Abstract
Pattern recognition via Toll-like receptors (TLR) by antigen-presenting cells is an important element of innate immunity. We report that wild-type measles virus but not vaccine strains activate cells via both human and murine TLR2, and this is a property of the hemagglutinin (H) protein. The ability to activate cells via TLR2 by wild-type MV H protein is abolished by mutation of a single amino acid, asparagine at position 481 to tyrosine, as is found in attenuated strains, which is important for interaction with CD46, the receptor for these strains. TLR2 activation by MV wild-type H protein stimulates induction of proinflammatory cytokines such as interleukin-6 (IL-6) in human monocytic cells and surface expression of CD150, the receptor for all MV strains. Confirming the specificity of this interaction, wild-type H protein did not induce IL-6 release in macrophages from TLR2−/− mice. Thus, the unique property of MV wild-type strains to activate TLR2-dependent signals might essentially contribute not only to immune activation but also to viral spread and pathogenicity by upregulating the MV receptor on monocytes.
In the course of acute measles, an efficient virus-specific immune response is generated which mediates viral clearance from the host and confers protection against reinfection. Paradoxically, a general immunosuppression is also induced favoring secondary infections, which are the major reason for the annual high morbidity and mortality rates associated with measles. The magnitude and duration of immune activation and immune suppression differ between natural or experimental infection and vaccination (20, 60). Studies addressing measles virus (MV)-induced immune suppression mainly have focused on alterations of T-cell functions and viability as a consequence of direct MV infection or contact-mediated signaling (53). In vitro observations suggest that MV infection also enhances apoptosis of monocytes and dendritic cells (DC) and affects their antigen-presenting capacity and cytokine release (31, 53). MV interaction with DC and monocytes is, however, also associated with their maturation or activation, respectively, and thus is important for induction of virus-specific immune responses (32, 39, 45, 54, 56). Strains expressing an MV wild-type-derived hemagglutinin (H) protein reveal a particular tropism for DC and are more efficient in inducing both DC maturation and immunosuppression (32, 48, 54). The mechanisms by which MV leads to these functional alterations are largely unknown. Downregulation of interleukin-12 (IL-12) production in monocytes was linked to MV- or antibody-mediated cross-linking of CD46, the receptor for certain MV strains (29). Lymphotropic MV wild-type strains and clinical isolates, with few known exceptions (43), fail to interact with CD46 but require CD150 for cell entry (15, 26, 49, 59). This molecule is absent from unstimulated monocytes and immature DC (33, 45, 48), and it is thus unknown how infection of these cells by CD150-dependent MV strains occurs.
Mammalian Toll-like receptors (TLRs) were implicated in the innate immune recognition of a variety of bacterial pathogens and bacterial products (2). Ten TLR family members were discovered, and several of these appear to play important roles in the activation of cells by various bacterial products. TLR2 is responsible for recognition of gram-positive bacteria (57, 65), bacterial lipoproteins (12, 42), and lipoteichoic acid (38, 55). TLR4 appears to be the main receptor for lipopolysaccharide (LPS) lipid A from gram-negative bacteria (41), TLR6 might be a coreceptor for TLR2 in recognizing certain bacterial structures (50, 58), and TLR9 and TLR3 mediate responses to CpG bacterial DNA and double-stranded RNA (dsRNA), respectively (3, 24). Hence, these receptors are able to discriminate between different bacteria and bacterial structures and thereby direct a proper immune response to a specific pathogen. Intracellular domains of the TLRs share motifs with the protein families of the IL-1 receptors, and a common intracellular pathway leading to activation of NF-κB and mitogen-activated protein kinases involves MyD88, IRAK, and TRAF6 (2). However, other signaling pathways upstream of NF-κB have been described which also include utilization of the phosphatidylinositol-3/Akt-kinase pathway by TLR2 (4). It has recently been demonstrated that mammalian TLR signaling can also be regulated by viral gene products. Vaccinia virus encodes gene products that interfere with proximal TLR signaling in the cytoplasm (11), and the fusion protein of respiratory syncytial virus (RSV) was found to activate cells via TLR4 and CD14 (35).
Using CHO cells stably overexpressing TLR2 or TLR4, we found that MV wild-type strains specifically activated cells via TLR2, and this was dependent on the expression of the H protein of the MV wild-type strain, WTF. The failure of attenuated MV strains to induce TLR2 activation correlated with a single amino acid exchange at position 481 which is, in turn, essential for the usage of CD46 as receptor by these strains. Importantly, MV expressing the wild-type H protein induced activation of TLR-responsive genes such as IL-1α/β, IL-6, and IL-12 p40 in monocytes and also the expression of CD150, the receptor for all MV strains. Activation of TLR signaling by wild-type MV H protein may thus essentially contribute to the immune activation during measles, and loss of the capability to activate TLR2 may be considered as an attenuation marker.
MATERIALS AND METHODS
Cells and viruses.
MV vaccine strains AIK-C and Moraten were grown on Vero cells (minimal essential medium with 5% fetal calf serum [FCS]); MV wild-type strains WTF (isolated on lymphoblastoid, Epstein-Barr virus-negative BJAB cells), Bilthoven (isolated on Epstein-Barr virus-transformed primary B cells), Wü5404, Wü5679, and Wü4797 (isolated on BJAB cells), the MV vaccine strain Edmonston (ED) and the recombinant viruses [MV(WTF-F)ED, MV(WTF-H)ED, MV(WTF-F/H)ED [28], and MV(WTF-H481N→Y)ED] [referred to as ED(WTF-F), ED(WTF-H), ED(WTF-F/H), and ED(WTF-H;N→Y) in this paper] on BJAB cells in RPMI 1640 with 10% FCS. For the generation of ED(WTF-H;N→Y), a point mutation was introduced in ED(WTF-H) at position 1441 (A to T), leading to amino acid Tyr (Y) instead of Asn (N) at position 481. Viruses and uninfected BJAB cells, as mock control, were harvested in phosphate-buffered saline and purified by discontinuous sucrose gradient ultracentrifugation. Viruses were titrated on marmoset lymphoblastoid B95a cells (maintained in RPMI 1640-5% FCS). Except for the experiments shown in Fig. 1a to c, virus preparations were UV inactivated (1.5 J/cm2), analyzed for their glycoprotein content by Western blotting using a rabbit serum raised against the cytoplasmic domains of MV F or H proteins, respectively, and adjusted to equal concentrations of glycoproteins. CHO cell clones (expressing functional hamster TLR4 but nonfunctional hamster TLR2) EL1 (expressing the NF-κB reporter plasmid ELAM.TAC), 3E10 (expressing ELAM.TAC and human CD14), 3E10 hTLR2 (expressing ELAM.TAC, CD14, and human TLR2), 3E10 hTLR4 (expressing ELAM.TAC, CD14, and human TLR4), CHO hTLR2 (only expressing human TLR2), EL1 mTLR2 (expressing ELAM.TAC and murine TLR2), and 3E10 mTLR2 (expressing ELAM.TAC, human CD14, and murine TLR2) (41, 42, 44, 65) were maintained in Ham's F-12-10% FCS containing 0.5 mg of G418 (Gibco BRL)/ml and/or 400 U of hygromycin B (Calbiochem)/ml, THP-1 cells in RPMI 1640-10% FCS, and matured with 100 mM vitamin D3 (Calbiochem) for 72 h. Human monocytes enriched from whole blood by density gradient centrifugation using Ficoll-Paque Plus and Percoll (Amersham Pharmacia) were maintained in RPMI 1640-10% FCS. Peritoneal macrophages were isolated from C3H/HeN, C3H/HeJ, and C3H/TL2−/− mice (64) after thioglycolate elicitation and were maintained in RPMI 1640-10% FCS-5 mM β-mercaptoethanol. For cell stimulation, LPS (Escherichia coli serotype 0111:B4; Sigma) (10 ng/ml), Pam3CysSerLys4 (PamCSK) (2.5 μg/ml) (EMC Microcollections, Tübingen, Germany) was used. All reagents, cell lines, and virus stocks were mycoplasma-free as determined by reverse transcription-PCR and contained less than 10 pg of endotoxin/ml as determined by a Limulus lysate assay (Cape Cod Associates).
