Abstract
The UL84 open reading frame encodes a protein that is required for origin-dependent DNA replication and interacts with the immediate-early protein IE2 in lytically infected cells. Transfection of UL84 expression constructs showed that UL84 localized to the nucleus of transfected cells in the absence of any other viral proteins and displayed a punctate speckled fluorescent staining pattern. Cotransfection of all the human cytomegalovirus replication proteins and oriLyt, along with pUL84-EGFP, showed that UL84 colocalized with UL44 (polymerase accessory protein) in replication compartments. Experiments using infected human fibroblasts demonstrated that UL84 also colocalized with UL44 and IE2 in viral replication compartments in infected cells. A nuclear localization signal was identified using plasmid constructs expressing truncation mutants of the UL84 protein in transient transfection assays. Transfection assays showed that UL84 failed to localize to the nucleus when 200 amino acids of the N terminus were deleted. Inspection of the UL84 amino acid sequence revealed a consensus putative nuclear localization signal between amino acids 160 and 171 (PEKKKEKQEKK) of the UL84 protein.
Transient transfection/infection assays identified one origin of lytic-phase DNA replication (oriLyt) for human cytomegalovirus (HCMV) (6, 24). HCMV oriLyt is a complex replicator consisting of multiple repeat regions and RNA-DNA hybrid structures that play an undetermined role in DNA replication (5, 31). Identification of the HCMV lytic origin paved the way for the development of a transient cotransfection replication assay, similar to the one used for herpes simplex virus type 1 (HSV-1) (9, 40). For HCMV, the transient cotransfection replication assay identified 11 distinct viral loci encoding trans-acting factors required for HCMV oriLyt-dependent DNA replication in human fibroblasts (28, 29). In addition to the six replication fork homologs common to all herpesviruses, four other loci were identified in the transient replication assay encoding proteins having unknown functions in DNA synthesis (28, 29). In human fibroblasts, as determined by the original cotransfection replication assay, these four additional HCMV-encoded loci (UL112-113, UL36-38, IE1 and IE2, and UL84) had not previously been implicated as having a role in DNA replication (14, 17, 34, 35). The gene products of UL36-38 were previously identified as four separate proteins, some of which act as transactivators, and are present at very early times in infection (4, 10). The UL112-113 locus encodes early kinetic class proteins that have been shown to be part of intranuclear replication compartments (3, 39). The immediate-early proteins IE1 and IE2 are well characterized as transactivators responsible for the up-regulation of viral and cellular transcription (1, 2, 23, 25, 26). The function of the UL84 gene product in viral growth or in DNA replication is an enigma.
UL84 is categorized as a “very early” gene in that mRNA can be detected as early as 2.5 h postinfection (p.i.) (18). It was shown previously that UL84 is necessary for origin-dependent DNA replication and also appears to inhibit viral infection in U373 cells when overexpressed (13, 34). These seemingly contradictory functions suggest that the UL84 protein may have both regulatory and replication functions.
An adaptation of the original cotransfection assay, using strong constitutive promoters to express HCMV replication proteins, identified different requirements for HCMV origin-dependent replication depending on the cell type used (34). Although the required factors were cell type dependent, it was clear that one protein, the gene product of UL84, was absolutely necessary in the replication assay (34). It was also demonstrated that UL84 facilitated the formation of replication compartments in cells cotransfected with HCMV plasmids encoding replication proteins and oriLyt. Replication compartments are large globular amorphous structures made up of factors involved in viral DNA replication (11, 12, 15, 22, 32, 38). It was demonstrated that UL84 was involved in the formation of replication compartments; however, it was not shown that UL84 itself was a component of these replication compartments. Therefore, it is important to determine if UL84 is part of the replication machinery. It was previously demonstrated that the protein product of UL84, pUL84, associates with the immediate-early protein IE2 (IE-580aa, IE86) in infected cells (36). Although the true nature of this association is unknown, it was postulated that this interaction with IE2 may allow for efficient targeting of UL84 to the nucleus (36).
In the study presented here, in an effort to characterize the role of UL84 in lytic replication, we investigated the subcellular localization of the UL84 protein in transfected and infected cells. We demonstrate for the first time that (i) UL84 is capable of nuclear localization in the absence of any additional viral proteins, (ii) UL84 protein colocalized with UL44 and is part of higher-order structures (replication compartments) in infected and cotransfected cells, and (iii) a nuclear localization sequence is located within the UL84 protein and is sufficient for efficient nuclear targeting.
The UL84-EGFP fusion protein complements oriLyt-dependent DNA replication.
