complex is either removed from circulation or degraded. Two intracellular signaling pathways control the expression of challenge-induced genes in the fat body, the predominant immuno-responsive tissue of Drosophila. DNA microarray data indicate that the infection-induced expression of several putative PPAE genes in Drosophila is under the control of the Toll pathway (De Gregorio et al., 2002a). Moreover, Spn27A transcription is also regulated in an immune-dependent manner (De Gregorio et al., 2001; P.Irving, personal communication). We wanted to examine whether the melanization observed in the Spn27Aex32 mutants could be correlated to the well-defined Toll-mediated host response in Drosophila. In Dif or spaetzle (spz) mutants, in which the Toll pathway is blocked (see Hoffmann and Reichhart, 2002), we observed that the serpin is not removed from the circulating hemolymph (Figure 4A; data not shown). Interestingly, the serpin is removed in kenny (key) mutants that block the Imd pathway (Figure 4A), which is not involved in the control of PPAE gene expression (De Gregorio et al., 2002a). A testable prediction that can be derived from these results is that in a Toll pathway mutant background PO enzymatic activity following bacterial challenge should be at very low levels. We indeed observed that Dif- and spz-infected flies have a PO activity comparable to the basal level of non-challenged wild-type flies in contrast to key-infected flies, which show normal PO levels (Figure 4B; data not shown). Our data furthermore imply that the protease (or the factor that triggers its activation), which removes Spn27A, may not be present as a zymogen activated by infection, but may need de novo protein synthesis dependent on Toll signal transduction. We confirmed this by infecting wild-type flies with bacteria in the presence of an inhibitor of translation (cycloheximide). We examined protein synthesis following bacterial infection by mass spectrometry and found that none of the induced antimicrobial peptides were synthesized in our experimental conditions indicating that protein synthesis was efficiently blocked (data not shown). Importantly, we noted that in these flies during the same infection procedures the serpin was not removed from circulation (Figure 4C). Taken together, our results on the requirement of Toll signaling pathway and protein synthesis for serpin removal show that the protease(s) removing Spn27A from circulation is synthesized de novo in response to Toll signaling. Alternatively, a component that activates it is dependent on Toll signaling-driven de novo protein synthesis.
Fig. 4. Spn27A depletion from the hemolymph and concomitant PO activation involves the Toll pathway and requires de novo protein synthesis. (A) In a Dif mutant background (or in a Dif-key double mutant) the serpin is not depleted in response to infection as opposed to key mutants where serpin removal occurs normally. We note that in a Dif-key double mutant, the serpin is detected at 0 h showing that basal levels of the serpin in non-infection conditions are not dependent on the Toll or the Imd pathway. (B) The above mentioned results correlate with measurements of PO activity. Triggering of the cascade leading to PO activation is dependent on Toll-mediated signal tranduction since, in a Dif mutant background, PO activity remains at basal levels 4 h post challenge. In contrast, key mutant flies exhibit wild-type levels of PO activity following infection. Basal levels of PO activity as well as PO activity following bacterial infection were measured in wild-type flies and used as controls. Each independent experiment was performed in triplicate. (C) Bacterial infection in the presence of inhibitors of translation (cycloheximide dissolved in DMSO) did not result in removal of Spn27A. As expected, immune challenge in the presence of DMSO did not have the same effect.
The question emerging from these data is whether the above-mentioned protease or activating component is directly involved in activating melanization or whether it is required solely for Spn27A removal. In other words, whether Toll signaling activates melanization directly. To address this issue, we generated an spn27A; spz double mutant. We noted that in larvae and flies homozygous for both mutations, constitutive melanization could still be observed (data not shown). This means that the components of the PO cascade are already in the hemolymph, and Toll pathway activation tips the balance from an inhibitory state to protease triggering by removal of Spn27A (see Discussion).
