Abstract
Recombinant retroviruses have been shown to bind to fibronectin (FN) and increase the efficiency of gene transfer to a variety of cell types. Despite recent work to optimize gene transfer on recombinant FN, the mechanism of retrovirus binding to FN and the interactions of target cells with the bound virus remain elusive. We investigated the roles of virus surface glycoprotein (gp70), cell-conditioned medium, and proteoglycans in mediating retrovirus binding to FN. We also examined the role of Polybrene (PB) in these interactions. We found that gp70 is not involved in retrovirus binding to FN. Immobilization of the virus, however, does not overcome its receptor requirement, and gp70 is still needed for successful gene transfer. Our results clearly show that retrovirus binds FN through virus-associated heparan sulfate (HS) and that binding is necessary for transduction without PB. Two distinct modes of gene transfer occur depending on PB: (i) in the presence of PB, retrovirus interacts directly with the target cells; and (ii) in the absence of PB, retrovirus binds to FN and target cells interact with the immobilized virus. PB may promote the former mode by interacting with the virus HS and reducing the negative charge of the viral particles. Interestingly, the latter mode is more efficient, leading to significantly enhanced gene transfer. A better understanding of these interactions may provide insight into virus-cell interactions and lead to a more rational design of transduction protocols.
The ability of retrovirus to bind to fibronectin (FN) has been utilized to transfer genes by colocalization of virus and target cells (17). This method, termed FN-assisted gene transfer (16, 17), improved retroviral transduction to T lymphocytes (10, 31) and hematopoietic stem cells (7, 9, 19, 29). Two clinical trials that employed this protocol showed promising initial results (1, 6).
Immobilization of recombinant retrovirus yielded significantly increased efficiency of gene transfer compared to transduction with retrovirus in solution (3). The amount of immobilized retrovirus was maximized by long times and low temperatures of incubation that preserve its biological activity. In addition, binding of concentrated virus to FN eliminated the effects of transduction inhibitors and toxic metabolic by-products, enhancing gene transfer by more than 10-fold (3).
The substrates most commonly employed to immobilize retrovirus are poly-l-lysine (2, 18) and peptides containing a high-affinity heparin-binding domain (1, 6, 7, 9, 10, 16, 17, 19, 29, 31). Poly-l-lysine binds the negatively charged retrovirus most likely through electrostatic interactions. FN binds retrovirus through a high-affinity heparin-binding domain and target cells via two cell-binding domains. One domain is made up of type III repeats containing the RGD and PHSRN sequences that bind integrins α5β1 and α3β1. The other domain contains the IIICS segment and binds integrin α4β1. Recombinant FN fragments used in transduction protocols contain the heparin-binding domain and one (CH271) or both (CH296) cell-binding domains (2).
Despite the increasing use of recombinant FN in transduction protocols, the mechanism of retrovirus binding to FN and the nature of the interaction of cells with immobilized virus remain elusive. Our results show that retrovirus binds to FN via its surface-associated proteoglycan heparan sulfate (HS) and elucidate some of the differences in the mechanism of interaction of target cells with free and immobilized retrovirus.
MATERIALS AND METHODS
Retroviral vectors and packaging cells.
The retroviral vector aSGC-LacZ encodes β-galactosidase under the control of an internal promoter and enhancer (α-globin promoter and cytomegalovirus enhancer). LacZ-encoding amphotropic retrovirus-producing cells (kindly provided by J. R. Morgan, Shriners Burns Hospital for Children) (27) were constructed by the introduction of the aSGC-LacZ vector into ΨCRIP packaging cells (8). The LXCGN retroviral vector was derived from the parent vector LXSN, originally described by Miller and Rosman (26). LXCGN encodes an enhanced green fluorescence protein (GFP)-neomycin phosphotransferase fusion protein (EGFP-Neor) transcriptionally controlled by the cytomegalovirus-derived promoter. GFP-encoding amphotropic retrovirus-producing cells (kindly provided by Steven J. Greenberg, Roswell Park Cancer Institute) (35) were produced by transfection of PA317 packaging cells (25) with the LXCGN retroviral vector. The BAG retroviral vector encodes β-galactosidase under the control of the viral long terminal repeat (32). LacZ-encoding ecotropic retrovirus-producing cells were constructed by transfection of ΨCRE packaging cells (8) with the BAG retroviral vector. The expression plasmid that was used to create glycoprotein 70 (gp70)-deficient retrovirus (Phoenix-gp) only encodes Gag-Pol under a Rous sarcoma virus promoter and hygromycin as a coselectable marker through the use of an internal ribosomal entry site (20). This expression plasmid was introduced into the 293T embryonic kidney cell line to produce Phoenix-gp retrovirus particles.
Cell culture and virus production.
NIH 3T3 cells (American Type Culture Collection [ATCC], Rockville, Md.), LacZ-encoding amphotropic retrovirus-producing cells, LacZ-encoding ecotropic retrovirus-producing cells (CRE BAG 2; ATCC), and Phoenix-gp retrovirus-producing cells (ATCC) were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco BRL) with 10% bovine calf serum (CS; Gibco BRL), 100 U of penicillin, and 100 μg of streptomycin (Gibco BRL)/ml at 37°C with 10% CO2. GFP-encoding amphotropic retrovirus-producing cells were cultured under similar conditions, except that 10% fetal bovine serum (Gibco BRL) was used. Human fibroblasts (HuFb) were isolated and cultured as previously described (3).
To harvest virus, fresh medium (32 ml in 182-cm2 tissue culture flasks [Marsh Biomedical, Rochester, N.Y.]) was added to confluent cultures of virus producer cells. After 24 h, the virus-containing medium was harvested, filtered through 0.45-μm-pore-size bottle top filters (Corning Costar, Corning, N.Y.), aliquoted, and stored at −75°C until use. The titers of LacZ-encoding amphotropic retrovirus stocks used in all experiments were determined as previously described (3) and ranged from 0.5 × 105 to 1 × 105 CFU/ml.
Transduction in TCP.