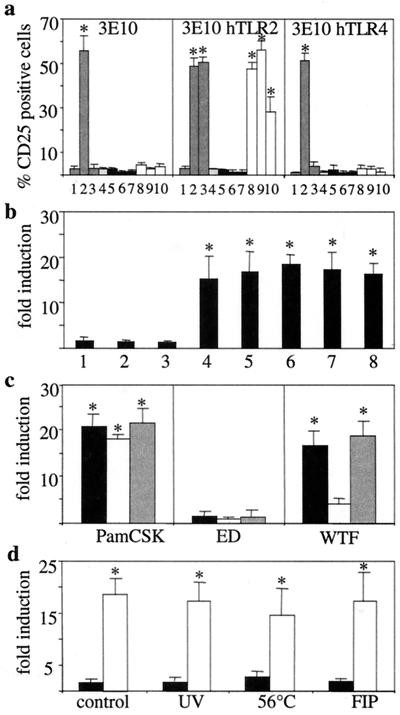
FIG. 1.
TLR2-mediated cell activation by MV. (a) CHO cells stably expressing an NF-κB-dependent reporter gene (ELAM.TAC) together with CD14 alone (3E10) (left panel) or with human TLR2 (3E10 hTLR2) (middle panel) or TLR4 (3E10 hTLR4) (right panel) were analyzed for CD25 expression after incubation with medium (lane 1), LPS (lane 2), PamCSK (lane 3), mock supernatant (lane 4), ED (black bars) (lane 5, MOI = 10; lane 6, MOI = 1; lane 7, MOI = 0.1), or WTF (white bars) (lane 8, MOI = 10; lane 9, MOI = 1; lane 10, MOI = 0.1). (b) 3E10 hTLR2 cells were incubated with MV vaccine strains ED (lane 1), AIK-C (lane 2), Moraten (lane 3), or lymphotropic wild-type strains WTF (lane 4), Bilthoven (lane 5), Wü5404 (lane 6), Wü5679 (lane 7), and Wü4797 (lane 8) (MOI = 1 for each). (c) 3E10 hTLR2 cells (black bars), EL1 mTLR2 cells (white bars), or 3E10 mTLR2 cells (grey bars) were incubated with ED or WTF (MOI = 1 for each), or with PamCSK. (d) 3E10 hTLR2 cells were incubated with ED (black bars) or WTF (white bars) (MOI = 1 for each) as live virus (control), inactivated by UV irradiation or heating at 56°C, or as live virus in the presence of FIP. Data shown were obtained in three (a, b, and d) or five (c) independent experiments, with P values of <0.01 (a to c) and <0.05 (d) with respect to the mock control. Statistically significant differences from the control are indicated by asterisks.
Reporter gene assay and IL-6 bioassay.
CHO cells (3 × 104/well) were stimulated in Ham's F12-10% FCS for 12 h and analyzed by flow cytometry for surface expression of CD25 using a phycoerythrin-conjugated antibody or an isotype control (both from Beckmann Coulter). When indicated, a fusion-inhibiting peptide (FIP) (Z-D-Phe-L-Phe-Gly-OH; Bachem) was added (final concentration, 0.2 mM). Supernatants were assayed for IL-6 content by the B9 cell proliferation assay (1). Results are shown in picograms per milliliter as means ± standard deviations of triplicate measurements.
RNase protection assay.
Total cellular RNA was extracted from vitamin D3-treated THP-1 cells 12 h following stimulation with TriFast (PeqLab, Heidelberg, Germany), DNase I digested, and purified on RNeasy columns (Qiagen, Hilden, Germany). RNase protection assays were performed using a Riboquant multiprime kit (hCK-2; Pharmingen).
Enzyme-linked immunosorbent assay and immunocytochemistry.
THP-1 cells, human primary monocytes, or peritoneal mouse macrophages (105/well) were stimulated for 12 h. For blocking experiments, cells were preincubated with monoclonal antibodies specific for either CD14 (clone 3C10), TLR2 (clone TL2.1; kindly provided by T. Espevik, Trondheim, Norway [18]), or TLR4 (clone HTA125; kindly provided by K. Miyake, Saga, Japan) and viruses with a pool of three monoclonal MV-H-specific antibodies (L77, K83, and K29) or an MV-N-specific antibody (F227) (all three antibodies were generated in our laboratory) at a final concentration of 10 μg/ml in RPMI 1640 for 30 min. IL-6 or IL-12 p70 levels were determined in supernatants obtained 12 h after stimulation by using an enzyme-linked immunosorbent assay (R & D). For detection of IL-12 p70, cells were prestimulated with 1,000 U of human gamma interferon (h-IFN-γ; Strathmann Biotech) for 30 min. Cells were analyzed for surface expression of HLA-DR (Pharmingen) and CD150 (clone 5C6; generated in our laboratory) (15) by using monoclonal antibodies or isotype-matched controls, respectively, followed by a secondary fluorescein-conjugated goat anti-mouse antibody.
Statistical analysis.
Statistical analysis was performed using Student's t test, and significance levels were determined based on the respective controls.
RESULTS
Wild-type MV but not vaccine strains activate TLR2 signaling.
To analyze whether MV acts as a TLR agonist, CHO cells stably transfected with a CD25 reporter gene driven by an NF-κB-dependent endothelial cell leukocyte adhesion molecule (ELAM) promoter together with human CD14 (3E10), human CD14/TLR2 (3E10 hTLR2), or CD14/TLR4 (3E10 hTLR4) were used in fluorescence-activated cell sorter reporter cell assays. As expected, LPS activated CHO cells expressing CD14, whereas a synthetic lipopeptide, PamCSK, required TLR2 coexpression (Fig. 1a). Corroborating the findings of Kurt-Jones et al. (35), RSV activated CD25 expression in 3E10 hTLR4 cells to about 20% (results not shown). The MV Edmonston (ED) vaccine strain did not induce CD25 expression in all CHO clones across a wide range of infectivities, whereas a lymphotropic MV strain, WTF, activated CD25 expression in 3E10 hTLR2 even at virus doses as low as a multiplicity of infection (MOI) of 0.1 (Fig. 1a). In this and all further experiments, activation of the reporter gene by all MV strains and mock preparations tested did not exceed 5% in 3E10 and 3E10 hTLR4 cells, also indicating that, in accordance with the Limulus lysate assay (data not shown), all reagents were essentially endotoxin free. Induction of reporter gene expression in the following experiments was thus normalized to the mock control on TLR2-expressing cells. While additional MV vaccine strains tested also failed to induce reporter gene activation (Fig. 1b, lanes 1 to 3), all MV wild-type strains efficiently stimulated CD25 expression in 3E10 hTLR2 cells (Fig. 1b, lanes 4 to 8). MV WTF also activated CD25 expression in CHO cells coexpressing murine TLR2 and human CD14, and less efficiently in the absence of CD14 (Fig. 1c). Similarly, MV WTF induced release of bioactive IL-6 from CHO cells expressing human TLR2, and this was enhanced by coexpression of CD14 (Table 1).
