Abstract
TVB receptors are death receptors of the tumor necrosis factor receptor (TNFR) family and serve as cellular receptors for cytopathic subgroups B and D and noncytopathic subgroup E of the avian leukosis viruses (ALVs). Although TVB is essential for ALV-B-mediated cell death, binding of the ALV-B envelope protein to its cognate receptor TVB activates cell death only in the presence of protein biosynthesis inhibitors, which presumably block the expression of protective factors. In the case of TNFR-1, the main antiapoptotic pathway depends upon nuclear factor kappa B (NF-κB)-activated survival factors. Here we show that overexpression of TVB receptors in human 293 cells activates NF-κB via a mechanism involving the cytoplasmic death domains of these receptors. NF-κB is also activated upon binding of a soluble ALV-B or ALV-E surface envelope-immunoglobulin fusion protein to the cognate TVB receptors and by ALV-B infection of a chicken embryo fibroblast cell line (DF1). Importantly, the cycloheximide requirement for TVB-dependent cell death was overcome by the expression of a transdominant form of IκB-α, and downregulation of NF-κB by the immunomodulator pyrrolidinedithiocarbamate enhanced the cytopathogenicity of ALV-B. These results demonstrate that TVB receptors trigger NF-κB-dependent gene expression and that NF-κB-regulated survival factors can protect against virus-induced cell death.
Cytopathic retroviruses are able to induce cell death (cytopathic effect) upon infection of their target cells. The viral determinants for cytopathic effects have been mapped to the viral surface (SU) Env protein of avian leukosis virus (ALV) and several additional retroviruses such as human immunodeficiency virus (HIV) (28), Cas-Br murine leukemia virus (20), avian hemangioma virus (22), and feline leukemia virus (13, 23). ALVs are divided into three cytopathic subgroups (B, D, and F) and six noncytopathic subgroups (A, C, E, G, H, and I). Infections by the cytopathic subgroup B of ALV lead to cell death during the acute phase of infection and are associated with a severe but transient anemia in newborn chickens (29). The induction of anemia has not been observed with infections by noncytopathic subgroups of ALV (29).
The determinants for cytopathogenicity of ALV-B colocalize with the determinants for receptor recognition, suggesting involvement of cellular receptors in ALV-B-mediated cell killing (14). The role of ALV-B receptors in virally induced cell killing was further supported by the fact that cytopathic ALV-B and ALV-D utilize TVBS3, a tumor necrosis factor receptor (TNFR), as a viral receptor (8). TVBS3 shares high sequence homology and structural features with mammalian death-receptors 4 and 5 (DR4 and DR5) (11). These shared features include three TNFR-like extracellular cysteine-rich domains, a single transmembrane region, and a putative cytoplasmic death domain (25). Activation of TNFR-1 by TNF-α leads to clustering of the cytoplasmic death domain, sequential recruitment of downstream proteins, and induction of diverse signaling pathways (3). TNFR-1 death domain clustering leads to binding of TRADD, a key adaptor protein, and association with FADD, which recruits and activates caspase 8 (3). We have shown that TVBS3 is a signaling-competent death receptor and is able to mediate cell death when it is activated upon binding to a soluble viral envelope-immunoglobulin (Ig) fusion protein (SUB-IgG) (7). SUB-IgG is comprised of the surface part of the ALV-B envelope protein fused in frame to the constant region of an immunoglobulin (1). This ability to activate cell death is shared with other TVB receptors: the turkey TVBT receptor specific for the noncytopathic subgroup E of ALV (1) and TVBS1, a chicken receptor for subgroups B, D, and E of ALV (2). Thus, cytopathic subgroups B and D and noncytopathic subgroup E viruses use TVB receptors that are competent for activating cell death pathways.
Although TVB receptors appear to be essential for ALV-B-induced cytopathic effects, these receptors do not trigger cell killing upon binding to cognate ALV envelope proteins in the absence of the protein biosynthesis inhibitor cycloheximide. Furthermore, cell death is not observed during the initial rounds of ALV-B infection. These observations suggest the existence of additional cofactors for cell death induction during ALV-B infections. One of these cofactors may be massive superinfection, since infections by cytopathic ALV-B lead to accumulation of multiple copies of unintegrated viral DNA in dying cells (38, 39), although this idea remains to be tested.
