Abstract
Previously, we reported that human papillomavirus (HPV) type 16 E6 binds to C/H1, C/H3, and the C-terminal domains of coactivators p300 and CBP, causing the modulation of the transcription of certain genes controlled by NF-κB (p65 or relA) and p53. To establish the biological significance of these observations, we have focused on the transcriptional regulation of interleukin-8 (IL-8), a potent chemoattractant for T lymphocytes and neutrophils, which is also essential for the initiation of the local immune response. The IL-8 promoter is regulated by NF-κB/p65 in response to tumor necrosis factor alpha and requires the cooperation of the coactivators CBP/p300 and steroid receptor coactivator 1 (SRC-1) and the p300/CBP-associated factor (P/CAF) for optimal activation. Here we report that, in the presence of HPV-16 E6, the promoter activity of IL-8 was repressed. Moreover, from the mutational analysis of the IL-8 promoter, we found that E6 down-regulates the IL-8 promoter activity through the NF-κB/p65 binding site. This inhibition appears to result from the ability of HPV-16 E6 to compete with NF-κB/p65 and SRC-1 for binding to the N terminus and C terminus of CBP, respectively. Reporter data also showed that E7 represses IL-8 promoter activity, though to a lesser extent than E6 but, like E6, the repression by E7 is through the NF-κB/p65 binding site. E7 was shown for the first time to bind to P/CAF, and the binding was necessary for the down regulation of the IL-8 promoter. E6 and E7 together inhibited transcription of the IL-8 promoter to a greater extent than either alone. Finally, by RNase protection assay, we showed that the synthesis of endogenous IL-8 mRNA was repressed in keratinocytes stably expressing E6 and E7. Taken together, the results provide evidence that E6 and E7 can cooperatively disrupt IL-8 transcription through disruption of transcriptional active complexes, and this may have important consequences for immune responses in infected hosts.
Human papillomaviruses (HPVs) are small DNA tumor viruses which exhibit distinct tropism for stratified epithelia in different parts of the body. Several genotypes infecting genital mucosa are known to cause cancers, and among them types 16, 18, 31, and 33 are found in more than 90% of cervical cancers. Two major viral proteins, E6 and E7, are found to exert a significant impact on cellular control mechanisms. E6 of HPV type 16 is 151 amino acids (aa) long with two zinc fingers comprised of four Cys-X-X-Cys motifs between aa 30 to 56 and 103 to 139. E6 has been shown to bind to p53 and promote p53 degradation through a ubiquitin-dependent pathway (63). It has also been demonstrated that E6 of HPV type 16 can bind to coactivators CBP and p300 and disrupt CBP/p300-dependent transcription (57, 83). E6 from high-risk HPV alone is sufficient to transform mammary epithelial cells (64), though efficient immortalization of primary human keratinocytes requires both E6 and E7 (25). E7 of HPV type 16 consists of 98 aa with an approximate molecular size of 13 kDa. It is comprised of three conserved regions, namely CR1, CR2, and CR3. Binding partners of E7 consist of many important regulatory proteins and transcription factors, such as the pRb family and AP-1 factors (2-4, 11, 47, 67). It has been previously determined that E7 has an important role during the G1 phase of the cell cycle and in facilitating cells to overcome the G1/S boundary (10, 24, 58).
The coactivator p300 was first identified through its direct interaction with adenovirus E1A protein (12, 80; Z. Arany, W. R. Sellers, D. M. Livingston, and R. Eckner, Letter, Cell 77:799-800, 1994). Its homologue, CBP (CREB binding protein), was initially described as a binding partner of the phosphorylated form of the cyclic AMP-responsive transcription factor CREB (8). There are several distinct regions within the protein sequence of CBP and p300 (20, 66). For example, two cystine-histidine-rich regions (C/H1 and C/H3) and a C-terminal domain adjacent to the C/H3 domain contribute to a large extent to the binding of CBP/p300 to transcription factors, basal transcription machinery elements, and viral proteins such as E1A and simian virus 40 large T protein and other cofactors (8, 9, 12-14, 19, 27, 37, 53, 61, 66, 81). The extreme N terminus binds to nuclear receptors such as retinoic acid receptor and estrogen receptor (26, 29). Finally, an intrinsic histone acetyltransferase (HAT) domain also exists between the C/H2 and C/H3 domains.
Both CBP and p300 are essential components of the transcription machinery. Firstly, CBP/p300 binds directly to both the basal transcription machinery and to some transcription factors, serving as a bridge to connect the two major elements of transcription (1, 33, 48). Secondly, as well as having intrinsic HAT activity, CBP/p300 can also recruit other HATs, such as the p300/CBP-associated factor (P/CAF), to modify the tail of histone proteins forming the nucleosome by adding an acetyl group. This will eventually lead to the uncoiling of chromatin structure and facilitate the entry of transcription factors to promoter regions (75). Finally, by utilizing either their own HAT domain or recruited HAT, CBP and p300 are able to acetylate transcription factors directly, resulting in optimal transcription (6, 21, 40, 60, 68).
One of the transcription factors activated by CBP/p300 is RelA/p65, which belongs to the NF-κB family. NF-κB transcriptional activation is essential for the synthesis of a variety of cytokines, chemokines, and inflammatory effectors which are crucial in initiating immune responses against viral or bacterial infections. Therefore, it would be beneficial for an infectious agent to evade the host immune system by down-regulating NF-κB transcriptional activation. Such disruption of NF-κB transcriptional activation can occur at several levels of NF-κB regulation. For instance, some enteric organisms have been shown to elicit immunosuppressive effects by down-regulating the synthesis of inflammatory effectors by preventing IκB-α degradation, thus avoiding the subsequent translocation of NF-κB family members to the nucleus (51). The interference of NF-κB transcription can also take place at the formation of the transcriptional complex. For example, SNIP1, a nuclear protein shown to be an inhibitor of the transforming growth factor β signal transduction pathway, inhibits NF-κB signaling by competing with RelA/p65 for binding to the C/H1 domain of CBP/p300 (28). It is now known that SRC-1 and P/CAF, along with CBP/p300, are required for the transcriptional activation by RelA/p65 and are recruited to an NF-κB-responsive promoter (65). Therefore, CBP/p300 serves as a platform to recruit these essential components to facilitate the transcription by p65. However, as shown recently by Chen et al. (7), the level of NF-κB transcriptional regulation is thought to be more complicated. They demonstrated for the first time that p65 is acetylated and that CBP/p300 and histone deacetylase-3 regulate the acetylation state of p65, which in turn determines the nuclear localization of p65 (7).
As mentioned above, NF-κB is involved in the transcriptional control of a number of cytokines and chemokines, including interleukin-8 (IL-8). IL-8 belongs to the CXC chemokine family and was originally identified as a potent activator and chemoattractant for neutrophils, basophils, and T lymphocytes (56), although T lymphocytes have been shown to be much more sensitive to IL-8 chemotaxis than neutrophils (34). IL-8 is secreted predominantly as a 72-aa protein and is strongly induced during viral infection and bacterial invasion. Moreover, IL-8 is secreted in a stimulus-specific manner, such as treatment with tumor necrosis factor alpha (TNF-α), by a wide variety of cell types, including keratinocytes, and is regulated at the level of gene transcription (16, 49, 69, 70, 76). The transcriptional control of IL-8 is mediated by the 100 bp of the 5′-flanking sequence upstream of the TATA box in its promoter. This region contains cis-acting elements for the binding of AP-1 factors, CCAAT/enhancer binding protein β (C/EBP-β), and NF-κB transcription factors. Previous reports have demonstrated that the IL-8 promoter is predominantly activated by the induction of NF-κB complex containing p65, although AP-1 factors and C/EBP-β may also play supporting roles (31, 32, 46, 55, 71).
HPVs may persist in their host for months, years, or even decades. To achieve this, the virus must have mechanisms to avoid inducing an effective immune response. Since we have previously shown that HPV-16 E6 inhibits the activity of an NF-κB consensus promoter (57), we explored the possibility that HPV-16 E6 down-regulates a more physiologically relevant NF-κB-induced promoter. In view of the fact that many cytokine and chemokine genes are regulated by NF-κB, we have chosen the promoter of IL-8, a chemokine predominantly regulated by NF-κB and induced by viral or bacterial infection in keratinocytes, as the subject of this study. In addition, since E7 is also known to modulate transcription and is synthesized concurrently with HPV-16 E6 during the course of viral infection, we also examined the effect of HPV-16 E7 on the IL-8 promoter. In this report, we show that E6 and E7 of HPV type 16 cooperatively repress IL-8 transcription. Furthermore, we offer a potential mechanism by which these viral proteins disrupt optimal NF-κB transcriptional activation, and the implications of these findings may help us understand how papillomaviruses cause persistent infections.
