Abstract
The paramyxovirus template for transcription and genome replication consists of the RNA genome encapsidated by the nucleocapsid protein (N protein). The activity of the complex, consisting of viral polymerase plus template, can be measured with minireplicons in which the genomic coding sequence is replaced by chloramphenical acetyltransferase (CAT) antisense RNA. Using this approach, we showed that the C-terminal 24 amino acids of the measles virus N protein are dispensable for transcription and replication, based upon the truncation of N proteins used to support minireplicon reporter gene expression. Truncation at the C-terminal or penultimate amino acid 524 resulted in no change in CAT expression, whereas larger truncations spanning residues 523 to 502 were accompanied by an approximately twofold increase in basal activity. Reporter gene expression was enhanced by supplementation with the major inducible 70-kDa heat shock protein (Hsp72) for minireplicons with the N protein or the N protein truncated at position 525 or 524 but not in systems with a truncation at position 523 or 522. Naturally occurring sequence variants of the N protein with variations at positions 522 and 523 were also shown to lack Hsp72 responsiveness independent of changes in basal activity. Since these residues lie within a linear sequence predicting a direct Hsp72 interaction, N protein-Hsp72 binding reactions were analyzed by using surface plasmon resonance technology. Truncation of the C-terminal portion of the N protein by protease digestion resulted in a reduced binding affinity between Hsp72 and the N protein. Furthermore, with synthetic peptides, we established a correlation between the functional responsiveness and the binding affinity for Hsp72 of C-terminal N protein sequences. Collectively, these results show that the C-terminal 24 amino acids of the N protein represent a regulatory domain containing a functional motif that mediates a direct interaction with Hsp72.
Measles virus (MV) is a negative-strand RNA virus within the Morbillivirus genus of the Paramyxoviridae family. The MV transcriptional complex consists of the virus-encoded RNA-dependent RNA polymerase (L), the polymerase cofactor (P), and a ribonucleoprotein template consisting of the single-stranded RNA genome and the nucleocapsid protein (N protein). N protein monomers assemble on the RNA genome during replication to form a single-start left-handed helix, protecting the genome from nuclease degradation. Encapsidation is initiated on specific sequences found within the leader RNA, drawing upon pools of soluble N-P heterodimers, whereas elongation of encapsidation occurs in an RNA sequence-independent manner (7, 24). Since genomic replication presumably is dependent upon concurrent encapsidation, functional motifs in the N protein required for genomic replication must include an RNA binding domain for the initiation of encapsidation, a binding site for P to form an N-P encapsidation complex, and N-N interaction sites required to drive nucleocapsid elongation and maintain nucleocapsid structural integrity. Additionally, N protein must contain a P binding site that is exposed on the nucleocapsid, thereby permitting the viral polymerase complex to interact with formed ribonucleoprotein templates during both transcription and genomic replication.
The expression of MV N protein deletion mutants demonstrates that only the amino-terminal three-fourths of the molecule (i.e., amino acids 1 to 398) is required for the formation of organized nucleocapsid-like particles, localizing the N-N interaction domain to this highly conserved portion of the protein (1, 16). This conclusion is supported by the observation that selective proteolysis of the MV nucleocapsid can remove the C-terminal 15 kDa of the N protein while leaving nucleocapsid structural integrity and the amino-terminal 45-kDa N protein fragment intact (12). Also identified within the latter region are two sites necessary for the formation of soluble N-P complexes, localized to amino acids 4 to 188 and 304 to 373 (1). Enhanced sensitivity of the C terminus of the N protein to proteolysis is consistent with its exposure on the surface of the formed nucleocapsid. Within this exposed hypervariable domain is a third binding site for P, tentatively localized between amino acids 457 and 525 (1). For Sendai virus, the C-terminal domain of the N protein is required for template function in RNA replication assays (5) and contains a P binding site (4). Given the essential role of P in supporting viral polymerase function, a model for paramyxoviruses emerges in which P provides the link between L and N protein domains that are exposed on the nucleocapsid and that are necessary for both transcription and replication.
The putative template function of the MV N protein C terminus makes it a potential mediator of changes in transcription and replication induced by cellular factors such as the major inducible 70-kDa heat shock protein (Hsp72). Hsp72 stimulates the transcriptional activities of both MV and the closely related canine distemper virus (CDV) (19, 20, 27); effects on genome replication have not been defined. For both MV and CDV, stimulation of transcription is associated with ATP-dependent reversible binding between Hsp72 and the nucleocapsid (19, 26). Exposed patches of hydrophobicity (i.e., accessible hydrophobicity) in native proteins are targets of ATP-dependent reversible low-affinity heat shock protein binding (6). The result of such heat shock protein-native protein interactions can be a change in the conformation and/or function of the protein substrate known as heat shock protein-mediated activity control (10). The C termini of the MV and CDV N proteins contain conserved hydrophobic patches (e.g., amino acids 517 to 524 of Edmonston MV) that are candidate Hsp72-interactive domains. Direct evidence supporting the MV N protein as a target of Hsp72-nucleocapsid interactions includes heat shock-induced colocalization of Hsp72 and N protein but not Hsp72 and P within CDV-infected cells (18). In addition, the stoichiometry of Hsp72-nucleocapsid complexes is consistent with a target with a copy number higher than can be accounted for by P or L (17).
The objective of the present work was to define the extent of the C terminus of the N protein that provides the template function supporting basal transcription and replication. For this approach, N proteins with C-terminal truncations were tested for their ability to support reporter gene expression from MV minireplicons (20, 21). Minireplicons were expressed from cDNA representing the genomic termini of Edmonston MV flanking the chloramphenicol acetyltransferase (CAT) coding sequence. The template function of N protein-encapsidated minireplicon RNA was thus measured by coexpressing P and L in transfected cells and subsequently analyzing CAT activity in cell extracts. Portions of the C terminus not necessary for template function were then tested for their ability to mediate the stimulation of minireplicon reporter gene expression by Hsp72. The N protein variants examined included naturally occurring sequence polymorphisms in the C-terminal four amino acids. Our results showed that these terminal residues influence the ability of Hsp72 to stimulate transcription and replication, where sequence-dependent responsiveness is proportional to the binding affinity of these sequences for Hsp72.
MATERIALS AND METHODS
Minireplicon reporter gene expression.
HEp-2 cells were transfected simultaneously with four plasmids encoding the Edmonston MV N protein (pT7MV-N), P (pT7MV-P), and L (pT7MV-L) and the MV minigenomic RNA representing genomic termini flanking CAT coding sequences (pMV107:CAT) (23). The T7 RNA polymerase promoter directed expression from the constructs; T7 RNA polymerase was produced by the replication-deficient recombinant vaccinia virus MVAT7, kindly provided by Bernard Moss (28). Analysis of reporter gene expression was based upon modifications of the procedure described by Parks et al. (20).
HEp-2 cells were maintained in 5% CO2 at 37°C in minimum essential medium with Earle's salts and supplemented with 10% fetal bovine serum. Calcium phosphate transfections (calcium phosphate transfection kit; Sigma) were performed with 80% confluent cell monolayers in six-well plates. Each transfection was done with 0.2 μg of pMV107:CAT, 0.8 μg of pT7MV-N, 0.6 μg of pT7MV-P, and 0.2 μg of pT7MV-L. Also included was 0.2 μg of pT7-GL3-Luc, derived by subcloning the insert of pGL3-Luc (Promega, Madison, Wis.) into the pT7 vector backbone, so that T7-mediated expression of luciferase could be used as a control for transfection efficiency. Cells were infected with MVAT7 at a multiplicity of infection of 2 concurrent with transfection. Medium was replaced after 6 h, and the cells were harvested 30 h posttransfection in 250 μl of reporter lysis buffer (Promega) for analysis of CAT and luciferase reporter gene expression.