The cycloheximide requirement of TVB-dependent cell death is highly reminiscent of a similar requirement of cell death pathways that are activated by TNFR-like receptors. In the case of TNFR-1, this protective pathway requires de novo protein biosynthesis induced by the transcription factor NF-κB. Activation of mammalian death receptors TNFR-1, DR3, DR4, and DR5 triggers translocation of NF-κB to the nucleus (reviewed in reference 27), where it drives the expression of cellular survival factors (10, 18, 40). Downregulation of NF-κB potentiates TNF-α-induced cell death (5), and mice lacking the NF-κB p65/RelA gene are defective in NF-κB signaling and die in utero from extensive cell death within their liver (24). NF-κB plays a pivotal role in many cellular responses to environmental changes (19). NF-κB exists in the cytoplasm as homo- and heterodimers and is sequestered by the specific inhibitory protein IκB (19). In response to a variety of extracellular stimuli, IκB is phosphorylated at serines at positions 32 and 36 and is subsequently ubiquitinylated and degraded (33). Consequently, NF-κB dissociates from IκB and translocates from the cytoplasm to the nucleus, where it induces target gene expression. Genes regulated by nuclear NF-κB include those involved in preventing cell death, such as TRAF1, TRAF2, c-IAP1, c-IAP2, A20, and IEX-1L (10, 18, 36, 40). Given the key role of NF-κB in cell survival we asked whether, like other TNFR-related death receptors, TVB receptors are competent in activating NF-κB and whether NF-κB-dependent pathways can protect against TVB-mediated cell death.
We show here that overexpression and cross-linking of TVB receptors lead to a striking increase in NF-κB activity. Furthermore, expression of the transdominant inhibitor of NF-κB (TD-IκB) overcomes the cycloheximide requirement for cell killing induced by soluble ALV envelope proteins, and a specific inhibitor of NF-κB, pyrrolidinedithiocarbamate (PDTC) (26), significantly enhanced the cytopathogenicity of ALV-B. These results indicate that an NF-κB-mediated protective pathway regulates the fate of cells during ALV-B infection. Therefore, the cytopathogenicity of ALV-B appears to be under the checkpoint control of NF-κB.
MATERIALS AND METHODS
Cell lines, plasmids, antibodies, and viruses.
Quail QT6 cells, chicken embryo fibroblast (DF1) cells, and human 293 cells have been described elsewhere (1, 8). The subgroup B-specific and subgroup E-specific SU-Ig fusion proteins (SUB-IgG and SUE-IgG, respectively) were described elsewhere (1). TVBS1, TVBS1ΔDD, TVBS1-F292A, TVBS1-R294A, TVBS1-L298A, TVBS1-L298N, TVBS1-W324A, TVBS3, TVBS3ΔDD, TVBS3-F292A, TVBS3-R294A, TVBS3-L298A, TVBS3-L298N, TVBS3-W324A, TVBT, and TVBTΔDD were described elsewhere (1, 8). The NF-κB-luciferase reporter plasmids, which contain either three wild-type NF-κB sites (3X-κB-L) or three mutant NF-κB sites (3X-mut-κB-L) were obtained from Bill Sugden (Madison, Wisc.). IκB-α (FL) (purchased from Santa Cruz Biotechnology, Inc.; catalog no. sc-847) was used as the antibody for transdominant IκB-α (TD-IκB). Anti-p50 and -p65 antibodies, used for supershift assays, were purchased from Santa Cruz Biotechnology, Inc. (catalog nos. sc-7178 and sc-109, respectively). TD-IκB was provided by Michael Karin (San Diego, Calif.). TD-IκB contains a hemagglutinin tag and serine-to-alanine substitutions of residues 32 and 36. PDTC was purchased from Sigma.
Measurements of NF-κB induction. (i) Luciferase assay.