TABLE 1.
Induction of IL-6 release from CHO transfectants by MV strains
| Strain or recombinant | IL-6 level after stimulation witha:
|
||||
|---|---|---|---|---|---|
| LPS | PamCSK | Mock | ED | WTF | |
| EL1 | 46.7 ± 2.8* | 12.1 ± 0.3 | 5.4 ± 6.5 | 22.6 ± 2.5 | 2.5 ± 4.8 |
| 3E10 | 152 ± 3.3* | 35.6 ± 8.2 | 22.3 ± 3.0 | 45.4 ± 1.6 | 22.5 ± 3.3 |
| 3E10 hTLR2 | 127.2 ± 1.8* | 199.0 ± 3.8* | 13.9 ± 7.4 | 46.9 ± 5.4 | 117.4 ± 2.4* |
| CHO hTLR2 | 32.2 ± 7 | 155.5 ± 4.7* | 6.3 ± 1.7 | 25.9 ± 1.7 | 19.3 ± 1.6* |
| 3E10 hTLR4 | 133.9 ± 3.3* | 22.5 ± 9.5 | 4.5 ± 1.4 | 22.1 ± 1.8 | 0.67 ± 2.7 |
Levels of bioactive IL-6 were determined in supernatants of EL1 cells (expressing the ELAM.TAC reporter plasmid alone), 3E10, 3E10 hTLR2, and 3E10 hTLR4 cells 12h following stimulation. Values are indicated in picograms per milliliter and represent means and standard deviations of two independent experiments, each performed in triplicate (P ≤ 0.05 compared to unstimulated cells). Statistically significant differences from the control are indicated by asterisks.
TLR2-dependent cell activation does not require virus entry and replication.
Heat or UV inactivation did not affect the ability of MV WTF to induce TLR2-dependent signaling, indicating that replication was not required (Fig. 1d). Stable expression of either entry receptor, CD46 or CD150, allows productive MV replication in CHO cells (14, 15, 26, 59). In contrast, neither TLR2 nor TLR4 supported viral entry into these cells, since only about 2% of MV nucleocapsid protein-positive cells were detected in all cultures and formation of syncytia did not occur (data not shown). Inclusion of a peptide inhibitor (FIP) previously found to inhibit mixing of the outer membrane leaflets and thereby preventing an early step of viral membrane fusion (63) did not affect CD25 induction, confirming that reporter gene activation resulted from a surface interaction of MV WTF (Fig. 1d).
The MV WTF H protein is the viral component triggering both human and mouse TLR2 activation, and this property is abolished upon a single amino acid exchange.
To identify the agonist responsible for MV-dependent TLR2 activation, we used purified UV-inactivated ED, WTF, and recombinant MV based on the ED strain backbone in which the F and H glycoproteins were singly or doubly swapped for those of the WTF strain (28). Since equal MOIs, as used in the experiments shown in Fig. 1, do not necessarily reflect equal amounts of viral glycoproteins applied, for this and all subsequent experiments the glycoprotein concentration of the individual viruses was adjusted by Western blotting (data not shown) prior to incubation with the cells. While the ED and the recombinant MV strain expressing the WTF-F protein [ED(WTF-F)] did not induce TLR2 activation (Fig. 2, lanes 1 and 2), recombinants containing the H protein of the WTF strain [ED(WTF-H) and ED(WTF-F/H)] stimulated reporter gene activation in 3E10 hTLR2 cells at equivalent levels to those seen with WTF (Fig. 2, lanes 3, 5, and 6). Activation with WTF-H-containing recombinants was not restricted to human TLR2, since CHO cells stably expressing the murine orthologue were also susceptible (Fig. 2). To define the potential basis for the failure of MV vaccine strains to activate TLR2 signaling, a recombinant MV expressing WTF-H [ED(WTF-H;N→Y)] was used in which the Asn (N) found at position 481 in lymphotropic wild-type viruses was replaced by Tyr (Y), which is important for the high-affinity interaction of MV with CD46 (47). In contrast to recombinants expressing the WTF-H protein, this mutant, as the ED-H-expressing MV strains, did not induce TLR2 activation (Fig. 2, lane 4). Thus, the abilities of MV H protein to use CD46 as a receptor and to activate TLR2 are apparently inversely correlated, and amino acid 481 plays an important role in this switch in activity.
FIG. 2.
A single amino acid exchange within the H protein discriminates whether TLR2 signaling is induced by MV. 3E10 hTLR2 cells (grey bars), EL1 mTLR2 cells (black bars), or 3E10 mTLR2 cells (white bars) were incubated with purified, UV-inactivated ED (lane 1), ED(WTF-F) (lane 2), ED(WTF-H) (lane 3), ED(WTF-H; N→Y) (lane 4), ED(WTF-F/H) (lane 5), or WTF (lane 6) and analyzed for CD25 induction. Values shown were obtained in three independent experiments (P < 0.01 with respect to the mock control). Statistically significant differences from the control are indicated by asterisks.
MV WTF triggers TLR signaling in monocytes and stimulates expression of CD150.