MATERIALS AND METHODS
Plasmids.
pGL2basic-IL-8(−135), pGL2basic-IL-8mAP1(−135), and pGL2basic-IL-8mNF-κB(−135) were generously provided by Lawrence Young and are described elsewhere (15). pGL2basic-IL-8mC/EBPβ(−135) was a gift from Gary Wu (78). E6.16, E6 C66G/C136G, E6Δ123-127, E7.16, and E7.16.2 were cloned in pCDNA3 as previously described (57). pCMV-p300 was kindly provided by David Livingston and was described elsewhere (57). pCX-Flag.P/CAF and pCX-Flag.P/CAF.ΔHAT were a generous gift from Tony Kouzarides (62). pGEX-CBP.1-771 was kindly provided by Dimitri Thanos (42). pGEX-CBP.CT, pGEX-E6.16, pGEX-E6.6, pGEX-E7.16, and pGEX-E7.16.2 were described previously (50, 57). pET-His-E6.16 and pQE30-His-E7.16 were generated and have been described elsewhere (50, 57). pSG5-SRC-1 and pGEM-RelA/p65 were gifts from David Heery and Sanjay Maggirwar, respectively. In addition, full-length P/CAF was cut from pCX-Flag-P/CAF by using KpnI and EcoRI and cloned in pGEM vector. The HAT domain of P/CAF (aa 352 to 658) was amplified from pCX-Flag.P/CAF by PCR and cloned in a pGEM vector.
Cell culture and establishment of cell lines.
Pooled primary keratinocytes were harvested from human neonatal foreskins (HFKs) and maintained in KGM (BioWhittaker) as previously described (52). Experiments involving HFKs were restricted to use of subconfluent early passage cells. COS-1 cells were maintained in Dulbecco modified Eagle medium (DMEM) (GIBCO-BRL) supplemented with 10% fetal calf serum (FCS) (HyClone). Cell lines stably expressing E6.16, E7.16, or E6.16/E7.16 were established by the pBABE amphotropic retroviral infection system and are described elsewhere (43, 52).
Reporter assays.
Reporter assays using HFKs were performed by seeding cells in 12-well dishes at a density of 6 × 104 cells/well. Cells were transfected 24 h later with 50 ng of IL-8WT, IL-8mAP1, IL-8mC/EBPβ, or IL-8mNF-κB along with 200 ng of E6.16, E6C66G/C136G, E7.16, or E7.16.2 and/or pCMV-p300, pCX-Flag.P/CAF, or pCX-Flag.P/CAF.ΔHAT by using FUGENE 6 (Roche) according to the manufacturer's instructions. After a 24-h incubation period, the transfection medium was removed and cells were treated with either vehicle or 2,000 U of TNF-α (Promega)/ml for an additional 6 h, at which time luciferase activity was measured and standardized as previously described (50, 57).
In vitro competition assays.
One microgram of purified GST-CBP 1-771 or GST-CBP.CT bound to glutathione beads was incubated with either His-E6.16 or His-E7.16 at various molar ratios in buffer A (50 mM Tris [pH 8.0], 150 mM NaCl, 5 mM EDTA, 0.5% NP-40) at 4°C overnight. Subsequently, radiolabeled in vitro-translated proteins were added to the solution and incubated for a further 2 h at 4°C. The pelleted beads were washed three times with buffer A, resuspended in 1× sample buffer, and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for analysis. The presence of the radiolabeled proteins was visualized by using a phosphorimager.
Glutathione S-transferase (GST) binding assays.
Radiolabeled P/CAF (80,000 cpm) was incubated in buffer B (25 mM Tris [pH 8], 60 mM KCl, 1 mM EDTA, 5% glycerol, 1 mM dithiothreitol, 0.2% NP-40) along with 2.5 μg of GST-E7.16, GST-E7.16.2, GST-E6.16, or GST-E6.6 bound to glutathione beads at 4°C overnight. Beads were then washed three times in buffer B, sample buffer was added, and the proteins were analyzed by SDS-PAGE and visualized by autoradiography or with a phosphorimager.
Coimmunoprecipitation.
For transient protein expression, COS-1 cells were plated at 50% confluence onto 100-mm plates the day before transfection. Subsequently, 10 μg of pSG5, pCX-Flag.P/CAF, or pSG5-E7.16 was transfected into cells by using Lipofectamine (Gibco-BRL) according to the manufacturer's protocol. After a 5-h incubation period, the transfection medium was removed and cells were maintained in DMEM with 10% fetal calf serum for 40 h. The cells were harvested and lysed in buffer A, and the cell lysates were rotated for 1 h at 4°C before the cell debris was removed via centrifugation. Total protein was determined using a Bradford protein assay (Bio-Rad) according to the manufacturer's instructions. The cell lysates (4 mg of protein per lysate) were precleared with 20 μl of anti-mouse immunoglobulin G (IgG) magnetic beads (Dynal Biotech) for 2 h at 4°C. The appropriate lysate was immunoprecipitated with the addition of anti-E7.16 (Zymed) and 20 μl of anti-mouse IgG magnetic beads. The immunocomplexes were washed three times in buffer A and then separated on a 4-to-20% gradient gel (Bio-Rad). Following protein transfer, immunodetection of E7.16 or Flag-P/CAF was achieved by blotting with anti-E7.16 or anti-Flag antibody (Sigma) and developing with Super Signal West Femto chemiluminescent reagent (Pierce).
RPA.
RNA was harvested from established cell lines treated with vehicle or TNF-α (2,000 U/ml) for 24 h. The RNase protection assay (RPA) was performed with the RiboQuant kit and Multi-Probe template hCK-5 (PharMingen), which contained probes for the chemokines RANTES, IP-10, IL-8, Ltn, MIP1-β, MIP1-α, MCP-1, and I-309, using 5 μg of total RNA, according to the manufacturer's protocol. Samples were loaded onto an acrylamide-urea gel and run at constant voltage (2,000 V) for approximately 3 h. After drying, protected mRNA species were visualized and quantified using a phosphorimager.
RESULTS
Characterization of IL-8 promoter activity in human keratinocytes.
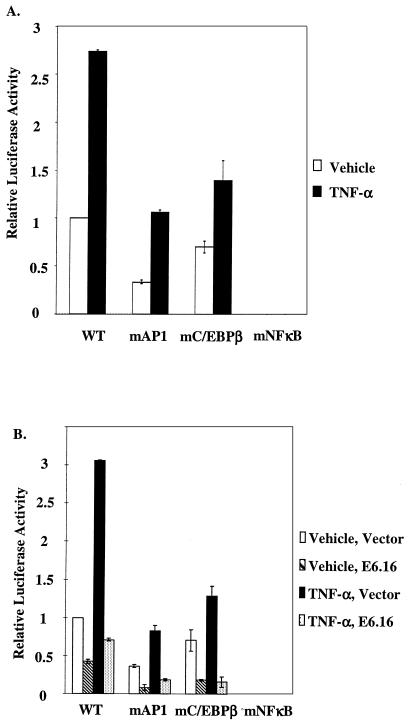
In order to examine IL-8 transcription regulation in human primary keratinocytes (HFKs), cells were transfected with various constructs of the IL-8 promoter upstream of the luciferase gene and then treated with TNF-α, a potent inducer of the IL-8 promoter. The wild-type IL-8 promoter (IL-8WT) or IL-8 promoter with mutations (IL-8mAP1, IL-8mCEBPβ, and IL-8mNFκB) that inhibit the binding site for each of three known transcription factors (AP-1 factors, C/EBP-β, and NF-κB, respectively) were transfected into HFKs. Approximately 20 h after the transfection, keratinocytes were treated with vehicle or 2,000 U of TNF-α/ml for 6 h. In the absence of exogenous TNF-α, keratinocytes were still able to maintain a basal level of IL-8 promoter activity (Fig. 1A), a phenomenon that has been observed by others (72). In this setting, the activities of IL-8mAP1 and IL-8mC/EBPβ were significantly reduced, while that of IL-8mNFκB was completely eliminated. Upon stimulation with TNF-α, IL-8mAP1 and IL-8mC/EBPβ were inducible by TNF-α. Importantly, even after stimulation with TNF-α, IL-8mNFκB exhibited no activity. Therefore, we concluded that although AP-1 factors, C/EBP-β, and NF-κB cooperatively activate IL-8 promoter activity in keratinocytes, NF-κB is absolutely required for the activation of the promoter by TNF-α.
FIG. 1.
NF-κB is required to maintain the IL-8 promoter activity in HFKs, and E6.16 down-regulates the IL-8 promoter activity. (A) Keratinocytes were seeded in 12-well dishes at a density of 6 × 104 cells/well. Cells were transfected 24 h later with 50 ng of ILWT, IL-8mAP1, IL-8mC/EBPβ, or IL-8mNF-κB using FUGENE 6. After a 24-h incubation period, the transfection medium was removed and cells were treated with either vehicle or 2,000 U of TNF-α/ml for an additional 6 h, at which time luciferase activity was measured and standardized as previously described (50, 57). Results are representative of three independent experiments. Error bars indicate standard deviations. (B) Wild-type E6.16 (200 ng) was cotransfected with 50 ng of IL-8WT, IL-8mAP1, IL-8mCEBPβ, or IL-8mNFκB into HFKs, and approximately 20 h after the transfection the keratinocytes were fed with fresh medium containing vehicle or 2,000 U of TNF-α/ml for 6 h, harvested, lysed, and measured for luciferase activity. Results are representative of three independent experiments. Error bars indicate standard deviations.
HPV-16 E6 oncoprotein down-regulates IL-8 promoter activity in keratinocytes.