Target cells (NIH 3T3 or HuFb, 5,000 cells/well) were incubated for ∼12 h at 37°C with 10% CO2 in 96-well tissue culture-treated plates (TCP) (Marsh). Next, LacZ-encoding amphotropic or ecotropic retrovirus (100 μl/well) was added to the cells for ∼12 h. Unless stated otherwise, Polybrene (PB) (Sigma, St. Louis, Mo.) was added at a concentration of 8 μg/ml. The virus was then replaced with fresh medium, the cells were allowed to grow until they reached confluence, and the transduction efficiency was determined by measuring the activity of β-galactosidase.
Transduction on fibronectin.
Non-tissue-culture-treated 96-well plates (Marsh) were coated with 100 μl of the CH296 fragment of human FN (Takara Biomedicals, Shiga, Japan)/well by overnight incubation at 4°C. Unless stated otherwise, the concentration of FN used for coating was 10 μg/ml. Unbound FN was removed, and target cells (5,000 cells/well) were added. The cells were incubated for ∼12 h at 37°C with 10% CO2. Next, LacZ-encoding amphotropic or ecotropic retrovirus (100 μl/well) was added to the cells for ∼12 h. Except for the HS-PB interaction experiments (see below), all the virus supernatant contained either 0 or 8 μg of PB/ml. The virus was then replaced with fresh medium, and the cells were allowed to grow until they reached confluence. The transduction efficiency was determined by measuring the activity of β-galactosidase.
Transduction efficiency assay (ONPG assay).
The activity of β-galactosidase was determined with a microplate assay as previously described (28). Briefly, once target cells in 96-well plates reached confluence, they were washed with 100 μl of phosphate-buffered saline (PBS) with 1 mM MgCl2. The wash solution was removed, and the cells were incubated with 50 μl of lysis buffer (PBS with 1 mM MgCl2 and 0.5% IGEPAL [Sigma])/well for 30 min at 37°C. After the cells were lysed, 50 μl of reaction mixture (lysis buffer with 6 mM o-nitrophenyl-β-d-galactopyranosidase [ONPG; Sigma])/well was added to the cell lysate and the plate was incubated at 37°C for another 15 min. The reaction was stopped by the addition of 20 μl of 1 M Na2CO3, and the optical density at 420 nm (OD420) was measured with an absorbance microplate reader (SpectraMax 340; Molecular Devices, Menlo Park, Calif.). The values were corrected for nonspecific background by subtracting the OD650. The absorbance of wells with nontransduced cells was subtracted as background.
Quantitation of retrovirus adsorption on FN by an ELISA for p30 capsid protein (virus binding assay).
An enzyme-linked immunosorbent assay (ELISA) for the matrix protein (p30) was used to quantitate the amount of bound virus. The wells of 96-well ELISA plates (Fisher Scientific, Agawan, Mass.) were coated with 100 μl of 10-μg/ml FN (unless otherwise indicated) by overnight incubation at 4°C. The next day, nonspecific binding sites were blocked by incubation with 250 μl of BLOTTO blocker in Tris-buffered saline (TBS) (Pierce, Rockford, Ill.) for 30 min at room temperature (RT). The virus samples were thawed and incubated (100 μl/well) in the FN-coated wells for ∼12 h (RT, 10% CO2). After washing with TBS (twice), the samples were fixed with 4% paraformaldehyde (Sigma) in deionized H2O (10 min, RT), and permeabilized with 0.2% Triton X-100 (Sigma) in deionized H2O (10 min, RT). After washing once with TBS, the nonspecific sites were blocked with 250 μl of BLOTTO blocker in TBS (30 min, RT). The primary antibody, goat polyclonal anti-p30 (78S221; Quality Biotech, Camden, N.J.), was added at a 1:400 dilution in BLOTTO (100 μl/well) for 1 h at 37°C. Following three washes with TBS, horseradish peroxidase-conjugated donkey anti-goat immunoglobulin G polyclonal secondary antibody (Jackson Immunoresearch Laboratories, West Grove, Pa.) was added at a concentration of 3.2 μg/ml in BLOTTO (100 μl/well) for 1 h at 25°C. The samples were washed three times, and substrate (100 μl/well) was added (SIGMA FAST o-phenylenediaminedihydrochloride tablet sets; Sigma). The reaction was allowed to proceed for 3 to 5 min before the addition of 50 μl per well of 8 N H2SO4 (stop solution). The OD490 was measured with an absorbance microplate reader (SpectraMax 340; Molecular Devices). The values were corrected for nonspecific background by subtracting the OD650. The ODs of wells loaded with DMEM plus 10% CS were subtracted as background.
gp70 blocking.
Amphotropic retrovirus was incubated with a polyclonal anti-gp70 antibody (1:20 dilution, 1 h, 37°C, 10% CO2 [79S834; Quality Biotech]). The virus was loaded on FN-coated wells (10 μg/ml) for ∼12 h at RT with 10% CO2. Following removal of the unbound virus, the wells were washed twice with TBS. The bound anti-gp70 antibody was blocked with a rabbit anti-goat Fab fragment (12 μg/ml; Jackson Immunoresearch Laboratories) for 1 h at 37°C, to prevent the horseradish peroxidase-conjugated polyclonal donkey anti-goat secondary antibody from binding to the anti-gp70 antibody. The bound virus was then quantified by using an ELISA for p30 as described above. The ODs of wells without anti-p30 were subtracted as background.
Enzymatic treatment of viruses and target cells.
The gp70-deficient virus and GFP (or LacZ)-encoding amphotropic virus were incubated with heparinase III (5 mIU/ml; Sigma and U.S. Biological, Swampscott, Mass.) or chondroitinase ABC (0.1 U/ml; Sigma and U.S. Biological) for 3 h at 37°C with 10% CO2. The treated samples were then loaded onto FN-coated wells to quantify binding or used to transduce untreated target cells.
We used enzymes from two sources to ensure that the effects of enzymatic treatment of retrovirus stocks were not due to the presence of impurities or other contaminating enzymes. While enzymes from Sigma have been used extensively, the manufacturer does not guarantee the absence of impurities from the preparations. On the other hand, heparinase III from U.S. Biological contained only trace amounts of chondroitinase A, B, and C and no keratanase. Similarly, chondroitinase ABC was reported to be free of proteases, keratanase, heparinase, and heparitinase.