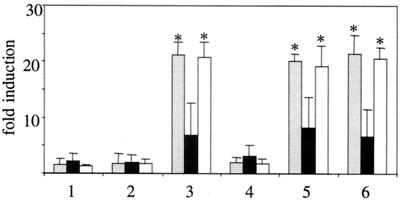
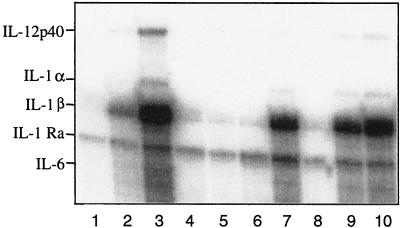
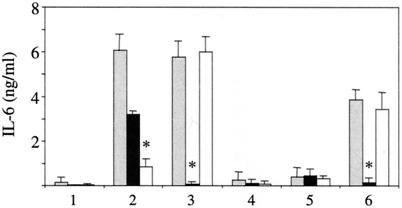
To establish whether MV also activates TLR2 signaling in monocytes, we analyzed the induction of monokines in RNase protection assays. Induction of transcripts specific for IL-1α, IL-1β, IL-12 p40, and IL-6 was detected with PamCSK and UV-inactivated WTF in vitamin D3-treated THP-1 cells, while ED had no effect (Fig. 3, lanes 3, 5, and 10, and Table 2). Induction of these transcripts was also seen with recombinants expressing the WTF-H protein (Fig. 3, lanes 7 and 9, and Table 2) but not with those containing the ED-H protein, and with ED(WTF-H;N→Y) (Fig. 3, lanes 5, 6, and 8, and Table 2). To investigate whether induction of TLR2-responsive genes by MV is also observed in primary human monocytes, these were treated with ED, WTF, and the recombinant viruses and the release of IL-6 was analyzed as a marker. Synthesis of IL-6 was significantly enhanced with viruses containing the WTF-H protein, but not with those expressing ED-H or WTF-H;N→Y protein (Fig. 4a). Although the overall levels of IL-6 were lower, similar observations were made in THP-1 cells (data not shown). Production of IL-12 p70 from monocytes was not detected with either virus strain, irrespective of IFN-γ priming (data not shown). To confirm that IL-6 induction in monocytes was dependent on both wild-type MV H protein and TLR2, antibody blocking experiments were performed. Pretreatment of WTF with anti-H antibodies or monocytes with TLR2-specific antibodies led to reduced efficiency of WTF-induced IL-6 production from these cells (Fig. 4c). MV-N-specific or TLR4-specific antibodies were used as irrelevant isotype controls and had no effect (Fig. 4c). Interestingly, pretreatment of monocytes with a CD14-specific antibody also interfered with the WTF-mediated IL-6 induction (Fig. 4c). Inhibition was, however, only partial and this might be explained for the TLR-specific antibodies by their known variable blocking efficiencies. Clearly supporting the importance of TLR2 activation by MV WTF-H protein, however, was the finding that peritoneal macrophages isolated from TLR2−/− mice did not show virus strain-specific differences in IL-6 production, while those isolated from C3H/HeN and C3H/HeJ mice released enhanced levels of this cytokine when treated with WTF (Fig. 5).
FIG. 3.
Induction of monokine-specific transcripts by MV expressing the WTF-H protein. THP-1 cells matured by vitamin D3 were incubated with medium (lane 1), LPS (lane 2), PamCSK (lane 3), mock supernatant (lane 4), ED (lane 5), ED(WTF-F) (lane 6), ED(WTF-H) (lane 7), ED(WTF-H; N→Y) (lane 8), ED(WTF-F/H) (lane 9), or WTF (lane 10), and RNAs were harvested 12 h later and analyzed by an RNase protection assay with 32P-labeled probes.
TABLE 2.
Induction of monokine-specific transcripts in TAP-1 and HL-60 cells by MV strains and recombinants
| Monokine | Monokine mRNA level after stimulation witha:
|
||||||
|---|---|---|---|---|---|---|---|
| Mock | ED | ED(WTF-F) | ED(WTF-H) | ED(WTF-H;N→Y) | ED(WTF-F/H) | WTF | |
| IL-12 p40 | 0.5 ± 1.3 | 1.8 ± 0.9 | 1.7 ± 1.1 | 12.1 ± 5.5 | 2.0 ± 2.2 | 9.9 ± 1.3* | 10.6 ± 2.7* |
| IL-1α | 0.9 ± 4.2 | −2.2 ± 3.7 | −1.1 ± 3.5 | 12.2 ± 2.2 | 0.2 ± 4.2 | 25.6 ± 3.9* | 29.6 ± 6.2* |
| IL-1β | 1.5 ± 0.6 | 0.0 ± 2.1 | −0.8 ± 2.4 | 36.6 ± 2.8* | 1.0 ± 2.9 | 40.2 ± 1.2* | 58.8 ± 1.5* |
| IL-1 Ra | 7.1 ± 3.3 | 2.6 ± 1.3 | 12.4 ± 1.7 | 66.4 ± 14.8 | 9.9 ± 1.2 | 67.4 ± 2.3* | 55.3 ± 5.4* |
| IL-6 | 1.7 ± 1.7 | 0.3 ± 0.7 | 0.4 ± 1.4 | 21.1 ± 1.3* | 1.7 ± 1.0 | 20.7 ± 2.7* | 30.1 ± 2.7* |
Levels of monokine-specific mRNAs were determined in vitamin D3-treated THP-1 cells 12 h after stimulation by RPA and normalized to those of the L32 housekeeping gene. Values were determined relative to induction by PamCSK (set as 100%) as means of three independent experiments (standard deviations are indicated [P < 0.01]). Statistically significant differences from the control are indicated by asterisks.
FIG. 4.
Activation of TLR2 by viruses expressing the WTF-H protein triggers release of IL-6 and expression of CD150 in human monocytes. (a and b) Cells were left untreated (lane 1) or were stimulated with LPS (lane 2), PamCSK (lane 3), mock supernatant (lane 4), ED (lane 5), ED(WTF-F) (lane 6), ED(WTF-H) (lane 7), ED(WTF-H; N→Y) (lane 8), ED(WTF-F/H) (lane 9), or WTF (lane 10). After 12 h, supernatants were harvested for determination of IL-6 (a), while cells were stained for CD150 surface expression (b). Values represent means of seven (a) or four (b) individual donors and were normalized to the IL-6 content of the inoculum (determined on the basis of the medium control; P ≤ 0.01) (a) or to the medium control (P ≤ 0.01) (b). (c and d) Cells (three donors) were pretreated with TLR2-, TLR4-, or CD14-specific antibodies and incubated with PamCSK (2.5, 0.5, and 0.1 μg/ml) (black bars) or WTF (10, 2 or 0.4 μg/ml of purified virus) (white bars). Alternatively, PamCSK or WTF (same concentrations as above) were treated with MV N- or MV H-specific antibodies prior to incubation with monocytes. Release of IL-6 (c) or induction of CD150 (d) was determined, and inhibition by the antibodies used (percent) was determined with respect to the control in the absence of antibody (P ≤ 0.01). Statistically significant differences from the control are indicated by asterisks.
FIG. 5.
IL-6 release from TLR2−/− murine macrophages cannot be stimulated by MV WTF. IL-6 concentrations (in nanograms per milliliter) were determined in supernatants of peritoneal macrophages from wild-type CH3/HeN (grey bars), C3H/TLR2−/− (black bars), or C3H/HeJ (white bars) (expressing nonfunctional TLR4) cells stimulated for 12 h with medium (lane 1), LPS (lane 2), PamCSK (lane 3), mock (lane 4), ED (lane 5), or WTF (lane 6). Values shown are means of two independent experiments (three animals of each genotype were used per experiment) (P ≤ 0.01). Statistically significant differences from the control are indicated by asterisks.
Since these viruses induced TLR2 signaling and monocyte functions consistent with activation, levels of surface molecules on these cells might also be stimulated. Expression levels of HLA-DR were augmented upon treatment of monocytes with LPS, PamCSK, and viruses containing the WTF-H protein, but not with those expressing the ED-H or WTF-H;N→Y protein (data not shown). Freshly isolated monocytes from healthy individuals do not express CD150. However, this molecule is upregulated after monocyte activation (45), possibly induced by TLR signals. Indeed, we observed upregulation of CD150 on monocytes by WTF-H-containing viruses and not with viruses containing the ED-H and WTF-H;N→Y protein (Fig. 4b). Supporting the importance of TLR2 and WTF-H in this process, induction of CD150 by WTF-H protein-expressing viruses was sensitive to antibodies specific to TLR2 and MV-H but not MV-N (Fig. 4d). Thus, lymphotropic wild-type MV activates human monocytes through TLR2 and triggers surface expression of the MV receptor CD150.
