Abstract
Simian varicella virus (SVV) infection of primates shares clinical, pathological, immunological, and virological features with varicella-zoster virus infection of humans. Natural varicella infection was simulated by exposing four SVV-seronegative monkeys to monkeys inoculated intratracheally with SVV, in which viral DNA and RNA persist in multiple tissues for more than 1 year (T. M. White, R. Mahalingam, V. Traina-Dorge, and D. H. Gilden, J. Neurovirol. 8:191-205, 2002). The four naturally exposed monkeys developed mild varicella 10 to 14 days later, and skin scrapings taken at the time of the rash contained SVV DNA. Analysis of multiple ganglia, liver, and lung tissues from the four naturally exposed monkeys sacrificed 6 to 8 weeks after resolution of the rash revealed SVV DNA in ganglia at multiple levels of the neuraxis but not in the lung or liver tissue of any of the four monkeys. This animal model provides an experimental system to gain information about varicella latency with direct relevance to the human disease.
Primary varicella-zoster virus (VZV) infection causes chicken pox (varicella). The virus then becomes latent in ganglia and reactivates decades later to produce shingles (zoster) and multiple other neurological complications (3). VZV causes disease only in humans. Attempts to produce the disease by experimental infection of animals with VZV have led to seroconversion but not to disease (14). However, clinical, pathological, immunological, and virological evidence suggests that simian varicella virus (SVV) infection of nonhuman primates is the counterpart of human VZV infection (10, 12).
Both the 1968 and 1974 outbreaks of varicella in Erythrocebus patas monkeys at the Tulane National Primate Research Center were attributed to reactivated SVV (11). Further, SVV is antigenically related to human VZV (2), and sequence analysis of the complete genome of SVV has revealed a high degree of homology between the two viruses at the nucleic acid and protein levels (5).
Studies with nonhuman primates inoculated intratracheally with SVV showed that multiple tissues, including blood mononuclear cells (MNCs), contain viral DNA and RNA for months after experimental infection (13). These findings differ from those for humans latently infected with VZV, in which case virus is present only in ganglia and viral transcription is limited (1). To develop a model of SVV latency that mimics VZV latency in humans, we simulated natural infection by exposing SVV-seronegative monkeys to monkeys that had been inoculated intratracheally with SVV, as primary infection is thought to occur through aerosol or droplet exposure of respiratory secretions from actively infected individuals. We observed the monkeys clinically for the development of varicella. Skin, blood MNCs, ganglia, and lung and liver tissue were analyzed for virus DNA.
MATERIALS AND METHODS
SVV and inoculation.
The deltaherpesvirus strain of SVV, isolated from a naturally infected monkey (E. patas), was propagated in Vero cells, and a virus stock was prepared as described previously (6). Two SVV-seronegative African green monkeys were inoculated intratracheally with 103 PFU of SVV as described previously (7). After 0 to 3 days, each of these monkeys was placed in a cage with two SVV-seronegative African green monkeys (monkeys 165 and 166 and monkeys 190 and 191). All monkeys were examined daily. Blood was obtained once a week from all monkeys until they were sacrificed 2 months later. Total body varicella developed 10 to 12 days later in the two monkeys that received the virus intratracheally. In the four monkeys that were naturally exposed to SVV, a mild rash occurred 10 to 14 days later, at which time the monkeys were anesthetized and the skin area containing the rash was cleaned and scraped with a sterile swab into sterile medium.
DNA isolation.
DNA was extracted by using a QIAamp blood mini kit (Qiagen, Valencia, Calif.) from blood MNCs, from the African green monkey kidney line BSC-1 infected with SVV (4), and from medium containing the skin rash scrapings. All monkeys were euthanized 6 to 8 weeks after intratracheal inoculation or natural exposure, and total DNA was extracted from either individual or pooled trigeminal, cervical, thoracic, lumbar, and sacral ganglia as well as from liver and lung tissue by using a DNeasy tissue kit (Qiagen) according to the manufacturer's instructions.
PCR.
