Abstract
Human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T-cell responses generated during acute infection play a critical role in the initial control of viremia. However, little is known about the viral T-cell epitopes targeted during acute infection or about their hierarchy in appearance and relative immunodominance over time. In this study, HIV-1-specific CD8+ T-cell responses in 18 acutely infected individuals expressing HLA-A3 and/or -B7 were characterized. Detailed analysis of CD8 responses in one such person who underwent treatment of acute infection followed by reexposure to HIV-1 through supervised treatment interruptions (STI) revealed recognition of only two cytotoxic T-lymphocyte (CTL) epitopes during symptomatic acute infection. HIV-1-specific CD8+ T-cell responses broadened significantly during subsequent exposure to the virus, ultimately targeting 27 distinct CTL epitopes, including 15 different CTL epitopes restricted by a single HLA class I allele (HLA-A3). The same few peptides were consistently targeted in an additional 17 persons expressing HLA-A3 and/or -B7 during acute infection. These studies demonstrate a consistent pattern in the development of epitope-specific responses restricted by a single HLA allele during acute HIV-1 infection, as well as persistence of the initial pattern of immunodominance during subsequent STI. In addition, they demonstrate that HIV-1-specific CD8+ T-cell responses can ultimately target a previously unexpected and unprecedented number of epitopes in a single infected individual, even though these are not detectable during the initial exposure to virus. These studies have important implications for vaccine design and evaluation.
Twenty years after the first clinical evidence of AIDS was reported, more than 60 million people have been infected with human immunodeficiency virus type 1 (HIV-1). AIDS is now the leading cause of death in sub-Saharan Africa and the fourth-biggest killer worldwide. The need to develop an effective HIV vaccine to either protect people from HIV-1 infection or attenuate the course of disease is urgent. Understanding the correlates of protective immunity is a logical first step in the development of immune-system-based approaches to the control of HIV-1 infection. Increasing evidence indicates that HIV-1-specific CD8+ cytotoxic T lymphocytes (CTL) and CD4+ T helper cells play a critical role in the control of viral replication in HIV-1 infection (reviewed in references 4, 14, 19, 25, 26, 36, and 40). The critical role of virus-specific CTL responses in control of viremia has been directly demonstrated by CD8+ T-cell depletion studies of simian immunodeficiency virus (SIV) infection in macaques, where CD8+ T cells are necessary to effectively suppress viral replication (22, 38). The immune activity generated during acute infection in particular appears to be a critical determinant of the ultimate speed of progression to disease (2, 10-12, 24, 35). Therefore, induction and maintenance of HIV-1-specific CD8+ T-cell responses are considered a key element in the development of effective HIV-1 vaccines (2, 8-10, 41).
Studies on acute retroviral infections in animal models have suggested that CD8+ T-cell responses directed against immunodominant CTL epitopes may be importantly involved in the initial control of viral replication (2, 9). Recent data on HIV-1-infected individuals indicate substantial differences between the viral CTL epitopes targeted by CD8+ T-cell responses during acute and chronic HIV-1 infection (5, 18). However, these studies have largely focused on the characterization of CD8+ T-cell responses directed against previously described epitopes during chronic HIV-1 infection. Very little is known about the immunodominant HIV-1-specific CD8+ T-cell responses during acute infection, since CD8+ T-cell responses directed against full-length HIV-1 during acute infection have never been comprehensively assessed. These early-recognized CTL epitopes might represent crucial targets, due to their early presentation, high immunogenicity, and apparent involvement in the initial control of viremia.
In this study, a large panel of 504 overlapping 15- to 18-mer peptides spanning the full-length sequences of HIV-1 clade B was used to comprehensively characterize CD8+ T-cell responses during acute HIV-1 infection. The evolution of the magnitude, breadth, and hierarchy of CD8+ T-cell responses was monitored longitudinally for individuals identified and treated during acute HIV-1 infection, and the impact of supervised treatment interruptions (STI) on the evolution and immunodominance of responses was assessed. The data show that a previously unexpected and unprecedentedly large number of HIV-1-specific CTL epitopes can be presented by a single HLA class I allele in an infected individual and that the temporal appearance of these responses follows similar patterns in persons of the same HLA type with acute HIV-1 infection.
MATERIALS AND METHODS
Subjects.
All 18 subjects were enrolled in Boston. Study subject AC-06 is an HIV-1-infected individual diagnosed with symptomatic acute HIV-1 infection and treated with highly active antiretroviral therapy (HAART), including two nucleoside analogs and one protease inhibitor, prior to HIV-1 seroconversion. At diagnosis, the viral load was 8.1 million copies of HIV-1 RNA per ml and the CD4+ T-cell count was 551 cells/μl. After 18 months of effective antiretroviral treatment, this individual underwent two cycles of STI. The study protocol required that during STI, treatment had to be reinitiated once the viral load rose above 50,000 copies of HIV-1 RNA per ml at a single time point or remained above 5,000 copies of HIV-1 RNA per ml for more than 3 consecutive weeks (35). The HLA type of this individual was homozygous in the HLA class I A, B, and Cw alleles (A3, B7, Cw7), as determined by sequence-specific primer PCR performed at the Massachusetts General Hospital (MGH) Tissue Typing Laboratory (15).
In addition to study subject AC-06, 17 other individuals identified during primary HIV-1 infection and expressing either HLA-A3 (n = 7), HLA-B7 (n = 4), or both alleles (n = 6) were enrolled in this study. The median initial viral load at presentation for these individuals was 750,000 (range, 22,000 to 1,100,000) copies of HIV-1 RNA per ml. HIV-1-specific CD8+ T-cell responses in these individuals were assessed during early HIV-1 infection, as well as after 1 year of treated infection and following STI for a subset of six individuals. The study was approved by the MGH Institutional Review Board, and each subject gave informed consent for participation in the study.
Cell lines and media.
Epstein-Barr virus-transformed B-lymphoblastoid cell lines (B-LCL) were established from peripheral blood mononuclear cells (PBMC) and maintained in R20 medium (RPMI 1640 medium [Sigma, St. Louis, Mo.] supplemented with 2 mM l-glutamine, 50 U of penicillin per ml, 50 μg of streptomycin per ml, 10 mM HEPES, and 20% heat-inactivated fetal calf serum [Sigma]) as previously described (45). For culture of CTL clones, RPMI medium containing 10% fetal calf serum (R10) supplemented with 50 U of recombinant interleukin-2 (rIL-2, kindly provided by M. Gately, Hoffmann-La Roche, Nutley, N.J.) per ml was used.
Synthetic HIV-1 peptides.
Five hundred and four overlapping peptides (15- to 18-mers with 10-amino-acid overlaps) spanning all the HIV-1 clade B Gag (p15, p17, and p24), Pol (Int, Prot, and RT), Env (gp120 and gp41), regulatory (Rev and Tat), and accessory (Vpr, Vpu, Vif, and Nef) protein sequences were synthesized on an automated peptide synthesizer (MBS 396; Advanced ChemTech, Louisville, Ky.) using fluorenylmethoxycarbonyl chemistry. In addition, peptides corresponding to optimal HIV-1 CTL epitopes described for the individual's HLA class I type (13) and truncated peptides for the fine mapping of novel optimal CTL epitopes were used.
Characterization of HIV-1-specific CD8+ T-cell responses by ELISPOT assay.
HIV-1-specific CD8+ T-cell responses were quantified by enzyme-linked immunospot (ELISPOT) assay as previously described (7). In brief, PBMC were plated out at 100,000 cells per well with peptides at a final concentration of 10−5 M in 96-well polyvinylidene difluoride-backed plates (MAIP S45; Millipore, Bedford, Mass.) precoated with 0.5 μg of the anti-gamma interferon (IFN-γ) monoclonal antibody (MAb) 1-DIK (Mabtech, Stockholm, Sweden)/ml overnight at 4°C. A total of 100,000 PBMC were incubated with R10 alone as a negative control or with phytohemagglutinin (PHA) as a positive control. Plates were incubated overnight (14 to 16 h) at 37°C under 5% CO2 and then processed as described previously (7). The number of specific IFN-γ-secreting T cells was counted by direct visualization, calculated by subtracting the negative-control value, and expressed as spot-forming cells (SFC) per 106 input cells. Negative-control values were always <30 SFC per 106 input cells. Results of 50 or more SFC per 106 input cells above background were considered positive. The CD8+ T-cell dependence of all responses to synthetic peptides was confirmed by CD4+/CD8+ T-cell depletion and enrichment studies using magnetic beads (MACS; Miltenyi Biotech, Cologne, Germany) according to the manufacturer's protocol (7).