DISCUSSION
Although crucial for overcoming acute measles and conferring long-lasting immunity, the mechanisms of immune induction, and particularly those concerning innate immunity, are largely unknown. The major findings provided by our study are (i) MV can activate cellular signaling via TLR2, (ii) this biological property is confined to wild-type strains, (iii) the H protein of MV wild-type strains is a TLR2 agonist, (iv) a single amino acid exchange is sufficient to abolish the agonistic activity of this protein, and (v) MV wild-type strains induce the expression of their cellular receptor, CD150, through TLR2 in monocytes.
After the first descriptions of viral proteins acting as TLR agonists, the F protein of RSV (35) and the env proteins of murine retroviruses (51), both of which activate TLR4, we have described that the H protein of MV, one of the most important human viral pathogens, recruits TLR2 for cell activation. Lymphotropic wild-type MV specifically activates cells through TLR2, but not TLR4. Thus, MV WTF-mediated TLR activation only occurred on CHO cells transfected to express either human or murine TLR2 and not on those expressing endogenous TLR4 without or together with human TLR4 (Fig. 1a). Confirming that TLR4 activation is not involved in our system, all our reagents were essentially free of endotoxin, as determined by Limulus lysate assays, and inclusion of polymyxin B did not inhibit MV WTF-induced activation of 3E10 hTLR2 cells (data not shown). TLR4 activation by the RSV F protein required coexpression of CD14, which is an essential coreceptor for most TLR2 and TLR4 agonists (2, 12), and this molecule also enhanced TLR2 activation by MV WTF. Thus, reporter gene activation and induction of IL-6 in CHO cells is more efficient in the presence of CD14 (Fig. 1c and 2; Table 1), and antibodies directed against CD14 reduce MV WTF-H protein-induced IL-6 production by monocytes (Fig. 4c). It is evident that the ability of MV wild-type strains to induce TLR2 signaling is independent of the MV receptors CD46 and CD150, which are not expressed by CHO cells. Additionally, both THP-1 cells and unstimulated primary human monocytes do not express CD150 (Fig. 4b) (45). Apparently, CD46 is also not involved, since viruses binding CD46 such as ED barely activate monokine and CD150 expression, whereas viruses not binding CD46 do (Fig. 4).
Most interestingly, the ability to activate TLR2 signaling in both CHO cells and monocytes was confined to lymphotropic MV wild-type strains. The failure of vaccine strains to induce this signaling is not likely to reflect the requirement of coexpression of a heterologous TLR such as TLR6, which is required for TLR2-dependent activation by certain agonists (40, 50, 58). This can be deduced, since ED and ED(WTF-F) fail to activate human monocytes expressing both TLR2 and TLR6 (61) (Fig. 4). The differential ability of MV strains to activate TLR-dependent signaling also argues against a role of TLR3, recently identified as a receptor for dsRNA (3). This is particularly so because induction of IFN-α/β as reported after TLR3 triggering with dsRNA might, if at all with MV, preferentially occur with vaccine and not with wild-type strains (46). The unique requirement for TLR2 in MV WTF monocyte activation was further confirmed with macrophages from TLR2−/− animals (Fig. 5). Since vaccine strains did not activate TLR2 even when applied at high doses and exchange of the H protein converted them into TLR2 agonists, amino acid differences within this protein were more likely to be important. We showed that a single amino acid exchange at position 481 within the WTF-H protein is sufficient to abolish activation of TLR2 signaling (Fig. 2). The structure of the MV H protein has not been resolved as yet; however, a model has been predicted (36). According to this model, amino acids 481 is located within one of the predicted six sheets of the most-membrane-distal domain, which has a propeller-like structure. Although amino acid changes are likely to alter the surface of the molecule, this can only be firmly established once the H protein structure is known. Remarkably, however, the amino acid at position 481 was also found to essentially determine binding to and usage of CD46 as an entry receptor for attenuated MV strains (9, 27, 37, 47). Thus, upon attenuation of wild-type strains in tissue culture and adaptation to utilization of CD46, the property to activate TLR2 signaling is concomitantly lost. This biological property may play an important role for the attenuation of MV vaccine viruses.
Monocyte activation in measles, as assessed by monokine induction, was addressed directly in one study where spontaneous release of IL-6 from peripheral blood mononuclear cells (PBMC) was found to be enhanced (21). Other studies applying clinical material or experimentally MV-infected macaques have relied on secondary cytokine responses in PBMC or monocyte cultures after restimulation in vitro (6, 7, 21). These cannot be compared to our experiments where primary consequences of MV interaction with directly challenged cells were investigated. In vaccinees, spontaneous production of IL-1α from PBMC was suppressed while levels of IL-1β were not affected, and serum levels of tumor necrosis factor alpha (TNF-α) were lower than those seen in controls (62). While MV wild-type strains can induce monocyte activation by triggering TLR2 signaling, attenuated, CD46-adapted strains are obviously, albeit to a more limited extent, also able to activate antigen-presenting cells (APC) in vivo and in vitro in a TLR2-independent manner. After infection of primary human monocytes and THP-1 cells with a Vero cell-adapted and thus CD46-utilizing wild-type strain, low-level secretion of IL-1β and TNF-α occurred (39), and in monocyte-derived DC cultures the replication-competent MV Halle strain, which is closely related to MV ED, induced low levels of IL-12 p40 and high levels of IL-1β transcripts (56). Mechanisms of TLR2-independent monocyte activation are unknown as yet but may include CD46 ligation. In T cells, activation of p120CBL, Vav, Rac, and ERK and stimulation of proliferation were seen upon CD3-CD46 coligation (5, 66), and in murine RAW264.7 monocytic cells ligation of transgenic CD46 enhanced IFN-γ-induced NO production after MV infection (30). In human macrophages, a CD46-specific antibody able to also block MV binding stimulated IL-12 p40 production, suggesting that positive signals can be induced via binding to this particular domain (34). Independent of CD46 ligation, MV replication can also stimulate NF-κB activation directly in certain cell types (13, 23).
Although activation of TLR signaling may play an important role for immune induction by MV wild-type strains, regulation of this pathway by MV wild-type viruses may also contribute to viral pathogenicity and induction of immunosuppression. Thus, CD150 was upregulated on monocytes by LPS, phytohemagglutinin (PHA), and two lymphotropic MV wild-type strains, both live and UV inactivated (45). Together with our observations that CD150 is induced on monocytes treated with WTF-H-containing viruses (Fig. 4b), this is, to the best of our knowledge, the first example of a virus triggering the expression of its own receptor. Partial suppression of this induction by TLR2-specific antibodies strongly supports the notion that TLR2 signaling is important for CD150 induction in monocytes (Fig. 4d). Our analyses do not allow us to distinguish whether CD150 upregulation results directly from TLR signaling or is induced indirectly via released cytokines such as IL-1α/β, which is also induced by this pathway (Fig. 3) and which is known to enhance CD150 expression on mature DC (33). Again in DC, upregulation of CD150 was seen after treatment with LPS but not with lipopeptides which activate TLR2 signaling (10). In contrast, we observed that TLR2-agonistic MV were able to enhance expression of CD150 in monocytes (Fig. 4b). This could reflect differences in the cell type used or a differential ability of TLR2 ligands to induce CD150 expression. Maturation of DC with TLR2 agonists such as PamCSK and the 19-kDa lipopeptide from Mycobacterium tuberculosis has also been described, although expression of CD150 was not addressed (25).