The UL84 open reading frame (ORF) encodes a 586-amino-acid protein with a calculated minimal molecular mass of 65 kDa without any modifications. Previous studies identified an infected cellular protein ranging in mass from 65 to 95 kDa, with the predominate protein species running at about the 80-kDa molecular size marker (18). In order to investigate the subcellular localization of UL84 protein, we constructed several expression constructs allowing us to detect the UL84 protein in infected and transfected cells. Although an antibody reacting to the UL84 protein was generated previously (18), we found that it was unsuitable for our cellular localization studies because of very high nonspecific background in infected and uninfected cells (Y. Xu and G. S. Pari, unpublished data). Consequently, we fused the UL84 ORF in frame with enhanced green fluorescence protein (EGFP) to generate pUL84-EGFP. This construct allowed for the visualization of the UL84 protein in infected and transfected cells. In addition, the UL84-EGFP fusion protein could be detected on a Western blot using an anti-EGFP antibody (data not shown). We performed the cotransfection replication assay substituting pUL84-EGFP for a construct encoding wild-type UL84 protein. Cellular DNA was harvested, cleaved with DpnI and EcoRI, and separated on an agarose gel, and Southern blots were performed using pGEM as a probe to detect replication products. The UL84-EGFP fusion construct was capable of complementing oriLyt-dependent DNA replication, indicating that this plasmid and wild-type UL84 functioned in an equivalent manner in the replication assay, therefore enabling us to use this construct for cellular localization studies (Fig. 1A, lane 1).
FIG. 1.
Functional analysis of the pUL84-EGFP expression construct and subcellular localization of UL84-EGFP in transiently transfected cells. Cells were seeded in 12-well plates on glass coverslips at a density of 2 × 105 cells per well 24 h prior to transfection. Transfections were performed using Lipofectamine 2000 reagent (Life Technologies) as recommended by the manufacturer or by the calcium phosphate coprecipitation method as described previously (29). Fluorescence microscopy was carried out at 48 h p.i. For the replication assays, plasmids encoding HCMV replication proteins (UL36-38, UL44, UL70, UL54, UL57, UL102, UL105, UL112/113, UL84, IE1/IE2, and IRS1) and oriLyt were used. (A) The UL84-EGFP fusion protein complements transient oriLyt-dependent DNA replication. Autoradiogram of a Southern blot of total cellular DNA from HFF cells cleaved with EcoRI and DpnI and hybridized with pGEM7zf(-) is shown. Lanes: 1, DNA from HFFs cotransfected with plasmids encoding HCMV replication proteins, plus oriLyt, where pUL84-EGFP was substituted for a wild-type UL84 plasmid construct; 2, DNA from HFFs cotransfected with all the wild-type HCMV replication plasmids plus oriLyt; 3, DNA from HFFs where the plasmid encoding UL84 was omitted from the cotransfection mixture. (B) COS7 cells were transfected with pUL84-EGFP or pEGFP-C1 and examined by epifluorescence microscopy. Left panel, pUL84-EGFP; Right panel, pEGFP-C1 (control).
UL84 localizes to the cell nucleus in the absence of any additional viral factors.
The pUL84-EGFP expression construct was transfected into COS7 cells, and the subcellular localization pattern of the fusion protein was visualized using the fluorescence microscope. When pUL84-EGFP was transfected alone, UL84 localized within the nucleus and a punctate speckled fluorescent pattern was observed (Fig. 1B, left panel). Transfection of the parent vector, pEGFP-C1, resulted in a diffuse fluorescent pattern distributed throughout the cell (Fig. 1B, right panel).
UL84 colocalizes with UL44 and is a component of DNA replication compartments in cotransfected and infected cells.
We also investigated if the fluorescent pattern of UL84 was influenced by the addition of the HCMV replication machinery. To this end, we cotransfected COS7 cells with pUL84-EGFP along with the other replication proteins identified from the original cotransfection replication assay and oriLyt (29). The UL84 fluorescent pattern was organized in globular structures within the nucleus of the cotransfected cell (Fig. 2A, panel 1). UL44, the polymerase accessory protein, known to be a component of replication compartments, showed the same fluorescent pattern as UL84 when reacted with a UL44-specific antibody (catalog no. 13-131-100; Advanced Biotechnologies) and secondary antibody conjugated with rhodamine (Fig. 2A, panel 2), and it colocalized with UL84 (Fig. 2A, panel 3). Omission of either IE1/IE2 or oriLyt from the cotransfection mixture resulted in the abrogation of the formation of replication compartments (data not shown). These experiments demonstrated that the UL84 protein is targeted to the nucleus and appears to be a component of replication compartments upon the addition of the replication machinery along with oriLyt and IE1/IE2.
FIG. 2.