CAT activity in 50 μl of extract was measured by using a FAST CAT yellow (deoxy) chloramphenicol acetyltransferase assay kit (Molecular Probes Inc., Eugene, Oreg.). The fluorescent deoxychloramphenicol substrate yields only a single acetylated product. Reaction products were resolved by silica gel thin-layer chromatography, and the UV fluorescence intensity of both the substrate and the acetylated product was quantified by using an AlphaImager 2000 gel documentation and analysis system supported by AlphaEase software. The signal was measured by using a rhodamine band-pass filter, and the camera aperture was adjusted so that the signal was in the linear range of detection. Line densitometry was used to compute percent conversion of the substrate to the acetylated product, and two-dimensional spot densitometry was used to quantify the yield of the acetylated product. Twenty microliters of product was analyzed for luciferase activity by using a luciferase assay system (Promega) and a Perkin-Elmer LS-5B luminescence spectrometer.
N protein mutagenesis.
Oligonucleotide-mediated site-directed mutagenesis of pT7MV-N was done by the method of Kunkel et al. (15). Nucleotide substitutions were designed to create premature stop codons that, in turn, supported the production of N protein C-terminal truncations. Mutagenic primers incorporated additional nucleotide substitutions that did not affect coding but did add or eliminate a unique restriction endonuclease site (i.e., the addition of a BsmAI or an EcoNI site; the deletion of a BsrGI, an XbaI, or an NcoI site). Alteration of these sites facilitated the screening of transformants by restriction analysis of plasmid DNA. In brief, dut ung mutant Escherichia coli RZ1032 was transformed to produce single-stranded pT7MV-N that contained uracil in place of thymidine. Mutagenic oligonucleotide primers were annealed to the single-stranded pT7MV-N, extended, and ligated in vitro, and the double-stranded plasmid was used to transform E. coli DH5α.
Mutagenic oligonucleotides for each of the truncations were as follows, with nucleotide substitutions underlined: NC-1 (5′-CGGCCTCTCGCACCTACTATAGAAGATTTC-3′); NC-2 (5′-CGCACCTAGTCTCAAAGATTTCTGTC-3′); NC-3 (5′-CGCACCTAGTCTAGTTAATTTCTGTCATTGTAC-3′); NC-4 (5′-CGCACCTAGTCTAGTAGTTATCTGTCATTGTAC-3′); NC-9 (5′-CTGTCATTGTACTATATAGGGGTGTCCG-3′); NC-16 (5′-GTCCGTGTCTCAGCCTTGTTCTTCCG-3′); NC-24 (5′-CCGAGATTCCTTACATGGCTTGCAGC-3′); NC-25 (5′-CGAGATTCCTGCCTAGGCTTGCAGCC-3′); NC-26 (5′-CCGAGATTCCTGCCATTCATTGCAGCCTAAGC-3′); and NC-31 (5′-GCTTGCAGCCTAAGCTAGGCGTCAGC-3′).
Bidirectional sequence analysis of the C terminus of the N coding region was used to confirm the presence of the desired mutation(s) and to rule out the presence of second site mutations. The upstream sequencing primer was located at positions 1094 to 1113 and the downstream primer was located at nucleotides 1622 to 1643 relative to the start of the N gene coding region. The parent plasmid pT7MV-N contains the N gene coding region of 1,575 nucleotides, a 63-nucleotide 3′ noncoding region (nucleotides 1576 to 1639), and a polyadenylation signal incorporated into the pT7 vector. Sequence analysis was done with a fluorescence automated 3700 DNA analyzer (Applied Biosystems Inc.) and BigDye Terminator cycle sequencing chemistry.
Coding mutations were also generated on the basis of naturally occurring MV sequence polymorphisms representing amino acid substitutions in the C-terminal four amino acids. Nucleotide substitutions included silent mutations that resulted in the loss of an XbaI site and/or the addition of a BsmAI or a BglII site. Oligonucleotide sequences used to generate the mutants were as follows, with nucleotide substitutions underlined: NΔ4D (5′-CCTAGTCTAGAAGGTCTCTGTCATTGTAC-3′); NΔ3P4D (5′-CGCACCTAGTCTAGGGGATCTCTGTCATTGTAC-3′); and NΔ2P4D (5′-CGCACCTAGTCTGGAAGATCTCTGTCATTGTAC-3′).
Resultant plasmids were used in support of minireplicon reporter gene expression as described above, with substitution of the mutant N protein plasmid (pT7MV-NC-n) for the parent plasmid pT7MV-N. Cell extracts used for CAT and luciferase assays were also analyzed by Western blot analysis for N protein expression. The primary mouse monoclonal antibody recognized the amino-terminal portion of the N protein and is described elsewhere (2). Total protein was resolved by sodium dodecyl sulfate (SDS)-12% polyacrylamide gel electrophoresis (PAGE), transferred to nitrocellulose membranes, and probed with primary antibody; bound primary antibody was detected by using alkaline phosphatase-conjugated secondary antibody with 5-bromo-4-chloro-3-indolylphosphate (BCIP)-nitroblue tetrazolium colorimetric signal development.
Hsp72-dependent transcription and replication.
The expression vector encoding an epitope-tagged Hsp72 protein was generated in a plasmid designed for T7 RNA polymerase-mediated expression. The vector backbone (pT7-VSV) was created by first excising the measles virus L gene from plasmid pEMC-La (21) and then replacing the excised gene with a double-stranded oligonucleotide (5′-CCATGGCTTACACCGACATCGAGATGAACCGGCTGGGCAAGGAGCTCACCTCGAGGGCCCGGGGATATCCAGATCT-3′) that encoded the vesicular stomatitis virus (VSV) G protein epitope tag and included sequence-specifying restriction sites suitable for subsequent cloning 3′ of the epitope coding sequence. Upon transfer of the Hsp72 cDNA sequence from a previously described clone, pT7-Hsp72 (20), the first two amino-terminal Hsp70 amino acid codons were replaced with a DNA sequence encoding MAYTIDIEMNRLGKELTSRAP (the VSV G protein epitope is underlined) to generate plasmid pT7VSV-Hsp72. The resultant construct was characterized by DNA sequencing.
To measure Hsp72-dependent stimulation of minireplicon reporter gene expression, 0, 1.25, or 2.5 μg of pT7VSV-Hsp72 was added to transfection mixtures containing 0.2 μg of pMV107:CAT, 0.8 μg of pT7MV-N, 0.6 μg of pT7MV-P, 0.2 μg of pT7MV-L, and 0.2 μg of pT7-GL3-Luc per well. The insert was deleted from the pT7VSV-Hsp72 construct to generate pT7-null, and the latter was added to each transfection mixture so that a total of 7 μg of plasmid was added to each cell monolayer. Infection with MVAT7, harvest, and analysis of CAT and luciferase reporter gene expression were performed as described above. The expression of Hsp72 was confirmed by Western blot analysis of cell extracts with a mouse monoclonal antibody recognizing either the VSV G protein epitope (P5D4; Boehringer Mannheim) or Hsp72 (SPA-810; StressGen Inc., Victoria, British Columbia, Canada).
Protein-peptide interaction analysis.