The requirement of CD150 as an entry receptor for MV wild-type strains has been clearly documented (15, 26, 49, 59), and there is compelling evidence for the crucial importance of this molecule in monocyte infection with MV wild-type but not vaccine strains (45). In this study, induction of CD150 on the surface of monocytes by compounds such as LPS, PHA and, interestingly, two MV wild-type strains was seen. While replication of MV vaccine strains, which can enter these cells via CD46 (which is also expressed on unstimulated monocytes), was unaffected by the presence of CD150-specific antibodies, replication of wild-type MV was efficiently blocked by these antibodies. Supporting the importance of CD150 as receptor for MV wild-type strains, the tropism of MV for DC was linked to the expression of the WTF-H protein (48). Thus, induction of CD150 on monocytes via TLR2 may play an essential role in pathogenicity by conferring susceptibility to lymphotropic MV wild-type strains and thereby enhancing not only viral spread but also depletion of monocytes by apoptosis (16, 17). In addition, deregulation of APC functions, such as loss of allostimulatory activity, has been documented after infection, particularly with MV wild-type strains (19, 22, 32, 54). MV strains strongly interacting with CD46 may even actively block TLR activation by bacterial cell wall components. In monocyte cultures, ligation of CD46 by antibodies, but also MV, efficiently prevented the induction of IL-12 by subsequent LPS or Staphylococcus aureus strain Cowan stimulation (29). Similarly, downregulation of stimulated IL-12 release was seen with replication competent but also, albeit less efficiently, with UV-inactivated MV (Halle strain) in DC T-cell cultures (19). Interestingly, surface contact with the MV glycoprotein complex abolishes the activation of the phosphoinositol-3/Akt kinase pathway by IL-2 in T cells (8). Since this particular pathway was found to be involved in TLR2 signaling in monocytes (4), MV could also possibly modulate TLR signaling via this pathway in these cells. Lastly, induction of cross-tolerance to restimulation by TLR agonists after initial activation of TLRs was observed with many bacterial components (38, 52). If triggering of TLR2 signaling by MV on APC were to induce tolerance to subsequent activation by bacterial cell wall components, this could play an important role in the high sensitivity to opportunistic infections associated with acute cases of measles.
Acknowledgments
We thank Michael Rehli, Bert Rima, Sieghart Sopper, and Stefan Niewiesk for helpful discussions, Ian Johnston for generating the MV recombinants, Douglas T. Golenbock for CHO transfectants, Terje Espevik and K. Miyake for providing antibodies, and Sieglinde Löffler, Maren Klett, Sylvia Fichte, Unni Nonstad, and Mari Sörensen for excellent technical assistance.
We also thank the Deutsche Forschungsgemeinschaft, the Bundesministerium für Bildung und Forschung, The Wellcome Trust, the Research Council of Norway, the Norwegian Cancer Society, and the European Communities for financial support.
REFERENCES
- 1.Aarden, L. A., E. R. De Groot, O. L. Schaap, and P. M. Lansdorp. 1987. Production of hybridoma growth factor by human monocytes. Eur. J. Immunol. 17:1411-1417. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., K. Takeda, and T. Kaisho. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675-680. [DOI] [PubMed] [Google Scholar]
- 3.Alexopoulou, L., A. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 4.Arbibe, L., J. P. Mira, N. Teusch, L. Kline, M. Guha, N. Mackman, P. J. Godowski, R. J. Ulevitch, and U. G. Knaus. 2000. Toll-like receptor 2-mediated NF-κB activation requires a RacI-dependent pathway. Nat. Immunol. 1:533-540. [DOI] [PubMed] [Google Scholar]
- 5.Astier, A., M. C. Trescol-Biemont, O. Azocar, B. Lamouille, and C. Rabourdin-Combe. 2000. CD46, a new costimulatory molecule for T cells, that induces p120CBL and LAT phosphorylation. J. Immunol. 164:6091-6095. [DOI] [PubMed] [Google Scholar]
- 6.Atabani, S. F., A. A. Byrnes, A. Jaye, I. M. Kidd, A. F. Magnusen, H. Whittle, and C. L. Karp. 2001. Natural measles causes prolonged suppression of interleukin-12 production. J. Infect. Dis. 184:1-9. [DOI] [PubMed] [Google Scholar]
- 7.Auwaerter, P. G., P. A. Rota, W. R. Elkins, R. J. Adams, T. DeLozier, Y. Shi, W. J. Bellini, B. R. Murphy, and D. E. Griffin. 1999. Measles virus infection in rhesus macaques: altered immune responses and comparison of the virulence of six different virus strains. J. Infect. Dis. 180:950-958. [DOI] [PubMed] [Google Scholar]
- 8.Avota, E., A. Avots, N. Niewiesk, L. P. Kane, U. Bommhardt, V. ter Meulen, and S. Schneider-Schaulies. 2001. Disruption of Akt kinase activation is important for immunosuppression induced by measles virus. Nat. Med. 7:725-731. [DOI] [PubMed] [Google Scholar]
- 9.Bartz, R., U. Brinckmann, L. M. Dunster, B. Rima, V. ter Meulen, and J. Schneider-Schaulies. 1996. Mapping amino acids of the measles virus hemagglutinin responsible for receptor (CD46) downregulation. Virology 224:334-337. [DOI] [PubMed] [Google Scholar]
- 10.Bleharski, J. R., K. R. Niazi, P. A. Sieling, G. Cheng, and R. L. Modlin. 2001. Signaling lymphocytic activation molecule is expressed on CD40 ligand-activated dendritic cells and directly augments production of inflammatory cytokines. J. Immunol. 167:3174-3181. [DOI] [PubMed] [Google Scholar]
- 11.Bowie, A., E. Kiss-Toth, J. A. Symons, G. L. Smith, S. K. Dower, and L. A. J. O'Neill. 2000. A46R and A52R from vaccinia virus are antagonists of host IL-1 and Toll-like receptor signaling. Proc. Natl. Acad. Sci. USA 97:10162-10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brightbill, H. D., and R. L. Modlin. 2000. Toll-like receptors: molecular mechanisms of the mammalian immune response. Immunology 101:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhib-Jalbut, S., J. Xia, H. Rangaviggula, Y.-Y. Fang, and T. Lee. 1999. Failure of measles virus to activate nuclear factor-κB in neuronal cells: implications on the immune response to viral infections in the central nervous system. J. Immunol. 162:4024-4029. [PubMed] [Google Scholar]
- 14.Doerig, R. E., A. Marcil, A. Chopra, and C. D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295-305. [DOI] [PubMed] [Google Scholar]