Discussion
In this study, we present evidence for the in vivo regulation of the melanization reaction and its activation through the Toll pathway, one of the major players of the antimicrobial host defence of Drosophila (Lemaitre et al., 1996). We show that Toll pathway mutants do not exhibit PO induction following bacterial challenge. In wild-type flies, PO activity reaches its maximum 3 h post-infection and decreases significantly at 17 h following immune challenge. Most of the larvae and a significant number of adults (see Table I) deficient for the serpin Spn27A show spontaneous melanization and increased PO activity. Our results further indicate that this circulating serpin disappears from the hemolymph 3 h following bacterial challenge and re-emerges at 17 h post-infection. This time window correlates with PO activation kinetics. Moreover, overexpression of Spn27A in fat body cells of adults prevents PO activation by immune challenge. Serpin depletion (as PO activation) relies on the Toll pathway, as in mutants of this pathway Spn27A is not removed from the hemolymph. Finally, we show that serpin depletion requires de novo protein synthesis, since infection in the presence of an inhibitor of translation (cycloheximide) does not result in its removal. We observed that among the serpin-deficient flies, only those with melanotic tumors showed a constitutive expression of the antimicrobial peptide genes drosomycin and diptericin. We do not yet have a clear explanation for their expression, which is dependent on Toll and Imd respectively. We believe that this could be an indirect effect of the presence of the melanotic capsules in the body cavity. It is relevant to note here that other mutations, which are not specifically linked to the immune response but result in melanotic tumors, activate antimicrobial peptide gene expression in the absence of an overt microbial infection (Manfruelli and Mathey-Prevot, 2002). An alternative model could be that Spn27A blocks both the Imd and Toll pathways and these pathways would be constitutively activated in flies deficient for the serpin. Although equally interesting, this possibility does not seem to be the case. If Spn27A negatively controlled these pathways, then all the serpin-deficient flies, and not only those with melanotic tumors, would exhibit expression of the peptides. Conversely, overexpression of the serpin would inhibit antimicrobial peptide induction following microbial challenge. Finally, bacterial infection of flies overexpressing the serpin (through a UAS-Spn27A transgene) results in expression of peptides at wild-type levels (P.Ligoxygakis, unpublished observations).
A surprising implication of our results is that at least one component of the melanization cascade is controlled by the Toll pathway and has to be synthesized de novo after infection. Two pieces of evidence support this hypothesis: (i) in a loss of function allele of Dif (Dif1), which encodes a protein able to translocate to the nucleus but unable to bind DNA (Rutschmann et al., 2000a), depletion of serpin27A and concomitant PO activation did not take place after bacterial challenge, and (ii) in the presence of protein synthesis inhibitors, Spn27A was not removed. Since the sequence found in the hinge region of serpin27A suggests that it is an active serine protease inhibitor (Irving et al., 2000), we propose that Spn27A is removed by its target protease, forming a covalent complex. Such complexes have a half-life of a few minutes and are rapidly cleared from the hemolymph, which could explain in a simple manner the observed depletion.
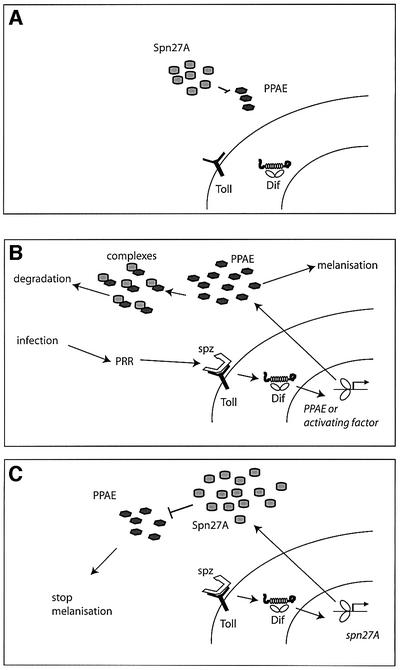
The model that we believe accommodates our data best is illustrated in Figure 5. In non-infection condi tions, Spn27A inhibits PPAE and blocks melanization (Figure 5A). Circulating pathogen recognition receptors sense the bacterial infection and signal to the Toll pathway. Intracellular transduction of the signal is mediated by the Rel transcription factor DIF (Rutschmann et al., 2000a), which initiates an acute phase transcription. This leads to de novo production of a further amount of PPAE, which induces PO cleavage and targets Spn27A for removal. Alternatively, Toll activation could lead to the production of serine protease homologs that are co-factors of PPAE, as demonstrated in a beetle (Kwon et al., 2000), or a modifying enzyme that triggers the cascade (like the Drosophila pipe gene; Sen et al., 1998; Figure 5B). Reappearance of the serpin in the hemolymph should inhibit any further melanization-associated proteolytic action (Figure 5C). We did not address the question whether this reappearance is a consequence of transcription. Nevertheless, DNA microarray data show that the Spn27A transcription level is strongly elevated after septic injury (De Gregorio et al., 2001, 2002a; P.Irving, personal communication). Finally, the fact that in the spn27A; spz double mutant spontaneous melanization is not suppressed evokes the possibility that the Toll pathway is activating a protease dedicated to the removal of Spn27A without taking part in the actual melanization cascade. Given that Spn27A has been shown to inhibit biochemically a PPAE of another insect (De Gregorio et al., 2002b) and that in turn Drosophila PPAEs are rapidly upregulated following infection (De Gregorio et al., 2001, 2002a; Irving et al., 2001), we favor the simpler scenario depicted in Figure 5. Analysis of an XP element insertion in a putative PPAE gene (N.Pelte and J.-M.Reichhart, unpublished data) will probably help to clarify the matter.