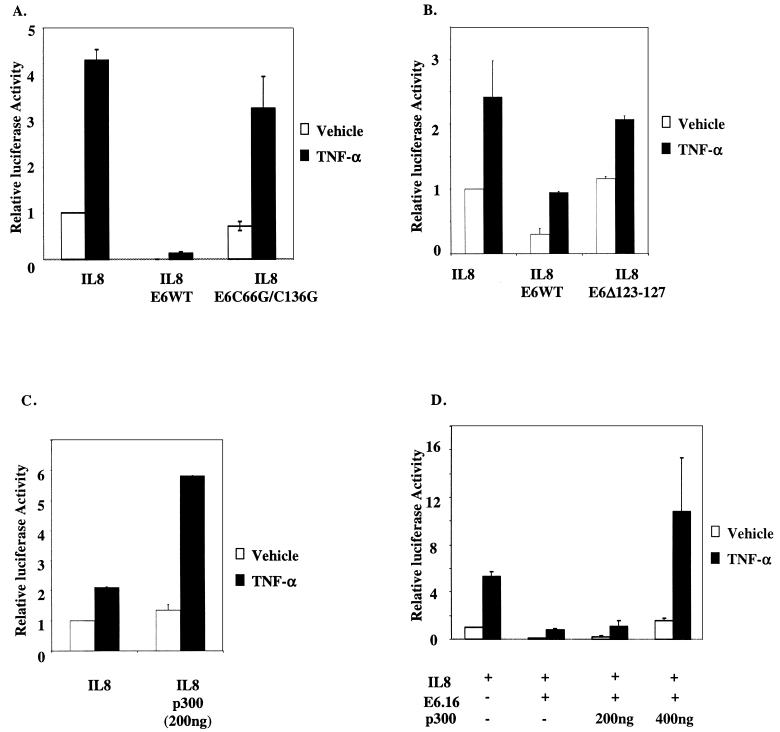
To assess the effect of the E6 protein of HPV 16 (E6.16) on the IL-8 promoter, we cotransfected wild-type E6.16 with IL-8WT, IL-8mAP1, IL-8mCEBPβ, or IL-8mNFκB into HFKs. As described above, approximately 20 h after the transfection keratinocytes were fed with fresh medium containing vehicle or 2,000 U of TNF-α/ml for 6 h. Interestingly, E6.16 repressed both the basal and TNF-α-inducible activity of IL-8WT, IL-8mAP1, and IL-8mCEBPβ (Fig. 1B). Since there was no activity from IL-8mNFκB to begin with, the presence of E6.16 did not pose any further effect on this construct. Our results suggested that E6.16 was disrupting IL-8 promoter activity by interfering with the optimal activation by NF-κB. Given that NF-κB activation requires the coactivators CBP and p300 for optimal transcriptional activation and that E6.16 is able to down-regulate NF-κB activation through CBP/p300 (57), we suspected that the repression of IL-8 promoter activity by E6.16 could be through CBP/p300. To this end, we cotransfected either E6.16 or an E6.16 mutant that was shown previously not to bind to CBP/p300, namely E6 C66G/C136G, along with IL-8WT into HFKs (57). While E6.16 showed a significant inhibition of the promoter activity, the C66G/C136G form failed to repress the activity to the same extent (Fig. 2A). Another E6 mutant which binds poorly to CBP/p300 (57) was also shown not to repress IL-8 promoter activity (Fig. 2B). Subsequently, we cotransfected an increasing amount of p300 into HFKs in the presence of both IL-8WT and E6.16. As expected, the exogenous p300 increased promoter activity on its own and could rescue the repression of IL-8 promoter activity by E6.16 (Fig. 2C and D). These observations indicated that the binding of E6.16 to CBP/p300 might play a major role in the suppression of IL-8 promoter activity.
FIG. 2.
E6.16 down-regulates IL-8 promoter activity through its interaction with CBP/p300. (A) E6.16 or E6 C66G/C136G (200 ng) was transfected along with IL-8WT (50 ng) into HFKs, and subsequent experimental procedures and assays were carried out as described in the legend for Fig. 1. (B) E6.16 or E6 Δ123-127 (100 ng) was transfected into keratinocytes along with IL-8WT (50 ng), and assays were carried out as described above. (C) IL-8WT (50 ng) was cotransfected with pCMV-p300 (200 ng). The subsequent experimental procedure and assays were carried out as described above. (D) E6.16 (200 ng) and IL-8WT (50 ng) were cotransfected with pCMV-p300 (200 or 400 ng). Subsequent experimental procedures and assays were carried out as described above.
HPV-16 E6 competes with p65 and SRC-1 for the N terminus and the C terminus of CBP, respectively.
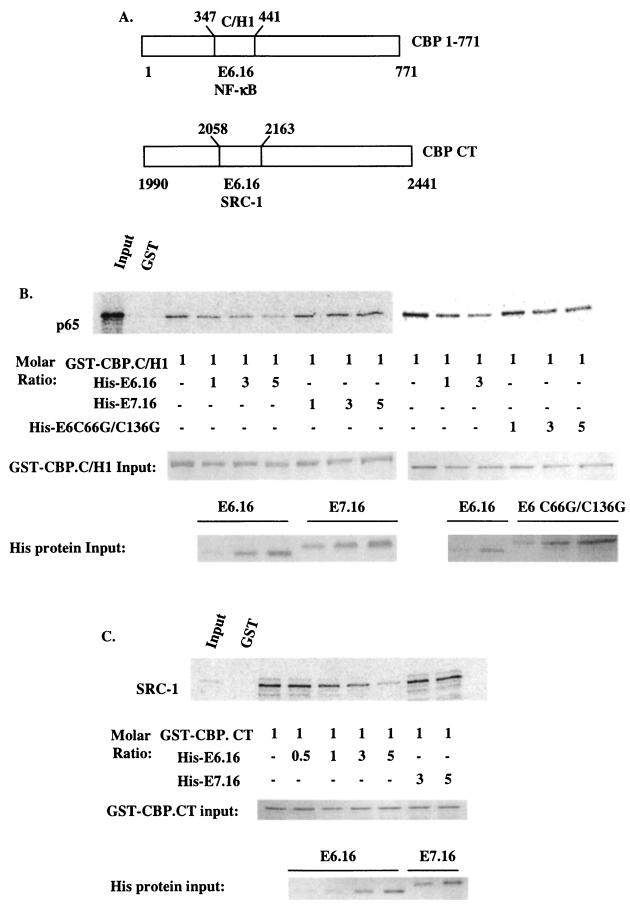
It has previously been shown by others that NF-κB (p65), SRC-1, and P/CAF bind to the C/H1, C-terminal, and C/H3 domains of CBP/p300, respectively (18, 23, 41, 59, 74, 79, 82). Also, it was reported that p65 requires CBP/p300, SRC-1, and the HAT activity of P/CAF to achieve its optimal activation (65). Since we previously demonstrated that E6.16 binds to the C/H1, C/H3, and the C-terminal domains of CBP/p300 (57) and that E6.16 does not inhibit the ability of NF-κB to translocate to the nucleus, nor does it inhibit the binding of NF-κB factors to the IL-8 promoter (data not shown), we wished to examine if E6.16 could affect the interactions between these proteins. In in vitro competition assays, the purified GST fusion protein GST-CBP 1-771 (the N terminus of CBP that includes the C/H1 domain) (Fig. 3A) was incubated with bacterially derived His-E6.16, His-E6C66G/C136G, or His-E7.16 in various molar ratios. Subsequently, radiolabeled in vitro-translated p65 was added to the mixture and incubated for a further 2 h at 4°C. Our results demonstrated that E6.16 was able to compete with p65 for the C/H1 domain in the N terminus of CBP (50 and 80% inhibition at 1:1 and 1:5 molar ratios, respectively); however, the E6 mutant and E7.16, which do not bind to the C/H1 domain of CBP/p300, did not inhibit the binding of CBP and p65 (Fig. 3B). A similar phenomenon was observed when using purified fusion protein GST-CBP C terminus (GST-CBP.CT) (Fig. 3A) and radiolabeled in vitro-translated SRC-1. GST-CBP.CT bound to SRC-1 with high affinity, pulling down 40 to 50% of SRC-1 input. Even so, the presence of His-E6.16, but not E7.16, almost completely abolished the binding of SRC-1 to the C terminus of CBP at the molar ratio of 1:5 (Fig. 3C) (50 and 80% inhibition with 1:1 and 1:5 molar ratios). Surprisingly, however, we found that E6.16 was not able to compete P/CAF away from the C/H3 domain of CBP/p300 (data not shown). Taken together, these results implicated a potential mechanism by which E6.16 inhibits the optimal transcriptional activation of NF-κB through CBP/p300.
FIG. 3.
HPV-16 E6 competes SRC-1 and p65 away from the C terminus and the N terminus of CBP, respectively. (A) Schematic of CBP 1-771 and CBP.CT. The binding domains of E6.16, RelA (p65), and SRC-1 are indicated. (B and C) 35S-radiolabeled in vitro-translated p65 or SRC-1 was synthesized using a TNT coupled rabbit reticulocyte system. One microgram of purified GST-CBP 1-771 or GST-CBP.CT bound to glutathione beads was incubated with His-E6.16, His-E7.16, or E6 C66G/C136G at various molar ratios at 4°C. Subsequently, 80,000 cpm of in vitro-translated p65 or SRC-1 was added and incubated for a further 2 h at 4°C. The pelleted beads were washed three times with buffer A, resuspended in 1× sample buffer, and subjected to SDS-PAGE for analysis. The presence of the radiolabeled proteins was visualized by using a PhosphorImager. At a 1:1 molar ratio, E6.16 inhibited binding of p65 to CBP 1-771 or SRC-1 to CBP.CT by approximately 50%, and at a 1:5 molar ratio the inhibition was 80%. The input levels of GST fusion proteins and His-tagged proteins utilized in each competition assay are indicated.