Fine mapping of epitopes was performed by ELISPOT assay using serial dilutions of truncated peptides (7). A total of 50,000 to 100,000 freshly isolated PBMC per well were incubated with peptides at concentrations ranging from 10−4 to 10−11 M overnight on the ELISPOT plate. All assays were run in duplicate. The optimal peptide was defined as the peptide that induced 50% maximal specific IFN-γ production by T cells at the lowest peptide concentration (7).
Generation of CTL clones.
CTL clones were isolated by limiting dilution as previously described (45), using the CD3-specific MAb 12F6 as a stimulus for T-cell proliferation. Developing clones were screened for HIV-1-specific CTL activity by a chromium-51 release assay against autologous B-cell lines (BCL) pulsed with the peptides recognized in the ELISPOT assays (45). HIV-1-specific clones were maintained by stimulation every 14 to 21 days with an anti-CD3 MAb and irradiated allogeneic PBMC (46). HLA restriction of CTL epitopes was determined by using a panel of target cells matched through only one of the HLA-A, HLA-B, or HLA-C class I alleles expressed by the effector cells, as described previously (7).
Generation of peptide-specific CD8+ T-cell lines.
CD8+ T cells were nonspecifically expanded from PBMC over 10 days by using a bispecific anti-CD3/CD4b MAb (48). Peptide-specific CD8+ T cells were subsequently isolated by using an IFN-γ capture assay, as described previously (3). Briefly, 107 CD8+ T cells were incubated with 20 μM peptide and the anti-CD28 and anti-CD49d MAbs (1 μg/ml each; Becton Dickinson, San Jose, Calif.) on 24-well plates at 37°C under 5% CO2 for 6 to 8 h. Cells were subsequently labeled with a bispecific CD45/IFN-γ Catch Reagent and incubated for 45 min at 37°C under 5% CO2. After several washes, IFN-γ producing cells were stained with a second, phycoerythrin (PE)-conjugated IFN-γ detection antibody and separated by use of anti-PE MicroBeads on a MACS separator. The isolated cells were then expanded for 10 days by using autologous irradiated feeders as described previously (3).
Flow cytometric detection of antigen-induced intracellular IFN-γ secretion.
Intracellular cytokine staining assays were performed as described elsewhere with minor modifications (20, 32). Briefly, 0.5 × 106 to 1 × 106 PBMC or CTL lines were incubated with 4 μM peptide and the anti-CD28 and anti-CD49d MAbs (1 μg/ml each; Becton Dickinson) at 37°C under 5% CO2 for 1 h before addition of 10 μg of brefeldin A (Sigma)/ml. Following a further 6 h of incubation at 37°C under 5% CO2, cells were placed at 4°C overnight. Cells were then washed and stained with the surface antibodies anti-CD8-PerCP and anti-CD4-APC (Becton Dickinson) at 4°C for 30 min. After a wash, cells were fixed and permeabilized by using a Caltag (Burlingame, Calif.) fixation or permeabilization kit, respectively, for 15 min at room temperature in the dark, and fluorescein isothiocyanate-conjugated anti-IFN-γ (Becton Dickinson) was added at 4°C for 30 min. Cells were then washed and analyzed on a FACScalibur flow cytometer (Becton Dickinson). Control conditions were established by use of autologous PBMC, which were treated identically but without peptide stimulation. Assays using HLA-matched or -mismatched BCL were run for the determination of HLA restriction of responses as described elsewhere (17): BCL that were pulsed with 10 μM peptide for 1 h were washed four times prior to incubation with effectors at 105 BCL and 4 × 105 effectors in 1 ml of R10. The anti-CD28 and anti-CD49d MAbs were then added, and the assay was run exactly as described above.
Statistical analysis.
Statistical analyses and graphic presentation were done using SigmaPlot 5.0 (SPSS Inc., Chicago, Ill.). Results are given as means ± standard deviations or medians with ranges. Statistical analysis of significance (P values) was based on two-tailed t tests.
RESULTS
Comprehensive characterization of HIV-1-specific CD8+ T-cell responses during STI in study subject AC-06.
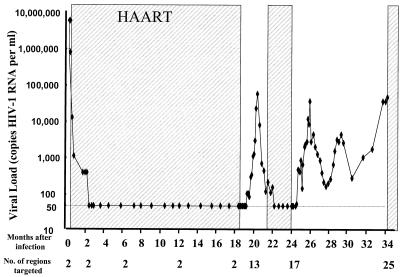
In order to examine the initial induction of immune responses in HIV-1 infection, we performed a detailed analysis of CD8 T-cell responses to all viral proteins expressed in the acute phase of infection. Early treatment followed by treatment interruption allowed for longitudinal analysis of the subsequent evolution of these responses. Initial studies were performed with subject AC-06, who was diagnosed during symptomatic acute HIV-1 infection with a viral load of 8.1 × 106 copies of HIV-1 RNA per ml of plasma. Following treatment with HAART prior to HIV-1 seroconversion, viremia dropped to undetectable levels within 6 weeks and remained undetectable for 18 months of treatment prior to initiation of STI. The evolution of viral loads in individual AC-06 during acute HIV-1 infection and two cycles of STI is shown in Fig. 1.
FIG. 1.
Viral loads (HIV-1 RNA copies per ml of plasma) in study subject AC-06 during the 34-month study period are shown on a logarithmic scale. Shading represents periods on HAART. The number of regions targeted by CD8+ T cells in AC-06 is given below the graph.
A comprehensive characterization of HIV-1-specific CTL responses during the study period of 34 months following acute infection was performed for individual AC-06 by using an ELISPOT assay and a panel of 504 individual overlapping 15- to 18-mer peptides spanning the full-length sequences of HIV-1 clade B. During acute infection, virus-specific T-cell responses were narrowly directed against one peptide in p24 Gag (TACQGVGGPGHKARVL) and one peptide in gp41 Env (HIPRRIRQGLERALL). The virus-specific T-cell responses were enhanced and broadened during reexposure to antigen, such that at the end of the second STI, 34 months after acute infection, subject AC-06 had developed T-cell responses against a total of 26 regions of HIV-1 proteins, including 15 responses against structural proteins and 11 responses against regulatory and accessory proteins (Fig. 1 and 2). Except for HIV-1 Tat and Vpu, all HIV-1 proteins were targeted by virus-specific T cells in this individual. All responses were CD8+ T-cell mediated, as confirmed by CD4+/CD8+ T-cell depletion and enrichment studies, except for one CD4+ T-cell-mediated response to an overlapping peptide in HIV-1 integrase (TKELQKQITKIQNFRVYY). The magnitude of responses to the individual overlapping peptides ranged from 60 to 1,800 SFC/106 PBMC (median, 710 SFC/106 PBMC). These data show that HIV-1-specific CD8+ T-cell responses are narrowly directed during acute HIV-1 infection, despite exposure to enormous levels of virus in the plasma, but that these responses broaden during reexposure to lower subsequent levels of viral antigen, ultimately targeting a large variety of different HIV-1 proteins.
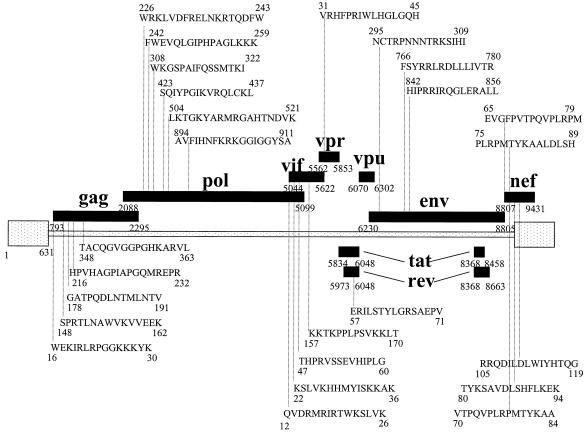
FIG. 2.
HIV-1-specific CD8+ T-cell responses to overlapping peptides in subject AC-06. The amino acid sequences and locations within HIV-1 proteins of all overlapping peptides targeted by virus-specific CD8+ T lymphocytes in subject AC-06 are shown. A total of 25 different regions within HIV-1 were targeted by CD8+ T cells.
Characterization of optimal CTL epitopes targeted by HIV-1-specific CD8+ T cells.