Because both transcriptional and translational products of its VZV homologue have been identified in human ganglia during latency (1, 8), we used oligonucleotide primers (Sigma-Genosys, St. Louis, Mo.) and probes specific for SVV open reading frame (ORF) 63 to amplify and detect SVV DNA as described previously (13). DNA (1 μg) extracted from tissue or MNCs was scored as positive if it was detectable in at least one of the triplicate PCRs carried out. As a control, DNA was omitted in one reaction tube.
RESULTS AND DISCUSSION
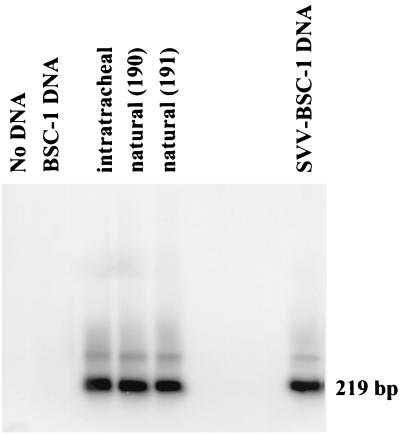
The two monkeys that had been inoculated intratracheally with SVV developed diffuse varicella 10 to 12 days later. The four monkeys that were caged with each of the intratracheally infected monkeys developed a mild rash 2 weeks later. Analysis of the DNA extracted from skin scrapings of one intratracheally inoculated monkey and monkeys 190 and 191, which were caged with the inoculated monkey, revealed SVV ORF 63 sequences in the skin of all three monkeys (Fig. 1), indicating that the rash in the simulated natural infection was caused by SVV.
FIG. 1.
Detection of SVV DNA in skin scrapings of rash areas from monkeys infected naturally or intratracheally with SVV. DNA samples extracted from scrapings of rash from a monkey intratracheally infected with SVV and from two monkeys (no. 190 and 191) exposed to the experimentally infected monkey were analyzed by nested PCR with primers and probes specific for SVV ORF 63 to amplify a 219-bp fragment. No DNA was included in one of the reactions (No DNA). DNA samples from uninfected (BSC-1 DNA) and SVV-infected (SVV-BSC-1 DNA) BSC-1 cells in culture were used as negative and positive controls, respectively.
In the four monkeys that developed varicella after natural exposure, blood MNCs were obtained once a week for the next 2 months. SVV DNA was detected in one monkey (no. 190) 10 days later (data not shown), consistent with the peak viremic phase seen days before and at the time of varicella infection (9). SVV DNA was not detected in blood MNCs at any other times or at necropsy from any of the four naturally infected monkeys (data not shown). It is unclear why the three other naturally infected monkeys that developed varicella were not viremic and why MNCs of only one monkey revealed SVV DNA. Perhaps the milder disease, indicated by minimal rash in the naturally infected monkeys compared with extensive rash in the intratracheally inoculated monkeys, accounts for this.
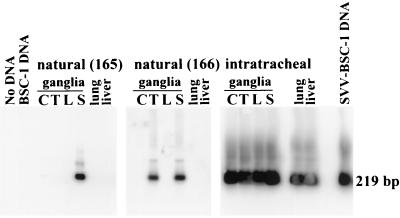
Six to 8 weeks after resolution of the rash, the four naturally infected monkeys were sacrificed and ganglionic and nonganglionic tissues were harvested. Pools of ganglia were prepared from multiple dermatomes (cervical, thoracic, lumbar, and sacral) of two naturally infected monkeys (no. 165 and 166) and from one intratracheally inoculated monkey. PCR analysis of DNA extracted from these pools of ganglia and from the lungs and livers of all three monkeys revealed sequences specific for SVV ORF 63 in all of the samples from the intratracheally inoculated monkey, consistent with earlier observations that SVV is found in multiple tissues months after experimental infection (13). SVV DNA was detected in pooled sacral ganglia of one naturally infected monkey (no. 165) and in pooled thoracic and pooled sacral ganglia of another naturally infected money (no. 166) but not in the lung or liver tissue of either monkey (Fig. 2 and Table 1).