For enzymatic treatment of target cells, NIH 3T3 cells (5,000 cells/well) were seeded either in TCP or in FN-coated plates and incubated for ∼12 h (37°C, 10% CO2). Medium containing heparinase III (5 mIU/ml) was added to the wells, and the wells were incubated for an additional 3 h at 37°C. The treated cells were then exposed to untreated LacZ-encoding amphotropic virus for ∼12 h at 37°C with 10% CO2.
HS competition and HS-PB interactions.
The gp70-deficient virus and GFP (or LacZ)-encoding amphotropic virus were mixed with different concentrations of HS (Sigma) and PB as indicated. The mixtures were then loaded onto FN-coated wells (5 μg/ml) to quantify binding or used to transduce target cells as described above.
RESULTS
Transduction on FN is more efficient in the absence of PB.
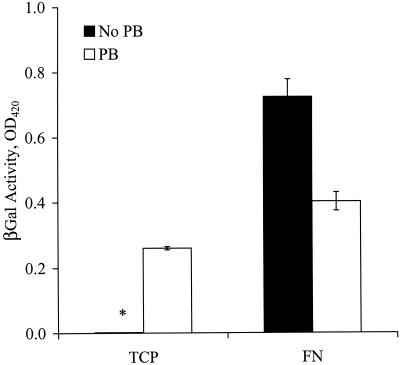
First we assessed the effects of FN on the efficiency of gene transfer. Cells were plated on TCP or on FN, and the next day the virus was introduced in the presence or absence of PB. Surprisingly, gene transfer on FN without PB (TFN) was approximately twofold higher than in the presence of 8 μg of PB/ml ( 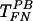 ) and threefold higher than on TCP (
) and threefold higher than on TCP ( 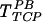 ) (Fig. 1). As expected, gene transfer on TCP was negligible in the absence of PB. These results show that virus-cell interactions are more efficient with immobilized retrovirus, especially in the absence of PB. Since PB promotes virus-cell interactions (37) but inhibits binding to FN (3), our results suggest two modes of virus-cell interactions. In the presence of PB the virus binds to the target cells directly, but in the absence of PB the virus binds to FN and cells interact with the immobilized virus.
) (Fig. 1). As expected, gene transfer on TCP was negligible in the absence of PB. These results show that virus-cell interactions are more efficient with immobilized retrovirus, especially in the absence of PB. Since PB promotes virus-cell interactions (37) but inhibits binding to FN (3), our results suggest two modes of virus-cell interactions. In the presence of PB the virus binds to the target cells directly, but in the absence of PB the virus binds to FN and cells interact with the immobilized virus.
FIG. 1.
Transduction on FN is more efficient in the absence of PB. NIH 3T3 cells were added to 96-well FN-coated plates or TCP at 5,000 cells/well. The next day, target cells were incubated (∼12 h) with amphotropic retrovirus stock in the presence or absence of PB. The transduction efficiency was quantified after the target cells reached confluence by measuring the activity of β-galactosidase (βGal). Values are means ± standard deviations of duplicate samples in a representative experiment. The asterisk denotes a very small value (close to zero).
gp70 is not required for virus immobilization but is necessary for successful transduction.
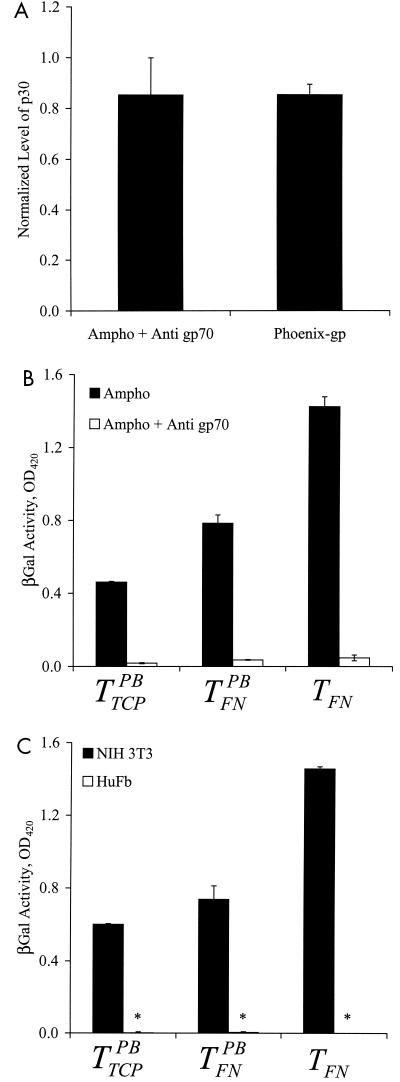
Although the retrovirus envelope glycoprotein gp70 is involved in binding to target cells, it was not clear whether gp70 is involved in the binding of retrovirus to FN. Preincubation of the virus with a polyclonal anti-gp70 antibody did not affect binding to FN (Fig. 2A). Furthermore, a gp70-deficient retrovirus (Phoenix-gp) also bound to FN, suggesting that viral surface motifs other than gp70 were responsible for mediating binding (Fig. 2A).
FIG. 2.
gp70 is required for transduction but not for binding to FN. Amphotropic retrovirus stock was incubated with a polyclonal goat anti-gp70 antibody (1:20 dilution) for 1 h at 37°C. Untreated amphotropic (Ampho) retrovirus (control) and gp-70 deficient (Phoenix-gp) retrovirus were incubated at 37oC for the same length of time in the absence of the blocking antibody. (A) All retrovirus samples were incubated in FN-coated 96-well plates for ∼12 h at 25oC, and binding was evaluated with an ELISA for the matrix protein p30. The OD was normalized to that of control retrovirus. (B) NIH 3T3 target cells in 96-well FN-coated plates or TCP (5,000 cells/well) were incubated with control and antibody-treated retrovirus in the presence or absence of PB for ∼12 h. (C) NIH 3T3 or HuFb target cells in 96-well FN-coated plates or TCP (5,000 cells/well) were incubated with ecotropic Mo-MuLV (not treated with anti-gp70) in the presence or absence of PB for ∼12 h. (B and C) The transduction efficiencies were quantified by measurements of β-galactosidase (βGal) activity once the target cells reached confluence. Values are means ± standard deviations of duplicate samples in a representative experiment. Asterisks denote very small values (close to zero).