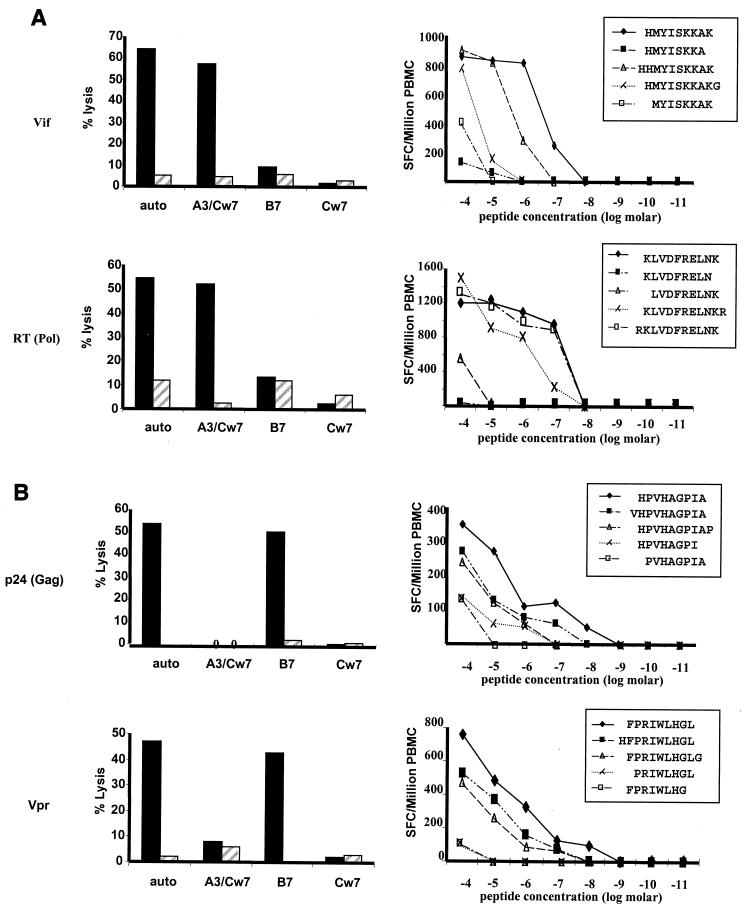
The data above describe the regions within HIV-1 targeted by CD8+ T cells in subject AC-06 but do not indicate the number of epitopes contained within each of the proteins (e.g., some overlapping peptides may contain the same minimal epitope in their overlap, or a single overlapping peptide may contain more than one epitope [5, 21]). In addition, they indicate recognition of a number of epitopes not previously described as presented by these HLA alleles. Optimal epitopes as well as restricting HLA alleles were therefore determined by an adaptation of previously described techniques. Following nonspecific polyclonal expansion of PBMC for 10 days using a bispecific anti-CD3/CD4b MAb, an adapted IFN-γ capture assay was performed to isolate peptide-specific CD8+ T-cell lines (3). By use of this approach, 13 different cytotoxic CD8+ T-cell lines specific for novel epitopes were isolated from PBMC. The HLA restriction of these CTL epitopes was determined by a chromium-51 release assay using partially HLA class I matched antigen-presenting cell lines (BCL). Subsequently, the optimal epitope sequences were determined by an ELISPOT assay using PBMC and serial dilutions of truncated peptides. This is exemplified for two novel HLA-A3-restricted and two novel HLA-B7-restricted epitopes in Fig. 3A and B, respectively. For one response to a subdominant HIV-1 Gag epitope, CD8+ T-cell frequencies were too low (80 SFC/106 PBMC) for isolation of peptide-specific lines by using the IFN-γ capture assay, and epitope-specific CD8+ T-cell lines could be isolated only by using standard limiting-dilution assays (data not shown). Overall, 10 novel HLA-A3-restricted and 3 novel HLA-B7-restricted optimal CTL epitopes were defined for subject AC-06, as well as the first HLA-Cw7-restricted CTL epitope described for HIV-1. This brought the total number of HLA-A3-restricted epitopes to 15, and the total number of HLA-B7-restricted epitopes to 11, in this individual (Table 1). The newly described epitopes included two epitopes which were previously reported to be restricted by HLA-A11, an allele closely related to HLA-A3 (39). The majority of the novel epitopes exactly fit the published HLA-A3, -B7, and -Cw7 peptide binding-motifs (Table 1). These results indicate a previously unexpected and unprecedented breadth of virus-specific CD8+ T-cell responses, including simultaneous recognition by CD8+ T cells of 15 HLA-A3-restricted, 11 HLA-B7-restricted, and 1 HLA-Cw7-restricted optimal HIV-1-specific CTL epitope.
FIG. 3.
Characterization of HLA-A3- and HLA-B7-restricted optimal HIV-1-specific CTL epitopes in subject AC-06. Graphs show the HLA class I restriction (left panels) and optimal amino acid sequences (right panels) of two novel HLA-A3-restricted CTL epitopes (A) and two novel HLA-B7-restricted CTL epitopes (B). HLA restriction was determined using peptides presented by autologous and partially HLA matched BCL in a 51Cr release assay. Solid bars, percent specific lysis of target cells pulsed with peptide; hatched bars, percent specific lysis of control target cells pulsed with no peptide. Fine mapping of the optimal epitope was done using serial dilutions of truncated peptides in an IFN-γ ELISPOT assay, and results are given as SFC per 106 PBMC.
TABLE 1.
Optimal CTL epitopes in AC-06
| Binding motif and HIV-1 protein | Optimal epitopea | HLA restriction | Designation |
|---|---|---|---|
| A3 binding motif | 2C terminus | ||
| LK | |||
| VY | |||
| MF | |||
| p17 Gag | KIRLRPGGK | A3 | A3-KK9 |
| p17 Gag | RLRPGGKKK | A3 | A3-RK9 |
| RT Pol | KLVDFRELNK | A3 | A3-KK10 |
| RT Pol | GIPHPAGLK | A3 | A3-GK9 |
| RT Pol | AIFQSSMTK | A3 | A3-ATK9 |
| RT Pol | QIYPGIKVR | A3 | A3-QR9 |
| RT Pol | RMRGAHTNDVK | A3 | A3-RK11 |
| Int Pol | AVFIHNFKRK | A3 | A3-AK10 |
| gp 41 Env | RLRDLLLIVTR | A3 | A3-RR11 |
| Nef | QVPLRPMTYK | A3 | A3-QK10 |
| Nef | AVDLSHFLK | A3 | A3-ALK9 |
| Rev | ERILSTYLGR | A3 | A3-ER10 |
| Vif | RIRTWKSLVK | A3 | A3-RK10 |
| Vif | HMYISKKAK | A3 | A3-HK9 |
| Vif | KTKPPLPSVKK | A3 | A3-KK11 |
| B7 binding motif | 123C terminus | ||
| PL | |||
| AR | |||
| RK | |||
| p24 Gag | SPRTLNAWV | B7 | B7-SV9 |
| p24 Gag | TPQDLNTML | B7 | B7-TL9 |
| p24 Gag | HPVHAGPIA | B7 | B7-HA9 |
| p24 Gag | GPGHKARVL | B7 | B7-GL9 |
| RT Pol | SPAIFQSSM | B7 | B7-SM9 |
| gp120 Env | RPNNNTRKSI | B7 | B7-RI10 |
| gp41 Env | IPRRIRQGL | B7 | B7-IL9 |
| Nef | TPQVPLRPM | B7 | B7-TM9 |
| Nef | RPMTYKAAL | B7 | B7-RL9 |
| Vpr | FPRIWLHGL | B7 | B7-FL9 |
| Vif | HPRVSSEVHI | B7 | B7-HI10 |
| Cw7 binding motif | 23456C terminus | ||
| YPDVVY | |||
| PGEYIF | |||
| RAVIL | |||
| Nef | RRQDILDLWIY | Cw7 | Cw7-RY11 |
Longitudinal evaluation of CTL responses.
Following the characterization of all optimal HIV-1-specific CTL epitopes targeted in subject AC-06, the evolution of HIV-1-specific CD8+ T-cell responses over time and the impact of reexposure to antigen on these responses were addressed. For this purpose, virus-specific CD8+ T-cell responses in this individual were determined at 11 different time points during the 34-month study period, starting from acute HIV-1-infection. This also allowed us to assess the hierarchy in the appearance of responses to CTL epitopes restricted by the same HLA class I allele over time.