Fig. 5. Linking blood-borne melanization to Toll pathway activation. In non-infectious conditions, the hemolymph is kept in an inhibitory state by an excess of Spn27A, which blocks PPAE activity. Presence of PPAE in the hemolymph along with the fact that in spn27A; spz double mutants melanization is not suppressed could explain why when Spn27A is absent melanization still occurs, tipping the balance to an active state without Toll activation (A). Circulating pathogen recognition receptors (PRRs) signal via the cytokine-like polypeptide Spaetzle (Spz) to the Toll receptor. Transduction of the signal leads to NF-κB-dependent transcription through the action of DIF (Rutschmann et al., 2000a). Transcriptional targets of DIF include the PPAE or a co-factor, which stimulates its activation. In turn, PPAE triggers PPO activation and is targeted for removal by Spn27A. Subsequently, the serpin– protease complex is removed from circulation (B). Re-entering of Spn27A in the hemolymph brings a halt to the process by inhibiting further proteolytic action (C).
Although a large body of evidence addressing the putative role of melanization in host defence has been available for some time, this is the first genetic study that establishes a link between melanization and the activation of a major mediator of antimicrobial responses, namely the Toll pathway in Drosophila. Our data point to the requirement of Toll-dependent de novo protein synthesis for the depletion of Spn27A and the subsequent activation of melanization. Further analysis is needed to identify the components that are synthesized and released into the hemolymph to trigger melanization. In a recent study, mutations in the serine protease persephone were found to suppress the loss-of-function phenotype of the serpin necrotic (Ligoxygakis et al., 2002). This study sets the stage for a similar suppressor screen on the melanization phenotype caused by the deficit of Spn27A in adult flies.
Materials and methods
Genetic stocks and procedures
From a modified P transposon library (XP), we selected to mobilize an XP element inserted in the 5′ UTR of the genomic region of Serpin27A, 200 bp upstream of the gene’s ATG. The XP element was mobilized using a w; CyO/Lobe; Δ2–3Ki strain as a source of transposase. The white-eyed CyO males (excision events) were individually crossed with a w; CyO/Gla strain to establish stocks. They were then checked by PCR to molecularly characterize the putative excision lines. Imprecise excisions were further characterized by sequencing. In the Spn27Aex32 strain, the XP element excision produced a 1.4 kb deletion comprising 1.2 kb of the intronless open reading frame of the serpin. The percentage of the melanized larvae was determined by repeated Cyo-GFP/Spn27Aex32 self crosses or Cyo-GFP/Spn27Aex32 with Cyo-GFP/Df(2L)6374 crosses. All the non-GFP larvae were counted. We found that ∼40% of the homozygous or hemizygous larvae exhibited a melanized phenotype. From the number of the homozygous or hemizygous adult progeny, lethality was inferred, and was confirmed by looking at non-GFP pupal cases. These contained melanized tissues that had obviously arrested their development, probably mid-way through pupariation. Other stocks used were described previously: Dif1 (Rutschmann et al., 2000a), key1 (Rutschmann et al., 2000b), Dif1-key1 (Rutschmann et al., 2002), spzrm7 (Lemaitre et al., 1996), yolkGAL4 and daGAL4 (Ligoxygakis et al., 2002). Survival experiments were performed as described previously (Rutschmann et al., 2000a; Ligoxygakis et al., 2002), using Cyo/spn27Aex32; TM6B/ spzrm7 (this study).