Binding between purified Hsp72 and peptides representing N protein C-terminal sequences or the MV nucleocapsid was analyzed by using BIAcore3000 (Pharmacia, Uppsala, Sweden), a system for real-time biomolecular interaction analysis that is based upon surface plasmon resonance technology. Purified human recombinant Hsp72 was obtained commercially (StressGen) and covalently bound to carboxy-methyl groups of CM5 sensor chips by using amine-coupling chemistry (Biosensor AB; Pharmacia). The levels of Hsp72 immobilized depended upon the size of the analyte; the binding of small peptide analytes required higher immobilization levels in order to detect binding events. In addition, higher levels of immobilized Hsp72 were used to establish binding specificity, whereas lower levels were used to characterize binding reactions. The latter facilitated kinetic analyses. Remaining flow channels on the sensor chip included a control for a nonspecific interaction with the sensor chip and a control for nonspecific interactions with irrelevant protein targets. Protein-peptide analytes were passed over the sensor chip in HBS-P buffer (0.01 M HEPES [pH 7.4], 0.15 M NaCl, 0.005% Surfactant P-20) containing 2.5 mM magnesium acetate and 2.5 mM ATP. Sensorgrams plotted changes in surface plasmon resonance (measured in response units [RU] and reflecting protein mass on the sensor surface) as a function of time. Multiple sensorgrams representing various analyte concentrations were analyzed by using BIAevaluation 3.1 software. Global fitting of experimental data to well-characterized binding reactions was used to define reaction rate constants.
Analytes included the Edmonston MV nucleocapsid purified from the cytoplasm of infected Vero cells as previously described (17, 26). In brief, Vero cells were infected at an multiplicity of infection of 0.1 and harvested at 60 h postinfection, the time of maximal cytopathic effect. The nucleocapsid was purified from clarified cytoplasmic extracts by CsCl isopycnic density centrifugation. Nucleocapsid-containing gradient fractions were pooled and dialyzed against HBS-P buffer, and the total protein concentration was measured by using a bicinchoninic acid assay. Nucleocapsid total protein was also evaluated by SDS-12% PAGE followed by Coomassie staining. Densitometric analysis of N, P, and L band intensities was compared to that of serial dilutions of bovine serum albumin to confirm the results of bicinchoninic acid analysis and to establish the proportion of each protein. The identity of protein bands was confirmed by Western blot immunoreactivity to an anti-MV polyclonal antiserum and an N protein-specific monoclonal antibody.
Selective proteolysis of N protein contained in the nucleocapsid was performed by using Staphylococcus aureus V8 protease (Pierce, Rockford, Ill.). Digestion was performed at 37°C with HBS-P buffer. Titration of the V8 protease concentration and digest time was performed in order to establish conditions for the selective proteolysis of only the C-terminal 15 kDa of the N protein while maintaining the integrity of the 45-kDa fragment and minimizing the levels of undigested N protein; 4.8 U of V8 protease/ml in a 20-min digest yielded optimal results. Digestion reaction products were analyzed by Western blot analysis for N protein expression as described above.
Synthetic peptide analytes were 15 amino acids long and represented the extreme C terminus of the MV N protein (N peptide: DTDTPIVYNDRNLLD), N peptide containing an asparagine (N)-to-D substitution at the fourth residue upstream from the C terminus (NΔ4D peptide: DTDTPIVYNDRDLLD), NΔ4D peptide containing an L-to-P substitution at the third residue upstream from the C terminus (NΔ3P4D peptide: DTDTPIVYNDRDPLD), a p53-derived peptide (p53 peptide: STSRHKKLMFKTEGP), and a myelin basic protein-derived peptide (MBP peptide: YGSLPQKAQRPQDEN). MBP peptide was from a commercial source (Sigma). The remaining peptides were custom synthesized (Genemed Synthesis Inc., San Francisco, Calif.). All peptides were purified to >95% and characterized by high-pressure liquid chromatography and mass spectral analyses.
RESULTS
C-terminal truncations of the N protein and template function.
The expression of intact N protein together with viral polymerase L and polymerase cofactor P was used to define baseline minireplicon reporter gene expression. The CAT activity in a given cell extract was adjusted for differences in transfection efficiency based upon T7 RNA polymerase-mediated luciferase reporter gene expression by pT7-GL3-Luc. Transfection of HEp-2 cells to express these products was performed in triplicate, and the resultant CAT activity was expressed as an average. Transfections excluding pT7MV-L and thus the expression of L served as a negative control to show that CAT expression was viral polymerase dependent (Fig. 1).
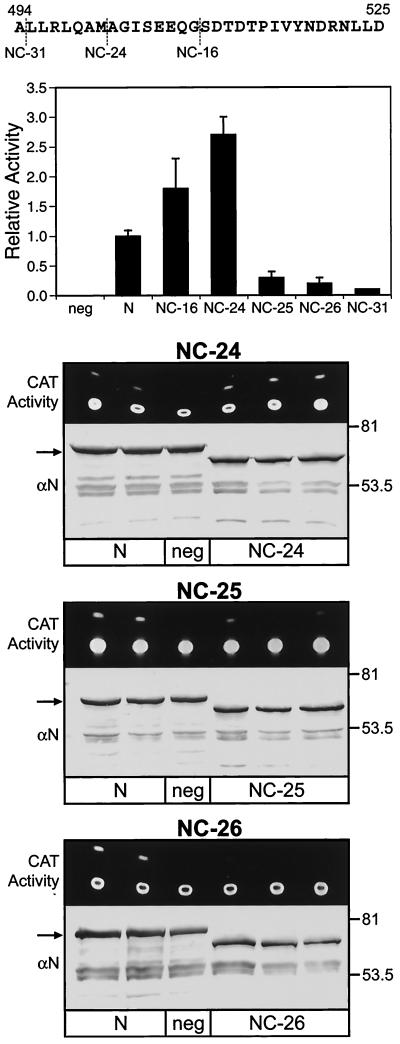
FIG. 1.
Effect of N protein C-terminal truncations on MV minireplicon CAT reporter gene expression. Duplicate or triplicate cell monolayers were transfected with plasmids directing the expression of minigenomic RNA and transcripts for P, L, and either parental N or N proteins lacking the C-terminal 16 (NC-16), 24 (NC-24), 25 (NC-25), 26 (NC-26), or 31 (NC-31) amino acids. The sequence of the C-terminal 32 amino acids of the 525-amino-acid Edmonston MV N protein is illustrated at the top. Cell extracts were processed separatelyfor measurement of CAT activity; results are expressed as an experimental average with correction for transfection efficiency, and that value was averaged for three trials. The latter average and standard error of the mean are presented in the histogram. Results were normalized to CAT activity supported by parental N protein, where that value equals 1.0; transfections with constructs expressing parental N protein were run in parallel with transfections with N protein truncation constructs. Raw data for one trial are presented for NC-24, NC-25, and NC-26. These data include the chromatogram for fluorescent CAT reaction products (i.e., substrate and monoacetylated product, UV illumination) and Western blot analysis of cell extracts with an anti-N protein monoclonal antibody (αN) as a probe. The positions of molecular size markers (kilodaltons) for the latter are indicated to the right. An arrow indicates the position of the 60-kDa N protein. The N protein truncation mutants exhibited greater electrophoretic mobility, corresponding to an apparent mass of 57 kDa, with degradation products migrating at between 45 and 55 kDa. Transfections lacking L constructs but expressing the template were used as negative controls (neg) for CAT activity.
Deletion of the C-terminal 16 and 24 amino acids of the N protein resulted in 1.8-and 2.7-fold increases in minireplicon CAT expression, respectively, relative to that supported by the N protein (Fig. 1). Deletion of the C-terminal 25, 26, and 31 amino acids resulted in 3-, 5-, and 10-fold reductions in basal transcription and replication. Cell extracts used in these CAT assays were analyzed by Western blot analysis with a monoclonal antibody specific to the amino-terminal portion of the N protein. Results showed that differences in minireplicon reporter gene expression were not correlated with differences in the expression of the N protein or truncated N protein. The expression of the N protein in controls lacking L expression was equivalent to that in reactions including L, indicating that the amount of the N protein available to provide template function was relatively constant. Truncations resulted in an electrophoretic mobility consistent with a mass of approximately 57 kDa; the mass of the N protein is 60 kDa. Significant differences in electrophoretic mobility between the truncated N proteins were not observed.