During acute HIV-1 infection, CD8+ T-cell responses in subject AC-06 were directed only against two HLA-B7-restricted epitopes, in p24 Gag (GPGHKARVL) and gp41 Env (IPRRIRQGL) (Table 2 and Fig. 4), with no detectable involvement of HLA-A3-restricted epitopes resulting from this initial exposure to the virus. During the short first treatment interruption, reexposure to antigen following viral rebound (see Fig. 1) was accompanied by enhancement of the magnitude of preexisting CD8+ T-cell responses, as well as by induction of 12 novel responses to different epitopes (Table 2 and Fig. 4). These included six HLA-B7-restricted epitopes, as well as five HLA-A3-restricted epitopes and one HLA-Cw7-restricted epitope. The initial HLA-B7-restricted p24 Gag epitope GPGHKARVL remained the immunodominant epitope. The HLA-A3-restricted p17 Gag epitope RLRPGGKKK was targeted for the first time after the first treatment interruption and evolved to become the immunodominant HLA-A3-restricted epitope at 29.5 months, prior to reinitiation of antiretroviral treatment (Table 2). Total HIV-1-specific CD8+ T-cell responses were augmented from 980 SFC/106 PBMC prior to the first cycle of STI to 5,100 SFC/106 PBMC at the end of the first cycle (P < 0.01). These responses were further enhanced in magnitude and breadth during the second treatment interruption, augmenting the total HIV-1-specific CD8+ T-cell responses to optimal CTL epitopes from 7,750 SFC/106 PBMC prior to the beginning of the second cycle of STI to 28,300 SFC/106 PBMC at the end of the second cycle (P < 0.00001). This enhancement of responses was due to the augmentation of the magnitude of responses to previously targeted epitopes, as well as to development of a total of 9 newly detectable CD8+ T-cell responses to optimal epitopes during this second cycle, raising the total number of CTL epitopes targeted to 27 (Table 2 and Fig. 4).
TABLE 2.
Longitudinal evolution of the magnitude of HIV-1-specific CD8+ T-cell responses in subject AC-06 at the single-epitope level
| HIV-1 protein | Epitope | No. of SFC/106 PBMC at the following mo after infection:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 6 | 12 | 18 | 20 | 24 | 29.5 | 34 | ||
| Gag | A3-RK9 | 0 | 0 | 0 | 0 | 0 | 420 | 590 | 1,600 | 1,100 |
| A3-KK9 | 0 | 0 | 0 | 0 | 0 | 220 | 370 | 1,400 | 1,000 | |
| B7-SV9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 680 | 120 | |
| B7-TL9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 740 | 1,300 | |
| B7-HA9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 700 | 360 | |
| B7-GL9 | 320 | 380 | 680 | 870 | 900 | 1,000 | 1,200 | 1,300 | 1,800 | |
| Pol | A3-KK10 | 0 | 0 | 0 | 0 | 0 | 0 | 70 | 1,200 | 1,800 |
| A3-GK9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 240 | 1,800 | |
| B7-SM9 | 0 | 0 | 0 | 0 | 0 | 90 | 130 | 1,400 | 1,000 | |
| A3-ATK9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 290 | 420 | |
| A3-QR9 | 0 | 0 | 0 | 0 | 0 | 760 | 1,000 | 1,200 | 840 | |
| A3-RK11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 840 | 900 | |
| A3-AK10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 260 | 120 | |
| Env | B7-IL9 | 60 | 60 | 60 | 70 | 80 | 840 | 1,000 | 1,600 | 1,400 |
| A3-RR11 | 0 | 0 | 0 | 0 | 0 | 0 | 260 | 230 | 320 | |
| B7-RI10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 800 | |
| Accessory | B7-TM9 | 0 | 0 | 0 | 0 | 0 | 150 | 200 | 320 | 1,300 |
| A3-QK10 | 0 | 0 | 0 | 0 | 0 | 110 | 70 | 1,100 | 1,000 | |
| B7-RL9 | 0 | 0 | 0 | 0 | 0 | 120 | 180 | 600 | 360 | |
| A3-ALK9 | 0 | 0 | 0 | 0 | 0 | 450 | 350 | 1,200 | 1,100 | |
| Cw7-RY11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 720 | 1,000 | |
| B7-FL9 | 0 | 0 | 0 | 0 | 0 | 0 | 210 | 380 | 960 | |
| B7-HI10 | 0 | 0 | 0 | 0 | 0 | 500 | 880 | 1,800 | 1,800 | |
| A3-RK10 | 0 | 0 | 0 | 0 | 0 | 180 | 270 | 1,100 | 1,600 | |
| A3-HK9 | 0 | 0 | 0 | 0 | 0 | 110 | 320 | 1,000 | 1,600 | |
| A3-KK11 | 0 | 0 | 0 | 0 | 0 | 150 | 340 | 600 | 1,200 | |
| Regulatory | A3-ER10 | 0 | 0 | 0 | 0 | 0 | 0 | 310 | 460 | 1,300 |
| Total | 380 | 440 | 740 | 940 | 980 | 5,100 | 7,750 | 22,960 | 28,300 | |
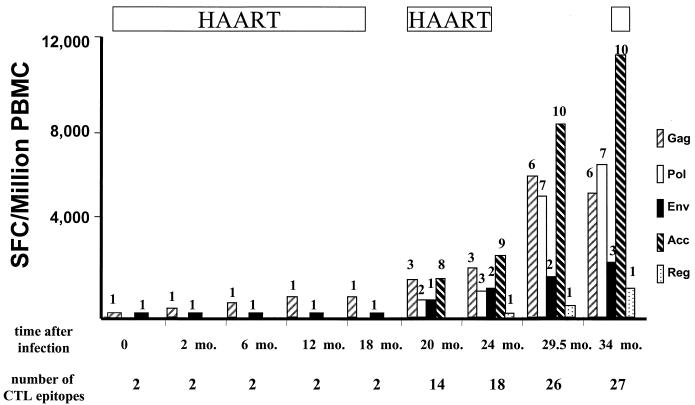
FIG. 4.
Longitudinal evolution of the magnitude and breadth of HIV-1-specific CD8+ T-cell responses on the single-epitope level during treated acute infection and STI in subject AC-06. The magnitudes of CD8+ T-cell responses (given as SFC/106 PBMC) directed against the HIV-1 proteins Gag, Pol, and Env, as well as against the accessory HIV-1 proteins (Vif, Vpr, and Vpu) and the regulatory HIV-1 proteins (Rev and Tat), in subject AC-06 during the 34-month study period are shown. The number of CTL epitopes targeted within each HIV-1 protein or protein group is indicated above each bar. The periods on treatment (HAART) are indicated above the graph; the time after infection and the total number of CTL epitopes targeted at each time point are shown below the graph. Only two CTL epitopes, one in HIV-1 Gag and one in HIV-1 Env, were targeted during acute infection, but virus-specific CD8+ T-cell responses targeted a total of 27 different CTL epitopes at the end of the second STI.
At the end of the second STI, CD8+ T-cell responses directed against epitopes within the accessory proteins (Vpr, Vif, and Nef) dominated total HIV-1-specific CD8+ T-cell responses (Table 2). Ten responses to different optimal epitopes in these proteins were defined, with a total magnitude of 11,920 SFC/106 PBMC (representing 42% of total HIV-1-specific CD8+ T-cell responses). CD8+ T-cell responses directed against optimal CTL epitopes within HIV-1 Gag and HIV-1 Pol were similar in both breadth and magnitude, with six responses to Gag (5,680 SFC/106 PBMC) and seven responses to Pol (6,880 SFC/106 PBMC). Only three responses to optimal CTL epitopes within HIV-1 Env (2,520 SFC/106 PBMC) and one response to HIV-1 Rev (1,300 SFC/106 PBMC) were detectable, respectively, and no CD8+ T-cell activity against the HIV-1 proteins Tat and Vpu was detectable.