- 15.Erlenhoefer, C., W. Wurzer, S. Loeffler, S. Schneider-Schaulies, V. ter Meulen, and J. Schneider-Schaulies. 2001. CD150 (SLAM) is a receptor for measles virus but is not involved in contact-mediated proliferation inhibition of lymphocytes. J. Virol. 75:4499-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esolen, L. M., B. J. Ward, T. R. Moench, and D. E. Griffin. 1993. Infection of monocytes during measles. J. Infect. Dis. 168:47-52. [DOI] [PubMed] [Google Scholar]
- 17.Esolen, M. E., S. W. Park, J. M. Hardwick, and D. E. Griffin. 1995. Apoptosis as a cause of death in measles virus-infected cells. J. Virol. 69:3955-3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flo, T. H., O. Halaas, E. Lien, L. Ryan, G. Teti, D. T. Golenbock, A. Sundan, and T. Espevik. 2000. Human Toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J. Immunol. 164:2064-2069. [DOI] [PubMed] [Google Scholar]
- 19.Fugier-Vivier, I., C. Servet-Delprat, P. Rivailler, M. Rissoan, Y. Liu, and C. Rabourdin-Combe. 1997. Measles virus suppresses cell-mediated immunity by interfering with the survival and function of dendritic cells. J. Exp. Med. 186:813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Griffin, D. E. 1995. Immune responses during measles virus infection. Curr. Top. Microbiol. Immunol. 191:117-134. [DOI] [PubMed] [Google Scholar]
- 21.Griffin, D. E., and J. B. Ward. 1993. Differential CD4 T cell activation in measles. J. Infect. Dis. 168:275-281. [DOI] [PubMed] [Google Scholar]
- 22.Grosjean, I., C. Caux, C. Bella, I. Berger, F. Wild, J. Banchereau, and D. Kaiserlian. 1997. Measles virus infects human dendritic cells and blocks their allostimulatory properties for CD4+ T cells. J. Exp. Med. 186:801-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Helin, E., R. Vanionpää, T. Hyypiä, I. Julkunen, and S. Matikainen. 2001. Measles virus activates NF-κB and STAT transcription factors and production of IFN-α/β and IL-6 in the human epithelial cell line A549. Virology 290:1-10. [DOI] [PubMed] [Google Scholar]
- 24.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 25.Hertz, C. J., S. M. Kiertschner, P. J. Godowski, D. A. Bouis, M. V. Norgard, M. D. Roth, and R. L. Modlin. 2001. Microbial lipopeptides stimulate dendritic cell maturation via Toll-like receptor 2. J. Immunol. 166:2444-2450. [DOI] [PubMed] [Google Scholar]
- 26.Hsu, E. C., C. Iorio, F. Sarangi, A. A. Khine, and C. D. Richardson. 2001. CDw150 (SLAM) is a receptor for a lymphotropic strain of measles virus and may account for the immunosuppressive properties of this virus. Virology 279:9-21. [DOI] [PubMed] [Google Scholar]
- 27.Hsu, E. C., F. Sarangi, C. Iorio, M. S. Sidhu, S. A. Udem, D. L. Dillehay, W. Xu, P. A. Rota, W. J. Bellini, and C. D. Richardson. 1998. A single amino acid change in the hemagglutinin protein of measles virus determines its ability to bind CD46 and reveals another receptor on marmoset B cells. J. Virol. 72:2905-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Johnston, I. C. D., V. ter Meulen, J. Schneider-Schaulies, and S. Schneider-Schaulies. 1999. A recombinant measles vaccine virus expressing wild-type glycoproteins: consequences for viral spread and cell tropism. J. Virol. 73:6903-6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karp, C. L., M. Wysocka, L. M. Wahl, J. M. Ahearn, P. J. Cuomo, B. Sherry, G. Trinchieri, and D. E. Griffin. 1996. Mechanism of suppression of cell-mediated immunity by measles virus. Science 273:228-231. [DOI] [PubMed] [Google Scholar]
- 30.Katayama, Y., A. Hirano, and T. C. Wong. 2000. Human receptor for measles virus (CD46) enhances nitric oxide production and restricts virus replication in mouse macrophages by modulating the production of alpha/beta interferon. J. Virol. 74:1252-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klagge, I. M., and S. Schneider-Schaulies. 1999. Virus interactions with dendritic cells. J. Gen. Virol. 80:823-833. [DOI] [PubMed] [Google Scholar]
- 32.Klagge, I. M., V. ter Meulen, and S. Schneider-Schaulies. 2000. Measles virus-induced promotion of dendritic cell maturation by soluble mediators does not overcome the immunosuppressive activity of viral glycoproteins on the cell surface. Eur. J. Immunol. 30:2741-2750. [DOI] [PubMed] [Google Scholar]
- 33.Kruse, M., E. Meinl, G. Henning, C. Kuhnt, S. Berchtold, T. Berger, G. Schuler, and A. Steinkasserer. 2001. Signaling lymphocyte activation molecule is expressed on mature CD83+ dendritic cells and is upregulated by IL-1β. J. Immunol. 167:1989-1995. [DOI] [PubMed] [Google Scholar]
- 34.Kurita-Taniguchi, M., A. Fukui, K. Hazeki, A. Hirano, S. Tsuji, M. Matsumoto, M. Watanabe, S. Ueda, and T. Seya. 2000. Functional modulation of human macrophages through CD46 (measles virus receptor): production of IL-12 p40 and nitric oxide in association with recruitment of protein-tyrosine phosphatase SHIP-1 to CD46. J. Immunol. 165:5143-5152. [DOI] [PubMed] [Google Scholar]
- 35.Kurt-Jones, E. A., L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Tripp, E. E. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. W. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398-401. [DOI] [PubMed] [Google Scholar]
- 36.Langendijk, J. P. M., F. J. Daus, and J. T. van Oirschot. 1997. Sequence and structure alignment of paramyxoviridae attachment proteins and discovery of enzymatic activity for a morbillivirus hemagglutinin. J. Virol. 71:6155-6167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lecouturier, V., J. Fayolle, M. Caballero, J. Carabana, M. L. Celma, R. Fernandez-Munoz, T. F. Wild, and R. Buckland. 1996. Identification of two amino acids in the hemagglutinin glycoprotein of measles virus (MV) that govern hemadsorption, HeLa cell fusion, and CD46 downregulation: phenotypic markers that differentiate vaccine and wild-type MV strains. J. Virol. 70:4200-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lehner, M. D., S. Morath, K. S. Michelsen, R. R. Schumann, and T. Hartung. 2001. Induction of cross-tolerance by lipopolysaccharide and highly purified lipoteichoic acid via different Toll-like receptors independent of paracrine mediators. J. Immunol. 166:5161-5167. [DOI] [PubMed] [Google Scholar]
- 39.Leopardi, R., R. Vainoonpaa, M. Hurme, P. Siljander, and A. A. Salmi. 1992. Measles virus infection enhances IL-1 beta but reduces tumor necrosis factor-alpha expression in human monocytes. J. Immunol. 149:2397-2401. [PubMed] [Google Scholar]