A total of 106 cells were transfected with 300 ng of each construct by using Lipofectamine (Gibco BRL). Thirty-six to 48 h later, cells were lysed in 250 μl of lysis buffer (Promega Lysis buffer). The luciferase assay was performed using 30 μl of luciferase substrate (Promega) in 90 μl of lysate, which was incubated for 5 min at room temperature and analyzed in a luminometer (Wallac Corporation). For the measurements of transient NF-κB induction, 5 × 104 DF1 cells expressing the NF-κB-luciferase reporter were cocultivated with 5 × 104 of either uninfected DF1 cells, DF1 cells chronically infected by ALV-A, or DF1 cells chronically infected by ALV-B. After incubation at 37°C for varied time intervals, the above-mentioned luciferase assay was performed. For the measurements of transient NF-κB induction by cocultivation in the presence of PDTC, 5 × 104 DF1 cells expressing the NF-κB-luciferase reporter were plated out. Approximately 5 h later, PDTC was added at different concentrations (0, 0.1, 0.3, 0.5, 0.7, 1, 2, and 5 μM). Two hours later, 5 × 104 of either uninfected DF1 cells, DF1 cells chronically infected by ALV-A, or DF1 cells chronically infected by ALV-B were added. Cells were incubated at 37°C for 15 h after the cocultivation was started and were lysed by the luciferase lysis buffer. The luciferase assay was performed as described above.
(ii) Electrophoretic mobility shift assay (EMSA).
Approximately 2 × 107 293 cells were transfected with 31.5 μg of DNA plasmids encoding different TVB proteins in the presence or absence of 5 μg of IκB with Lipofectamine (Gibco BRL). After incubation at 37°C for 48 h (293 cells), cells were washed twice with phosphate-buffered saline (PBS) and centrifuged at 600 × g at 4°C. Pellets were lysed in low-salt buffer containing 10 mM HEPES at pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol (DTT), 0.5 mM phenylmethylsulfonyl fluoride (PMSF), protein inhibitor cocktail pill, and 0.5% Nonidet P-40. The cytoplasmic proteins in the supernatant were collected after centrifugation at 13,400 × g at 4°C. The cytoplasmic fraction was used for the immunoblotting assay. The pellets containing the nuclear fraction were resuspended and extracted in high-salt buffer containing 20 mM HEPES at pH 7.9, 400 mM KCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM PMSF, and protein inhibitor cocktail. Protein concentrations of both the cytoplasmic fraction and nuclear fraction were determined by a Bio-Rad protein assay. The NF-κB double-stranded oligonucleotides corresponding to the NF-κB consensus sequence of the κ light chain enhancer in B cells (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) (Promega) were end labeled with [γ-32P]ATP (3,000 Ci/mmol) (Amersham) by the T4 polynucleotide kinase (Promega) and purified on a G-25 column. Samples were prepared by mixing 10 μg of extracts with 0.1 pmol of [γ-32P]ATP end-labeled double-stranded NF-κB probe in binding buffer containing 10 mM Tris at pH 7.5, 50 mM NaCl, 1 mM EDTA, 5% glycerol, 1 mM DTT, and 1 mg of poly(dI-dC). The competition assay included 5 pmol of NF-κB oligo. Samples were resolved on a 6.5% polyacrylamide gel that was run at 200 V for 3 h in 0.5× TBE (45 mM Tris-borate and 1 mM EDTA). Gels were vacuum dried at 80°C for 2 h and exposed to Kodak film at −80°C for 10 to 24 h.
Immunoblotting.
Fifty micrograms of the protein extracts from the above low-salt buffer (see EMSA assay) was applied to a 10% polyacrylamide-sodium dodecyl sulfate gel under reducing conditions and transferred to nitrocellulose membranes. The membranes were first probed with SUB-IgG (for TVBS1 and TVBS3 receptors) or IκB-α (FL) (for TD-IκB) and then with horseradish peroxidase-conjugated donkey antibody specific for rabbit Ig (Amersham). Bound antibodies were detected by enhanced chemiluminescence (Amersham).
Cell killing analysis. (i) Cell killing by SU-IgG.
Approximately 105 QT6 or DF1 cells stably expressing TD-IκB were incubated at 37°C with 50 ng of SUB-IgG or SUE-IgG. Three days later, cells were washed with PBS, trypsinized, and replated. Four hours later live cells were trypsinized and counted under a microscope using a hemocytometer. Apoptotic cells were stained after 2 days with Hoechst 33342 stain (Sigma) and counted.