Hsp72-dependent transcription and replication.
An Hsp72 expression construct (pT7VSV-Hsp72) was added to transfections that included plasmids expressing N, P, L, and luciferase transcripts and MV minigenomic RNA. Increasing the amount of pT7VSV-Hsp72 from 0 to 1.0 or 2.5 μg per transfection resulted in a dosage-dependent increase in the amount of Hsp72 detected in cell extracts by Western blot analysis (Fig. 2). Immunoblotting was done with probes directed at Hsp72-specific epitopes, detecting both endogenous and transgenic Hsp72, or the VSV G protein epitope. The latter clearly distinguished transgenic from endogenous Hsp72. Quantification of CAT activity in cell extracts showed progressive increases in minireplicon reporter gene expression with 1.0 and 2.5 μg of pT7VSV-Hsp72 supplementation. Transfections were performed in duplicate, and the average CAT activity per treatment was compared to that in controls lacking Hsp72 supplementation. The Hsp72-dependent increase in minireplicon reporter gene expression was not associated with increases in viral N protein concentrations in cell extracts, as determined by Western blot analysis. In addition, Hsp72 supplementation did not affect transfection efficiency, as defined by luciferase reporter gene expression from pT7-GL3-Luc (data not shown). At higher levels of pT7VSV-Hsp72 supplementation (i.e., 5 μg/transfection), the levels of N protein were markedly reduced and CAT activity relative to that in transfections lacking Hsp72 supplementation was reduced by an average of 13-fold. An analysis of interassay variability showed differences in the level of Hsp72 supplementation that supported maximal stimulation of CAT reporter gene expression and in the magnitude of that stimulation (data not shown). Similar results were obtained when Hsp72 was expressed by pT7-Hsp72, indicating that the biphasic dose response was not specific to the pT7VSV-Hsp72 construct (data not shown). Accordingly, all subsequent analyses were done with two levels of pT7VSV-Hsp72 supplementation, and the results of multiple consecutive trials were averaged.
FIG. 2.
Hsp72 dosage-dependent stimulation of MV minireplicon CAT reporter gene expression. Cell monolayers were transfected in duplicate with plasmids directing the expression of minigenomic RNA, transcripts for N, P, and L proteins, and an Hsp72 fusion protein containing the VSV G protein epitope. The histogram indicates average CAT activity in transfections with 1.0, 2.5, or 5.0 μg of the Hsp72 expression plasmid pT7VSV-Hsp72 relative to CAT activity in transfections lacking the Hsp72 expression plasmid. The average CAT activity in the latter was defined as 1.0. Transfections lacking the L protein expression plasmid were used as negative controls (neg). Corresponding chromatograms of CAT reaction products visualized under UV light are shown. Western blot analysis of cell extracts used for CAT assays was done with a monoclonal antibody recognizing either Hsp72-specific epitopes (α-Hsp), the VSV G protein tag of transgenic Hsp72 (α-VSV-G), or the MV N protein (α-N). The positions of molecular size markers are indicated to the right (kilodaltons).
Preliminary studies showed that the addition of Hsp72 to transfections failed to enhance minireplicon reporter gene expression when the NC-16 and NC-24 truncation mutants provided the template function, tentatively localizing N protein determinants of functional Hsp72 responsiveness to amino acids 510 to 525. To identify the extent of the C terminus required to support Hsp72 responsiveness, sequential C-terminal N protein truncations were generated beginning with the terminal amino acid (NC-1) and increasing the size of the truncation in a stepwise manner (i.e., NC-2, NC-3,… NC-8, etc.). A comparison of minireplicon reporter gene expression supported by truncated N protein versus parental N protein was carried out as described above. Both NC-1 and NC-2 supported basal and Hsp72-dependent enhanced CAT reporter gene expression that was equivalent to that seen with parental N protein (Fig. 3). The level of Hsp72 supplementation yielding the greatest increase in CAT activity was used for purposes of comparison. The results of three consecutive experimental trials were averaged, and the stimulation of CAT reporter gene expression was shown to be statistically significant (P < 0.05; one-tailed Student's t test).
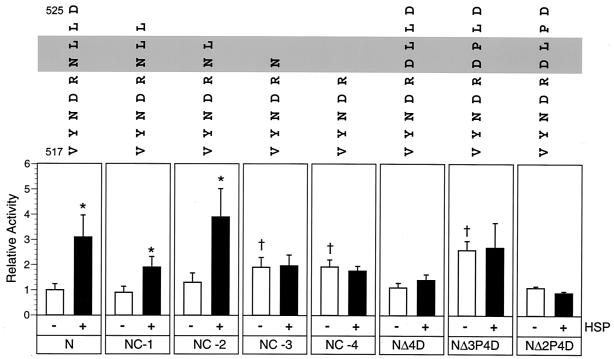
FIG. 3.
Hsp72-dependent stimulation of MV minireplicon CAT reporter gene expression when N protein or N protein mutants provide the template function. Cell monolayers were transfected in triplicate with plasmids directing the expression of minigenomic RNA and transcripts for P, L, parental N, and mutant N proteins. The N protein mutants included C-terminal truncations or amino acid substitutions representing naturally occurring sequence polymorphisms. Truncated proteins lacked the last one (NC-1), two (NC-2), three (NC-3), or four (NC-4) amino acids. Amino acid substitutions replaced the asparagine at amino acid 522 of the N protein with aspartic acid (NΔ4D). The NΔ3P4D and NΔ2P4D mutants contained an additional leucine-to-proline substitution at amino acids 523 and 524, respectively. Hsp72 supplementation was done by adding either 1.0 or 2.5 μg of pT7VSV-Hsp72 per transfection. Cell extracts were processed separately for measurement of CAT activity; results are expressed as an experimental average with correction for transfection efficiency, and that value was averaged for three separate but consecutive trials. The mean and standard error of the mean are presented relative to the activity supported by parental N protein, where the latter equals 1.0. The Hsp72 supplementation level yielding the greatest stimulation of reporter gene expression (black bars) was compared to results for treatment groups lacking Hsp72 supplementation (white bars). Western blot analyses showed constant levels of N antigen expression between treatment groups when those levels were adjusted for transfection efficiency (data not shown). Significant differences in reporter gene expression in Hsp72-supplemented versus nonsupplemented reactions were identified by using Student's t test (P < 0.05) and are indicated by an asterisk. Similarly, significant differences between basal activities supported by mutant N protein and parental N protein are indicated by a dagger. The C-terminal sequence of each N construct is indicated above the corresponding histogram, beginning at amino acid 517. Amino acids 522 to 523 (grey) significantly influence either basal or Hsp72-dependent minireplicon reporter gene expression.
Deletion of the C-terminal three or four amino acids of the N protein resulted in an approximately 1.8-fold increase in minireplicon reporter gene expression relative to that supported by parental N protein (Fig. 3), a difference that was statistically significant (P < 0.05). In addition, Hsp72 supplementation failed to stimulate activity when NC-3 or NC-4 provided the template function, whereas Hsp72 responsiveness with parental N protein was demonstrated in the same experimental trial. The increase in basal activity and the lack of responsiveness to Hsp72 were thus analogous to results obtained with NC-16 and NC-24 and with truncations of an intermediate size, including NC-8 (data not shown).