Colocalization of UL84 and UL44 in replication compartments. (A) Cotransfection of UL84 and HCMV plasmids encoding replication factors and oriLyt. Panel 1, direct fluorescence of UL84-EGFP, shown as green; panel 2, transfected cells stained with a rhodamine-labeled mouse monoclonal antibody to HCMV UL44 protein, shown as red; panel 3, merged image of panels 1 and 2 (colocalization of UL44 and UL84 is shown as yellow). (B) Nuclear colocalization of UL84-EGFP and UL44 proteins in replication compartments in HCMV-infected HFF cells. HFF cells were transfected with pUL84-EGFP followed by infection with HCMV and assayed for UL44 protein expression by immunofluorescence assay at 48 h p.i. Panel 1, UL84-EGFP fusion protein, showed as green; panel 2, immunodetection of UL44, shown as red; panel 3, merged image of panels 1 and 2 showing the colocalization of UL84-EGFP and UL44 in the replication compartments.
Transient transfection experiments have demonstrated that UL84 is one of the proteins required for origin-dependent DNA replication (28, 33). We demonstrated that in cotransfected cells UL84 and UL44 colocalized in DNA replication compartments. We next wanted to determine if UL84 and UL44 colocalized in infected cells. We transfected pUL84-EGFP into human foreskin fibroblast (HFF) cells that were subsequently infected with HCMV using an approximate multiplicity of infection of 1 and determined the pattern of fluorescence for UL44 and UL84. UL84 and UL44 formed a globular fluorescent pattern consistent with previously described replication compartments in infected cells (Fig. 2B, panels 1 and 2, respectively). The merged image of the two patterns demonstrated that the two proteins colocalized in infected cells (Fig. 2B, panel 3). These data are the first demonstration, in infected cells, that UL84 partitions into replication compartments and colocalizes with UL44, an enzyme known to participate in HCMV DNA replication.
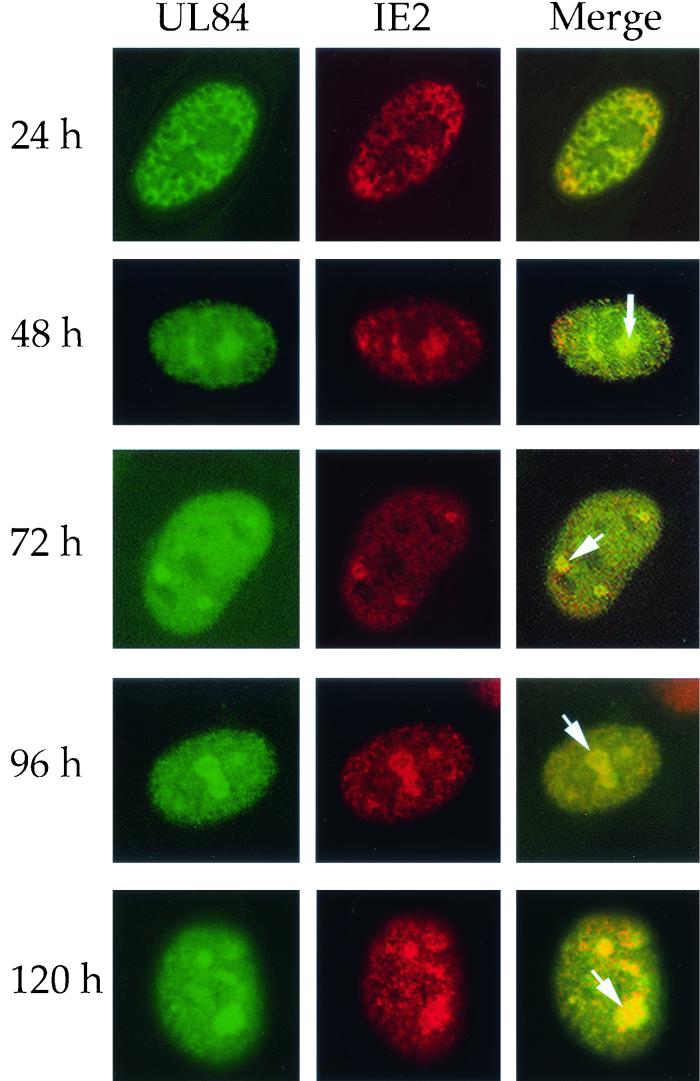
IE2 and UL84 colocalize in replication compartments as early as 48 h p.i.
Since it was previously shown that UL84 protein interacts with IE2 protein in lytically infected cells (36), we sought to determine if this association correlated with the colocalization of these two proteins in infected cells. HFF cells were transfected with pUL84-EGFP and subsequently infected with HCMV using a multiplicity of infection of 1. Cells were fixed at 24, 48, 72, 96, and 120 h p.i. and reacted with a monoclonal antibody recognizing IE2 (#G13-12E2; Vancouver Biotech Ltd.), and cells were then reacted with a secondary antibody conjugated with rhodamine to visualize IE2. The merged images of UL84-EGFP fusion protein (green) and the IE2 protein (red) are seen as yellow, indicating that the two proteins colocalize. At 24 h p.i., some colocalization was observed within the nucleus (Fig. 3, 24 h panel); however, both UL84 and IE2 were distributed throughout the nucleus, and very little structural organization was observed. This fluorescent pattern for IE2 and UL84 is consistent with the pattern observed for UL44 at early times p.i. (3, 34).