(ii) Cell killing by infection.
A total of 7 × 104 DF1 cells was incubated at 37°C for 6 h with 50 μl of ALV-A-green fluorescent protein (GFP) (titer of 106) or ALV-B-GFP (titer of 106) in the absence or presence of varied concentrations of PDTC. PDTC was added 2 h before the infection. At different time points, cells were washed with PBS, trypsinized, and counted.
Flow cytometric analysis of infectious activities of ALVs.
The infection status of ALV-A-GFP- and ALV-B-GFP-infected DF1 cells was determined at different time points. Cells were washed with PBS, trypsinized, and resuspended in PBS, and GFP expression was measured by flow cytometry.
RESULTS
NF-κB activation by overexpression of TVB receptors in human 293 cells.
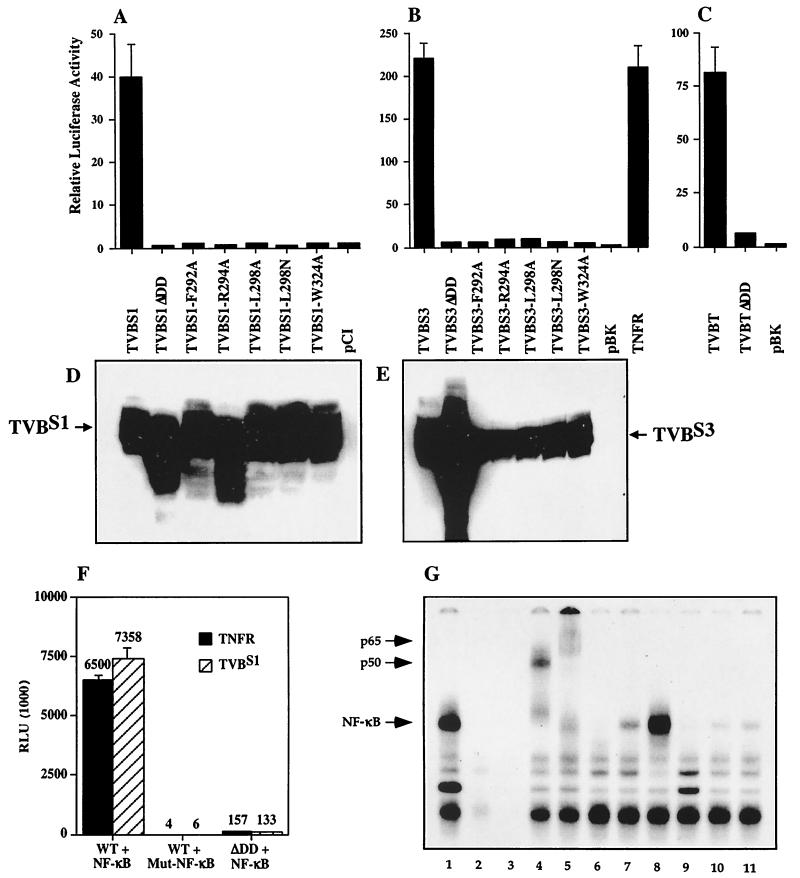
To test whether TVB receptors are able to activate NF-κB, we took advantage of the fact that overexpression of specific TNFR-related receptors in human 293 cells can activate this transcription factor. Different TVB constructs were cotransfected with an NF-κB-luciferase reporter plasmid into 293 cells. Overexpression of full-length TVBS1, TVBS3, and TVBT led to 40-, 220-, and 80-fold increases in NF-κB activation, respectively (Fig. 1A, B, and C). The results obtained with TVBS3 were comparable to that seen with overexpression of human TNFR-1 (Fig. 1B). We confirmed that the cytoplasmic death domains of the TVB receptors were required for NF-κB activation by overexpressing mutant TVBS1 and TVBS3 that either lacked these domains (ΔDD) or contained inactivating amino acid substitutions (F292A, L298A, L298N, W324A) within this domain (Fig. 2). These three residues are highly conserved among TNFR-like death domains and are required for NF-κB signaling (32) and TVB-induced cell death (7). Western blot analysis of lysates derived from transfected 293 cells confirmed that full-length and mutant TVB receptors were expressed at approximately equal levels (Fig. 1D and E) with the exception of TVBS1-R294A, TVBS1ΔDD, and TVBS3ΔDD (Fig. 1D and E). Expression levels of wild-type and death domain deletion mutants of TVBT and TNFR-1 have been described previously (1, 32). The mutant TVB receptors were unable to activate NF-κB (Fig. 1A, B, and C). In addition, overexpression of full-length TVBS3 and TNFR in the presence of mutant NF-κB-luciferase reporter plasmids, which contain mutant NF-κB elements, did not result in the induction of luciferase activity (Fig. 1F). This result supports the hypothesis that full-length TVB receptors are able to specifically induce NF-κB.