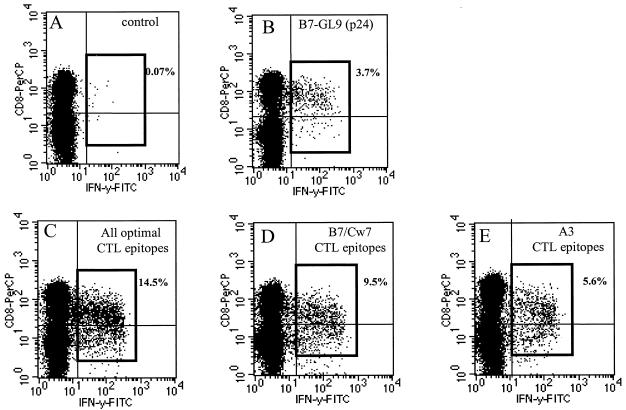
One limitation of the IFN-γ ELISPOT assay is a potential underestimation of the frequency of high-magnitude responses, due to the limited number of SFC per well that can be quantified. We therefore used intracellular IFN-γ quantification by flow cytometry to more accurately quantify the contributions of HLA-A3- and HLA-B7- or -Cw7-restricted CD8+ T-cell responses to total HIV-1-specific CD8+ T-cell responses. Autologous BCL were pulsed with pools of peptides containing either the 15 HLA-A3-restricted or the 12 HLA-B7- or -Cw7-restricted optimal HIV-1 CTL epitopes targeted by AC-06, or all 27 epitope peptides. A total of 14.5% of CD8+ T cells were specific for the tested epitopes at the end of the second treatment interruption (Fig. 5C). Virus-specific responses were dominated by HLA-B7- or -Cw7-restricted CD8+ T-cell responses (9.5%), which contributed more than 60% to total HIV-1-specific CD8+ T-cell response (Fig. 5D). The p24 Gag epitope GPGHKARVL, the first epitope targeted during acute infection, induced peptide-specific IFN-γ production by 3.7% of CD8+ T cells, contributing more than 24% to total virus-specific responses (Fig. 5B). These data demonstrate on the single-epitope level that HIV-1-specific CD8+ T-cell responses can be sequentially enhanced and broadened by reexposure to antigen and can ultimately target 15 different CTL epitopes (an unexpectedly high number) restricted by a single HLA class I allele in an infected individual homozygous for the HLA class I A, B, and Cw alleles. These data also indicate that the first HIV-1 epitope targeted during acute HIV-1 infection (GPGHKARVL) can remain the immunodominant epitope during the broadening of virus-specific CD8+ T-cell responses following STI.
FIG. 5.
Quantification of the contributions of HLA-A3-restricted and HLA-B7- or -Cw7-restricted CD8+ T-cell responses to total HIV-1-specific CD8+ T-cell responses in subject AC-06. PBMC of AC-06 were incubated with autologous BCL pulsed with either the HLA-B7-restricted immunodominant p24 Gag epitope GPGHKARVL (B), all 27 targeted optimal CTL epitopes (C), the 12 HLA-B7- or -Cw7-restricted optimal CTL epitopes (D), or the 15 HLA-A3-restricted optimal CTL epitopes (E) recognized by AC-06. BCL pulsed without peptide were used as negative controls (A). Percentages of peptide-specific IFN-γ-producing CD8+ T cells after subtraction of background activity are given in the individual plots.
Hierarchy of HLA-A3- and HLA-B7-restricted CD8+ T-cell responses during acute HIV-1 infection and after STI.
The studies of subject AC-06 demonstrate that only a limited number of HIV-1-specific CD8+ T-cell epitopes are detectable in acute infection and that additional responses appear following reexposure to viral antigen. We next studied whether the appearance and hierarchy of responses to CTL epitopes restricted by a particular HLA class I allele follow a common pattern in individuals expressing the same allele, by using a cohort of 17 additional individuals diagnosed and treated during acute HIV-1 infection and expressing either HLA-A3 (n = 7), HLA-B7 (n = 4), or both (n = 6). HIV-1-specific CD8+ T-cell responses directed against all optimal CTL epitopes described for the HLA class I types of the study subjects, including all newly defined HLA-A3 and -B7 CTL epitopes, were tested for these 18 individuals (including study subject AC-06) during acute infection, as well as after 1 year of treatment with HAART. Furthermore, in addition to subject AC-06, HIV-1-specific CD8+ T-cell responses in a subset of six additional individuals (three expressing A3 and three expressing A3 and B7) were studied after their reexposure to viral antigen during one cycle of STI.
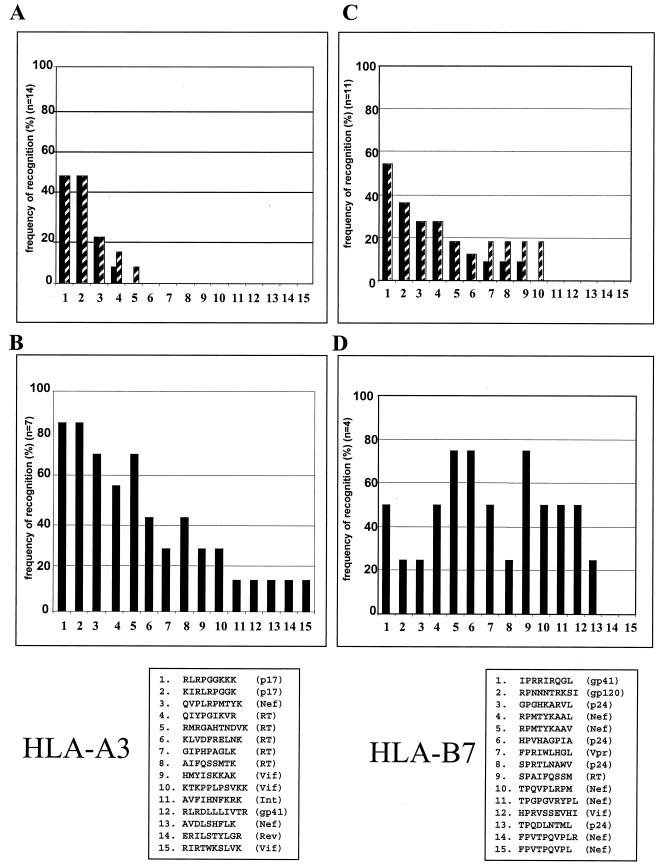
Eight out of the 14 individuals expressing HLA-A3 had detectable HIV-1-specific CD8+ T-cell responses restricted by HLA-A3 during acute infection. Only 5 out of 15 HLA-A3-restricted CTL epitopes tested were targeted following acute HIV-1 infection in these individuals (Fig. 6A). Of the HLA-A3-restricted epitopes, two within p17 Gag (p17-RK9 and p17-KK9) were targeted by 50% (7 of 14) of the individuals tested (Fig. 6A), representing 88% (7 of 8) of the individuals with detectable HLA-A3-restricted responses during acute infection. The p17 Gag epitope RLRPGGKKK was the immunodominant A3-restricted CTL response during acute infection. Both p17 Gag epitopes remained the most frequently targeted HLA-A3-restricted CTL epitopes after 1 year of treatment with HAART (Fig. 6A), as well as after reexposure following STI (Fig. 6B), for the seven individuals studied. In addition, for the four of seven individuals with no detectable HLA-A3-restricted responses during acute infection, the two p17 Gag epitopes RLRPGGKKK and KIRLRPGGK again represented the first HLA-A3 epitopes targeted (data not shown), as described for subject AC-06 above. Following reexposure to antigen, all 15 HLA-A3-restricted CTL epitopes were targeted by at least one individual. However, five epitopes were recognized only by subject AC-06 and not by the other individuals expressing HLA-A3 (Fig. 6B and Table 1).
FIG. 6.
Frequency of recognition of optimal CTL epitopes during acute HIV-1 infection and after STI. (A) Percentages of individuals expressing HLA-A3 (n = 14) who recognize the individual HLA-A3-restricted optimal CTL epitopes 1 to 15 during acute HIV-1 infection (solid bars) and after 12 months of treatment with HAART (hatched bars). (B) Percentages of individuals expressing HLA-A3 (n = 7) who recognize the individual HLA-A3-restricted optimal CTL epitopes 1 to 15 following STI. (C) Percentages of individuals expressing HLA-B7 (n = 11) who recognize the individual HLA-B7-restricted optimal CTL epitopes 1 to 15 during acute HIV-1 infection (solid bars) and after 12 months of treatment with HAART (hatched bars). (D) Percentages of individuals expressing HLA-B7 (n = 7) who recognize the individual HLA-B7-restricted optimal CTL epitopes 1 to 15 following STI. The amino acid sequences and corresponding HIV-1 proteins for the CTL epitopes tested are given at the bottom of the figure.
All 11 individuals expressing HLA-B7 had detectable HIV-1-specific CD8+ T-cell responses during acute HIV-1 infection. Among the HLA-B7-restricted epitopes, two (the gp41 epitope IPRRIRQGL and the gp120 epitope RPNNNTRKSI) were targeted by more than 50% (6 of 11) and 36% (4 of 11) of individuals with acute infection, respectively (Fig. 6C). However, B7-restricted responses were directed against a larger variety of different epitopes (10 of 15 tested epitopes) following acute infection. Following STI for 4 individuals expressing HLA-B7, the majority (13 of 15) of described HLA-B7 CTL epitopes were targeted by at least 1 individual (Fig. 6D).