HPV-16 E7 also down-regulates IL-8 promoter activity.
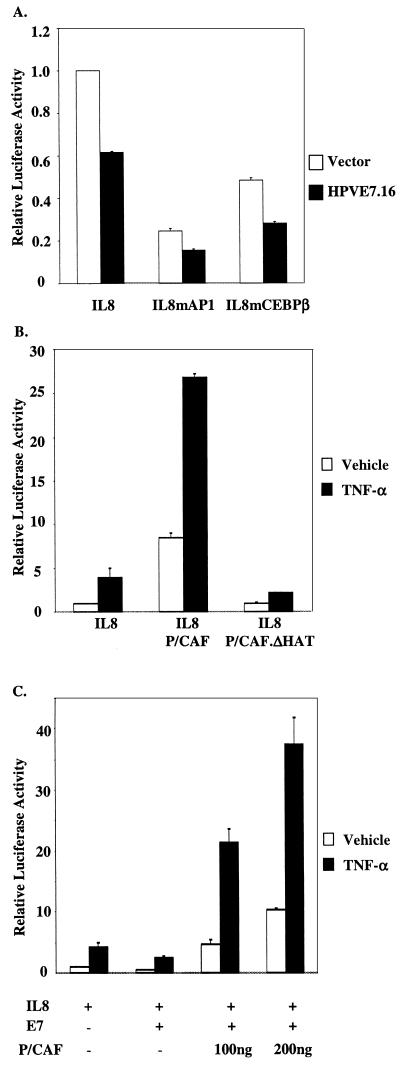
Since E6 and E7 are both expressed in a normal infection, we investigated if E7.16 had any effect on IL-8 transcription. To assess the effect of E7.16 on IL-8 promoter activity, we cotransfected E7.16 with IL-8WT, IL-8mAP1, or IL-8mCEBPβ into HFKs. As previously described, approximately 20 h after the transfection HFKs were fed fresh medium containing 2,000 U of TNF-α/ml for 6 h. We found that E7.16 alone was able to repress IL-8 promoter activity by approximately 40%, which was not as significant as the repression by E6.16 (Fig. 4A ). Interestingly, similar to the data for E6.16, the results also showed that E7.16 repressed the activity of IL-8WT, IL-8mAP1, and IL-8mCEBPβ, implying that E7.16 inhibited the activation of the IL-8 promoter by altering the optimal function of NF-κB. Since it has previously been shown that the presence of P/CAF is required for NF-κB transcriptional activation, we wished to further examine the interplay between E7.16 and P/CAF on the IL-8 promoter. Interestingly, when IL-8WT was cotransfected with the wild-type P/CAF, the activation of the promoter was significantly enhanced. However, when the same amount of the mutant P/CAF, which has a 30-aa (aa 497 to 526) deletion in the HAT domain of P/CAF and has no HAT activity, was cointroduced into HFKs with IL-8WT, the enhancing effect of the wild-type P/CAF on the activation of the IL-8 promoter was no longer observed (Fig. 4B). Subsequently, we cotransfected increasing amounts of P/CAF into HFKs in the presence of both IL-8WT and E7.16. Interestingly, the exogenous P/CAF rescued the repression of IL-8 promoter activity by E7.16 (Fig. 4C). These results suggested that E7.16 might contribute to the repression of the IL-8 promoter by disrupting the function of P/CAF in NF-κB transcriptional activation.
FIG.4.
HPV-16 E7 down-regulates IL-8 promoter activity. (A) Results with the same treatment shown in Fig. 2, except that 200 ng of E7.16 was transfected with IL-8WT, IL-8mAP1, or IL-8mCEBPβ into HFKs. Results are representative of three independent experiments. (B) Results with the same treatment shown in panel A, except that 50 ng of IL-8WT was cotransfected with either 200 ng of pCX-Flag.P/CAF or pCX-Flag.P/CAFΔHAT. (C) E7.16 (200 ng) and IL-8WT (50 ng) were cotransfected with either 100 or 200 ng of pCX-Flag.P/CAF. The subsequent experimental process was carried out as described for panel A.
HPV-16 E7 binds to P/CAF both in vitro and in vivo.
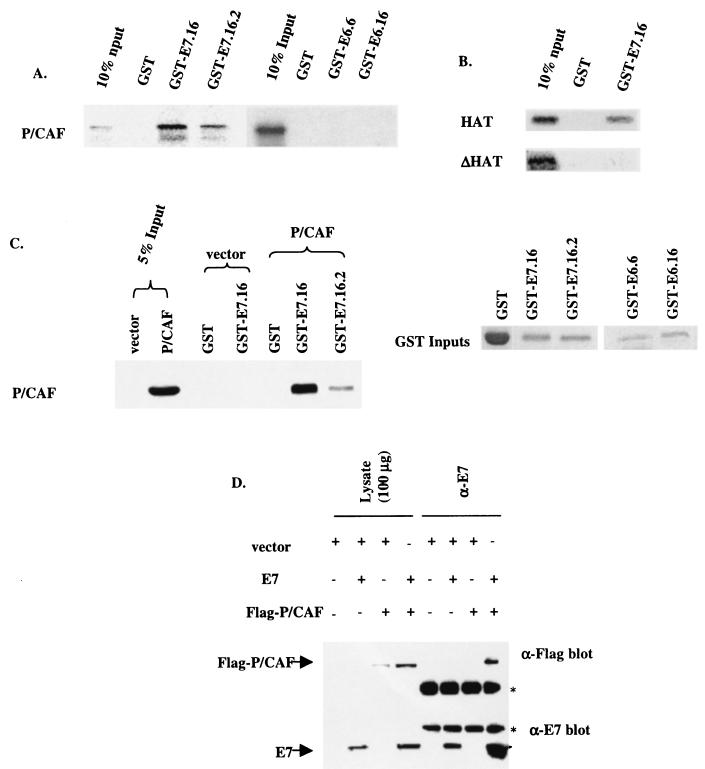
To further clarify the interplay between E7.16 and P/CAF, we postulated that E7.16 associates with P/CAF. Firstly, in vitro GST pull-down assays were performed. The purified fusion protein GST-E7.16 was incubated with radiolabeled in vitro-transcribed and -translated P/CAF in buffer B for 2 h at 4°C. GST-E7.16 was able to interact with radiolabeled in vitro-translated P/CAF, and it pulled down approximately 40% of the P/CAF input (Fig. 5A, left panel). Interestingly, E7.16.2, which contains a mutation at the second amino acid of E7.16, resulting in inhibition of many of the biological properties of wild-type E7.16, bound to P/CAF at only 30% of that for E7.16. However, this mutation is still able to bind to the retinoblastoma protein (Rb) (3). In contrast, neither E6.16 nor E6 of HPV type 6 (E6.6) was able to interact with any radiolabeled P/CAF (Fig. 5A, right panel). In addition, to further map the E7-P/CAF binding on P/CAF, the purified fusion protein GST-E7.16 was incubated with either the radiolabeled in vitro-transcribed and -translated HAT domain (aa 352 to 658) of P/CAF or the HAT domain with a 30-aa deletion (aa 497 to 526) (ΔHAT) that completely abolishes the HAT activity. The results demonstrated that E7.16 bound to the HAT domain of P/CAF, while the 30-aa deletion that eliminates the HAT activity inhibited the binding of E7.16 to P/CAF (Fig. 5B). These results imply a possible means by which the E7-P/CAF interaction may affect the HAT activity of P/CAF. To further assess the binding of E7.16 to P/CAF, we transfected either Flag-P/CAF or the empty vector into COS-1 cells and harvested the transfected cells 48 h after the transfection. The cellular extracts were incubated with GST, GST-E7.16, or GST-E7.16.2 overnight at 4°C. The pulled down proteins were separated by SDS-PAGE and assayed by Western blotting using α-Flag antibody. We showed that E7.16 was able to associate with the Flag-P/CAF expressed in the COS-1 cellular extract (Fig. 5C) and that E7.16.2 exhibited reduced binding capacity to P/CAF. Moreover, to demonstrate that E7.16 and P/CAF associate in vivo, Flag-P/CAF and E7.16 were either transfected with the empty vector or cotransfected into COS-1 cells. The cell lysates were immunoprecipitated with anti-E7 antibody and subsequently blotted with either anti-E7 or anti-Flag antibody (Fig. 5D). The results confirmed that E7.16 indeed associated with P/CAF in vivo, since the anti-Flag antibody was able to detect the presence of Flag-P/CAF in the E7-antibody precipitated complex. Nevertheless, when in vitro competition assays were performed to examine the effect of E7.16 on P/CAF binding to CBP/p300, E7.16 did not disrupt the binding (data not shown). Therefore, we examined the effect of E7.16-P/CAF interaction on the HAT activity of P/CAF. The HAT domain of P/CAF (aa 352 to 658) was purified as a GST fusion protein and used in an in vitro HAT assay with a commonly used substrate, histone H3. E7.16 was able to exert only a moderate level of repression, approximately 50%, on the acetylation of H3 (data not shown). These data established the interaction between E7.16 and P/CAF. However, despite this interaction, E7.16 did not seem to exert a profound effect on the HAT activity of P/CAF on histone H3.