On the other hand, preincubation with the anti-gp70 antibody reduced gene transfer by all three protocols (TFN, 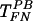 , and
, and 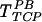 ) (Fig. 2B). Additionally, an ecotropic Moloney murine leukemia virus (Mo-MuLV) transduced NIH 3T3 mouse cells by TFN but failed to transduce human cells (HuFb) (Fig. 2C). These data suggest that although gp70 is not required for retrovirus binding to FN, gene transfer with immobilized retrovirus does not overcome the requirement for gp70 binding to cell surface receptors.
) (Fig. 2B). Additionally, an ecotropic Moloney murine leukemia virus (Mo-MuLV) transduced NIH 3T3 mouse cells by TFN but failed to transduce human cells (HuFb) (Fig. 2C). These data suggest that although gp70 is not required for retrovirus binding to FN, gene transfer with immobilized retrovirus does not overcome the requirement for gp70 binding to cell surface receptors.
HS mediates retrovirus binding to FN.
Since retrovirus binds to the heparin-binding domain of FN (2), it is conceivable that virus surface proteoglycans may bind directly to FN or that free proteoglycans may act as a bridge to mediate this binding (both HS and chondroitin sulfate have been shown to bind to the heparin-binding domain of FN) (34). To test these hypotheses, we incubated viral supernatant with either heparinase III or chondroitinase ABC to digest any free and/or viral surface proteoglycans and then we evaluated binding to FN and gene transfer to target cells.
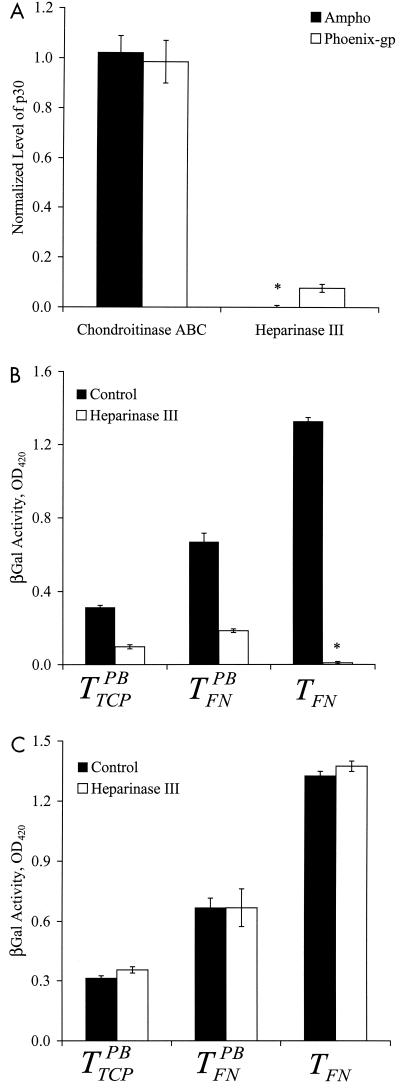
Treatment with chondroitinase ABC had no effect on virus binding to FN (Fig. 3A). We used the same concentration of chondroitinase ABC (0.1 U/ml) that was previously reported to remove chondroitin sulfate from the cell surface and increase the efficiency of gene transfer (22). In agreement with these investigators, we observed a twofold increase in gene transfer when target cells were treated with 0.1 U of chondroitinase ABC/ml (data not shown).
FIG. 3.
Enzymatic digestion of HS eliminates binding to FN and gene transfer in the absence of PB. Amphotropic (Ampho) or gp70-deficient (Phoenix-gp) Mo-MuLV was incubated with either heparinase III (5 mIU/ml) or chondroitinase ABC (0.1 U/ml) for 3 h at 37°C. Control samples were incubated at 37°C for the same length of time in the absence of the enzyme. (A) The virus was incubated in 96-well FN-coated plates for ∼12 h at 25°C, and binding was evaluated with an ELISA for the matrix protein p30. The OD was normalized to that of control virus. (B) NIH 3T3 target cells in 96-well FN-coated plates or TCP (5,000 cells/well) were incubated with the enzyme-treated virus in the presence or absence of PB. (C) NIH 3T3 cells were added to 96-well FN-coated plates or TCP at 5,000 cells/well. The next day, the target cells were exposed to either culture medium (control) or heparinase III (5 mIU/ml) for 3 h at 37°C. The cells were washed with PBS and exposed to untreated virus stock. (B and C) The transduction efficiencies were quantified by measurements of β-galactosidase(βGal) activity once the target cells reached confluence. Values are means ± standard deviations of duplicate samples in a representative experiment. Asterisks denote very small values (close to zero).
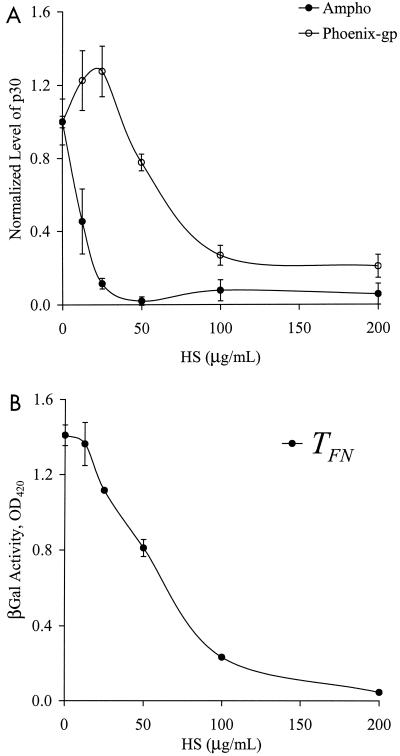
In contrast, treatment with 5.0 mIU of heparinase III/ml reduced binding substantially for both amphotropic and Phoenix-gp retrovirus (Fig. 3A). Experiments with different enzyme concentrations showed that 5.0 mIU/ml inhibited binding significantly and that higher concentrations had no additional effect (data not shown). Since heparinase III inhibited the binding of gp70-deficient retrovirus as well, these results suggest that retrovirus binding to FN may be mediated by HS and not by sugar residues associated with gp70.
Interestingly, treatment with heparinase III eliminated transduction by TFN, and decreased gene transfer with 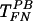 and
and 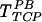 (Fig. 3B). However, pretreatment of target cells (but not the virus) with heparinase III did not affect TFN,
(Fig. 3B). However, pretreatment of target cells (but not the virus) with heparinase III did not affect TFN, 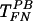 , or
, or 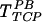 (Fig. 3C), suggesting that HS on the target cells is not involved in direct virus-target cell interaction.