It was demonstrated above for study subject AC-06 that the earliest targeted CTL epitope, B7-GPGHKARVL in p24 Gag, remained the immunodominant CTL epitope during the entire study period (Fig. 5). We extended these studies to the five of six individuals who had detectable CD8+ T-cell responses during acute infection and for whom follow-up samples during STI were available. In the sixth individual, no CD8+ T-cell responses against optimal CTL epitopes were detectable during acute infection. HIV-1-specific CD8+ T-cell responses broadened in all individuals following reexposure to antigen during STI. Nevertheless, in all five study subjects, the immunodominant CD8+ T-cell response during acute infection remained the dominant response at the last available time point following reexposure to antigen. In three of five individuals this response was restricted by HLA-A3; in the other two individuals it was restricted by HLA-B8 and HLA-B60, respectively (data not shown). In addition, the p17 Gag epitope RLRPGGKKK remained the immunodominant HLA-A3-restricted response in all five of six individuals targeting this epitope after treatment interruptions. Taken together, these data show that a subset of epitopes is preferentially targeted during acute HIV-1 infection and that these early targeted epitopes remain immunodominant during subsequent enhancement of HIV-1-specific CD8+ T-cell responses following reexposure to viral antigen.
DISCUSSION
Accumulating data indicate that the antiviral immune responses generated during acute infection may be crucial for initial control of viral replication and the outcome of chronic infection (2, 10-12, 24, 35). However, to date very little is known about the specificity of CTL epitopes targeted during acute HIV-1 infection or the evolution of these epitope-specific responses during later stages of infection. In this study, the hierarchy in the development of epitope-specific CD8+ T-cell responses restricted by selected HLA class I alleles and their immunodominance during acute HIV-1 infection was assessed. The evolution of these epitope-specific CD8+ T-cell responses was also monitored longitudinally during STI. Our data suggest that for selected HLA alleles the hierarchy in immunodominance of epitope-specific CD8+ T-cell responses is determined early during acute infection and that this hierarchy is little modified during the subsequent broadening of responses following reexposure to antigen. These early treatment studies also show that the initial high-level viremia does not induce detectable broadly directed responses, but that these become detectable following repeated exposure to much lower levels of viral antigen, ultimately targeting an unprecedented number of HIV-1-specific CTL epitopes restricted by a single HLA class I allele.
Recent technical advances in the assessment of antigen-specific CD8+ T-cell responses, including the IFN-γ ELISPOT assay and flow cytometry-based assays, have allowed for much more rapid and detailed characterization of HIV-1-specific CD8+ T-cell responses in infected individuals. In this study we used these sensitive techniques to comprehensively assess HIV-1-specific CD8+ T-cell responses in an infected individual identified during acute infection, using a panel of 504 overlapping peptides spanning the full-length sequence of HIV-1. The regions within HIV-1 recognized by CD8+ T cells were determined, and all optimal CTL epitopes targeted, as well as their restricting HLA class I alleles, were characterized. Longitudinal studies demonstrated that only two CTL epitopes were targeted by HIV-1-specific CD8+ T cells during acute infection but that CD8+ T-cell responses broadened significantly during subsequent exposure to antigen. Following two cycles of STI, 27 distinct CTL epitopes were ultimately recognized by virus-specific CD8+ T cells of the study subject, including 14 novel epitopes (10 of which are restricted by HLA-A3) described for the first time in this report. All novel HLA-A3-, HLA-B7-, and HLA-Cw7-restricted CTL epitopes fit the peptide-binding motifs described for the corresponding HLA class I allele very well (13, 33). The large number of reported novel HLA-A3-restricted epitopes is impressive, taking into account that only eight CTL epitopes restricted by HLA-A3 had previously been described in the literature (13). These findings are in line with previous studies showing the enhancement of virus-specific CD8+ T-cell responses in infected individuals during reexposure to viral antigen (6, 16, 30, 31, 35, 37; J. Lisziewicz, E. Rosenberg, J. Lieberman, H. Jessen, L. Lopalco, R. Siliciano, B. Walker, and F. Lori, Letter, N. Engl. J. Med. 340:1683-1684, 1999). They also emphasize the importance of a comprehensive approach assessing CD8+ T cells directed against full-length HIV-1 in order to determine the entire breadth and magnitude of virus-specific immune responses.
These data indicate that numerous epitopes can be presented simultaneously by a single HLA class I allele. Fifteen distinct CTL epitopes were restricted by HLA-A3 in one individual, including two epitopes previously reported as restricted by HLA-A11, a closely related HLA class I allele belonging to the HLA-A3-superfamily (39, 43). In addition, 11 distinct epitopes were restricted by HLA-B7 and one by HLA-Cw7. Several factors may have contributed to this unprecedented breadth of detectable CTL responses. To our knowledge, this individual represented the first HIV-1-infected person for whom HIV-1-specific CD8+ T-cell responses were comprehensively assessed on the single-epitope level, including responses directed against all HIV-1 proteins. In addition, the study subject was homozygous for the HLA class I A, B, and Cw locus. Therefore, presentation of viral antigen was necessarily limited to these three HLA class I alleles. In contrast, in heterozygous individuals expressing six different major HLA class I alleles, a broader variety of differently restricted epitopes might be presented. Furthermore, different HLA class I molecules may compete with one another for binding and presentation of peptide epitopes (29). However, data from the SIV/macaque model have shown that an animal can simultaneously recognize as many as 15 different epitopes restricted by a single major histocompatibility complex (MHC) class I molecule (Mamu-A∗01) (1), and preliminary data on additional HIV-1-infected individuals screened comprehensively for all expressed HIV-1 proteins by using overlapping peptides demonstrate that as many as 42 different regions within the virus can be targeted in a single infected individual heterozygous for all three major HLA class I alleles (M. M. Addo, unpublished data). However, these studies may still underestimate the real breadth of responses, since virus-specific CD8+ T-cell responses were assessed by using HIV-1 clade B consensus sequences and not autologous virus sequences (34, 42, 47). Taken together, these data suggest that HIV-1-specific CD8+ T-cell responses may be much more broadly directed in infected individuals than has been expected to date.
During acute HIV-1 infection, only two CTL epitopes were targeted by the subject studied in most detail (AC-06), including the immunodominant HLA-B7-restricted epitope GPGHKARVL in p24 Gag. Interestingly, this earliest targeted CTL epitope remained the immunodominant response, even as virus-specific CD8+ T-cell responses broadened significantly to a total of 27 epitopes targeted. We extended these studies to another 17 individuals with acute HIV-1 infection expressing HLA-A3 and/or HLA-B7. For HLA-A3, an immunodominant CTL epitope within HIV-1 p17 Gag was identified (RLRPGGKKK). This epitope was targeted during acute infection by 88% of HLA-A3-expressing individuals with detectable CD8+ T-cell responses restricted by this allele. In addition, RLRPGGKKK also represented the first HLA-A3-restricted epitope targeted during reexposure to the virus in those individuals who lacked detectable HLA-A3-restricted CD8+ T-cell responses during acute infection. In contrast, HLA-B7-restricted CD8+ T-cell responses were directed against a larger variety of different epitopes, including the early-targeted p24 Gag epitope GPGHKARVL described for AC-06, but also nine additional epitopes within different HIV-1 proteins. Differences in the CTL epitopes restricted by HLA-A2 have also been noted in acute and chronic HIV-1 infection (18). These data indicate that immunodominant viral epitopes consistently targeted during acute HIV-1 infection can be identified for certain HLA class I alleles but that differences in the pattern of development of epitope-specific CTL responses exist among different HLA class I alleles in HIV-1 infection.
Following reexposure to antigen during treatment interruptions, CD8+ T-cell responses to novel epitopes became detectable in all individuals. These responses might represent either newly primed specificities or preexisting low-frequency responses that were boosted to detectable levels. However, the immunodominant CD8+ T-cell response identified during acute infection remained immunodominant after treatment interruptions in all individuals studied. The studies of these additional individuals with acute HIV-1 infection were limited by the use of described optimal CTL epitopes, due to limited numbers of available samples, and responses to additional epitopes may have been missed. Nevertheless, these data demonstrate a consistent pattern in the development and immunodominance of epitope-specific CD8+ T-cell responses and suggest that the immunodominance and hierarchy of virus-specific CD8+ T-cell responses restricted by HLA-A3 are determined early in HIV-1 infection and persist during reexposure to viral antigen later in infection. This early determination of epitope hierarchy during acute HIV-1 infection is in line with recent observations in animal models suggesting that the majority of antigen-specific CD8+ T-cell responses are primed during the initial phase of infection (23, 27, 44).