FIG. 5.
HPV-16 E7 binds to P/CAF both in vitro and in vivo. (A) The purified fusion proteins GST, GST-E7.16, GST-E7.16.2, GST-E6.16, or GST-E6.6 were incubated with 35S-radiolabeled in vitro-transcribed and -translated P/CAF for 2 h at 4°C. The pelleted beads were washed three times with buffer A and subjected to SDS-PAGE for analysis. The presence of the radiolabeled proteins was visualized by using a PhosphorImager. (B) The purified fusion protein GST-E7.16 was incubated with either the radiolabeled in vitro-transcribed and -translated HAT domain (aa 352 to 658) of P/CAF (HAT) or the HAT domain with a 30-aa deletion (aa 497 to 526) that completely abolishes the HAT activity (ΔHAT) in buffer B. The subsequent experimental procedure was carried out as described above for panel A. (C) Either pCX-Flag.P/CAF or the empty vector was transfected into COS-1 cells, and the transfected cells were harvested 48 h after the transfection. Two milligrams of cellular extracts was incubated with GST, GST-E7.16, or GST-E7.16.2 overnight at 4°C. The proteins pulled down were separated by SDS-PAGE and assayed by Western blotting using anti-Flag antibody. The levels of GST fusion protein inputs in these pull-down experiments are shown on the right panel. (D) COS-1 cells were plated at 50% confluency onto 100-mm-diameter plates the day before transfection. Subsequently, 10 μg of pSG5, pCX-Flag.P/CAF, or pSG5-E7.16 was transfected into cells by using Lipofectamine. The cells were harvested and lysed in buffer A. The cell lysates (4 mg of protein per lysate) were precleared with 20 μl of anti-mouse IgG magnetic beads, and proteins were immunoprecipitated with the addition of anti-E7.16 and 20 μl of anti-mouse IgG magnetic beads. Following protein transfer, immunodetection of E7.16 or Flag-P/CAF was achieved by blotting with anti-E7.16 or anti-Flag antibody and developing with Super Signal West Femto chemiluminescent reagent. Asterisks denote the heavy chain (upper) and light chain (lower).
Mutation E7.16.2 does not inhibit IL-8 promoter activity.
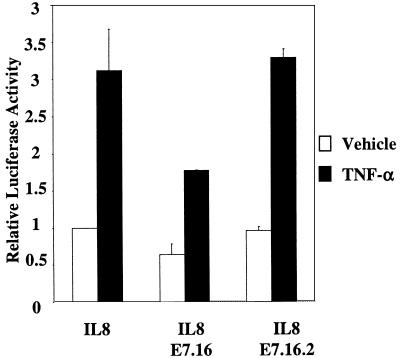
Since E7.16.2 exhibited significantly less binding to P/CAF than E7.16, we next examined the effect of this mutant on IL-8 promoter activity. As described previously, we cotransfected either E7.16 or E7.16.2 along with IL-8WT into HFKs. Approximately 20 h after transfection, cells were fed with fresh medium containing either vehicle or 2,000 U of TNF-α/ml. We found that while E7.16 inhibited IL-8 promoter activity as shown previously, E7.16.2 did not have any effect (Fig. 6). The results suggested that the binding of E7.16 to P/CAF might play a role in the suppression of IL-8 promoter activity.
FIG. 6.
Mutation E7.16.2 does not inhibit the IL-8 promoter activity. The experiment was performed as described in the legend for Fig. 2, except that 50 ng of IL-8WT was cotransfected with 200 ng of either pCDNA-E7.16 or pCDNA-E7.16.2.
HPV-16 E6 and HPV-16 E7 cooperatively achieve maximum repression of IL-8 promoter activity and endogenous gene expression.
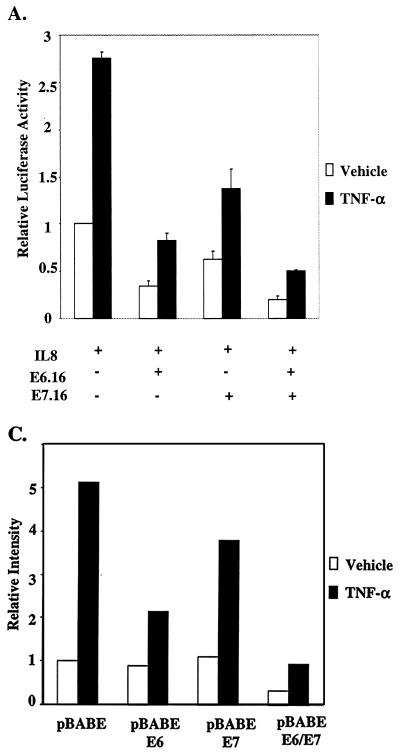
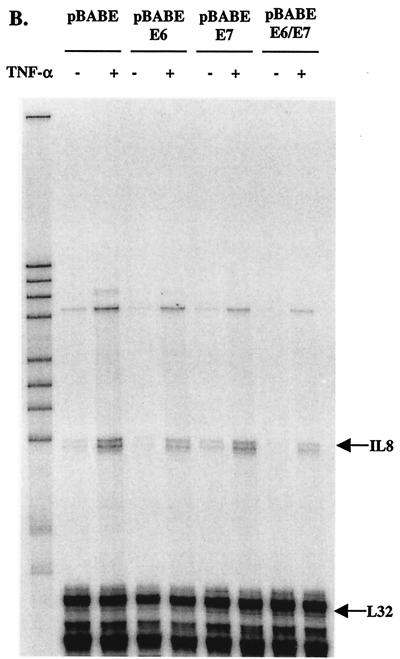
After observing the individual effect of E6.16 and E7.16 on IL-8 promoter activity, we next examined the combined effect of E6.16 and E7.16 on the activation of IL-8WT. Interestingly, we found that in the presence of both E6.16 and E7.16 the activity of IL-8WT can be further reduced approximately twofold compared to that with E6.16 alone (Fig. 7A), demonstrating that E6.16 and E7.16 were able to cooperatively repress IL-8 promoter activity. To further scrutinize the effect of E6.16 and E7.16 on endogenous IL-8 expression in keratinocytes, we produced keratinocytes constitutively expressing E6, E7, or E6 and E7 by infecting keratinocytes with retroviruses that contain pBABE-E6, pBABE-E7, or pBABE-E6/E7 constructs, respectively, and selecting cells expressing viral proteins with puromycin. Cells were used for experiments at an early passage after puromycin selection. These selected cells were treated with fresh medium containing vehicle or 2,000 U of TNF-α/ml at approximately 50% confluence for 24 h. Subsequently, total RNA was harvested and a standard RPA was performed. The intensity of the IL-8 transcripts was measured using a phosphorimager and normalized against the transcription of a housekeeping gene, L32. Our results showed a similar trend to that demonstrated by the reporter assay data (Fig. 7B). pBABE keratinocytes were able to maintain a basal level of IL-8 transcription, and the protected RNA showed up as a doublet. However, after TNF-α stimulation, the intensity of IL-8 transcript was increased approximately 5.5-fold (Fig. 7B and C). The introduction of E6.16 reduced IL-8 mRNA synthesis in the presence of TNF-α by approximately 50%, while the reduction by E7.16 was approximately 20%. More importantly, in the presence of both E6 and E7, the intensity of the IL-8 mRNA was reduced by almost 82% (Fig. 7B and C), further demonstrating the ability of E6.16 and E7.16 to cooperatively down-regulate IL-8 transcription. At least two sets of cell lines were produced for each construct, with similar effects on theendogenous IL-8 promoter. The bands in the upper part of the gel represent the chemokines RANTES (upper faint band) and IP-10, which have also been shown to be regulated by NF-κB (17, 35, 36, 38, 39, 44, 54, 73, 77). Therefore, HPV-16 E6 and E7 had profound effects on IL-8 transcription and possibly also on the transcription of RANTES and IP-10, but not on other chemokines detected in this assay system. Since these chemokines have important roles in recruiting immune cells to sites of infection, our results suggest that E6 and E7 may affect the ability of host keratinocytes to induce an effective immune response after HPV infection.
FIG. 7.
HPV-16 E6 and HPV-16 E7 cooperatively achieve maximum repression of the IL-8 promoter activity and endogenous gene expression. (A) The experiment was performed as described in the legend for Fig. 2, except that 50 ng of IL-8WT was cotransfected with 200 ng of pCDNA-E6.16 and/or pCDNA-E7.16. (B and C) Keratinocytes constitutively expressing E6, E7, or E6 and E7 were treated with fresh medium containing vehicle or 2,000 U of TNF-α/ml at approximately 50% confluence for 24 h. Subsequently, total RNA was harvested with an RNeasy Mini kit, and a standard RPA was performed (B). The intensity of the IL-8 transcripts was measured by using a PhosphorImager and normalized against the transcription of the housekeeping gene L32 (C).