Antibody production
A chimeric protein composed of glutathione S-transferase (GST) fused to the Spn27A gene product was produced using a GST–SPN27A expression vector: a BamHI–XhoI 690 bp fragment of Spn27 cDNA was subcloned into the BamHI–XhoI sites of the pGEX2T expression vector (Pharmacia). The GST–SPN27A fusion protein was expressed in the Escherichia coli strain LE 392. One liter of bacterial culture was grown to an OD of 0.6 at 37°C. After induction with IPTG (0.1 mM) and 4 h of culture at 37°C, cells were pelleted by centrifugation, washed with PBS and resuspended in 30 ml PBS and 1% Triton X-100. Bacteria were centrifuged at 12 000 g for 10 min at 4°C. The recombinant proteins were localized in inclusion bodies and these were extracted using a sarkosyl-based method: the pellet was resuspended in 10 ml of extraction buffer (25 mM Tris–HCl pH 8 and 1.5% sarkosyl) and incubated 45 min at 4°C with constant stirring. The extract was then centrifuged at 12 000 g for 10 min at 4°C. The supernatant was equilibrated in 1% Triton X-100 and applied to a 3 ml glutathione–Sepharose 4B column (Pharmacia). The fusion protein was purified according to the supplier’s recommendations. Fractions of 1 ml were collected and proteins were quantified with a Bradford colorimetric assay (Bio-Rad). Antibodies were obtained by inoculating 100 µg of the recombinant protein in rabbits using standard methods.
Western blot analysis
Flies were challenged using a mixture of Gram-negative (E.coli) and Gram-positive (Micrococcus luteus) bacteria. Hemolymph was collected from flies using specially prepared thin glass capillaries adjusted to a Nanoject apparatus (Drummond Scientific) and these extracts were recovered in PBS with protease inhibitors (Complete cocktail tablets, Roche). Protein concentration was determined using a Bradford assay.
All hemolymph extracts, containing 4 µg of protein were equilibrated in 2× Laemmli solution and denatured at 95°C for 5 min prior to loading on a 10% SDS–ployacrylamide gel. Following SDS–PAGE, proteins were blotted to hybond enhanced chemiluminesence (ECL) nitrocellulose membranes (Amersham) and blocked for 1 h in TBS-T (0.1% Tris buffer saline–Tween) and 3% of non-fat dry milk (Bio-Rad). Blots were incubated overnight at 4°C with a 1/5000 dilution of the anti-GST– SPN27A serum. After washing with TBS-T, the blots were incubated for 1 h at room temperature with a 1/10 000 dilution of horseradish peroxidase (HRP)–conjugated donkey anti-rabbit secondary antibody (Amersham). After washing with TBS-T, the blots were developed using the ECL system. For inhibition of translation studies, cycloheximide (100 mg/ml in DMSO, Sigma) was injected into flies prior to bacterial challenge. Hemolymph was collected 4 h following infection, and the presence of the Spn27A protein was assessed.
Measurements of PO activity
PO activity measurements 4 h after bacterial challenge. Hemolymph was collected 4 h following infection (when Spn27A is absent from the hemolymph; see Figure 3A) and the protein concentration was determined as described for western blotting (see above). Measure ments were performed in 96-well plates as follows: 10 µg of protein were suspended in 40 µl of PBS and protease inhibitors. One hundred and twenty microliters of a saturated solution of l,3-4-dihydroxyphenyl alanine (l-DOPA) were added in each sample. OD measurements were taken after 5 min at 492 nm. For each genotype, we performed at least three independent measurements. Mean values are shown with their respective error bars. Flies were challenged using a mixture of Gram-negative (E.coli) and Gram-positive (M.luteus) bacteria.
PO kinetics experiments in wild-type flies. Wild-type flies were infected with the above mentioned bacterial mix and hemolymph was collected at selected time points. Each hemolymph sample was divided in two parts. One was used for PO activity measurements and the other for western blot analysis (detection of Spn27A as described in the relevant section). This procedure was carried out in three independent experiments. A western blot representative of our results is shown in Figure 3A. Mean values (with their respective error bars) of PO measurements are presented in Figure 3B.
Molecular biology
Northern blot analysis was performed as described previously (Ligoxygakis et al., 2002). PCR primers were designed to cover 4 kb comprising the genomic region of serpin27A. Details and sequences available on request. Imprecise excisions were sequenced. Sequencing was performed using a CEQ 2000 Beckman DNA analysis system. The predicted serpin27A (CG11331) gene matches clone GH02428 from the BDGP EST project. This clone was sequenced and introduced as an EcoRI–XhoI fragment into pUAST (Brand and Perrimon, 1993). Transgenic flies were obtained using standard techniques (Spradling, 1986).