(Fig. 3C), suggesting that HS on the target cells is not involved in direct virus-target cell interaction.
The moiety that promotes retrovirus binding to FN is associated with the viral particle.
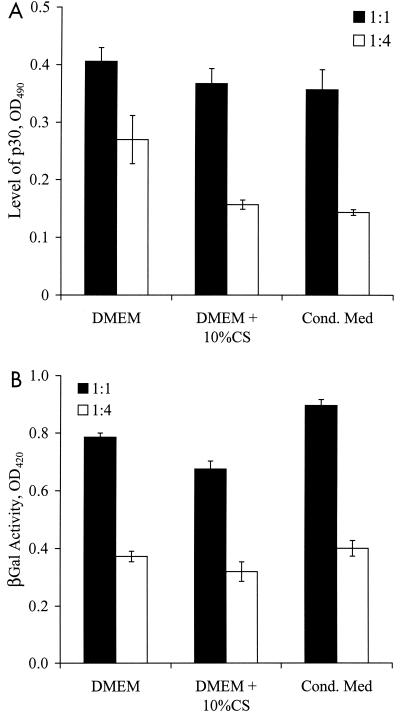
Next we investigated whether the heparinase-sensitive moiety that mediated binding to FN was free in solution or associated with the viral particles. To determine if the conditioned medium contained a binding-promoting activity, we diluted the virus stock (virus-to-medium ratio of 1:1 or 1:4) with DMEM, DMEM plus 10% CS, or medium conditioned by NIH 3T3 mouse fibroblasts and then we evaluated virus binding to FN and gene transfer to target cells.
Binding to FN was maximal when the virus was diluted with DMEM, and it decreased upon dilution with DMEM plus 10% CS and conditioned medium. The effect was more pronounced for higher dilutions of the virus (Fig. 4A). On the other hand, gene transfer by TFN was independent of serum or conditioned medium (Fig. 4B). These results suggest that the serum and conditioned medium contain factor(s) that inhibit rather than promote binding of retrovirus to FN.
FIG. 4.
Serum and conditioned medium do not promote retrovirus binding to FN. Amphotropic retrovirus stock was diluted (virus-to-medium ratio of 1:1 or 1:4) in DMEM, DMEM plus 10% CS, or NIH 3T3-conditioned medium. (A) The diluted virus samples were incubated in 96-well FN-coated plates for ∼12 h at 25°C, and binding was evaluated with an ELISA for the matrix protein p30. (B) NIH 3T3 target cells in 96-well FN-coated plates (5,000 cells/well) were incubated with the diluted virus in the absence of PB. The transduction efficiency was quantified by measurements of β-galactosidase (βGal) activity once the target cells reached confluence. Values are means ± standard deviations of duplicate samples in a representative experiment.
In addition, increasing concentrations of HS in the viral supernatant decreased binding of regular (amphotropic) and gp70-deficient (Phoenix-gp) retrovirus in a dose-dependent fashion (Fig. 5A). The efficiency of TFN followed a similar trend (Fig. 5B). Likewise, the addition of heparin to the virus stock resulted in a similar dose-dependent inhibition of binding and TFN (data not shown) (5). Collectively, these data support the hypothesis that HS associated with the viral particles mediates binding of retrovirus to FN.
FIG. 5.
HS competes with retrovirus for binding to FN. Amphotropic (Ampho) or gp70-deficient (Phoenix-gp) retrovirus was treated with different concentrations of HS as indicated. (A) The HS-treated virus was incubated in 96-well FN (5 μg/ml)-coated plates for ∼12 h at 25°C, and binding was evaluated with an ELISA for the matrix protein p30. The OD was normalized to that of the untreated samples. (B) NIH 3T3 target cells in 96-well FN (5 μg/ml)-coated plates (5,000 cells/well) were incubated with the HS-treated virus in the absence of PB. The transduction efficiency was quantified by measurements of β-galactosidase (βGal) activity once the target cells reached confluence. Values are means ± standard deviations of duplicate samples in a representative experiment.
HS interacts with PB.
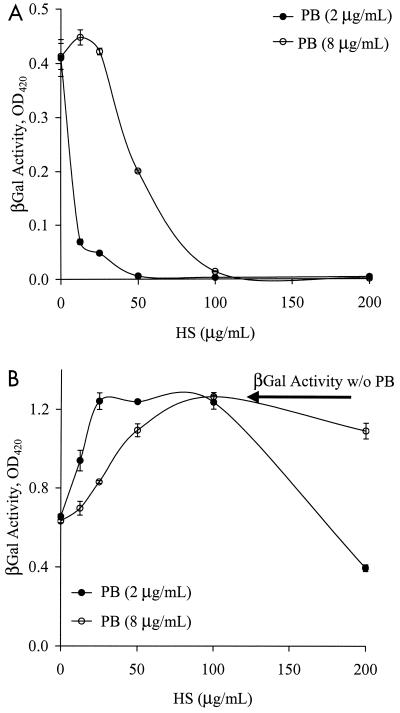
Transduction in the presence of PB (on TCP or FN) should not be affected by the presence of HS in the viral supernatant, since the virus interacts directly with the cells and not with FN. However, the efficiency of 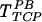 decreased with increasing concentrations of HS (Fig. 6A). Interestingly, higher concentrations of HS were needed to counteract the effect of higher concentrations of PB. Specifically, 100 μg of HS/ml neutralized the effect of 8 μg of PB/ml, while only 25 μg of HS/ml was needed to neutralize 2 μg of PB/ml, suggesting that negatively charged HS may interact with the positively charged PB.
decreased with increasing concentrations of HS (Fig. 6A). Interestingly, higher concentrations of HS were needed to counteract the effect of higher concentrations of PB. Specifically, 100 μg of HS/ml neutralized the effect of 8 μg of PB/ml, while only 25 μg of HS/ml was needed to neutralize 2 μg of PB/ml, suggesting that negatively charged HS may interact with the positively charged PB.
FIG. 6.