DISCUSSION
In this study, we investigated the effect of E6 and E7 on the transcription of the NF-κB-dependent IL-8 promoter and we showed that both viral proteins can significantly reduce the activity of the endogenous IL-8 promoter in human primary keratinocytes. IL-8 is a potent chemoattractant for T cells, neutrophils, and basophils (56) and is also essential in initiating a local immune response. Moreover, it was shown by others that keratinocytes, the natural host of HPV, synthesize IL-8 under the stimulation of various cytokines (16) produced in response to infectious agents, resulting in the initiation of a local immune response. Interestingly, it has been demonstrated that HPV-immortalized exocervical cell lines synthesize reduced amounts of IL-8 (76), and there is a strong association between persistent infection by HPV and the development of squamous intraepithelial lesions (30, 45). Taken together, the reduced IL-8 synthesis due to the inhibition of NF-κB transcription by E6 and E7 may create a more favorable environment for persistent HPV infection and subsequent oncogenesis.
Using reporter assays, we demonstrated that the NF-κB transcription factor is necessary to maintain activity of the IL-8 promoter in keratinocytes. Although the predominant NF-κB dimer that binds the IL-8 promoter is still unknown (the existing data suggest that it is possibly not the conventional p65/p50 dimer), the presence of the p65 member of the NF-κB family in these putative NF-κB complexes has been documented (55, 71). We found that E6.16 down-regulates the IL-8 promoter activity and that this repression is via the NF-κB transcription factor binding site. We have shown that E6.16 does not inhibit binding of NF-κB factors to the IL-8 promoter (data not shown) but does disrupt the coactivation of NF-κB by CBP/p300 (57). Moreover, the fact that E6 C66G/C136G, a mutant E6.16 that does not bind to CBP/p300, did not yield significant repression strongly implies that the repression of NF-κB transcriptional activation by E6.16 is associated with the binding of E6.16 to CBP/p300. In addition, the repressed IL-8 promoter activity was inhibited when p300 was exogenously expressed. We also showed that E6.16 was capable of disrupting the binding of p65 and SRC-1 to the N terminus and C terminus of CBP, respectively. Since both CBP and SRC-1 are essential in NF-κB transcriptional activation these data suggested that E6.16, by binding to CBP, inhibits the binding of p65 and SRC-1 and in turn suppresses NF-κB activation.
During the course of these investigations, we also revealed the possible role of E7 in the down regulation of NF-κB-dependent transcription. We found that, like E6, E7 was able to repress the IL-8 promoter activity and that this repression was also through the NF-κB binding site. However, we found that the repression was moderate, exhibiting approximately 40 to 50% reduction on the IL-8 promoter activity. This result led us to search for other essential components in the NF-κB activation complex that could be targeted by E7. Interestingly, one of the components required in the NF-κB activation complex is P/CAF. Through a series of GST pull-down assays and coimmunoprecipitation assays, we elucidated a novel interaction between E7 and P/CAF. We have also found that E7.16.2, which contains a mutation at the second amino acid of E7.16 and inhibits most of the functions of E7.16, bound to P/CAF at a significantly reduced level compared to E7.16 and was unable to inhibit TNF-α-stimulated IL-8 promoter activity. In addition, deletion analysis revealed that E7 bound to the HAT domain (aa 352 to 658) of P/CAF. Despite the strong interaction between E7 and P/CAF, E7 did not seem to compete P/CAF away from the C/H3 domain of CBP, and it only reduced the HAT activity by at most 50% with histone H3 as substrate (data not shown). However, since histone H3 was utilized in this assay, it is possible that the substrate specificity of the P/CAF HAT activity in the NF-κB transcriptional complex was not accurately reflected. Adenovirus E1A has also been shown to bind P/CAF, but the outcome of the interaction is confusing. In three different studies, E1A had either no effect (62) or actually reduced HAT activity (5, 22). The variable outcomes probably reflect a situation where E1A may have an effect on specific substrates at certain periods in the cell cycle. Furthermore, the fact that E7.16.2 had no effect at all on IL-8 promoter activity and had significantly reduced binding to P/CAF further demonstrated the need for binding and the specificity of the inhibitory effect of the E7-P/CAF interaction on IL-8 transcription.
When the combined effects of E6 and E7 were investigated, we observed that the presence of both E6 and E7 repressed IL-8 promoter activity more than either one of them alone. Subsequently, when endogenous IL-8 mRNA synthesis was determined by RPAs with retrovirus-infected keratinocytes stably expressing E6, E7, or E6 and E7, we observed similar trends in the reduction of IL-8 mRNA synthesis. Endogenous IL-8 promoter activity in the presence of E6.16, E7.16, or E6.16/E7.16 was reduced by 50, 20, and 80%, respectively. Therefore, with the results for IL-8 promoter activity in the reporter assays together with endogenous IL-8 mRNA quantification in RPAs, we have demonstrated that E6.16 and E7.16 cooperatively inhibit the transcription of IL-8 through disruption of optimal activation by NF-κB.
IL-8 and IL-6 are two inflammatory chemokines or cytokines which help to stimulate the initial phase of an immune response to infectious agents. Any limit on their production during infection may reduce the host's ability to mount an effective immune response. We have elucidated a potential mechanism by which the HPV-16 proteins E6 and E7 inhibit NF-κB-dependent activation of the IL-8 promoter, which compromises the ability of the host to respond to HPV infection. In addition, from the RPA data, we have preliminary evidence that transcription of two other chemokines, RANTES and IP-10, are also reduced. The combined effects of E6 and E7 may therefore reduce the ability of the host to respond in HPV infection, leading to the establishment of persistent HPV infection and subsequent tumorigenesis.
Acknowledgments
We thank Don Nguyen, Thomas Westbrook, Laurel Baglia, Daksha Patel, and Helene McMurray for helpful discussions and reviews of the manuscript. In addition, we are also indebted to people mentioned in the paper for kindly providing us plasmids and reagents.
This work was funded by grants NIH DE13526 and NIH PO AI-99-008 to D.J.M.
REFERENCES
- 1.Abraham, S. E., S. Lobo, P. Yaciuk, H. G. Wang, and E. Moran. 1993. p300, and p300-associated proteins, are components of TATA-binding protein (TBP) complexes. Oncogene 8:1639-1647. [PubMed] [Google Scholar]
- 2.Antinore, M. J., M. J. Birrer, D. Patel, L. Nader, and D. J. McCance. 1996. The human papillomavirus type 16 E7 gene product interacts with and trans-activates the AP1 family of transcription factors. EMBO J. 15:1950-1960. [PMC free article] [PubMed] [Google Scholar]
- 3.Banks, L., C. Edmonds, and K. H. Vousden. 1990. Ability of the HPV16 E7 protein to bind RB and induce DNA synthesis is not sufficient for efficient transforming activity in NIH 3T3 cells. Oncogene 5:1383-1389. [PubMed] [Google Scholar]
- 4.Barbosa, M. S., C. Edmonds, C. Fisher, J. T. Schiller, D. R. Lowy, and K. H. Vousden. 1990. The region of the HPV E7 oncoprotein homologous to adenovirus E1a and SV40 large T antigen contains separate domains for Rb binding and casein kinase II phosphorylation. EMBO J. 9:153-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakravarti, D., V. Ogryzko, H.-Y. Kao, A. Nash, H. Chen, Y. Nakatani, and R. M. Evans. 1999. A viral mechanism for inhibition of p300 and PCAF acetyltransferase activity. Cell 96:393-403. [DOI] [PubMed] [Google Scholar]
- 6.Chan, H. M., M. Krstic-Demonacos, L. Smith, C. Demonacos, and N. B. La Thangue. 2001. Acetylation control of the retinoblastoma tumour-suppressor protein. Nat. Cell Biol. 3:667-674. [DOI] [PubMed] [Google Scholar]
- 7.Chen, L., W. Fischle, E. Verdin, and W. C. Greene. 2001. Duration of nuclear NF-κB action regulated by reversible acetylation. Science 293:1653-1657. [DOI] [PubMed] [Google Scholar]
- 8.Chrivia, J. C., R. P. Kwok, N. Lamb, M. Hagiwara, M. R. Montminy, and R. H. Goodman. 1993. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature 365:855-859. [DOI] [PubMed] [Google Scholar]
- 9.Dai, P., H. Akimaru, Y. Tanaka, D. X. Hou, T. Yasukawa, C. Kanei-Ishii, T. Takahashi, and S. Ishii. 1996. CBP as a transcriptional coactivator of c-Myb. Genes Dev. 10:528-540. [DOI] [PubMed] [Google Scholar]