Acknowledgments
Acknowledgements
We thank Drs Kanost and Zhu for making available to us the serpin-3 sequence and their unpublished results. The technical help of Anny Meunier and Yang Wang is gratefully acknowledged. P.L. was supported by an EMBO Long Term Postdoctoral Fellowship. This work was supported in part by National Institute of Health Grant (GM58634) to H.J. Research in Strasbourg was financed by the CNRS, the National Institute of Health (1PO1 AI44220), Entomed (Strasbourg, in which J.A.H. acts as a scientific advisor) and Exelixis (South San Francisco).
References
- Ashida M. and Brey,P. (1995) Role of the integument in insect defence: prophenoloxidase cascade in the cuticular matrix. Proc. Natl Acad. Sci. USA, 92, 10698–10702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashida M. and Brey,P. (1997) Recent advances in research on the insect prophenoloxidase cascade. In Brey,P.T. and Hultmark,D. (eds), Molecular Mechanisms of Immune Responses in Insects. Chapman and Hall, London, UK, pp. 133–172.
- Ashida M. and Sasaki,T. (1994) A target protease activity of serpins in insect hemolymph. Insect Biochem. Mol. Biol., 24, 1037–1041. [Google Scholar]
- Brand A.H. and Perrimon N. (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development, 118, 401–415. [DOI] [PubMed] [Google Scholar]
- Chosa N., Fukumitsu,T., Fujimoto,K. and Ohnishi,E. (1997). Activation of prophenoloxydase A1 by an activating enzyme in Drosophila melanogaster. Insect Biochem. Mol. Biol., 27, 61–68. [DOI] [PubMed] [Google Scholar]
- De Gregorio E., Spellman,P.T., Rubin,G.M. and Lemaitre,B. (2001) Genome-wide analysis of the Drosophila immune response by using oligonucleotide microarrays. Proc. Natl Acad. Sci. USA, 98, 12590–12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio E., Spellman,P.T., Tzou,P., Rubin,G.M. and Lemaitre,B. (2002a) The Toll and Imd pathways are the major regulators of immune response in Drosophila. EMBO J., 21, 2568–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio E. et al. (2002b) An immune-responsive serpin regulates the melanization cascade in Drosophila. Dev. Cell, 3, 1–20. [DOI] [PubMed] [Google Scholar]
- Gerttula S., Jin,Y. and Anderson,K.V. (1988) Zygotic expression and activity of the Drosophila Toll gene, a gene required maternally for embryonic dorsal–ventral pattern formation. Genetics, 119, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind S. (1996) Rel signalling pathway and the melanotic tumour phenotype of Drosophila. Biochem. Soc. Trans, 24, 39–44. [DOI] [PubMed] [Google Scholar]
- Hoffmann J.A. and Reichhart,J.-M. (2002) Drosophila innate immunity: an evolutionary perspective. Nat. Immunol., 3, 121–126. [DOI] [PubMed] [Google Scholar]
- Irving J.A., Pike,R.N., Lesk,A.M. and Whisstock,J.C. (2000) Phylogeny of the serpin superfamily: implications of patterns of amino acid conservation for structure and function. Genome Res., 10, 1845–1864. [DOI] [PubMed] [Google Scholar]
- Irving P., Troxler,L., Heuer,T.S., Belvin,M., Kopczynski,C., Reichhart,J.-M., Hoffmann,J.A. and Hetru,C. (2001) A genome-wide analysis of immune responses in Drosophila. Proc. Natl Acad. Sci. USA, 98, 15119–15124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H. and Kanost,M.R. (1997) Characterisation and functional analysis of 12 naturally occurring reactive site variants of serpin-1 from Manduca sexta. J. Biol. Chem., 272, 1082–1087. [DOI] [PubMed] [Google Scholar]
- Jiang H. and Kanost,M.R. (2000) The clip-domain family of serine proteinases in arthropods. Insect Biochem. Mol. Biol., 30, 95–105. [DOI] [PubMed] [Google Scholar]
- Jiang H., Wang,Y. and Kanost,M.R. (1998) Pro-phenoloxydase activating proteinase from an insect, Manduca sexta: a bacteria-inducible protein similar to Drosophila easter. Proc. Natl Acad. Sci. USA, 95, 12220–12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon T.H., Kim,M.S., Choi,H.W., Joo,C.H., Cho,M.Y. and Lee,B.L. (2000) A masquerade-like serine protease homologue is necessary for PO activation in the coleopteran insect Holotrichia diomphalia larvae. Eur. J. Biochem., 267, 6188–6196. [DOI] [PubMed] [Google Scholar]
- Lemaitre B., Meister,M., Govind,S., Georgel,P., Steward,R., Reichhart,J.M. and Hoffmann,J.A. (1995) Functional analysis and regulation of nuclear import of dorsal during the immune response of Drosophila. EMBO J., 14, 536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B., Nicolas,E., Michaut,L., Reichhart,J.-M. and Hoffmann,J.A. (1996) The dorsoventral regulatory gene cassette spaetzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell, 86, 973–983. [DOI] [PubMed] [Google Scholar]
- Ligoxygakis P., Pelte,N., Hoffmann,J.A. and Reichhart,J.-M. (2002) Activation of Drosophila Toll during fungal infection by a blood serine protease. Science, 297, 114–116. [DOI] [PubMed] [Google Scholar]
- Manfruelli P. and Mathey-Prevot,B. (2002) Induction of antimicrobial peptide genes in the absence of any pathogens in mutants exhibiting melanotic masses. 43rd Annual Drosophila Research Conference, Genetics Society of America, Bethesda, MD.