HS interacts with PB and negates it charge. Amphotropic virus stock was treated with increasing concentrations of HS as indicated. NIH 3T3 target cells plated in TCP (A) or 96-well FN-coated plates (B) were incubated with the virus in the presence of 2 or 8 μg of PB/ml. The transduction efficiency was quantified by measurements of β-galactosidase (βGal) activity once the target cells reached confluence. Values are means ± standard deviations of duplicate samples in a representative experiment. w/o, without.
Interestingly, low HS concentrations increased the efficiency of 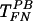 to the level of TFN (Fig. 6B), possibly by counteracting the action of PB and hence promoting virus binding to FN. After
to the level of TFN (Fig. 6B), possibly by counteracting the action of PB and hence promoting virus binding to FN. After 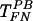 had reached the level of TFN, additional HS reduced the efficiency of
had reached the level of TFN, additional HS reduced the efficiency of 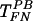 , possibly by competing with the virus for binding to FN (Fig. 6B). Therefore, at low concentrations, HS interacts with PB, eliminating direct virus-cell interactions. Gene transfer then occurs only with virus that binds to FN, as in the case of TFN. At higher concentrations, a fraction of HS interacts with PB and the rest competes with the virus for binding to FN, thus decreasing gene transfer with immobilized virus as well.
, possibly by competing with the virus for binding to FN (Fig. 6B). Therefore, at low concentrations, HS interacts with PB, eliminating direct virus-cell interactions. Gene transfer then occurs only with virus that binds to FN, as in the case of TFN. At higher concentrations, a fraction of HS interacts with PB and the rest competes with the virus for binding to FN, thus decreasing gene transfer with immobilized virus as well.
DISCUSSION
Retroviral gene transfer to cells grown on FN is significantly higher than transfer to cells grown on TCP, especially in the absence of PB. We have previously shown that PB inhibits binding of retrovirus to FN in a dose-dependent fashion (3). In the absence of PB, retroviral gene transfer on TCP is negligible but binding to FN and gene transfer to cells grown on FN is maximized. These results suggest that, in the absence of PB, virus-cell interactions diminish while virus-FN interactions are optimized. Since gene transfer on FN is higher in the absence than in the presence of PB, these data also suggest that virus-cell interactions are more efficient with immobilized retrovirus rather than retrovirus approaching the cells from solution. Although the mechanism for this behavior is not known, arguments based on the physical forces that act between viruses and cells in each setting may provide an explanation.
Retroviruses are submicrometer particles (100-nm diameter) subject to small thermal forces (on the order of 10−3 pN), and they approach target cells by diffusion (30). As they approach target cells, small repulsive forces between the negatively charged viruses and cells may be enough to repel the virus away from the cell surface. The addition of PB neutralizes the electrostatic repulsion, thus promoting binding and internalization of retroviral particles. However, even when PB neutralizes the electrostatic repulsion, other repulsive steric or osmotic forces may limit binding of the virus to the receptor and internalization (30). In the absence of PB, retroviruses are repelled from the cells but instead they bind to FN and are immobilized on the surface. In this case, cells may interact with immobilized retroviral particles as they migrate on FN. Traction forces that the migrating cells exert on the substrate (104 to 106 pN) (21) may be considerably larger than the repulsive forces, ultimately promoting virus binding and entry into the target cell.
Our results suggest that retrovirus binds to FN through virus-associated HS. First, we show that the gp70 envelope glycoprotein is not required for retrovirus binding to FN. A polyclonal anti-gp70 antibody does not inhibit binding, and even more convincingly, viral particles that lack gp70 bind to FN with high efficiency. Second, treatment of the virus—but not of target cells—with heparinase eliminates binding to FN and gene transfer. Third, cell-conditioned medium does not promote binding to FN. Finally, soluble HS competes with the virus for binding to FN. Collectively, these data suggest that retrovirus binds to FN via virus-associated HS, which, upon degradation, leads to a substantial decrease in binding. Since HS is ubiquitously expressed on the surface of most mammalian cells, including fibroblasts (11, 14, 15, 38, 39), it is possible that retroviruses acquire HS with the plasma membrane as they bud from the surface of the producer cells. Although heparin also competes with the virus for binding to FN (data not shown) (5), it is unlikely that heparin mediates retrovirus binding and TFN, since it is only produced by mast cells (11, 14) and not expressed by mouse fibroblasts (NIH 3T3, ΨCRIP, PA317, CRE BAG2) or human kidney cells (Phoenix-gp) (15).
These results can explain previous data, which showed that treatment of virus producer cells with tunicamycin C resulted in production of viral particles that did not bind to FN (23). Since tunicamycin C is a competitive inhibitor of glycosylation, it was assumed that lack of binding was due to improper glycosylation or decreased production of gp70. However, our data clearly show that gp70 is not involved in binding to FN and suggest that the decline in binding may be due to reduced production of HS proteoglycans.
Our data shed light on the role of PB in virus-cell interactions. Although previous investigators have suggested that polycations such as PB or protamine sulfate may act by neutralizing the electrostatic repulsion between the negatively charged viruses and target cells, the molecules that are involved in such interactions are not known. We show that PB interacts with HS in solution with a stoichiometric ratio of 1:8 (PB/HS ratio). Therefore, PB may also bind to the HS on the surface of the virus, thus neutralizing the negative charges and promoting virus-cell interactions.
Other viruses, including herpes simplex virus types 1 and 2, cytomegalovirus, adeno-associated virus, and adenovirus types 2 and 5, have evolved to take advantage of the ubiquitous presence of HS and other proteoglycans to anchor to the cell surface before binding to specific receptors and internalization (12, 13). Interestingly, these viruses do not need PB or other polycations to mediate binding and entry into the cells. In contrast, our data suggest that recombinant retroviruses may be decorated with HS molecules that mediate binding to positive surfaces rather than target cells, suggesting that binding to the extracellular matrix may be the first step in a series of interactions of retrovirus with the host cells in vivo. In support of this hypothesis, human immunodeficiency virus (HIV) type 1 has been shown to bind to FN, and heparin, HS, or antibodies against FN blocked binding. Additionally, bronchoalveolar lavage fluid from children with HIV type 1 infections contained significantly higher concentrations of FN than fluids from healthy children (24), suggesting that the extracellular matrix may play an important role in retroviral infection by binding and harboring viral particles. However, contrary to the case of Mo-MuLV, the binding of HIV to FN is mediated by the envelope glycoproteins gp120 and gp160 (4, 33, 36).