FIG. 3.
Nuclear localization of UL84-EGFP and IE2 in transfected/infected HFF cells. HFF cells were transfected with pUL84-EGFP and subsequently infected with HCMV. Cells were reacted with antibody specific for IE2 and visualized by IFA. UL84-EGFP fusion protein was visualized by direct fluorescence microscopy at 24 to 120 h p.i. Arrows indicate the colocalization of UL84 and IE2 (yellow) at 48, 72, 96, and 120 h p.i.
At 48 h p.i., UL84 was present within the nucleus in globular structures (Fig. 3, 48 h panel, see arrow) similar to those shown in Fig. 2, as well as a speckled pattern throughout the nucleus. IE2 protein was also distributed throughout the nucleus and colocalized with UL84 in replication compartments (Fig. 3, 48 h panel). This pattern was observed at all subsequent time points (Fig. 3, 72 h to 120 h panels). Based on this observed pattern, we concluded that UL84 and IE2 are both present in replication compartments as early as 48 h p.i., which is consistent with UL44-UL84 colocalization patterns and kinetics.
Identification of a nuclear localization signal (NLS) for UL84.
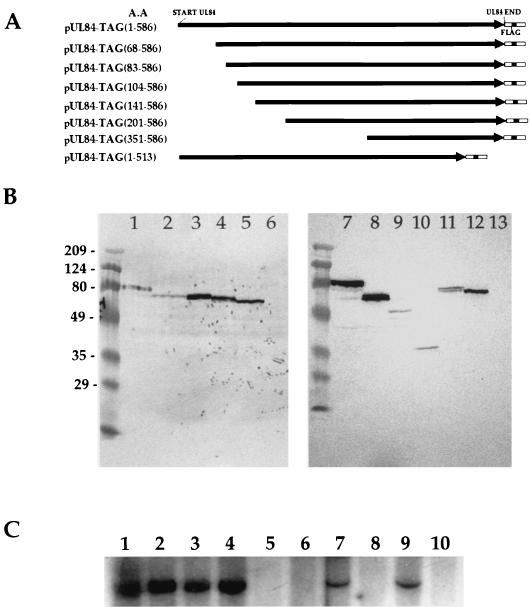
Transient transfections clearly demonstrated that UL84 localized to the nucleus in the absence of any other viral proteins. Therefore, we postulated that a signal sequence must be present within the protein that is capable of targeting this protein to the nucleus. In order to investigate this, we constructed a series of UL84 expression constructs where increasing amounts of the N terminus (in addition to one C-terminal truncation) of the UL84 protein were deleted. These seven truncation mutants, shown as a schematic representation in Fig. 4A, were made from the parent plasmid construct, pUL84-Tag(1-586), where the full-length UL84 ORF was fused in frame with the FLAG epitope. Each of these UL84 truncation mutants was individually transfected into COS7 cells and analyzed by a Western blot for protein production. All seven plasmid constructs generated the correct translation products corresponding to various calculated molecular weights, which were detected on a Western blot reacted with the anti-FLAG epitope antibody (Fig. 4B).
FIG. 4.
UL84 truncation mutants. (A) Schematic representation of the deletion constructs of UL84. Each mutant was subcloned in frame with the FLAG epitope, which can be detected by anti-FLAG antibody. The orientation of transcription is indicated by the arrow, and the number of the amino acids (A.A.) remaining in each truncation mutant is indicated at left along with the construct name. (B) Western blot of total cellular protein from COS7 cells transfected with UL84-Tag truncation mutants from panel A showing correct reading frame expression of all the UL84-Tag recombinant proteins. Blots were reacted with the anti-FLAG M2 antibody specific for the FLAG epitope (Stratagene). Lanes: 1 and 7, intact UL84-Tag fusion protein (1-586) (∼80 kDa); 2, pUL84-Tag(68-586) truncation mutant; 3, pUL84-Tag(83-586) truncation mutant; 4, pUL84-Tag(104-586) truncation mutant; 5 and 12, control Tag vector; 6 and 13, untransfected COS7 cells (negative control); 8, pUL84-Tag(141-586) truncation mutant; 9, pUL84-Tag(201-586) truncation mutant; 10, pUL84-Tag(351-586); 11 pUL84-Tag(1-513). Molecular weight markers, in thousands, are shown at the left of the blot. (C) Replication assay using UL84 truncation mutants cotransfected with plasmids encoding HCMV replication proteins plus oriLyt. Each UL84 truncation mutant was substituted for the wild-type UL84 plasmid construct. Autoradiogram of a Southern blot of total cellular DNA from HFF cells cleaved with EcoRI and DpnI and hybridized with pGEM7zf(-) is shown. Lanes: 1, intact pUL84-Tag(1-586); 2, pUL84-Tag(68-586) truncation mutant; 3, pUL84-Tag(83-586) truncation mutant; 4, pUL84-Tag(104-586) truncation mutant; 5, pUL84-Tag(141-586) truncation mutant; 6, pUL84-Tag(201-586) truncation mutant; 7, pUL84-Tag(1-513) truncation mutant; 8, pUL84-Tag(351-586); 9, wild-type UL84 plasmid; 10, control Tag vector (negative control) substituted for the UL84 plasmid.