FIG. 1.
TVBS1, TVBS3, and TVBT receptors induce NF-κB upon transfection of human 293 cells. Human 293 cells were transfected with NF-κB-luciferase reporter plasmid and wild-type TVBS1, TVBS1ΔDD, TVBS1-F292A, TVBS1-R294A, TVBS1-L298A, TVBS1-L298N, TVBS1-W324A, and control plasmid pCI-neo (Promega) (A); wild-type TVBS3, TVBS3ΔDD, TVBS3-F292A, TVBS1-R294A, TVBS3-L298A, TVBS3-L298N, TVBS3-W324A, control plasmid pBK-CMV (Stratagene), and TNFR (B); or TVBT, TVBTΔDD, and control plasmid pBK-CMV (Stratagene) (C). The representative experiments were performed in triplicate, and the standard deviations of the data are indicated with error bars. Wild-type and mutant TVBS1 (D) or TVBS3 (E) are expressed in human 293 cells. Protein lysates from cells transfected with corresponding plasmids (panels A to C) were immunoblotted using SUB-IgG. (F) Overexpression of TVBS3 and TNFR in 293 cells triggers NF-κB. Transfection of TVBS3 and TNFR in 293 cells triggers luciferase activity in the presence of wild-type but not mutant NF-κB-luciferase reporter plasmids. (G) TVB receptors activate NF-κB. Human 293 cells were transfected with wild-type TVBS3 in the absence (lane 1) or presence (lane 6) of TD-IκB or with TVBS3-F292A (lane 7), TNFR (lane 8), TNFRΔDD (lane 9), or control plasmids pBK and GFP (lanes 10 and 11, respectively) and incubated with a radiolabeled wild-type NF-κB or a mutant NF-κB (lane 3) oligonucleotide. Supershift assays were performed with anti-NF-κB p50 and anti-NF-κB p65 antibodies of TVBS3-transfected cells (lanes 4 and 5, respectively). Lane 2 corresponds to lysates from 293 cells transfected with TVBS3 and supplemented with an excess unlabeled competitor NF-κB oligonucleotide. Nuclear lysates were subjected to EMSA.
FIG. 2.
Death domain mutations of TVBS3. Amino acid substitutions of highly conserved residues within the cytoplasmic death domain of TVBS3 are indicated, and deletions are demonstrated by dashes.
To demonstrate more directly that TVB receptors activate NF-κB, an EMSA was used. Consistent with the results obtained with the luciferase assay, overexpression of wild-type TVBS3 strongly increased levels of free NF-κB, whereas mutant TVBS3 (TVBS3-F292A), TNFRΔDD, and control plasmids (pBK and GFP) failed to do so (Fig. 1G, lanes 7, 9, 10, and 11). NF-κB activation by TVBS3 was similar to levels seen with overexpression of TNFR-1 (Fig. 1G, lanes 1 and 8). The specificity of the reaction was shown with mutant NF-κB probes, which were unable to give a signal (Fig. 1G, lane 3). Furthermore, supershift assays were performed to characterize the DNA-protein complexes. Anti-NF-κB p50 and anti-NF-κB p65 antibodies supershifted the complex (Fig. 1G, lanes 4 and 5), indicating that the NF-κB family members p50 and p65 are present in the induced complex. These results confirm that NF-κB is activated in cells transiently transfected with TVB proteins. Taken together, TVB receptors are capable of activating NF-κB by a mechanism requiring a functional death domain.