Naturally occurring MV sequence polymorphisms affecting the base composition of the last four (C-terminal) amino acids were identified (14), and the changes were incorporated into the parental N protein backbone. The mutations included an asparagine-to-aspartic acid substitution at amino acid 522 (corresponding to the NC-4 position and designated NΔ4D), a change found in 67% of the reported sequences. Additional mutants had the substitution at position 522 plus a leucine-to-proline substitution at position 523 (NΔ3P4D) or 524 (NΔ2P4D); these polymorphisms were identified in only 3 isolates, from a total of 100 examined. The NΔ4D construct supported minireplicon CAT expression to a degree equivalent to that observed with parental N protein, although stimulation following Hsp72 supplementation was not observed (Fig. 3). The additional leucine-to-proline substitution at amino acid 524 in NΔ2P4D did not affect the pattern of minireplicon CAT expression seen with NΔ4D. However, substitution of proline for leucine at amino acid 523 in NΔ3P4D resulted in a 2.6-fold increase in basal activity relative to that supported by parental N protein in addition to the loss of Hsp72 responsiveness.
Hsp72 binding reactions.
Evidence supporting the direct binding of Hsp72 to the C terminus of the N protein came from an analysis of binding between purified human recombinant Hsp72 and synthetic peptides. The peptide sequences were based upon the C-terminal 15 amino acids of the N protein (N peptide). Commercially prepared Hsp72 was produced from the same coding sequence as that used for the construction of pT7VSV-Hsp72. Binding reactions were analyzed by using BIAcore3000 instrumentation, where changes in surface plasmon resonance were monitored in real time as the peptide was passed over sensor chips to which Hsp72 was covalently coupled. This analytic approach is ideally suited to reversible low-affinity protein-protein interactions that typify biologically relevant Hsp72 binding to native protein substrates. Moreover, reaction rate and equilibrium constants were calculated for reactions with different protein substrates, allowing differences in Hsp72 binding affinities to be readily quantified. Changes in protein mass on the sensor surface (e.g., a result of ligand immobilization or analyte-ligand binding) induced changes in surface plasmon resonance that were recorded as RU; 1,000 RU equal a change in mass of 1 ng/mm2. Flow buffers containing the peptide analyte also contained 2.5 mM ATP, allowing for reversible Hsp72-substrate interactions that reproduce physiologic conditions.
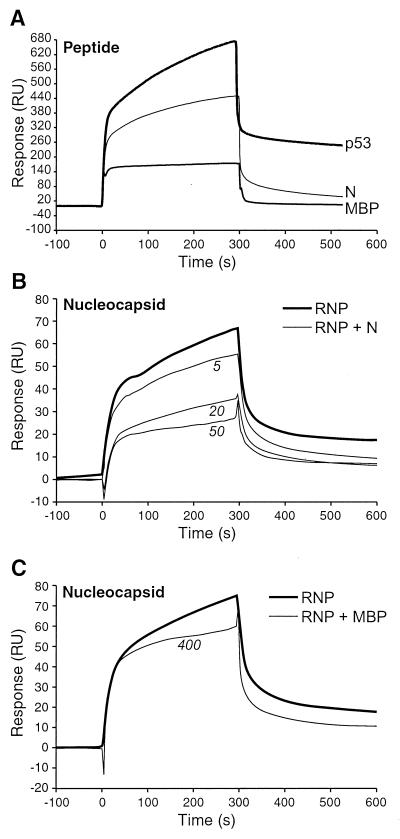
The specificity of binding between N peptide and Hsp72 was shown by using a 1 mM peptide solution and 20,000 RU of immobilized Hsp72 (Fig. 4A). A 15-amino-acid synthetic peptide derived from the myelin basic protein sequence (MBP peptide) was used as a negative-control analyte, having an amino acid composition predicted not to bind to the 70-kDa heat shock protein (11). The MBP peptide binding curve was identical to that of N peptide passed over negative-control flow channels. These channels contained either no ligand, a control for nonspecific interactions between the analyte and the sensor surface, or a flow channel to which 6,300 RU of immunoglobulin were conjugated (a control for nonspecific protein-protein interactions). The maximal response recorded for these negative controls was 180 RU, in contrast to 460 RU of maximal binding between N peptide and Hsp72, a 280 RU increase over the background. A 15-amino-acid peptide representing the 70-kDa heat shock protein binding motif of p53 (p53 peptide) was used as a positive control for these experiments (9), generating a response that was 480 RU above the background. The demonstration of specific binding was repeatable, although the amplitude of maximal specific binding varied.
FIG. 4.
BIAcore3000 sensorgrams illustrating the binding of synthetic peptides to immobilized Hsp72 or peptide competitive inhibition of nucleocapsid-Hsp72 binding. (A) Binding assays. Solutions of peptides (1 mM) representing the Hsp72-interactive domain of p53 (p53), the C terminus of the MV N protein (N), or an immunogenic domain of myelin basic protein (MBP) were passed over sensor chips to which Hsp72 was conjugated. All peptides were 15 amino acids long. Retention of a peptide on the sensor chip was indicated by a change in RU over the course of the 300-s injection interval. Background peptide-sensor chip interactions were defined by the negative-control MBP peptide; these sensorgrams were identical to those of N peptide passed over negative-control sensor flow channels (data not shown). (B and C) Competitive inhibition assays. (B) MV ribonucleoprotein core particles (RNP or nucleocapsid) representing 1 nM N protein were passed over sensor chips in the presence or absence of N peptide. N peptide concentrations of 5, 20, and 50 μM decreased the magnitude of RNP binding to immobilized Hsp72 in a peptide dosage-dependent manner. (C) The same competitive inhibition experiment was performed to compare RNP binding to Hsp72 with or without the addition of the negative-control MBP peptide. Minimal inhibition was observed at peptide concentrations of as high as 400 μM.
N peptide mediated competitive inhibition of nucleocapsid-Hsp72 binding reactions, establishing the relevance of analyses done with peptide alone. The Edmonston MV nucleocapsid purified from the cytoplasm of infected Vero cells had a density of 1.32 g/ml, a value consistent with published reports (25). The protein composition of nucleocapsid preparations, defined by SDS-PAGE and densitometric analysis of Coomassie blue staining intensity, was 29:3:1 for N-P-L. Nucleocapsid preparations were sheared to generate the uniform small particle size required to support binding curves free of particulate-induced artifacts (e.g., sensorgram spikes). The addition of 5, 20, and 50 μM N peptide to nucleocapsid solutions representing 1 nM N protein readily inhibited nucleocapsid binding to 20,000 RU of Hsp72 (Fig. 4B). The inhibition was dose dependent, decreasing nucleocapsid binding by 15, 46, and 62%, respectively. In contrast, nucleocapsid binding was diminished by only 20% when 400 μM MBP peptide was used as the competitive inhibitor (Fig. 4C).
The affinity of binding between N peptide and Hsp72 was established by using 10,000 RU of immobilized Hsp72 and peptide concentrations of 25, 50, 100, 200, 500, and 1,000 μM (Fig. 5). Dosage-dependent binding was observed in this range. A signal above the background was not detected for peptide concentrations of less than 25 μM when Hsp72 was immobilized at this lower level. Reactions conformed to a 1:1 ligand-substrate (Langmuir) binding model, exhibiting the best fit (i.e., a χ2 value of <1 and residuals within the range of ±2) when data were combined for the four peptide solution concentrations closest to the calculated equilibrium dissociation constant (KD) of 1 μM (i.e., 25, 50, 100, and 200 μM peptide concentrations). This binding affinity was within the range observed for peptide p53-Hsp72 binding reactions (8). Analysis of nucleocapsid-Hsp72 binding affinity was calculated by using 250 RU of immobilized Hsp72 and nucleocapsid N protein concentrations of 5, 10, 20, 40, and 80 nM (Fig. 6). The binding affinity determined by global analysis of all binding reactions was 16 nM, a 60-fold increase in affinity relative to that seen with N peptide. Deletion of the C-terminal 15 kDa of the N protein was performed by selective proteolysis of nucleocapsid total protein to show the contribution of the N protein C terminus to nucleocapsid-Hsp72 binding (Fig. 6). The binding affinity was calculated for V8-digested nucleocapsid, where >90% of the N protein had been converted to the 45-kDa form. Analysis with the same molar concentrations of the N protein indicated a KD of 428 nM, a 27-fold decrease in binding affinity relative to that seen with parental nucleocapsid.