Western blot data served to confirm that the correct proteins were being translated in the same reading frame context as the FLAG epitope. We also wanted to determine the ability of the various UL84-FLAG fusion proteins to complement transient oriLyt-dependent DNA replication. Each plasmid construct was transfected into HFF cells along with plasmids encoding the remaining required HCMV replication proteins plus oriLyt. An autoradiogram of a Southern blot of the transient cotransfection assay determined that the intact pUL84-Tag(1-586), the N-terminally truncated mutants pUL84-Tag(68-586), pUL84-Tag(83-586), and pUL84-Tag(104-586), and the C-terminal truncation mutant pUL84-Tag(1-513) were capable of complementing oriLyt-dependent DNA replication, whereas three truncation mutants, pUL84-Tag(141-586), pUL84-Tag(201-586), and pUL84-Tag(351-586), failed to complement (Fig. 4C).
Transfections were again performed, but this time UL84 truncation mutants were transfected alone (without any other viral factors) into COS7 cells and evaluated for their ability to localize to the nucleus. Transfected cells were incubated with the anti-FLAG antibody followed by incubation with a secondary antibody conjugated to fluorescein isothiocyanate. This antibody allowed us to detect the UL84-FLAG fusion protein and determine if any change in subcellular localization occurred. This change corresponded directly to how much protein sequence was eliminated from the wild-type UL84 protein. As shown in Fig. 5, intact pUL84-Tag localized in the nucleus and displayed a punctate nuclear pattern of fluorescence (Fig. 5A). The N-terminal truncation mutants pUL84-Tag(68-586), pUL84-Tag(83-586), pUL84-Tag(104-586), and pUL84-Tag(141-586) also localized to the nucleus; however, the pattern of fluorescence became more diffuse as more amino acids were removed from the N terminus (compare Fig. 5B, C, D, and E). Interestingly, the plasmid construct pUL84-Tag(141-586) also localized to the nucleus but was not able to complement oriLyt-dependent DNA replication (Fig. 4C, lane 5). The plasmid construct pUL84-Tag(201-586) failed to produce a protein product that was capable of localizing to the nucleus (Fig. 5 panel F). This mutant has 200 amino acids deleted from the N terminus of the UL84 protein (Fig. 4A). Instead of a speckled nuclear fluorescent pattern, transfection of this plasmid resulted in a speckled cytoplasmic distribution of the protein. This pattern was also observed upon transfection of pUL84-Tag(351-586) (Fig. 5G). Transfection of the C-terminal mutant pUL84-Tag(1-513) did not show any appreciable difference from pUL84-Tag(1-586) in fluorescent staining pattern (Fig. 5H).
FIG. 5.
Subcellular localization of UL84-Tag truncation mutants in transfected cells. COS7 cells were transfected with each of the UL84-Tag mutants, respectively, and detected by fluorescein isothiocyanate-labeled anti-FLAG M2 antibody specific for the FLAG epitope. (A) Intact UL84-Tag(1-586); (B) UL84-Tag(68-586) truncation mutant; (C) UL84-Tag(83-586) truncation mutant; (D) UL84-Tag(104-586) truncation mutant; (E) UL84-Tag(141-586) truncation mutant; (F) UL84-Tag(201-586) truncation mutant (arrow indicates the exclusion of UL84 from the nucleus); (G) UL84-Tag(351-586) truncation mutant; (H) UL84-Tag(1-513) truncation mutant.
The data from the transfection of the truncation mutants revealed that a possible nuclear localization signal exists between amino acids 141 and 201. Inspection of the amino acid sequence of UL84 in this region revealed a consensus NLS between amino acids 160 and 171. This UL84 basic amino acid sequence, PEKKKEKQEKK, is similar to the NLS for the simian virus 40 large T antigen (PKKKRKV).