- 10.Dyson, N., P. Guida, K. Munger, and E. Harlow. 1992. Homologous sequences in adenovirus E1A and human papillomavirus E7 proteins mediate interaction with the same set of cellular proteins. J. Virol. 66:6893-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dyson, N., P. M. Howley, K. Munger, and E. Harlow. 1989. The human papillomavirus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243:934-937. [DOI] [PubMed] [Google Scholar]
- 12.Eckner, R., M. E. Ewen, D. Newsome, M. Gerdes, J. A. DeCaprio, J. B. Lawrence, and D. M. Livingston. 1994. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adaptor. Genes Dev. 8:869-884. [DOI] [PubMed] [Google Scholar]
- 13.Eckner, R., J. W. Ludlow, N. L. Lill, E. Oldread, Z. Arany, N. Modjtahedi, J. A. DeCaprio, D. M. Livingston, and J. A. Morgan. 1996. Association of p300 and CBP with simian virus 40 large T antigen. Mol. Cell. Biol. 16:3454-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eckner, R., T. P. Yao, E. Oldread, and D. M. Livingston. 1996. Interaction and functional collaboration of p300/CBP and bHLH proteins in muscle and B-cell differentiation. Genes Dev. 10:2478-2490. [DOI] [PubMed] [Google Scholar]
- 15.Eliopoulos, A. G., N. J. Gallagher, S. M. Blake, C. W. Dawson, and L. S. Young. 1999. Activation of the p38 mitogen-activated protein kinase pathway by Epstein-Barr virus-encoded latent membrane protein 1 coregulates interleukin-6 and interleukin-8 production. J. Biol. Chem. 274:16085-16096. [DOI] [PubMed] [Google Scholar]
- 16.Fujisawa, H., B. Wang, D. N. Sauder, and S. Kondo. 1997. Effects of interferons on the production of interleukin-6 and interleukin-8 in human keratinocytes. J. Interferon Cytokine Res. 17:347-353. [DOI] [PubMed] [Google Scholar]
- 17.Genin, P., M. Algarte, P. Roof, R. Lin, and J. Hiscott. 2000. Regulation of RANTES chemokine gene expression requires cooperativity between NF-κB and IFN-regulatory factor transcription factors. J. Immunol. 164:5352-5361. [DOI] [PubMed] [Google Scholar]
- 18.Gerritsen, M. E., A. J. Williams, A. S. Neish, S. Moore, Y. Shi, and T. Collins. 1997. CREB-binding protein/p300 are transcriptional coactivators of p65. Proc. Natl. Acad. Sci. USA 94:2927-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giles, R. H., D. J. Peters, and M. H. Breuning. 1998. Conjunction dysfunction: CBP/p300 in human disease. Trends Genet. 14:178-183. [DOI] [PubMed] [Google Scholar]
- 20.Glass, C. K., D. W. Rose, and M. G. Rosenfeld. 1997. Nuclear receptor coactivators. Curr. Opin. Cell Biol. 9:222-232. [DOI] [PubMed] [Google Scholar]
- 21.Gu, W., and R. G. Roeder. 1997. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell 90:595-606. [DOI] [PubMed] [Google Scholar]
- 22.Hamamori, Y., V. Sartorelli, V. Ogryzko, P. L. Puri, H.-Y. Wu, J. Y. J. Wang, Y. Nakatani, and L. Kedes. 1999. Regulation of histone acetyltransferases p300 and PCAF by the bHLH protein Twist and adenoviral oncoprotein E1A. Cell 96:405-413. [DOI] [PubMed] [Google Scholar]
- 23.Heery, D. M., E. Kalkhoven, S. Hoare, and M. G. Parker. 1997. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387:733-736. [DOI] [PubMed] [Google Scholar]
- 24.Huang, P. S., D. R. Patrick, G. Edwards, P. J. Goodhart, H. E. Huber, L. Miles, V. M. Garsky, A. Oliff, and D. C. Heimbrook. 1993. Protein domains governing interactions between E2F, the retinoblastoma gene product, and human papillomavirus type 16 E7 protein. Mol. Cell. Biol. 13:953-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hudson, J. B., M. A. Bedell, D. J. McCance, and L. A. Laiminis. 1990. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J. Virol. 64:519-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamei, Y., L. Xu, T. Heinzel, J. Torchia, R. Kurokawa, B. Gloss, S. C. Lin, R. A. Heyman, D. W. Rose, C. K. Glass, and M. G. Rosenfeld. 1996. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell 85:403-414. [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki, H., R. Eckner, T. P. Yao, K. Taira, R. Chiu, D. M. Livingston, and K. K. Yokoyama. 1998. Distinct roles of the co-activators p300 and CBP in retinoic-acid-induced F9-cell differentiation. Nature 393:284-289. [DOI] [PubMed] [Google Scholar]
- 28.Kim, R. H., K. C. Flanders, S. Birkey Reffey, L. A. Anderson, C. S. Duckett, N. D. Perkins, and A. B. Roberts. 2001. SNIP1 inhibits NF-κB signaling by competing for its binding to the C/H1 domain of CBP/p300 transcriptional co-activator. J. Biol. Chem. 276:46297-46304. [DOI] [PubMed] [Google Scholar]
- 29.Korzus, E., J. Torchia, D. W. Rose, L. Xu, R. Kurokawa, E. M. McInerney, T. M. Mullen, C. K. Glass, and M. G. Rosenfeld. 1998. Transcription factor-specific requirements for coactivators and their acetyltransferase functions. Science 279:703-707. [DOI] [PubMed] [Google Scholar]
- 30.Koutsky, L. A., K. K. Holmes, C. W. Critchlow, C. E. Stevens, J. Paavonen, A. M. Beckmann, T. A. DeRouen, D. A. Galloway, D. Vernon, and N. B. Kiviat. 1992. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N. Engl. J. Med. 327:1272-1278. [DOI] [PubMed] [Google Scholar]
- 31.Kunsch, C., R. K. Lang, C. A. Rosen, and M. F. Shannon. 1994. Synergistic transcriptional activation of the IL-8 gene by NF-κB p65 (RelA) and NF-IL-6. J. Immunol. 153:153-164. [PubMed] [Google Scholar]
- 32.Kunsch, C., and C. A. Rosen. 1993. NF-κB subunit-specific regulation of the interleukin-8 promoter. Mol. Cell. Biol. 13:6137-6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwok, R. P., J. R. Lundblad, J. C. Chrivia, J. P. Richards, H. P. Bachinger, R. G. Brennan, S. G. Roberts, M. R. Green, and R. H. Goodman. 1994. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature 370:223-226. [DOI] [PubMed] [Google Scholar]
- 34.Larsen, C. G., A. O. Anderson, E. Appella, J. J. Oppenheim, and K. Matsushima. 1989. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science 243:1464-1466. [DOI] [PubMed] [Google Scholar]
- 35.Lebovic, D. I., V. A. Chao, J. F. Martini, and R. N. Taylor. 2001. IL-1β induction of RANTES (regulated upon activation, normal T cell expressed and secreted) chemokine gene expression in endometriotic stromal cells depends on a nuclear factor-κB site in the proximal promoter. J. Clin. Endocrinol. Metab. 86:4759-4764. [DOI] [PubMed] [Google Scholar]
- 36.Lee, A. H., J. H. Hong, and Y. S. Seo. 2000. Tumour necrosis factor-alpha and interferon-gamma synergistically activate the RANTES promoter through nuclear factor κB and interferon regulatory factor 1 (IRF-1) transcription factors. Biochem. J. 350:131-138. [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, J. S., X. Zhang, and Y. Shi. 1996. Differential interactions of the CREB/ATF family of transcription factors with p300 and adenovirus E1A. J. Biol. Chem. 271:17666-17674. [PubMed] [Google Scholar]
- 38.Li, Q. Q., C. T. Bever, D. R. Burt, S. I. Judge, and G. D. Trisler. 2001. Induction of RANTES chemokine expression in human astrocytic cells is dependent upon activation of NF-κB transcription factor. Int. J. Mol. Med. 7:527-533. [DOI] [PubMed] [Google Scholar]
- 39.Majumder, S., L. Z. Zhou, P. Chaturvedi, G. Babcock, S. Aras, and R. M. Ransohoff. 1998. Regulation of human IP-10 gene expression in astrocytoma cells by inflammatory cytokines. J. Neurosci. Res. 54:169-180. [DOI] [PubMed] [Google Scholar]
- 40.Martinez-Balbas, M. A., U. M. Bauer, S. J. Nielsen, A. Brehm, and T. Kouzarides. 2000. Regulation of E2F1 activity by acetylation. EMBO J. 19:662-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McInerney, E. M., D. W. Rose, S. E. Flynn, S. Westin, T. M. Mullen, A. Krones, J. Inostroza, J. Torchia, R. T. Nolte, N. Assa-Munt, M. V. Milburn, C. K. Glass, and M. G. Rosenfeld. 1998. Determinants of coactivator LXXLL motif specificity in nuclear receptor transcriptional activation. Genes Dev. 12:3357-3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merika, M., A. J. Williams, G. Chen, T. Collins, and D. Thanos. 1998. Recruitment of CBP/p300 by the IFN beta enhanceosome is required for synergistic activation of transcription. Mol. Cell 1:277-287. [DOI] [PubMed] [Google Scholar]
- 43.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 18:3587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moriuchi, H., M. Moriuchi, and A. S. Fauci. 1997. Nuclear factor-κB potently up-regulates the promoter activity of RANTES, a chemokine that blocks HIV infection. J. Immunol. 158:3483-3491. [PubMed] [Google Scholar]
- 45.Moscicki, A. B., S. Shiboski, J. Broering, K. Powell, L. Clayton, N. Jay, T. M. Darragh, R. Brescia, S. Kanowitz, S. B. Miller, J. Stone, E. Hanson, and J. Palefsky. 1998. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J. Pediatr. 132:277-284. [DOI] [PubMed] [Google Scholar]