FIG. 5.
BIAcore3000 sensorgrams illustrating dosage-dependent binding of synthetic peptides to 10,000 RU of immobilized Hsp72. Peptides were 15 amino acids long and represented the C terminus of Edmonston MV N protein (N peptide), the N peptide with an asparagine-to-aspartic acid substitution at amino acid 4 from the C terminus (NΔ4D peptide), and the NΔ4D peptide with a leucine-to-proline substitution at amino acid 3 from the C terminus (NΔ3P4D peptide). Peptide-Hsp72 responses were corrected for nonspecific interactions with the sensor surface by subtracting the signal recorded when peptides were passed over sensor flow channels lacking Hsp72. The corrected signal was then expressed as a response differential (Resp. Diff.) in RU. Sensorgrams for the interaction between Hsp72 and N peptide concentrations of 25, 50, 100, and 200 μM allowed high-quality global fitting to a 1:1 Langmuir binding model. Calculated equilibrium dissociation constants (KD) and rate constants ka and kd are shown. High-quality global fitting for NΔ4D peptide and NΔ3P4D peptide was based upon sensorgams generated with 100, 200, 500, and 1,000 μM peptide concentrations.
FIG. 6.
BIAcore3000 sensorgram illustrating dosage-dependent binding of MV ribonucleoprotein core particles (RNP or nucleocapsid) to 250 RU of immobilized Hsp72. The RNP-Hsp72 responses were corrected for nonspecific interactions with the sensor surface by subtracting the signal recorded when RNP was passed over sensor flow channels lacking Hsp72. The corrected signal was then expressed as a response differential (Resp. Diff.) in RU. Sensorgrams for the interaction between Hsp72 and RNP N protein concentrations of 5, 10, 20, 40, and 80 nM allowed high-quality global fitting to a 1:1 Langmuir binding model, allowing the computation of KD and association and dissociation rate constants ka and kd. This binding reaction was compared to that of RNP subjected to selective proteolysis with S. aureus V8 protease. Western blot analysis of RNP and V8-digested RNP (RNP+V8) was done with an MV N protein-specific monoclonal antibody as a probe to show the conversion of the 60-kDa N protein to the 45-kDa amino-terminal three-fourths of the N protein (panel at lower right; numbers to the right of the panel are in kilodaltons). The affinity of binding between V8-digested RNP and Hsp72 was calculated as described for RNP.
Peptides that incorporated naturally occurring sequence polymorphisms, corresponding to mutant N proteins that failed to respond to heat shock protein stimulation of minireplicon transcription and replication, had binding affinities that were several hundredfold lower than that seen with parental N peptide (Fig. 5). A synthetic peptide of 15 amino acids was prepared that incorporated the asparagine-to-aspartic acid substitution at amino acid 4 from the C terminus (NΔ4D peptide). A second peptide was generated that incorporated the additional leucine-to-proline substitution three amino acids from the C terminus (NΔ3P4D peptide). An analysis of binding reactions was performed in parallel with the N peptide binding reaction analysis described above. Reactions for NΔ4D peptide and NΔ3P4D peptide conformed to the Langmuir binding model, exhibiting the best fit when data were combined for the four peptide solution concentrations closest to the calculated KDs of 782 and 329 μM (i.e., 100, 200, 500, and 1,000 μM peptide concentrations), respectively. Examination of reaction rate constants showed that a decrease in the association rate constant (ka) was most responsible for the lower level of Hsp72 binding exhibited by mutant peptides relative to N peptide. The results were repeatable, with no change in the relative affinities for Hsp72 and N peptide or Hsp72 and the NΔ4D and NΔ3P4D sequence variants. However, Hsp72 binding affinity for positive-control and test peptides diminished with repeated use; subsequent analysis of the binding reaction for Hsp72 and N peptide yielded a KD of 4 μM, in contrast to 15 and 4 mM for NΔ4D peptide and NΔ3P4D peptide, respectively.
DISCUSSION
The results of this study show that the C-terminal 24 amino acids of the MV N protein are not required as a template during minigenome transcription and replication by viral P and L. Deletion of the C-terminal or penultimate amino acid had no effect on basal minireplicon CAT expression. In contrast, N protein truncations spanning NC-3 through NC-24 supported basal activity that was significantly elevated over that supported by parental N protein, the difference being 1.8- to 2.7-fold. Western blot analysis showed that the differences in minireplicon reporter gene expression did not reflect changes in the amount of the N protein providing the template function. This finding suggests that amino acids 502 to 523 of the N protein dampen basal transcription and replication, possibly by interacting with cellular and/or viral modulatory elements or by limiting access of the viral polymerase to the genomic RNA template. A precipitous decline in CAT expression was observed when C-terminal truncations included amino acids 501 through 495 of the N protein. Whereas truncations at amino acid 502 resulted in a 2.7-fold increase in basal activity, truncations at amino acid 501 resulted in a 70% decrease in activity relative to that supported by parental N protein. Elimination of sequences required for N-P interactions on the nucleocapsid should reduce basal transcription and replication, and such a domain has been tentatively localized to amino acids 457 to 525 of the N protein by Bankamp et al. (1). Our results suggest that such localization can be refined to amino acids 457 to 501. Moreover, amino acids 495 to 501 represent a sequence that is strictly conserved in 99 out of a total of 100 MV strains reported by Kreis et al. (14). Conservation of such a motif in an otherwise hypervariable domain of the N protein is consistent with such an essential function.
The C-terminal and penultimate amino acids of the N protein are dispensable for both basal and Hsp72-dependent transcription and replication. This conclusion is based upon the observation that Hsp72 enhanced minireplicon reporter gene expression when N protein truncation mutants NC-1 and NC-2 formed the RNP template. The response to Hsp72 was similar to that recorded when the parental N construct provided the template function. Hsp72 supplementation does not affect the level of the N protein expressed from the plasmid, consistent with our observation that Hsp72 supplementation does not affect transfection efficiency and the observation of Sedger et al. that Hsp72 does not influence vaccinia virus replication (22). These findings support the conclusion that it is the activity and not the amount of the N protein that is the basis for Hsp72-dependent increases in minireplicon reporter gene expression.
Since minireplicon reporter gene expression reflects both genomic transcription and replication, selective effects of Hsp72 on transcription must await the recovery of cytoplasmic nucleocapsid from recombinant infectious virus expressing Hsp72-responsive and non-Hsp72-responsive C-terminal motifs. The cell-free transcriptional activity of these nucleocapsids can then be measured in the presence or absence of Hsp72. Using this approach, it was shown that Hsp72 directly enhances CDV nucleocapsid transcriptional activity (19) and that MV nucleocapsid-Hsp72 complexes exhibit enhanced transcriptional activity relative to that of an equal amount of nucleocapsid lacking Hsp72 (26, 27). Although Hsp72 stimulates the cell-free transcriptional activity of purified CDV nucleocapsid, supplementation at or above 20 μg/ml (0.29 μM) results in a dramatic loss in activity (19), a result attributed to destabilization of the nucleocapsid helix. Parks et al. (20) reported a similar biphasic dose response of MV minireplicon reporter gene expression to Hsp72 supplementation. In the present work, however, the reduced CAT activity at high levels of Hsp72 supplementation were correlated with reductions in the levels of N protein present in cell extracts. A reduction of N protein expression could readily account for decreased minireplicon reporter gene expression, and a role for Hsp72 in the posttranscriptional regulation of non-heat shock protein expression is recognized (3, 13).