We investigated whether this sequence was sufficient to target an otherwise non-nucleus-associated protein to the nucleus. We designed oligonucleotides encoding the UL84 putative NLS sequence and annealed and ligated the double-stranded sequence in frame with EGFP (Fig. 6A). This construct, p84NLS-EGFP, was transfected into COS7 cells, which were subsequently visualized at 48 h posttransfection. Transfection of p84NLS-EGFP resulted in a fluorescent staining pattern predominantly within the nucleus, whereas EGFP alone localized throughout the cell (Fig. 6B, compare left and right panels).
FIG. 6.
Subcellular localization of p84NLS-EGFP. (A) Diagram of p84NLS-EGFP. The NLS of UL84 was subcloned in frame with EGFP gene in pEGFP-N1 vector. The HCMV major immediate-early promoter was used to initiate expression of the recombinant protein. (B) The subcellular localization of p84NLS-EGFP and pEGFP-N1. COS7 cells transfected with p84NLS-EGFP or pEGFP-N1 were examined by epifluorescence microscopy. Left panel, p84NLS-EGFP; right panel, pEGFP-N1.
Herpesvirus DNA replication appears to involve two functional components. The first, common to all herpesviruses, involves those factors that carry out the enzymatic synthesis of viral DNA. It has been demonstrated that these enzymes perform their functions independent of the specific virus they originate from. A specific example of this was demonstrated by the fact that the core replication machinery from HCMV can be used to replicate an Epstein-Barr virus oriLyt provided that a specific Epstein Barr Virus-encoded initiator protein (Zta) is present (34). This initiator protein is representative of the second functional component of herpesvirus replication, promoting the initiation of DNA synthesis. This class of proteins probably orchestrates very early events in DNA synthesis such that a complete set of replication proteins are able to recognize a defined cis-acting region. In the case of HSV-1, the UL9 initiator protein possesses an associated helicase function (8, 21, 27, 37). For HCMV, the replication process appears to be more complex than those of other herpesvirus systems characterized to date. In human fibroblasts, HCMV oriLyt-dependent DNA replication requires the gene products of UL36-38, IE1/IE2, and UL84. The requirement of these additional factors, coupled with the fact that the HCMV oriLyt is more complex than other characterized herpesvirus lytic origins, may reflect a different mode of replication for this virus.
In the study presented here, we demonstrate that UL84 is a nuclear protein, capable of nuclear localization in the absence of any additional viral factors, and is part of higher-order structures previously described as replication compartments (3, 11, 22). The formation of replication compartments was originally described for HSV-1 (32). For HCMV, several viral replication proteins have been shown to locate to replication compartments (3). In an earlier report, Sarisky and Hayward demonstrated that UL84 was obligatory for intranuclear replication compartment formation (34). However, they did not identify UL84 as a component of these replication compartments. The study presented here demonstrates that UL44, a replication protein known to be a component of replication compartments, and UL84 colocalized in infected cells. This colocalization is strong evidence that UL84 participates directly in DNA replication. UL84 also partitioned into large higher-order structures in the nucleus in cotransfected cells, but only upon the addition of all the necessary replication proteins, IE1/IE2, and oriLyt. This result is consistent with the observation that UL44 also partitioned into replication compartments under similar conditions in cotransfection assays (34).
The association of UL84 with IE2 may have some impact on the regulatory function of IE2. Alternatively, UL84 may have an as yet undetermined regulatory function. Consistent with a possible regulatory nature for UL84, we observed that UL84 is phosphorylated in uninfected cells (7; K. Colletti and G. S. Pari, unpublished data). In IE2, multiple domains are phosphorylated by cellular kinases (16, 19). Specifically, mitogen-activated protein kinase and extracellular signal-regulated kinase 2 were shown to phosphorylate the IE2 protein (16). The fact that over-expression of UL84 decreases IE2-mediated regulation of some early promoters (13) may suggest that UL84 itself has activation states determined by phosphorylation.
We demonstrated that UL84 contains an NLS beginning at amino acid 160 and ending at amino acid 171. This sequence, PEKKKEKQEKK, closely resembles the consensus sequences of simian virus 40 large T antigen and IE2 nuclear localization signals (20, 30). The possibility that this basic amino acid sequence could function as an NLS was first reported by He et al. (18). We have now confirmed that this sequence alone is sufficient to direct the nuclear localization of UL84 protein and EGFP. Also of interest is the observation that the truncation mutant pUL84-TAG(141-586) localized to the nucleus but failed to complement origin-dependent DNA replication. This deletion removes the leucine zipper motif within the protein, indicating that this region is essential for the DNA replication function of UL84.
Acknowledgments
These studies were supported by Public Health Service research grant RO1 AI45096.