- 46.Mukaida, N., Y. Mahe, and K. Matsushima. 1990. Cooperative interaction of nuclear factor-κB and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J. Biol. Chem. 265:21128-21133. [PubMed] [Google Scholar]
- 47.Munger, K., B. A. Werness, N. Dyson, W. C. Phelps, E. Harlow, and P. M. Howley. 1989. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 8:4099-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakajima, T., C. Uchida, S. F. Anderson, C. G. Lee, J. Hurwitz, J. D. Parvin, and M. Montminy. 1997. RNA helicase A mediates association of CBP with RNA polymerase II. Cell 90:1107-1112. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura, H., K. Yoshimura, H. A. Jaffe, and R. G. Crystal. 1991. Interleukin-8 gene expression in human bronchial epithelial cells. J. Biol. Chem. 266:19611-19617. [PubMed] [Google Scholar]
- 50.Nead, M. A., L. A. Baglia, M. J. Antinore, J. W. Ludlow, and D. J. McCance. 1998. Rb binds c-Jun and activates transcription. EMBO J. 17:2342-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neish, A. S., A. T. Gewirtz, H. Zeng, A. N. Young, M. E. Hobert, V. Karmali, A. S. Rao, and J. L. Madara. 2000. Prokaryotic regulation of epithelial responses by inhibition of IκB-α ubiquitination. Science 289:1560-1563. [DOI] [PubMed] [Google Scholar]
- 52.Nguyen, D. X., T. F. Westerbrook, and D. J. McCance. 2002. Human papillomavirus type 16 E7 maintains elevated levels of the cdc25A tyrosine phosphatase during deregulation of cell cycle arrest. J. Virol. 76:619-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oelgeschlager, M., R. Janknecht, J. Krieg, S. Schreek, and B. Luscher. 1996. Interaction of the co-activator CBP with Myb proteins: effects on Myb-specific transactivation and on the cooperativity with NF-M. EMBO J. 15:2771-2780. [PMC free article] [PubMed] [Google Scholar]
- 54.Ohmori, Y., and T. A. Hamilton. 1993. Cooperative interaction between interferon (IFN) stimulus response element and kappa B sequence motifs controls IFN gamma- and lipopolysaccharide-stimulated transcription from the murine IP-10 promoter. J. Biol. Chem. 268:6677-6688. [PubMed] [Google Scholar]
- 55.Okamoto, S., N. Mukaida, K. Yasumoto, H. Horiguchi, and K. Matsushima. 1993. Molecular mechanism of interleukin-8 gene expression. Adv. Exp. Med. Biol. 351:87-97. [DOI] [PubMed] [Google Scholar]
- 56.Oppenheim, J. J., C. O. Zachariae, N. Mukaida, and K. Matsushima. 1991. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu. Rev. Immunol. 9:617-648. [DOI] [PubMed] [Google Scholar]
- 57.Patel, D., S. M. Huang, L. A. Baglia, and D. J. McCance. 1999. The E6 protein of human papillomavirus type 16 binds to and inhibits co-activation by CBP and p300. EMBO J. 18:5061-5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Patrick, D. R., A. Oliff, and D. C. Heimbrook. 1994. Identification of a novel retinoblastoma gene product binding site on human papillomavirus type 16 E7 protein. J. Biol. Chem. 269:6842-6850. [PubMed] [Google Scholar]
- 59.Perkins, N. D., L. K. Felzien, J. C. Betts, K. Leung, D. H. Beach, and G. J. Nabel. 1997. Regulation of NF-κB by cyclin-dependent kinases associated with the p300 coactivator. Science 275:523-527. [DOI] [PubMed] [Google Scholar]
- 60.Polesskaya, A., A. Duquet, I. Naguibneva, C. Weise, A. Vervisch, E. Bengal, F. Hucho, P. Robin, and A. Harel-Bellan. 2000. CREB-binding protein/p300 activates MyoD by acetylation. J. Biol. Chem. 275:34359-34364. [DOI] [PubMed] [Google Scholar]
- 61.Puri, P. L., M. L. Avantaggiati, C. Balsano, N. Sang, A. Graessmann, A. Giordano, and M. Levrero. 1997. p300 is required for MyoD-dependent cell cycle arrest and muscle-specific gene transcription. EMBO J. 16:369-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reid, J. L., A. J. Bannister, P. Zegerman, M. A. Martinez-Balbas, and T. Kouzarides. 1998. E1A directly binds and regulates the P/CAF acetyltransferase. EMBO J. 17:4469-4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scheffner, M., J. M. Huibregtse, R. D. Vierstra, and P. M. Howley. 1993. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 75:495-505. [DOI] [PubMed] [Google Scholar]
- 64.Shay, J. W., W. E. Wright, D. Brasiskyte, and B. A. Van der Haegen. 1993. E6 of human papillomavirus type 16 can overcome the M1 stage of immortalization in human mammary epithelial cells but not in human fibroblasts. Oncogene 8:1407-1413. [PubMed] [Google Scholar]
- 65.Sheppard, K. A., D. W. Rose, Z. K. Haque, R. Kurokawa, E. McInerney, S. Westin, D. Thanos, M. G. Rosenfeld, C. K. Glass, and T. Collins. 1999. Transcriptional activation by NF-κB requires multiple coactivators. Mol. Cell. Biol. 19:6367-6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shikama, N., J. Lyon, and N. B. La Thangue. 1997. The p300/CBP family: integrating signals with transcription factors and chromatin. Trends Cell Biol. 7:230-236. [DOI] [PubMed] [Google Scholar]
- 67.Smith-McCune, K., D. Kalman, C. Robbins, S. Shivakumar, L. Yuschenkoff, and J. M. Bishop. 1999. Intranuclear localization of human papillomavirus 16 E7 during transformation and preferential binding of E7 to the Rb family member p130. Proc. Natl. Acad. Sci. USA 96:6999-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Spilianakis, C., J. Papamatheakis, and A. Kretsovali. 2000. Acetylation by PCAF enhances CIITA nuclear accumulation and transactivation of major histocompatibility complex class II genes. Mol. Cell. Biol. 20:8489-8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Standiford, T. J., S. L. Kunkel, M. A. Basha, S. W. Chensue, J. P. D. Lynch, G. B. Toews, J. Westwick, and R. M. Strieter. 1990. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J. Clin. Investig. 86:1945-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Standiford, T. J., S. L. Kunkel, S. H. Phan, B. J. Rollins, and R. M. Strieter. 1991. Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J. Biol. Chem. 266:9912-9918. [PubMed] [Google Scholar]
- 71.Stein, B., and A. S. Baldwin, Jr. 1993. Distinct mechanisms for regulation of the interleukin-8 gene involve synergism and cooperativity between C/EBP and NF-κB. Mol. Cell. Biol. 13:7191-7198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takematsu, H., and H. Tagami. 1993. Mode of release of interleukin-8 from proliferating human epidermal keratinocytes in vitro. Exp. Dermatol. 2:121-124. [DOI] [PubMed] [Google Scholar]
- 73.Thomas, L. H., J. S. Friedland, M. Sharland, and S. Becker. 1998. Respiratory syncytial virus-induced RANTES production from human bronchial epithelial cells is dependent on nuclear factor-κB nuclear binding and is inhibited by adenovirus-mediated expression of inhibitor of κB alpha. J. Immunol. 161:1007-1016. [PubMed] [Google Scholar]
- 74.Torchia, J., D. W. Rose, J. Inostroza, Y. Kamei, S. Westin, C. K. Glass, and M. G. Rosenfeld. 1997. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature 387:677-684. [DOI] [PubMed] [Google Scholar]
- 75.Wade, P. A., D. Pruss, and A. P. Wolffe. 1997. Histone acetylation: chromatin in action. Trends Biochem. Sci. 22:128-132. [DOI] [PubMed] [Google Scholar]
- 76.Woodworth, C. D., and S. Simpson. 1993. Comparative lymphokine secretion by cultured normal human cervical keratinocytes, papillomavirus-immortalized, and carcinoma cell lines. Am. J. Pathol. 142:1544-1555. [PMC free article] [PubMed] [Google Scholar]
- 77.Wu, C., Y. Ohmori, S. Bandyopadhyay, G. Sen, and T. Hamilton. 1994. Interferon-stimulated response element and NF-κB sites cooperate to regulate double-stranded RNA-induced transcription of the IP-10 gene. J. Interferon Res. 14:357-363. [DOI] [PubMed] [Google Scholar]
- 78.Wu, G. D., E. J. Lai, N. Huang, and X. Wen. 1997. Oct-1 and CCAAT/enhancer-binding protein (C/EBP) bind to overlapping elements within the interleukin-8 promoter. The role of Oct-1 as a transcriptional repressor. J. Biol. Chem. 272:2396-2403. [PubMed] [Google Scholar]
- 79.Yang, X. J., V. V. Ogryzko, J. Nishikawa, B. H. Howard, and Y. Nakatani. 1996. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature 382:319-324. [DOI] [PubMed] [Google Scholar]
- 80.Yee, S. P., and P. E. Branton. 1985. Detection of cellular proteins associated with human adenovirus type 5 early region 1A polypeptides. Virology 147:142-153. [DOI] [PubMed] [Google Scholar]
- 81.Yuan, W., G. Condorelli, M. Caruso, A. Felsani, and A. Giordano. 1996. Human p300 protein is a coactivator for the transcription factor MyoD. J. Biol. Chem. 271:9009-9013. [DOI] [PubMed] [Google Scholar]
- 82.Zhong, H., R. E. Voll, and S. Ghosh. 1998. Phosphorylation of NF-κB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell 1:661-671. [DOI] [PubMed] [Google Scholar]
- 83.Zimmermann, H., R. Degenkolbe, H.-U. Bernard, and M. J. O'Connor. 1999. The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP/p300. J. Virol. 73:6209-6219. [DOI] [PMC free article] [PubMed] [Google Scholar]