FIG. 2.
Detection of SVV DNA in ganglia from monkeys naturally infected with SVV. DNA samples extracted from pooled cervical (C), thoracic (T), lumbar (L), and sacral (S) ganglia and from the lungs and livers of two monkeys (no. 165 and 166) exposed to an intratracheally infected monkey (no. 164) were analyzed by nested PCR with primers and probes specific for SVV ORF 63 to amplify a 219-bp fragment. No DNA was included in one of the reactions (No DNA). DNA samples from uninfected (BSC-1 DNA) and SVV-infected (SVV-BSC-1 DNA) BSC-1 cells in culture were used as negative and positive controls, respectively.
TABLE 1.
PCR detection of SVV DNA in ganglia of naturally infected monkeys
| Monkey no. | No. positive/total no. examined of ganglion type
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Trigeminal (Inda) | Cervical
|
Thoracic
|
Lumbar
|
Sacral
|
|||||
| Pooled | Ind | Pooled | Ind | Pooled | Ind | Pooled | Ind | ||
| 165 | NDb | 0/1 | ND | 0/1 | ND | 0/1 | ND | 1/1 | ND |
| 166 | ND | 0/1 | ND | 1/1 | ND | 0/1 | ND | 1/1 | ND |
| 190 | 1/2 | 1/2 | 5/8 | 4/4 | ND | 0/3 | 2/4 | ND | 0/3 |
| 191 | 0/2 | 0/2 | 0/8 | 3/6 | ND | 0/3 | 2/4 | ND | 0/6 |
Ind, individual ganglia.
ND, not done.
Because pooling multiple ganglia for DNA extraction might have diluted any low-abundance SVV DNA present in individual ganglia from each monkey, we examined DNA extracted from both individual and pooled ganglia of two naturally infected monkeys (no. 190 and 191) (Table 1). When analyzed individually, one of the trigeminal ganglia from monkey no. 190, but neither of the two trigeminal ganglia from monkey no. 191, revealed SVV DNA. Five of eight cervical ganglia from monkey no. 190, compared to none of eight cervical ganglia from monkey no. 191, contained SVV DNA. Because of their small size, individual thoracic ganglia were not tested. In both monkeys, SVV DNA was found in two of four lumbar ganglia, but not in sacral ganglia. Overall, SVV DNA was detected in 8 of 17 ganglia in monkey no. 190 and in 2 of 20 ganglia of monkey no. 191, both of which were naturally infected.
In pools of two to four ganglia from monkeys 190 and 191, SVV DNA was detected in cervical ganglia in one of two pools from monkey no. 190 but not in either of two pools from monkey no. 191 (Table 1). In thoracic ganglia, SVV DNA was present in four of four pools from monkey no. 190 and in three of six pools from monkey no. 191. None of the three pools of lumbar ganglia from either monkey revealed SVV DNA. Together, the results obtained from PCR analysis of individual and pooled ganglia indicate that SVV DNA was distributed along the trigeminal, cervical, thoracic, and lumbar regions in monkey no. 190 and only in the thoracic and lumbar regions in monkey no. 191. Overall, the detection of SVV DNA in ganglia but not in the lung or liver tissue from any of four naturally infected monkeys suggests the presence of latent infection in the ganglia.
It was previously shown that intratracheal inoculation of SVV results in persistence of viral DNA in multiple tissues for many months (13). In contrast, in the naturally infected monkeys described herein, virus DNA was restricted to the ganglia. The virological differences could reflect the large amount of virus administered intratracheally compared to a presumed smaller aerosol inoculum in the naturally exposed monkeys. Taken together, SVV infection of monkeys provides a unique animal model that closely resembles human VZV infection. Our model will allow analysis of the physical state of varicella nucleic acid in latently infected ganglia (e.g., cell type and extent of transcription and translation) and experiments to reactivate the virus from latency.
Acknowledgments
This work was supported in part by NIH grants NS 32623 and AG 06127.
We thank Marina Hoffman for editorial review and Cathy Allen for preparing the manuscript.