REFERENCES
- 1.Ahn, J. H., and G. S. Hayward. 2000. Disruption of PML-associated nuclear bodies by IE1 correlates with efficient early stages of viral gene expression and DNA replication in human cytomegalovirus infection. Virology 274:39-55. [DOI] [PubMed] [Google Scholar]
- 2.Ahn, J. H., and G. S. Hayward. 1997. The major immediate-early proteins IE1 and IE2 of human cytomegalovirus colocalize with and disrupt PML-associated nuclear bodies at very early times in infected permissive cells. J. Virol. 71:4599-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn, J. H., W. J. Jang, and G. S. Hayward. 1999. The human cytomegalovirus IE2 and UL112-113 proteins accumulate in viral DNA replication compartments that initiate from the periphery of promyelocytic leukemia protein-associated nuclear bodies (PODs or ND10). J. Virol. 73:10458-10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Barazi, H. O., and A. M. Colberg-Poley. 1996. The human cytomegalovirus UL37 immediate-early regulatory protein is an integral membrane N-glycoprotein which traffics through the endoplasmic reticulum and Golgi apparatus. J. Virol. 70:7198-7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anders, D. G., M. A. Kacica, G. Pari, and S. M. Punturieri. 1992. Boundaries and structure of human cytomegalovirus oriLyt, a complex origin for lytic-phase DNA replication. J. Virol. 66:3373-3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anders, D. G., and S. M. Punturieri. 1991. Multicomponent origin of cytomegalovirus lytic-phase DNA replication. J. Virol. 65:931-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blom, N., S. Gammeltoft, and S. Brunak. 1999. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 294:1351-1362. [DOI] [PubMed] [Google Scholar]
- 8.Bruckner, R. C., J. J. Crute, M. S. Dodson, and I. R. Lehman. 1991. The herpes simplex virus 1 origin binding protein: a DNA helicase. J. Biol. Chem. 266:2669-2674. [PubMed] [Google Scholar]
- 9.Challberg, M. D. 1986. A method for identifying the viral genes required for herpesvirus DNA replication. Proc. Natl. Acad. Sci. USA 83:9094-9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colberg-Poley, A. M., L. D. Santomenna, P. P. Harlow, P. A. Benfield, and D. J. Tenney. 1992. Human cytomegalovirus UL3 and UL36-38 immediate-early proteins regulate gene expression. J. Virol. 66:95-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Bruyn Kops, A., and D. M. Knipe. 1988. Formation of DNA replication structures in herpes virus-infected cells requires a viral DNA binding protein. Cell 55:857-868. [DOI] [PubMed] [Google Scholar]
- 12.de Bruyn Kops, A., and D. M. Knipe. 1994. Preexisting nuclear architecture defines the intranuclear location of herpesvirus DNA replication structures. J. Virol. 68:3512-3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gebert, S., S. Schmolke, G. Sorg, S. Floss, B. Plachter, and T. Stamminger. 1997. The UL84 protein of human cytomegalovirus acts as a transdominant inhibitor of immediate-early-mediated transactivation that is able to prevent viral replication. J. Virol. 71:7048-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldmacher, V. S., L. M. Bartle, A. Skaletskaya, C. A. Dionne, N. L. Kedersha, C. A. Vater, J. W. Han, R. J. Lutz, S. Watanabe, E. D. Cahir McFarland, E. D. Kieff, E. S. Mocarski, and T. Chittenden. 1999. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl. Acad. Sci. USA 96:12536-12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodrich, L. D., P. A. Schaffer, D. I. Dorsky, C. S. Crumpacker, and D. S. Parris. 1990. Localization of the herpes simplex virus type 1 65-kilodalton DNA-binding protein and DNA polymerase in the presence and absence of viral DNA synthesis. J. Virol. 64:5738-5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harel, N. Y., and J. C. Alwine. 1998. Phosphorylation of the human cytomegalovirus 86-kilodalton immediate-early protein IE2. J. Virol. 72:5481-5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayajneh, W. A., A. M. Colberg-Poley, A. Skaletskaya, L. M. Bartle, M. M. Lesperance, D. G. Contopoulos-Ioannidis, N. L. Kedersha, and V. S. Goldmacher. 2001. The sequence and antiapoptotic functional domains of the human cytomegalovirus UL37 exon 1 immediate early protein are conserved in multiple primary strains. Virology 279:233-240. [DOI] [PubMed] [Google Scholar]
- 18.He, Y. S., L. Xu, and E.-S. Huang. 1992. Characterization of human cytomegalovirus UL84 early gene and identification of its putative protein product. J. Virol. 66:1098-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermiston, T. W., C. L. Malone, P. R. Witte, and M. F. Stinski. 1987. Identification and characterization of the human cytomegalovirus immediate-early region 2 gene that stimulates gene expression from an inducible promoter. J. Virol. 61:3214-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalderon, D., W. D. Richardson, A. F. Markham, and A. E. Smith. 1984. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature 311:33-38. [DOI] [PubMed] [Google Scholar]