- Nappi A.J. and Vass,E. (1993) Melanogenesis and the generation of cytotoxic molecules during insect cellular immune reactions. Pigment Cell Res., 6, 117–126. [DOI] [PubMed] [Google Scholar]
- Nappi A.J., Vass,E., Frey,F. and Carton,Y. (1995) Superoxide anion generation in Drosophila during melanotic encapsulation of parasites. Eur. J. Cell Biol., 68, 450–456. [PubMed] [Google Scholar]
- Park D.S., Shin,S.W., Hong,S.D. and Park,H.Y. (2000) Immunological detection of serpin in the fall webworm, Hyphandria cunea and its inhibitory activity on the prophenoloxidase system. Mol. Cells, 10, 186–192. [DOI] [PubMed] [Google Scholar]
- Pye A.E. (1974) Microbial activation of prophenoloxidase from immune insect larvae. Nature, 251, 610–613. [DOI] [PubMed] [Google Scholar]
- Qui P., Pan,P.C. and Govind,S. (1998) A role for the Drosophila Toll/Cactus pathway in larval hematopoiesis. Development, 125, 1909–1920. [DOI] [PubMed] [Google Scholar]
- Rutschmann S., Jung,A.C., Hetru,C., Reichhart,J.-M., Hoffmann,J.A. and Ferrandon,D. (2000a) The Rel protein DIF mediates the antifungal but not the antibacterial host defence in Drosophila. Immunity, 12, 569–580. [DOI] [PubMed] [Google Scholar]
- Rutschmann S., Jung,A.C., Zhou,R., Silverman,N., Hoffmann,J.A. and Ferrandon,D. (2000b) Role of Drosophila IKKγ in a Toll-independent antibacterial immune response. Nat. Immunol., 1, 342–347. [DOI] [PubMed] [Google Scholar]
- Rutschmann S., Kilinc,A. and Ferrandon,D. (2002) Cutting edge: the Toll pathway is required for resistance to Gram-positive bacterial infections in Drosophila. J. Immunol., 168, 1542–1546. [DOI] [PubMed] [Google Scholar]
- Sen J. Goltz,J.S., Stevens,L. and Stein,D. (1998) Spatially restricted expression of pipe in the Drosophila egg chamber defines embryonic dorsal–ventral polarity. Cell, 95, 471–481. [DOI] [PubMed] [Google Scholar]
- Silverman G.A. et al. (2001) The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions and a revised nomenclature. J. Biol. Chem., 276, 33293–33296. [DOI] [PubMed] [Google Scholar]
- Spradling A.C. (1986) P element-mediated transformation. In Roberts,D.B. (ed.), Drosophila: A Practical Approach, IRL Press, Oxford, UK, pp. 175–197.
- Söderhäll K. and Cerenius,L. (1998) Role of the prophenoloxidase-activating system in invertebrate immunity. Curr. Opin. Immunol., 10, 23–28. [DOI] [PubMed] [Google Scholar]
- Zhu Y. (2001) Identification of immune-related genes from the tobacco hornworm, Manduca sexta, and characterization of two immune-inducible proteins, serpin-3 and leureptin. PhD thesis, Kansas State University, Manhattan, KS.