Interestingly, the observed immunodominance of HLA-A3-restricted HIV-1 Gag (p17) epitopes in both acute and chronic infection is very similar to data from the SIV/macaque model showing that the early-targeted SIV Gag CM9 epitope persists as the immunodominant epitope during chronic SIV infection (2, 10, 28) and that the virus mutates to escape this response only late in infection (9). However, other immunodominant epitopes targeted during acute infection have been shown to escape earlier, and responses to these epitopes subsequently decrease (2). The early initiation of antiretroviral treatment for the study subjects may have modified the natural course of infection and prevented early viral escape, resulting in the persistence of immunodominance and hierarchy of virus-specific CD8+ T-cell responses. Further studies during both acute infection and STI, including analysis of responses directed against the autologous virus, are needed in order to better understand the interaction between HIV-1-specific CD8+ T-cell responses and viral escape from CTL-mediated immune pressure.
In conclusion, this study represents the first comprehensive characterization of HIV-1-specific CD8+ T-cell responses in an acutely infected individual, assessing responses directed against all expressed HIV-1 proteins. It demonstrates that detectable virus-specific CD8+ T-cell responses are narrowly directed against a limited number of CTL epitopes during acute HIV-1 infection, despite exposure to enormous levels of viral antigen. These responses can be sequentially enhanced in magnitude and breadth during reexposure to viral antigen, reaching a previously unprecedented number of targeted optimal CTL epitopes. The temporal hierarchy in the development of epitope-specific responses restricted by HLA-A3 and their immunodominance during acute infection and subsequent treatment interruptions followed similar patterns in the individuals studied. These data indicate consistent early targeting of specific immunodominant epitopes, which may be important for the design of a highly immunogenic HIV-1 vaccine.
Acknowledgments
This study was supported by the Doris Duke Charitable Foundation (M.A., E.S.R., and B.D.W.), the National Institutes of Health (R37 AI128568, R01 AI50429, R01 AI30914, R01 AI44656, and R01 AI40873), the Foundation for AIDS & Immune Research (M.A.), the German Research Council (DFG, Emmy-Noether grant AD-171) (M.M.A.), the American Foundation for AIDS Research (M.M.A.), and the Partners/Fenway/Shattuck Center for AIDS Research (X.G.Y.). B.D.W. is the recipient of a Doris Duke Distinguished Clinical Scientist Award.
REFERENCES
- 1.Allen, T. M., B. R. Mothe, J. Sidney, P. Jing, J. L. Dzuris, M. E. Liebl, T. U. Vogel, D. H. O'Connor, X. Wang, M. C. Wussow, J. A. Thomson, J. D. Altman, D. I. Watkins, and A. Sette. 2001. CD8+ lymphocytes from simian immunodeficiency virus-infected rhesus macaques recognize 14 different epitopes bound by the major histocompatibility complex class I molecule Mamu-A∗01: implications for vaccine design and testing. J. Virol. 75:738-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386-390. [DOI] [PubMed] [Google Scholar]
- 3.Altfeld, M., M. M. Addo, R. L. Eldridge, X. G. Yu, S. Thomas, A. Khatri, D. Strick, M. N. Phillips, G. B. Cohen, S. A. Islam, S. A. Kalams, C. Brander, P. J. R. Goulder, and B. D. Walker. 2001. Vpr is preferentially targeted by cytotoxic T lymphocytes during HIV-1 infection. J. Immunol. 167:2743-2752.. [DOI] [PubMed] [Google Scholar]
- 4.Altfeld, M., and E. S. Rosenberg. 2000. The role of CD4+ T helper cells in the cytotoxic T lymphocyte response to HIV-1. Curr. Opin. Immunol. 12:375-380. [DOI] [PubMed] [Google Scholar]
- 5.Altfeld, M., E. S. Rosenberg, R. Shankarappa, J. S. Mukherjee, F. M. Hecht, R. L. Eldridge, M. M. Addo, S. H. Poon, M. N. Phillips, G. K. Robbins, P. E. Sax, S. Boswell, J. O. Kahn, C. Brander, P. J. Goulder, J. A. Levy, J. I. Mullins, and B. D. Walker. 2001. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J. Exp. Med. 193:169-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altfeld, M., and B. D. Walker. 2001. Less is more? STI in acute and chronic HIV-1 infection. Nat. Med. 7:881-884. [DOI] [PubMed] [Google Scholar]
- 7.Altfeld, M. A., A. Trocha, R. L. Eldridge, E. S. Rosenberg, M. N. Phillips, M. M. Addo, R. P. Sekaly, S. A. Kalams, S. A. Burchett, K. McIntosh, B. D. Walker, and P. J. Goulder. 2000. Identification of dominant optimal HLA-B60- and HLA-B61-restricted cytotoxic T-lymphocyte (CTL) epitopes: rapid characterization of CTL responses by enzyme-linked immunospot assay. J. Virol. 74:8541-8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 9.Barouch, D. H., J. Kunstman, M. J. Kuroda, J. E. Schmitz, S. Santra, F. W. Peyerl, G. R. Krivulka, K. Beaudry, M. A. Lifton, D. A. Gorgone, D. C. Montefiori, M. G. Lewis, S. M. Wolinsky, and N. L. Letvin. 2002. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature 415:335-339. [DOI] [PubMed] [Google Scholar]
- 10.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, and M. G. Lewis. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 11.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borrow, P., H. Lewicki, X. Wei, M. S. Horwitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3:205-211. [DOI] [PubMed] [Google Scholar]
- 13.Brander, C., and P. Goulder. 2000. The evolving field of HIV CTL epitope mapping: new approaches for the identification of novel epitopes, p. I-1-I-20. In B. T. M. Korber, C. Brander, B. D. Walker, R. A. Koup, J. Moore, B. Haynes, and G. Meyer (ed.), HIV molecular database. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 14.Bucy, R. P., and J. M. Kilby. 2001. Perspectives on inducing efficient immune control of HIV-1 replication—a new goal for HIV therapeutics? AIDS 15(Suppl. 2):S36-S42. [DOI] [PubMed] [Google Scholar]
- 15.Bunce, M., G. C. Fanning, and K. I. Welsh. 1995. Comprehensive, serologically equivalent DNA typing for HLA-B by PCR using sequence-specific primers (PCR-SSP). Tissue Antigens 45:81-90. [DOI] [PubMed] [Google Scholar]
- 16.Garcia, F., M. Plana, G. M. Ortiz, S. Bonhoeffer, A. Soriano, C. Vidal, A. Cruceta, M. Arnedo, C. Gil, G. Pantaleo, T. Pumarola, T. Gallart, D. F. Nixon, J. M. Miro, and J. M. Gatell. 2001. The virological and immunological consequences of structured treatment interruptions in chronic HIV-1 infection. AIDS 15:F29-F40. [DOI] [PubMed] [Google Scholar]
- 17.Goulder, P. J., M. M. Addo, M. A. Altfeld, E. S. Rosenberg, Y. Tang, U. Govender, N. Mngqundaniso, K. Annamalai, T. U. Vogel, M. Hammond, M. Bunce, H. M. Coovadia, and B. D. Walker. 2001. Rapid definition of five novel HLA-A∗3002-restricted human immunodeficiency virus-specific cytotoxic T-lymphocyte epitopes by Elispot and intracellular cytokine staining assays. J. Virol. 75:1339-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goulder, P. J., M. A. Altfeld, E. S. Rosenberg, T. Nguyen, Y. Tang, R. L. Eldridge, M. M. Addo, S. He, J. S. Mukherjee, M. N. Phillips, M. Bunce, S. A. Kalams, R. P. Sekaly, B. D. Walker, and C. Brander. 2001. Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection. J. Exp. Med. 193:181-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goulder, P. J., S. L. Rowland-Jones, A. J. McMichael, and B. D. Walker. 1999. Anti-HIV cellular immunity: recent advances towards vaccine design. AIDS 13:S121-S136. [PubMed] [Google Scholar]