To test whether an NF-κB-dependent cellular pathway might protect against TVB-induced cell death, a dominant-negative form of the mammalian IκB (TD-IκB) (33), a specific inhibitor of NF-κB, was employed. TD-IκB lacks serine 32 and 36 and can no longer be phosphorylated and degraded upon TNFR activation. Thus, it constitutively binds to NF-κB and specifically inhibits its function. As expected, cotransfection of TVBS1 with TD-IκB resulted in a significant reduction in receptor-activated free NF-κB levels as demonstrated by EMSA (Fig. 1G, lane 6). Thus, activation of the NF-κB pathway by TVBS1 was specifically blocked by TD-IκB.
Cross-linking of TVB receptors with ALV-B SU-Ig proteins leads to NF-κB induction in chicken DF1 cells.
To determine whether binding of ALV envelope protein to the endogenous TVB receptor TVBS3 expressed in avian cells can activate NF-κB, we treated DF1 cells, which are homozygous for this tvb allele, with a soluble ALV-B SU-Ig fusion protein (SUB-IgG).
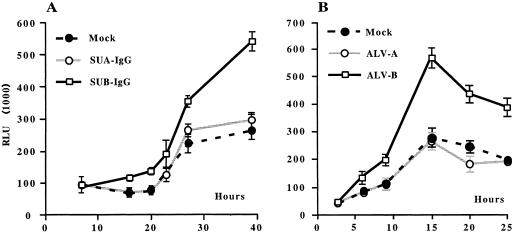
To determine induction of NF-κB after binding of the ALV-B envelope protein to TVBS3, we treated DF1 cells stably expressing the NF-κB reporter construct (DF1:NF-κB-luc) with SUB-IgG or SUA-IgG. As expected, SUB-IgG but not SUA-IgG triggered NF-κB in DF1:NF-κB-luc cells (Fig. 3A). No signal was detected for the first 15 h, and a twofold increased induction of NF-κB was detected over the period of 15 to 40 h. 293 cells expressing the ALV-B envelope protein were also able to activate NF-κB when cocultivated with DF1:NF-κB-luc cells (data not shown).
FIG. 3.
Binding of the ALV-B surface envelope fusion proteins to TVBS3 and cocultivation of DF1 cells with ALV-B-infected cells triggers NF-κB. (A) DF1 cells expressing NF-κB-luciferase reporter plasmids were left untreated (•) or were treated with SUA-IgG (○) or SUB-IgG (□) for 40 h. (B) Transient NF-κB induction upon cocultivation of DF1 cells with ALV-B-infected cells. DF1 cells expressing NF-κB-luciferase reporter plasmids were cocultivated with uninfected DF1 cells (•), ALV-A (○) chronically infected DF1 cells, or ALV-B (□) chronically infected DF1 cells, at a ratio of 1:1. The representative experiments were performed in triplicate, and the standard deviations of the data are indicated with error bars.
Cocultivation of DF1 cells with ALV-B-infected cells triggers NF-κB activation.
To determine whether NF-κB induction occurs during ALV-B infections, DF1 cells stably expressing the NF-κB reporter system (DF1:NF-κB-luc) were cocultivated with DF1 cells that were either uninfected or chronically infected by ALV-A or ALV-B. Cocultivation of DF1:NF-κB-luc cells with ALV-B-infected cells led to an approximately threefold increase in NF-κB activity over cells that were either cocultivated with uninfected cells or ALV-A-infected cells (Fig. 3B). NF-κB activation by ALV-B was transient and peaked 15 h after the start of the cocultivation. ALV-A, which utilizes the low density lipoprotein receptor (LDLR)-related TVA receptors and presumably does not activate NF-κB (4), was used as a negative control. As expected, no NF-κB induction over background was detected in DF1:NF-κB-luc cells cocultivated with ALV-A-infected cells. NF-κB activation after cocultivation with ALV-B-infected cells could be caused by several factors, such as binding of free ALV-B to TVB receptors by interactions of the ALV-B envelope proteins expressed on infected cells with TVB receptors expressed on uninfected cells and by postentry events (Fig. 3B). Subsequently, we wanted to investigate whether the induction of NF-κB by TVB receptors triggers an antiapoptotic pathway.
Expression of TD-IκB renders avian cells susceptible to ALV envelope protein-mediated cell death.