The asparagine at position 522, a feature of all group A MV genotypes, is required for the Hsp72-mediated enhancement of minireplicon reporter gene expression. Replacement of the asparagine at amino acid 522 with aspartic acid substitutes a polar uncharged side group with one that is acidic, a nonconservative substitution found in 67% of MV isolates representing all non-group A genotypes. This substitution does not affect the support of basal minireplicon CAT expression but does negate the Hsp72-dependent stimulation. The same result was obtained with NΔ2P4D peptide, a finding consistent with the previous observation that the leucine at amino acid 524 (i.e., the NC-2 position) does not influence basal reporter gene expression. The leucine-to-proline substitution at position 524 is nonconservative in that the size of the hydrophobic side group is significantly reduced. The failure of Hsp72 to enhance reporter gene expression with NΔ4D protein and NΔ2P4D peptide indicates that the stimulatory effect of Hsp72 is mediated through the N protein and not by any direct influence on the enzymatic activity of L. When NΔ3P4D protein provides the template function, basal activity is increased by 2.6-fold, a finding consistent with the results obtained with NC-3 and the role of leucine at amino acid 523 in supporting a level of transcription and replication that is characteristic of Edmonston MV. The NΔ3P4D sequence variant and all truncations that increased basal transcription also failed to respond to Hsp72 supplementation. This finding suggests that one effect of Hsp72 supplementation is to reverse any suppressive effects mediated by amino acids 502 to 522 of the N protein.
Binding studies established a correlation between the Hsp72 functional responsiveness of C-terminal N protein sequences and the binding affinity of those sequences for Hsp72. Synthetic peptides representing the C-terminal 15 amino acids of the N protein specifically bound purified Hsp72. The equilibrium dissociation constant describing these binding reactions was 1 to 4 μM. This value is within the range reported for peptides representing the Hsp72-interactive domain of p53 (i.e., <200 μM) (8, 9). An analysis of binding reactions involving the prokaryotic homologue of Hsp72, DnaK, distinguishes between the roles of heat shock proteins as chaperones versus mediators of activity control. In their role as chaperones, heat shock proteins bind exposed hydrophobic residues in folding intermediates, denatured proteins, or hydrophobic monomers with a high affinity, maintaining the solubility of the heat shock protein-substrate complex until the substrate is properly folded or incorporated into a stable multimeric complex (i.e., chaperone-mediated translational or stability control). For DnaK, the equilibrium dissociation constant for unfolded protein substrates is 0.01 to 1 μM (6). In contrast, low-affinity binding of heat shock proteins to native protein substrates describes activity control, including DnaK-mediated activity control of λ phage replication complexes and cellular transcription factors (i.e., σ32). Here, the KDs are between 1 and 50 μM (6), values within the range of most intracellular protein concentrations. Thus, the KD describing Hsp72-N peptide binding is characteristic of reactions mediating activity control, albeit of a relatively high affinity for this category of reaction. It is this type of interaction that is relevant to the association between Hsp72 and preformed nucleocapsid.
Direct evidence that N peptide binding is relevant to Hsp72-nucleocapsid interactions comes from the competitive inhibition of Hsp72-nucleocapsid binding reactions by N peptide. The fact that the Hsp72 binding affinity for nucleocapsid is greater than the affinity for N peptide most likely reflects the fact that nucleocapsid segments support multivalent interactions with Hsp72, in contrast to the monovalent interaction between N peptide and Hsp72. This possibility is supported by the similarity in association rate constants for nucleocapsid and N peptide, where differences in dissociation rate constants contribute the most to differences in KD values. In addition, conformational degrees of freedom are greater in peptides than in the corresponding domains of a protein; should a specific conformation favor Hsp72 binding, then greater binding affinities would be calculated for a peptide domain of a protein than for a free peptide. Selective proteolysis of nucleocapsid with V8 protease significantly reduced the binding affinity for Hsp72, providing further support for a role of the N protein C terminus in Hsp72 binding reactions. NΔ4D peptide and NΔ3P4D peptide exhibited binding affinities that were several hundredfold lower than that of N peptide. The KDs, being greatly in excess of 200 μM, suggest that binding would not occur under physiologic conditions, a conclusion consistent with the failure of mutant N proteins incorporating these motifs to support the Hsp72-mediated stimulation of minireplicon transcription and replication.
The asparagine at position 522 of the Edmonston MV N protein lies within a linear sequence of eight amino acids that are predicted to bind Hsp72. The prediction was based upon an analysis of the compositions of peptides known to bind 70-kDa heat shock protein family members (i.e., BiP, Hsp72, and Hsp73) (11). Essential elements of this sequence conform to the consensus sequence V-Y-X-X-R/K-X-L-L (amino acids 518 to 524), being maintained in 94 of 100 MV strains. The peptide binding site of 70-kDa heat shock proteins contains four pockets that accommodate large hydrophobic (e.g., V or L) or aromatic (Y) residues; stable binding between a 70-kDa heat shock protein and a peptide is favored when at least two of these pockets are occupied. Specific binding preferences are observed among the 70-kDa heat shock protein family members, with selective binding to Hsp72 (Hsp72 > Hsp73 ≫ BiP) being predicted by the incorporation of basic residues (e.g., R and K) into the motif. Acidic side groups are tolerated, but increasing their number can significantly reduce the binding affinity for Hsp72, a result consistent with the reduction in Hsp72 binding affinity observed with NΔ4D peptide (VYNDRDLL) relative to the N protein (VYNDRNLL).
From these and published data, a model for the stimulation of MV nucleocapsid transcription and replication by Hsp72 can be constructed. Hsp72 binding to the C terminus of the N protein may alter the N protein conformation in such a manner as to enhance the exposure of the encapsidated genome to the viral polymerase, an effect analogous to chromatin remodeling mediated by histone acetylation. The model draws upon the observation that Hsp72-nucleocapsid complexes of CDV exhibiting enhanced transcriptional activity have an expanded nucleocapsid helical structure that is associated with increased exposure of the RNA genome (17, 19). Group A genotypes contain a responsive motif, and Hsp72 supplementation has been shown to enhance viral gene expression and cytopathic effects within infected-cell monolayers (20, 26, 27). However, the effect of Hsp72 on infection by MV genotypes containing the NΔ4D polymorphism has yet to be characterized. Work in progress is designed to confirm such a model by rescuing infectious virus that incorporates non-Hsp72-responsive sequences into the C terminus of the N protein, with subsequent analysis of cell-free transcriptional activity and ultrastructural conformation. More importantly, this approach will permit the biological significance of variations in the Hsp72 responsiveness of the MV N protein to be established.
Acknowledgments
This work was supported by funds from the National Institute of Neurological Disorders and Stroke (R01 NS31693).
We thank Martin Billeter for providing plasmid components of the minireplicon system.