- 21.Kroff, A., J. F. Schwedes, and P. Tegtmeyer. 1991. Herpes simplex virus origin-binding protein (UL9) loops and distorts the viral replication origin. J. Virol. 65:3284-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liptak, L. M., S. L. Uprichard, and D. M. Knipe. 1996. Functional order of assembly of herpes simplex virus DNA replication proteins into prereplicative site structures. J. Virol. 70:1759-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malone, C. L., D. H. Vesole, and M. F. Stinski. 1994. Transactivation of human cytomegalovirus early promoter by gene products form the immediate-early gen IE2 and augmentation by IE1: mutational analysis of the viral proteins. J. Virol. 64:1498-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masse, M. J. O., S. Karlin, G. A. Schachtel, and E. S. Mocarski. 1992. Human cytomegalovirus origin of replication (oriLyt) resides within a highly complex repetitive region. Proc. Natl. Acad. Sci. USA 89:5246-5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monick, M. M., L. J. Geist, M. F. Stinski, and G. W. Hunninghake. 1992. The immediate early genes of human cytomegalovirus upregulate expression of the cellular genes myc and fos. Am. J. Respir. Cell Mol. Biol. 7:251-256. [DOI] [PubMed] [Google Scholar]
- 26.Murphy, E. A., D. N. Streblow, J. A. Nelson, and M. F. Stinski. 2000. The human cytomegalovirus IE86 protein can block cell cycle progression after inducing transition into the S phase of permissive cells. J. Virol. 74:7108-7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olivo, P. D., N. J. Nelson, and M. D. Challberg. 1988. Herpes simplex virus DNA replication: the UL9 gene encodes an origin-binding protein. Proc. Natl. Acad. Sci. USA 85:5414-5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pari, G. S., and D. G. Anders. 1993. Eleven loci encoding trans-acting factors are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA replication. J. Virol. 67:6979-6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pari, G. S., M. A. Kacica, and D. G. Anders. 1993. Open reading frames UL44, IRS1/TRS1, and UL36-38 are required for transient complementation of human cytomegalovirus oriLyt-dependent DNA synthesis. J. Virol. 67:2575-2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pizzorno, M. C., M. A. Mullen, Y. N. Chang, and G. S. Hayward. 1991. The functionally active IE2 immediate-early regulatory protein of human cytomegalovirus is an 80-kilodalton polypeptide that contains two distinct activator domains and a duplicated nuclear localization signal. J. Virol. 65:3839-3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prichard, M. N., S. Jairath, M. E. Penfold, S. St Jeor, M. C. Bohlman, and G. S. Pari. 1998. Identification of persistent RNA-DNA hybrid structures within the origin of replication of human cytomegalovirus. J. Virol. 72:6997-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quinlan, M. P., L. B. Chen, and D. M. Knipe. 1984. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell 36:857-868. [DOI] [PubMed] [Google Scholar]
- 33.Sarisky, R. T., Z. Gao, P. M. Lieberman, E. D. Fixman, G. S. Hayward, and S. D. Hayward. 1996. A replication function associated with the activation domain of the Epstein-Barr virus Zta transactivator. J. Virol. 70:8340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarisky, R. T., and G. S. Hayward. 1996. Evidence that the UL84 gene product of human cytomegalovirus is essential for promoting oriLyt-dependent DNA replication and formation of replication compartments in cotransfection assays. J. Virol. 70:7398-7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skaletskaya, A., L. M. Bartle, T. Chittenden, A. L. McCormick, E. S. Mocarski, and V. S. Goldmacher. 2001. A cytomegalovirus-encoded inhibitor of apoptosis that suppresses caspase-8 activation. Proc. Natl. Acad. Sci. USA 98:7829-7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spector, D. J., and M. J. Tevethia. 1994. Protein-protein interactions between human cytomegalovirus IE2-5f80aa and pUL84 in lytically infected cells. J. Virol. 68:7549-7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weir, H., and N. D. Stow. 1990. Two binding sites for the herpes simplex type 1 UL9 protein are required for efficient activity of the oris replication origin. J. Gen. Virol. 71:1379-1385. [DOI] [PubMed] [Google Scholar]
- 38.Wilcock, D., and D. P. Lane. 1991. Localization of p53, retinoblastoma and host replication proteins at sites of viral replication in herpes-infected cells. Nature 349:429-431. [DOI] [PubMed] [Google Scholar]
- 39.Wright, D. A., and D. H. Spector. 1989. Posttranscriptional regulation of a class of human cytomegalovirus phosphoproteins encoded by an early transcription unit. J. Virol. 63:3117-3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu, C. A., N. J. Nelson, D. J. McGeoch, and M. D. Challberg. 1988. Identification of herpes simplex virus type 1 genes required for origin-dependent DNA synthesis. J. Gen. Virol. 62:435-443. [DOI] [PMC free article] [PubMed] [Google Scholar]