- 20.Goulder, P. J., Y. Tang, C. Brander, M. R. Betts, M. Altfeld, K. Annamalai, A. Trocha, S. He, E. S. Rosenberg, G. Ogg, C. A. O'Callaghan, S. A. Kalams, R. E. McKinney, Jr., K. Mayer, R. A. Koup, S. I. Pelton, S. K. Burchett, K. McIntosh, and B. D. Walker. 2000. Functionally inert HIV-specific cytotoxic T lymphocytes do not play a major role in chronically infected adults and children. J. Exp. Med. 192:1819-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goulder, P. J., Y. Tang, S. I. Pelton, and B. D. Walker. 2000. HLA-B57-restricted cytotoxic T-lymphocyte activity in a single infected subject toward two optimal epitopes, one of which is entirely contained within the other. J. Virol. 74:5291-5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaech, S. M., and R. Ahmed. 2001. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2:415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMichael, A. J., G. Ogg, J. Wilson, M. Callan, S. Hambleton, V. Appay, T. Kelleher, and S. Rowland-Jones. 2000. Memory CD8+ T cells in HIV infection. Philos. Trans. R. Soc. Lond. B 355:363-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMichael, A. J., and S. L. Rowland-Jones. 2001. Cellular immune responses to HIV. Nature 410:980-987. [DOI] [PubMed] [Google Scholar]
- 27.Mercado, R., S. Vijh, S. E. Allen, K. Kerksiek, I. M. Pilip, and E. G. Pamer. 2000. Early programming of T cell populations responding to bacterial infection. J. Immunol. 165:6833-6839. [DOI] [PubMed] [Google Scholar]
- 28.Miller, M. D., H. Yamamoto, A. L. Hughes, D. I. Watkins, and N. L. Letvin. 1991. Definition of an epitope and MHC class I molecule recognized by gag-specific cytotoxic T lymphocytes in SIVmac-infected rhesus monkeys. J. Immunol. 147:320-329. [PubMed] [Google Scholar]
- 29.Nowak, M. A., R. M. May, R. E. Phillips, S. Rowland-Jones, D. G. Lalloo, S. McAdam, P. Klenerman, B. Koppe, K. Sigmund, C. R. Bangham, et al. 1995. Antigenic oscillations and shifting immunodominance in HIV-1 infections. Nature 375:606-611. [DOI] [PubMed] [Google Scholar]
- 30.Ortiz, G. M., D. F. Nixon, A. Trkola, J. Binley, X. Jin, S. Bonhoeffer, P. J. Kuebler, S. M. Donahoe, M. A. Demoitie, W. M. Kakimoto, T. Ketas, B. Clas, J. J. Heymann, L. Zhang, Y. Cao, A. Hurley, J. P. Moore, D. D. Ho, and M. Markowitz. 1999. HIV-1-specific immune responses in subjects who temporarily contain virus replication after discontinuation of highly active antiretroviral therapy. J. Clin. Investig. 104:R13-R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ortiz, G. M., M. Wellons, J. Brancato, H. T. Vo, R. L. Zinn, D. E. Clarkson, K. Van Loon, S. Bonhoeffer, G. D. Miralles, D. Montefiori, J. A. Bartlett, and D. F. Nixon. 2001. Structured antiretroviral treatment interruptions in chronically HIV-1-infected subjects. Proc. Natl. Acad. Sci. USA 98:13288-13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitcher, C. J., C. Quittner, D. M. Peterson, M. Connors, R. A. Koup, V. C. Maino, and L. J. Picker. 1999. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 5:518-525. [DOI] [PubMed] [Google Scholar]
- 33.Rammensee, H. G., T. Friede, and S. Stevanoviic. 1995. MHC ligands and peptide motifs: first listing. Immunogenetics 41:178-228. [DOI] [PubMed] [Google Scholar]
- 34.Ray, S. C., N. Lubaki, B. R. Dhruva, R. F. Siliciano, and R. C. Bollinger. 1998. Autologous strain-specific cytolytic T lymphocyte responses directed against human immunodeficiency virus type 1 Env. AIDS Res. Hum. Retrovir. 14:3-13. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg, E. S., M. Altfeld, S. H. Poon, M. N. Phillips, B. M. Wilkes, R. L. Eldridge, G. K. Robbins, R. T. D'Aquila, P. J. Goulder, and B. D. Walker. 2000. Immune control of HIV-1 after early treatment of acute infection. Nature 407:523-526. [DOI] [PubMed] [Google Scholar]
- 36.Rowland-Jones, S., S. Pinheiro, and R. Kaul. 2001. New insights into host factors in HIV-1 pathogenesis. Cell 104:473-476. [DOI] [PubMed] [Google Scholar]
- 37.Ruiz, L., G. Carcelain, J. Martinez-Picado, S. Frost, S. Marfil, R. Paredes, J. Romeu, E. Ferrer, K. Morales-Lopetegi, B. Autran, and B. Clotet. 2001. HIV dynamics and T-cell immunity after three structured treatment interruptions in chronic HIV-1 infection. AIDS 15:F19-F27. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 39.Sette, A., and J. Sidney. 1998. HLA supertypes and supermotifs: a functional perspective on HLA polymorphism. Curr. Opin. Immunol. 10:478-482. [DOI] [PubMed] [Google Scholar]
- 40.Sewell, A. K., D. A. Price, A. Oxenius, A. D. Kelleher, and R. E. Phillips. 2000. Cytotoxic T lymphocyte responses to human immunodeficiency virus: control and escape. Stem Cells 18:230-244. [DOI] [PubMed] [Google Scholar]
- 41.Shiver, J. W., T. M. Fu, L. Chen, D. R. Casimiro, M. E. Davies, R. K. Evans, Z. Q. Zhang, A. J. Simon, W. L. Trigona, S. A. Dubey, L. Huang, V. A. Harris, R. S. Long, X. Liang, L. Handt, W. A. Schleif, L. Zhu, D. C. Freed, N. V. Persaud, L. Guan, K. S. Punt, A. Tang, M. Chen, K. A. Wilson, K. B. Collins, G. J. Heidecker, V. R. Fernandez, H. C. Perry, J. G. Joyce, K. M. Grimm, J. C. Cook, P. M. Keller, D. S. Kresock, H. Mach, R. D. Troutman, L. A. Isopi, D. M. Williams, Z. Xu, K. E. Bohannon, D. B. Volkin, D. C. Montefiori, A. Miura, G. R. Krivulka, M. A. Lifton, M. J. Kuroda, J. E. Schmitz, N. L. Letvin, M. J. Caulfield, A. J. Bett, R. Youil, D. C. Kaslow, and E. A. Emini. 2002. Replication-incompetent adenoviral vaccine vector elicits effective anti-immunodeficiency-virus immunity. Nature 415:331-335. [DOI] [PubMed] [Google Scholar]
- 42.Sipsas, N. V., S. A. Kalams, A. Trocha, S. He, W. A. Blattner, B. D. Walker, and R. P. Johnson. 1997. Identification of type-specific cytotoxic T lymphocyte responses to homologous viral proteins in laboratory workers accidentally infected with HIV-1. J. Clin. Investig. 99:752-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Threlkeld, S. C., P. A. Wentworth, S. A. Kalams, B. M. Wilkes, D. J. Ruhl, E. Keogh, J. Sidney, S. Southwood, B. D. Walker, and A. Sette. 1997. Degenerate and promiscuous recognition by CTL of peptides presented by the MHC class I A3-like superfamily: implications for vaccine development. J. Immunol. 159:1648-1657. [PubMed] [Google Scholar]
- 44.van Stipdonk, M. J., E. E. Lemmens, and S. P. Schoenberger. 2001. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2:423-429. [DOI] [PubMed] [Google Scholar]
- 45.Walker, B. D., S. Chakrabarti, B. Moss, T. J. Paradis, T. Flynn, A. G. Durno, R. S. Blumberg, J. C. Kaplan, M. S. Hirsch, and R. T. Schooley. 1987. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature 328:345-348. [DOI] [PubMed] [Google Scholar]
- 46.Walker, B. D., C. Flexner, K. Birch-Limberger, L. Fisher, T. J. Paradis, A. Aldovini, R. Young, B. Moss, and R. T. Schooley. 1989. Long-term culture and fine specificity of human cytotoxic T-lymphocyte clones reactive with human immunodeficiency virus type 1. Proc. Natl. Acad. Sci. USA 86:9514-9518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson, C. C., S. A. Kalams, B. M. Wilkes, D. J. Ruhl, F. Gao, B. H. Hahn, I. C. Hanson, K. Luzuriaga, S. Wolinsky, R. Koup, S. P. Buchbinder, R. P. Johnson, and B. D. Walker. 1997. Overlapping epitopes in human immunodeficiency virus type 1 gp120 presented by HLA A, B, and C molecules: effects of viral variation on cytotoxic T-lymphocyte recognition. J. Virol. 71:1256-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong, D. K., D. D. Dudley, N. H. Afdhal, J. Dienstag, C. M. Rice, L. Wang, M. Houghton, B. D. Walker, and M. J. Koziel. 1998. Liver-derived CTL in hepatitis C virus infection: breadth and specificity of responses in a cohort of persons with chronic infection. J. Immunol. 160:1479-1488. [PubMed] [Google Scholar]