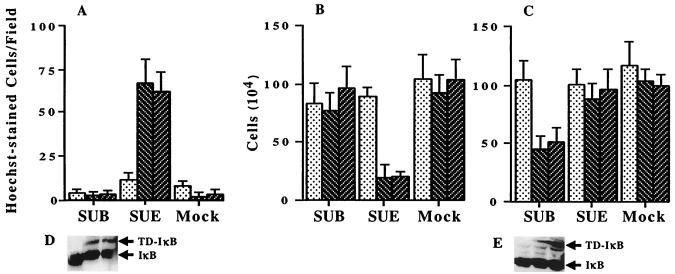
To investigate whether the cycloheximide requirement for TVB-dependent cell death is due to an NF-κB-mediated survival pathway, the transdominant NF-κB inhibitor TD-IκB was stably expressed in DF1 cells (DF1-IκB-1 and DF1-IκB-2) and QT6 cells (QT6-IκB-1 and QT6-IκB-2). Expression of TD-IκB in QT6 and DF1 cells was confirmed by Western blot analysis (Fig. 4D and E). Parental and TD-IκB-expressing cell lines were treated with SUB-IgG or SUE-IgG and the numbers of apoptotic and live cells were determined. Quail QT6-IκB cells, which express an endogenous ALV-E receptor and stably express TD-IκB (Fig. 4A and B), were killed in the presence of the soluble ALV-E but not the ALV-B envelope protein. An approximately 30-fold increase in cell death induction of SUE-IgG-treated QT6-IκB cells over untreated or SUB-IgG-treated cells was measured by Hoechst staining after 48 h (Fig. 4A). By contrast, no apoptosis induction was observed in the parental QT6 cells in the presence of SUE-IgG. In addition, we observed a three- to fourfold reduction in the number of live QT6 cells expressing TD-IκB after 3 days of incubation with SUE-IgG (Fig. 4B). Accordingly, DF1-IκB cells, which express the receptor for ALV-B and TD-IκB, were killed in the presence of the soluble ALV-B but not the ALV-E envelope protein (Fig. 4C). DF1 cell lines that did not express TD-IκB were not susceptible to cell killing by SUB-IgG. Therefore, expression of TD-IκB rendered QT6 and DF1 cells susceptible to SU-IgG-mediated cell death in a cycloheximide-independent fashion. These results indicate that cycloheximide is required for TVB-dependent cell death by preventing the expression of NF-κB-regulated survival factors.
FIG. 4.
Inhibition of NF-κB by dominant-negative IκB renders cells susceptible to killing by SUB-IgG or SUE-IgG. (A) Hoechst-stained apoptotic cells were counted per field of parental QT6 cells (dots) or QT6 cells stably expressing TD-IκB (slashes) 2 days after incubation with SUB-IgG, SUE-IgG, and Mock. Shown are cell counts of parental QT6 (B) and DF1 (C) cells (dots) or cells stably expressing TD-IκB (slashes) 3 days after incubation with SUB-IgG, SUE-IgG, and Mock. The representative experiments were performed in triplicate. (D and E) Western blot analysis of TD-IκB expression in parental and TD-IκB-expressing QT6 (D) and DF1 (E) cells.
Downregulation of NF-κB by PDTC enhances the cytopathic potential of ALV-B.
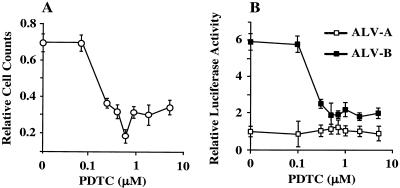
We have shown that downregulation of NF-κB levels renders cells susceptible to cell death induction upon binding of a soluble viral envelope protein. To understand whether reduced NF-κB levels enhance the apoptotic potential of ALV-B, we treated DF1 cells with the NF-κB inhibitor PDTC (26) for 2 h and then infected cells with ALV-B at a multiplicity of infection of 0.1. PDTC treatment enhanced cell killing by ALV-B compared with untreated cells (Fig. 5A). Maximal cell killing was observed at 0.7 μM PDTC (Fig. 5A), in correlation with a threefold reduction of NF-κB activation levels below levels in the absence of PDTC (Fig. 5B). ALV-B infection was not impaired in DF1 cells when treated with PDTC at the concentrations used (data not shown). These results suggest that levels of free NF-κB regulate the cytopathogenicity of ALV-B, and factors induced by NF-κB may keep cells alive during the initial rounds of ALV-B infection and during the chronic phase of infections.