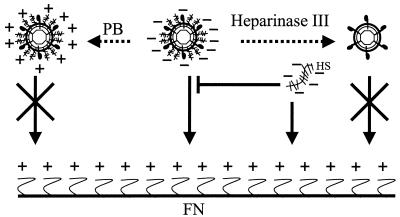
Our findings are summarized in Fig. 7, which depicts the molecules that mediate virus-FN interactions. Binding of retrovirus to FN is mediated by virus-associated HS and inhibited by PB, heparinase, and soluble HS. The positively charged PB may interact with the negatively charged HS on the viral particle and prevent virus binding to FN. Heparinase inhibits binding to FN by degrading virus-associated HS. Likewise, free HS competes with the virus for binding to the heparin-binding domain of FN. Surprisingly, the target cells interact more efficiently with bound retrovirus particles than with free retrovirus particles, although the receptor is still required for internalization and gene transfer. Understanding how the cells find the immobilized virus and what mechanism facilitates their interaction may provide ways to increase virus-cell encounters, ultimately increasing the probability of gene transfer.
FIG. 7.
Molecules that mediate binding of retrovirus to FN. This schematic depicts the role of virus-associated HS, free HS, heparinase, and PB in retrovirus binding to FN. See Discussion for details. Dashed arrows indicate treatment, and regular arrows indicate interaction with FN. Blunt arrows represent inhibition of binding, and crossed arrows represent no interaction.
Acknowledgments
Pedro Lei and Bharat Bajaj contributed equally to this work.
This work was supported by the Whitaker Foundation for Biomedical Engineers.
REFERENCES
- 1.Abonour, R., D. A. Williams, L. Einhorn, K. M. Hall, J. Chen, J. Coffman, C. M. Traycoff, A. Bank, I. Kato, M. Ward, S. D. Williams, R. Hromas, M. J. Robertson, F. O. Smith, D. Woo, B. Mills, E. F. Srour, and K. Cornetta. 2000. Efficient retrovirus-mediated transfer of the multidrug resistance 1 gene into autologous human long-term repopulating hematopoietic stem cells. Nat. Med. 6:652-658. [DOI] [PubMed] [Google Scholar]
- 2.Asada, K., T. Uemori, T. Ueno, K. Hashino, N. Koyama, A. Kawamura, and I. Kato. 1998. Enhancement of retroviral gene transduction on a dish coated with a cocktail of two different polypeptides: one exhibiting binding activity toward target cells, and the other toward retroviral vectors. J. Biochem. (Tokyo) 123:1041-1047. [DOI] [PubMed] [Google Scholar]
- 3.Bajaj, B., P. Lei, and S. T. Andreadis. 2001. High efficiencies of gene transfer with immobilized recombinant retrovirus: kinetics and optimization. Biotechnol. Prog. 17:587-596. [DOI] [PubMed] [Google Scholar]
- 4.Bozzini, S., V. Falcone, P. G. Conaldi, L. Visai, L. Biancone, A. Dolei, A. Toniolo, and P. Speziale. 1998. Heparin-binding domain of human fibronectin binds HIV-1 gp120/160 and reduces virus infectivity. J. Med. Virol. 54:44-53. [DOI] [PubMed] [Google Scholar]
- 5.Carstanjen, D., P. Dutt, and T. Moritz. 2001. Heparin inhibits retrovirus binding to fibronectin as well as retrovirus gene transfer on fibronectin fragments. J. Virol. 75:6218-6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cavazzana-Calvo, M., S. Hacein-Bey, G. de Saint Basile, F. Gross, E. Yvon, P. Nusbaum, F. Selz, C. Hue, S. Certain, J. L. Casanova, P. Bousso, F. L. Deist, and A. Fischer. 2000. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 288:669-672. [DOI] [PubMed] [Google Scholar]
- 7.Conneally, E., C. J. Eaves, and R. K. Humphries. 1998. Efficient retroviral-mediated gene transfer to human cord blood stem cells with in vivo repopulating potential. Blood. 91:3487-3493. [PubMed] [Google Scholar]
- 8.Danos, O., and R. C. Mulligan. 1988. Safe and efficient generation of recombinant retroviruses with amphotropic and ecotropic host ranges. Proc. Natl. Acad. Sci. USA 85:6460-6464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dao, M. A., K. Hashino, I. Kato, and J. A. Nolta. 1998. Adhesion to fibronectin maintains regenerative capacity during ex vivo culture and transduction of human hematopoietic stem and progenitor cells. Blood 92:4612-4621. [PubMed] [Google Scholar]
- 10.Dardalhon, V., N. Noraz, K. Pollok, C. Rebouissou, M. Boyer, A. Q. Bakker, H. Spits, and N. Taylor. 1999. Green fluorescent protein as a selectable marker of fibronectin-facilitated retroviral gene transfer in primary human T lymphocytes. Hum. Gene Ther. 10:5-14. [DOI] [PubMed] [Google Scholar]
- 11.David, G. 1993. Integral membrane heparan sulfate proteoglycans. FASEB J. 7:1023-1030. [DOI] [PubMed] [Google Scholar]
- 12.Dechecchi, M. C., P. Melotti, A. Bonizzato, M. Santacatterina, M. Chilosi, and G. Cabrini. 2001. Heparan sulfate glycosaminoglycans are receptors sufficient to mediate the initial binding of adenovirus types 2 and 5. J. Virol. 75:8772-8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dechecchi, M. C., A. Tamanini, A. Bonizzato, and G. Cabrini. 2000. Heparan sulfate glycosaminoglycans are involved in adenovirus type 5 and 2-host cell interactions. Virology 268:382-390. [DOI] [PubMed] [Google Scholar]
- 14.Esko, J. D. 1999. Proteoglycans and glycosaminoglycans, p. 145-159. In A. Varki, R. Cummings, J. Esko, H. Freeze, G. Hart, and J. Marth (ed.), Essentials of glycobiology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 15.Gallagher, J. T., M. Lyon, and W. P. Steward. 1986. Structure and function of heparan sulphate proteoglycans. Biochem. J. 236:313-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanenberg, H., K. Hashino, H. Konishi, R. A. Hock, I. Kato, and D. A. Williams. 1997. Optimization of fibronectin-assisted retroviral gene transfer into human CD34+ hematopoietic cells. Hum. Gene Ther. 8:2193-2206. [DOI] [PubMed] [Google Scholar]
- 17.Hanenberg, H., X. L. Xiao, D. Dilloo, K. Hashino, I. Kato, and D. A. Williams. 1996. Colocalization of retrovirus and target cells on specific fibronectin fragments increases genetic transduction of mammalian cells. Nat. Med. 2:876-882. [DOI] [PubMed] [Google Scholar]
- 18.Hennemann, B., J. Y. Chuo, P. D. Schley, K. Lambie, R. K. Humphries, and C. J. Eaves. 2000. High-efficiency retroviral transduction of mammalian cells on positively charged surfaces. Hum. Gene Ther. 11:43-51. [DOI] [PubMed] [Google Scholar]
- 19.Kiem, H. P., R. G. Andrews, J. Morris, L. Peterson, S. Heyward, J. M. Allen, J. E. Rasko, J. Potter, and A. D. Miller. 1998. Improved gene transfer into baboon marrow repopulating cells using recombinant human fibronectin fragment CH-296 in combination with interleukin-6, stem cell factor, FLT-3 ligand, and megakaryocyte growth and development factor. Blood 92:1878-1886. [PubMed] [Google Scholar]
- 20.Kinsella, T. M., and G. P. Nolan. 1996. Episomal vectors rapidly and stably produce high-titer recombinant retrovirus. Hum. Gene Ther. 7:1405-1413. [DOI] [PubMed] [Google Scholar]
- 21.Lauffenburger, D. A., and J. J. Linderman. 1993. Receptors. Oxford University Press, New York, N.Y.