REFERENCES
- 1.Cohrs, R. J., M. Barbour, and D. H. Gilden. 1996. Varicella-zoster virus (VZV) transcription during latency in human ganglia: detection of transcripts mapping to genes 21, 29, 62, and 63 in a cDNA library enriched for VZV RNA. J. Virol. 70:2789-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felsenfeld, A. D., and N. J. Schmidt. 1977. Antigenic relationships among several simian varicella-like viruses and varicella-zoster virus. Infect. Immun. 15:807-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilden, D. H., B. K. Kleinschmidt-DeMasters, J. J. LaGuardia, R. Mahalingam, and R. J. Cohrs. 2000. Neurologic complications of the reactivation of varicella-zoster virus. N. Engl. J. Med. 342:635-645. [DOI] [PubMed] [Google Scholar]
- 4.Gilden, D. H., Y. Shtram, A. Friedmann, M. Wellish, M. Devlin, A. Cohen, N. Fraser, and Y. Becker. 1982. Extraction of cell-associated varicella-zoster virus DNA with Triton X-100-NaCl. J. Virol. Methods 4:263-275. [DOI] [PubMed] [Google Scholar]
- 5.Gray, W. L., B. Starnes, M. W. White, and R. Mahalingam. 2001. The DNA sequence of the simian varicella virus genome. Virology 284:123-130. [DOI] [PubMed] [Google Scholar]
- 6.Mahalingam, R., P. Clarke, M. Wellish, A. N. Dueland, K. F. Soike, D. H. Gilden, and R. Cohrs. 1992. Prevalence and distribution of latent simian varicella virus DNA in monkey ganglia. Virology 188:193-197. [DOI] [PubMed] [Google Scholar]
- 7.Mahalingam, R., D. Smith, M. Wellish, W. Wolf, A. N. Dueland, R. Cohrs, K. Soike, and D. Gilden. 1991. Simian varicella virus DNA in dorsal root ganglia. Proc. Natl. Acad. Sci. USA 88:2750-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahalingam, R., M. Wellish, R. Cohrs, S. Debrus, J. Piette, B. Rentier, and D. H. Gilden. 1996. Expression of protein encoded by varicella-zoster virus open reading frame 63 in latently infected human ganglionic neurons. Proc. Natl. Acad. Sci. USA 93:2122-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozaki, T., T. Ichikawa, Y. Matsui, T. Nagai, Y. Asano, K. Yamanishi, and M. Takahashi. 1984. Viremic phase in nonimmunocompromised children with varicella. J. Pediatr. 104:85-87. [DOI] [PubMed] [Google Scholar]
- 10.Roberts, E. D., G. B. Baskin, K. Soike, and S. V. Gibson. 1984. Pathologic changes of experimental simian varicella (Delta herpesvirus) infection in African green monkeys (Cercopithecus aethiops). Am. J. Vet. Res. 45:523-530. [PubMed] [Google Scholar]
- 11.Soike, K. F. 1992. Simian varicella virus infection in African and Asian monkeys. The potential for development of antivirals for animal diseases. Ann. N. Y. Acad. Sci. 653:323-333. [DOI] [PubMed] [Google Scholar]
- 12.Wenner, H. A., D. Abel, S. Barrick, and P. Seshumurty. 1997. Clinical and pathogenetic studies of Medical Lake macaque virus infections in cynomolgus monkeys (simian varicella). J. Infect. Dis. 135:611-622. [DOI] [PubMed] [Google Scholar]
- 13.White, T. M., R. Mahalingam, V. Traina-Dorge, and D. H. Gilden. 2002. Simian varicella virus DNA is present and transcribed months after experimental infection of adult African green monkeys. J. Neurovirol. 8:191-205. [DOI] [PubMed] [Google Scholar]
- 14.Wroblewska, Z., M. Devlin, K. Reilly, H. van Trieste, M. Wellish, and D. H. Gilden. 1982. The production of varicella zoster virus antiserum in laboratory animals. Arch. Virol. 74:233-238. [DOI] [PubMed] [Google Scholar]