REFERENCES
- 1.Bankamp, B., S. M. Horikami, P. D. Thompson, M. Huber, M. Billeter, and S. A. Moyer. 1996. Domains of the measles virus N protein required for binding to P protein and self-assembly. Virology 216:272-277. [DOI] [PubMed] [Google Scholar]
- 2.Birrer, M., B. R. Bloom, and S. Udem. 1981. Characterization of measles virus polypeptides by monoclonal antibodies. Virology 108:381-390. [DOI] [PubMed] [Google Scholar]
- 3.Brostrom, C. O., and M. A. Brostrom. 1998. Regulation of translational initiation during cellular responses to stress. Prog. Nucleic Acid Res. Mol. Biol. 58:79-125. [DOI] [PubMed] [Google Scholar]
- 4.Buchholz, C. J., C. Retzler, H. E. Homann, and W. J. Neubert. 1994. The carboxy-terminal domain of Sendai virus nucleocapsid protein is involved in complex formation between phosphoprotein and nucleocapsid-like particles. Virology 204:770-776. [DOI] [PubMed] [Google Scholar]
- 5.Curran, J., H. Homann, C. Buchholtz, S. Rochat, W. Neubert, and D. Kolakofsky. 1993. The hypervariable C-terminal tail of the Sendai paramyxovirus nucleocapsid protein is required for template function but not for RNA encapsidation. J. Virol. 67:4358-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Crouy-Chanel, A., M. Kohiyama, and G. Richarme. 1999. Interaction of DnaK with native proteins and membrane proteins correlates with their accessible hydrophobicity. Gene 230:163-170. [DOI] [PubMed] [Google Scholar]
- 7.Fooks, A. R., J. R. Stephenson, A. Warnes, A. B. Dowsett, B. K. Rima, and G. W. G. Wilkinson. 1993. Measles virus nucleocapsid protein expressed in insect cells assembles into nucleocapsid-like structures. J. Gen. Virol. 74:1439-1444. [DOI] [PubMed] [Google Scholar]
- 8.Fourie, A. M., T. R. Hupp, D. P. Lane, B.-C. Sang, M. S. Barbosa, J. F. Sambrook, and M.-J. H. Gething. 1997. Hsp70 binding sites in the tumor suppressor protein p53. J. Biol. Chem. 272:19471-19479. [DOI] [PubMed] [Google Scholar]
- 9.Fourie, A. M., J. F. Sambrook, and M.-J. H. Gething. 1994. Common and divergent peptide binding specificities of hsp70 molecular chaperones. J. Biol. Chem. 269:30470-30478. [PubMed] [Google Scholar]
- 10.Gamer, J., G. Multhaup, T. Tomoyasu, J. S. McCarty, S. Rüdiger, H.-J. Schönfeld, C. Schirra, H. Bujard, and B. Bukau. 1996. A cycle of binding and release of the DnaK, DnaJ, and GrpE chaperones regulates activity of the E. coli heat shock transcription factor sigma 32. EMBO J. 157:607-617. [PMC free article] [PubMed] [Google Scholar]
- 11.Gething, M.-J., S. Blond-Elguindi, J. Buchner, A. Fourie, G. Knarr, S. Modrow, L. Nanu, M. Segal, and J. Sambrook. 1995. Binding sites for hsp70 molecular chaperones in natural proteins. Cold Spring Harbor Symp. Quant. Biol. 60:417-418. [DOI] [PubMed] [Google Scholar]
- 12.Giraudon, P., M. F. Jackquier, and T. F. Wild. 1988. Antigenic analysis of African measles virus field isolates: identification and localization of one conserved and two variable epitope sites on the NP protein. Virus Res. 18:137-152. [DOI] [PubMed] [Google Scholar]
- 13.Henics, T., E. Nagy, H. J. Oh, P. Csermely, A. von Gabain, and J. R. Subjeck. 1999. Mammalian Hsp70 and Hsp110 proteins bind to RNA motifs involved in mRNA stability. J. Biol. Chem. 274:17318-17324. [DOI] [PubMed] [Google Scholar]
- 14.Kreis, S., E. Vardas, and T. Whistler. 1997. Sequence analysis of the nucleocapsid gene of measles virus isolates from South Africa identifies a new genotype. J. Gen. Virol. 78:1581-1587. [DOI] [PubMed] [Google Scholar]
- 15.Kunkel, T. A., K. Bebenek, and J. McClary. 1991. Efficient site directed mutagenesis using uracil containing DNA. Methods Enzymol. 204:125-139. [DOI] [PubMed] [Google Scholar]
- 16.Liston, P., R. Batal, C. DiFlumeri, and D. J. Briedis. 1997. Protein interaction domains of the measles virus nucleocapsid protein. Arch. Virol. 142:305-321. [DOI] [PubMed] [Google Scholar]
- 17.Oglesbee, M., L. Tatalick, J. Rice, and S. Krakowka. 1989. Isolation and characterization of canine distemper virus nucleocapsid variants. J. Gen. Virol. 70:2409-2419. [DOI] [PubMed] [Google Scholar]
- 18.Oglesbee, M., and S. Krakowka. 1993. Cellular stress response induces selective intranuclear trafficking and accumulation of morbillivirus major core protein. Lab. Investig. 68:109-117. [PubMed] [Google Scholar]
- 19.Oglesbee, M. J., Z. Liu, H. Kenney, and C. Brooks. 1996. The highly inducible member of the 70 kDa family of heat shock proteins increases canine distemper virus polymerase activity. J. Gen. Virol. 77:2125-2135. [DOI] [PubMed] [Google Scholar]
- 20.Parks, C. L., R. A. Lerch, P. Walpita, M. S. Sidhu, and S. A. Udem. 1999. Enhanced measles virus cDNA rescue and gene expression after heat shock. J. Virol. 73:3560-3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radecke, F., P. Spielhofer, H. Schneider, K. Kaelin, M. Huber, C. Dötsch, G. Christiansen, and M. A. Billeter. 1995. Rescue of measles virus from cloned DNA. EMBO J. 14:5773-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sedger, L., I. Ramshaw, A. Condie, J. Medveczzky, A. Braithwaite, and J. Ruby. 1996. Vaccinia virus replication is independent of cellular HSP72 expression which is induced during virus infection. Virology 225:423-427. [DOI] [PubMed] [Google Scholar]
- 23.Sidhu, M. S., J. Chan, K. Kaelin, P. Spielhofer, F. Radecke, H. Schneider, M. Masurekar, P. C. Dowling, M. A. Billeter, and S. A. Udem. 1995. Rescue of synthetic measles virus minireplicons: measles virus genomic termini direct efficient expression and propagation of a reporter gene. Virology 208:800-807. [DOI] [PubMed] [Google Scholar]
- 24.Sphener, D., A. Kirn, and R. Drillien. 1991. Assembly of nucleocapsid-like structures in animal cells infected with a vaccinia virus recombinant encoding the measles virus nucleoprotein. J. Virol. 65:6296-6300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thorne, H. V., and E. Dermott. 1976. Circular and elongated linear forms of measles virus nucleocapsid. Nature 264:473-474. [DOI] [PubMed] [Google Scholar]
- 26.Vasconcelos, D., E. Norrby, and M. Oglesbee. 1998. The cellular stress response increases measles virus-induced cytopathic effect. J. Gen. Virol. 79:1769-1773. [DOI] [PubMed] [Google Scholar]
- 27.Vasconcelos, D., X.-H. Cai, and M. J. Oglesbee. 1998. Constitutive expression of the major inducible 70 kDa heat shock protein mediates large plaque formation by measles virus. J. Gen. Virol. 79:2239-2247. [DOI] [PubMed] [Google Scholar]
- 28.Wyatt, L. S., B. Moss, and S. Rozenblatt. 1995. Replication deficient vaccinia virus encoding bacteriophage T7 RNA polymerase for transient gene expression in mammalian cells. Virology 210:202-205. [DOI] [PubMed] [Google Scholar]