- 22.LeDoux, J. M., J. R. Morgan, and M. L. Yarmush. 1996. Proteoglycans secreted by packaging cell lines inhibit retrovirus infection. J. Virol. 70:6468-6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacNeill, E. C., H. Hanenberg, K. E. Pollok, J. C. van der Loo, M. F. Bierhuizen, G. Wagemaker, and D. A. Williams. 1999. Simultaneous infection with retroviruses pseudotyped with different envelope proteins bypasses viral receptor interference associated with colocalization of gp70 and target cells on fibronectin CH-296. J. Virol. 73:3960-3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Midulla, F., P. Strappini, T. Sandstrom, L. Bjermer, C. Falasca, P. Capocaccia, S. Catania, E. Soldi, M. Pia Villa, and R. Ronchetti. 2001. Cellular and noncellular components of bronchoalveolar lavage fluid in HIV-1-infected children with radiological evidence of interstitial lung damage. Pediatr. Pulmonol. 31:205-213. [DOI] [PubMed] [Google Scholar]
- 25.Miller, A. D., and C. Buttimore. 1986. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol. Cell. Biol. 6:2895-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller, A. D., and G. J. Rosman. 1989. Improved retroviral vectors for gene transfer and expression. BioTechniques 7:980-982, 984-986, 989-990. [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan, J. R., J. M. LeDoux, R. G. Snow, R. G. Tompkins, and M. L. Yarmush. 1995. Retrovirus infection: effect of time and target cell number. J. Virol. 69:6994-7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgan, J. R., J. Lee, R. G. Tompkins, and M. L. Yarmush. 1994. Rapid quantitation of recombinant retroviruses. Biotechnol. Prog. 10:441-446. [DOI] [PubMed] [Google Scholar]
- 29.Moritz, T., P. Dutt, X. Xiao, D. Carstanjen, T. Vik, H. Hanenberg, and D. A. Williams. 1996. Fibronectin improves transduction of reconstituting hematopoietic stem cells by retroviral vectors: evidence of direct viral binding to chymotryptic carboxy-terminal fragments. Blood 88:855-862. [PubMed] [Google Scholar]
- 30.Palsson, B. O., and S. Andreadis. 1997. The physico-chemical factors that govern retrovirus-mediated gene transfer. Exp. Hematol. 25:94-102. [PubMed] [Google Scholar]
- 31.Pollok, K. E., H. Hanenberg, T. W. Noblitt, W. L. Schroeder, I. Kato, D. Emanuel, and D. A. Williams. 1998. High-efficiency gene transfer into normal and adenosine deaminase-deficient T lymphocytes is mediated by transduction on recombinant fibronectin fragments. J. Virol. 72:4882-4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price, J., D. Turner, and C. Cepko. 1987. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc. Natl. Acad. Sci. USA 84:156-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pugliese, A., A. Savarino, C. Cantamessa, and D. Torre. 1996. Influence of fibronectin on HIV-1 infection and capability of binding to platelets. Cell Biochem. Funct. 14:291-296. [DOI] [PubMed] [Google Scholar]
- 34.Romberger, D. J. 1997. Fibronectin. Int. J. Biochem. Cell Biol. 29:939-943. [DOI] [PubMed] [Google Scholar]
- 35.Selkirk, S. M., S. J. Greenberg, R. J. Plunkett, T. A. Barone, A. Lis, and P. O. Spence. 2002. Syngeneic central nervous system transplantation of genetically transduced mature, adult astrocytes. Gene Ther. 9:432-443. [DOI] [PubMed] [Google Scholar]
- 36.Tellier, M. C., G. Greco, M. Klotman, A. Mosoian, A. Cara, W. Arap, E. Ruoslahti, R. Pasqualini, and L. M. Schnapp. 2000. Superfibronectin, a multimeric form of fibronectin, increases HIV infection of primary CD4+ T lymphocytes. J. Immunol. 164:3236-3245. [DOI] [PubMed] [Google Scholar]
- 37.Toyoshima, K., and P. K. Vogt. 1969. Enhancement and inhibition of avian sarcoma viruses by polycations and polyanions. Virology 38:414-426. [DOI] [PubMed] [Google Scholar]
- 38.Woods, A., M. Hook, L. Kjellen, C. G. Smith, and D. A. Rees. 1984. Relationship of heparan sulfate proteoglycans to the cytoskeleton and extracellular matrix of cultured fibroblasts. J. Cell Biol. 99:1743-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanagishita, M., and V. C. Hascall. 1992. Cell surface heparan sulfate proteoglycans. J. Biol. Chem. 267:9451-9454. [PubMed] [Google Scholar]