- 40.Li, M., D. F. Carpio, Y. Zheng, P. Bruzzo, V. Singh, F. Ouaaz, R. M. Medzhitov, and A. A. Beg. 2001. An essential role of the NF-κB/Toll like receptor pathway in induction of inflammatory and tissue repair gene expression by necrotic cells. J. Immunol. 166:7128-7135. [DOI] [PubMed] [Google Scholar]
- 41.Lien, E., T. K. Means, H. Heine, A. Yoshimura, S. Kusumoto, K. Fukase, M. J. Fenton, M. Oikawa, N. Qureshi, B. Monks, R. W. Finberg, R. R. Ingalls, and D. T. Golenbock. 2000. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J. Clin. Investig. 105:497-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lien, E., J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 43.Manchester, M., D. S. Eto, A. Valsamakis, P. B. Liton, R. Fernandez-Munoz, P. A. Rota, W. J. Bellini, D. N. Forthal, and M. B. A. Oldstone. 2000. Clinical isolates of measles virus use CD46 as a cellular receptor. J. Virol. 74:3967-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Means, T. K., B. W. Jones, A. B. Schromm, B. A. Shurtleff, J. A. Smith, J. Keane, D. T. Golenbock, S. N. Vogel, and M. J. Fenton. 2001. Differential effects of a Toll-like receptor antagonist on Mycobacterium tuberculosis induced macrophage responses. J. Immunol. 166:4074-4082. [DOI] [PubMed] [Google Scholar]
- 45.Minagawa, H., K. Tanaka, N. Ono, H. Tatsuo, and Y. Yanagi. 2001. Induction of the measles virus receptor SLAM (CD150) on monocytes. J. Gen. Virol. 82:2913-2917. [DOI] [PubMed] [Google Scholar]
- 46.Naniche, D., A. Yeh, D. Eto, M. Manchester, R. M. Friedman, and M. B. A. Oldstone. 2000. Evasion of host defenses by measles virus: wild-type measles virus infection interferes with induction of alpha/beta interferon production. J. Virol. 74:7478-7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nielsen, L., M. Blixenkrone-Moller, M. Thylstrup, N. J. V. Hansen, and G. Bolt. 2001. Adaptation of wild-type measles virus to CD46 receptor usage. Arch. Virol. 146:197-208. [DOI] [PubMed] [Google Scholar]
- 48.Ohgimoto, S., K. Ohgimoto, S. Niewiesk, I. M. Klagge, J. Pfeuffer, I. C. D. Johnston, J. Schneider-Schaulies, A. Weidmann, V. ter Meulen, and S. Schneider-Schaulies. 2001. The hemagglutinin protein is an important determinant for measles virus tropism for dendritic cells in vitro and immunosuppression in vivo. J. Gen. Virol. 82:1835-1844. [DOI] [PubMed] [Google Scholar]
- 49.Ono, N., H. Tatsuo, Y. Hidaka, T. Aoki, H. Minagawa, and Y. Yanagi. 2001. Measles viruses of throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not 46 as a cellular receptor. J. Virol. 75:4399-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between Toll-like receptors. Proc. Natl. Acad. Sci. USA 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rassa, J. C., J. L. Meyers, Y. Zahng, R. Kudaravalli, and S. R. Ross. 2002. Murine retroviruses activate B cells via interaction with Toll-like receptor 4. Proc. Natl. Acad. Sci. USA 99:2281-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schade, F. U., S. Flash, M. Flohe, M. Majetschak, E. Kreuzfelder, E. Dominguez-Fernandez, J. Borgermann, M. Reuter, and U. Obertacke. 1999. Endotoxin tolerance, p. 50-67. In H. Brade, S. M. Opal, S. N. Vogel, and D. C. Morrison (ed.), Endotoxin in health and disease. Dekker, New York, N.Y.
- 53.Schneider-Schaulies, S., S. Niewiesk, J. Schneider-Schaulies, and V. ter Meulen. 2001. Measles virus induced immunosuppression: targets and effector mechanisms. Curr. Mol. Med. 1:163-182. [DOI] [PubMed] [Google Scholar]
- 54.Schnorr, J. J., S. Xanthakos, P. Keikavoussi, E. Kaempgen, V. ter Meulen, and S. Schneider-Schaulies. 1997. Induction of maturation of human blood dendritic cell precursors by measles virus is associated with immunosuppression. Proc. Natl. Acad. Sci. USA 94:5326-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 56.Servet-Delprat, C., O. Vidalain, H. Bausinger, O. Manie, F. Le Deist, O. Azocar, A. Fischer, and C. Rabourdin-Combe. 2000. Measles virus induces abnormal differentiation of CD40-ligand activated human dendritic cells. J. Immunol. 164:1753-1760. [DOI] [PubMed] [Google Scholar]
- 57.Takeuchi, O., K. Hoshino, T. Kawai, H. Sanjo, H. Takada, T. Ogawa, K. Takeda, and S. Akira. 1999. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 11:443-451. [DOI] [PubMed] [Google Scholar]
- 58.Takeuchi, O., T. Kawai, P. F. Mühlradt, M. Morr, J. D. Radolf, A. Zychlinsky, K. Takeda, and S. Akira. 2001. Discrimination of bacterial lipopropteins by Toll-like receptor 6. Int. Immunol. 13:933-940. [DOI] [PubMed] [Google Scholar]
- 59.Tatsuo, H., N. Ono, and Y. Yanagi. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406:893-897. [DOI] [PubMed] [Google Scholar]
- 60.Van Binnendijk, R. S., R. W. J. van der Heijden, and A. D. M. E. Osterhaus. 1995. Monkeys in measles research. Curr. Top. Microbiol. Immunol. 191:135-148. [DOI] [PubMed] [Google Scholar]
- 61.Visintin, A., A. Mazzoni, J. H. Spitzer, D. H. Wyllie, S. K. Dower, and D. M. Segal. 2001. Regulation of Toll-like receptors in human monocytes and dendritic cells. J. Immunol. 166:249-255. [DOI] [PubMed] [Google Scholar]
- 62.Ward, B. J., and D. E. Griffin. 1993. Changes in cytokine production after measles virus vaccination: predominant production of IL-4 suggests induction of a Th2 response. Clin. Immunol. Immunopathol. 67:171-177. [DOI] [PubMed] [Google Scholar]
- 63.Weidmann, A., C. Fischer, S. Ohgimoto, C. Rueth, V. ter Meulen, and S. Schneider-Schaulies. 2000. Measles virus-induced immunosuppression in vitro is independent of complex glycosylation of viral glycoproteins and hemifusion. J. Virol. 74:7548-7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wooten, R. M., Y. Ma, R. A. Yoder, J. P. Brown, J. H. Weis, J. F. Zachary, C. J. Kirschning, and J. J. Weis. 2002. Toll-like receptor 2 is required for innate, but not acquired host defense to Borrelia burgdorferi. J. Immunol. 168:348-355. [DOI] [PubMed] [Google Scholar]
- 65.Yoshimura, A., E. Lien, R. R. Ingalls, E. Tuomanen, R. Dziarksi, and D. Golenbock. 1999. Recognition of gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J. Immunol. 163:1-5. [PubMed] [Google Scholar]
- 66.Zaffran, Y., O. Destaing, A. Roux, S. Ory, T. Nheu, P. Jurdic, C. Rabourdin-Combe, and A. L. Astier. 2001. CD46/CD3 costimulation induces morphological changes of human T cells and activation of Vav, Rac, and extracellular signal-related kinase mitogen-activated protein kinase. J. Immunol. 167:6780-6785. [DOI] [PubMed] [Google Scholar]