FIG. 5.
Reduction of NF-κB activation by PDTC enhances the cytopathogenicity of ALV-B. (A) Ratio of cell counts of ALV-B-infected to uninfected DF1 cells, which were pretreated with various doses of PDTC. (B) Ratio of relative light units (RLU) for NF-κB-luciferase reporter-expressing DF1 cells pretreated with various doses of PDTC induced by cocultivation with chronically ALV-A (□) or ALV-B (▪) infected to uninfected DF1 cells. The representative experiments were performed in triplicate, and the standard deviations are indicated.
DISCUSSION
Here we have shown that TVB receptors (TVBS3, TVBS1, and TVBT), like mammalian TNFR-1, are able to trigger NF-κB upon activation and that a functional cytoplasmic death domain is essential for this activity. We demonstrated that TVB receptors activate NF-κB upon binding of soluble viral envelope protein (SU-IgG) and upon ALV-B infection of target cells. The ability to activate NF-κB is shared by TVB receptors for cytopathic ALV-B and -D and by TVB receptors for noncytopathic ALV-E. Therefore, it appears that the difference in the cytopathogenicities of ALV-B and ALV-E is not due to whether or not their receptors are able to trigger NF-κB.
NF-κB activation upon ALV-B infections might influence viral and cellular activities. For example, NF-κB activation in ALV-B-infected cells might promote ALV replication. Many viral proteins are able to activate NF-κB, including the human T-cell leukemia virus type 1 Tax protein (17), the HIV type 1 (HIV-1) Tat protein (12), and the Epstein-Barr virus LMP-1 (15). In many cases, NF-κB activation enhances viral replication and is therefore beneficial for these viruses. The HIV long terminal repeat (LTR), for example, contains NF-κB sites that render HIV highly responsive to NF-κB, and NF-κB activation enhances HIV gene expression and replication. In contrast, the ALV-LTR does not contain NF-κB sites; however, the ALV-LTR has been shown to be indirectly activated by NF-κB-binding proteins (6).
In addition, NF-κB activation orchestrates a host inflammatory response by inducing the expression of numerous cytokines, chemokines, growth factors, and immunoregulatory proteins, some of which might promote viral replication as well. The activation of NF-κB represents a double-edged sword in infected animals, however, since NF-κB could stimulate an immune response against the invading viruses. Numerous viruses have developed strategies to counter a strong immune response by blocking host-induced cell killing. For instance, several viruses encode Bcl-2 homologues, proteins that inactivate p53 (reviewed in reference 34), or potent inhibitors of caspases (CrmA [21]). Baculoviruses encode two apoptotic inhibitors, p35 and the inhibitors of apoptosis proteins (IAP).
Cytopathic ALV-B appears to have adopted at least two strategies to evade the immune response, including downregulation of the cognate receptors and activation of a protective antiapoptotic pathway. Chronic infections by ALV-B lead to a blockade of the TVB cognate receptors, presumably by downregulation of TVB receptors from the cellular surface. This blockade of TVB receptors might protect cells from being eliminated by the immune system. Death receptors like Fas or TNFR have been implicated in playing a key role in the immune response, and mice lacking either TNFR or Fas succumb to infections by specific pathogens (37). Accordingly, the downregulation of TVB receptors might help ALV-infected cells to evade the immune response. However, it is not yet known whether TVB receptors play a role in the host defense. It remains to be shown whether chickens that are defective for the TVB locus or are chronically infected by ALV-B are immunocompromised. In addition, NF-κB activation might stimulate resting cells, thereby creating an additional reservoir for the virus.
TNFR is able to activate an NF-κB-mediated antiapoptotic pathway (3) by induction of protective factors such as TRAF1, TRAF2, c-IAP, A20, and IEX-1L (10, 18, 40). The presence of an NF-κB-mediated antiapoptotic pathway that controls apoptosis induction by TVB receptors is supported by our findings that (i) SUB/E-mediated cell killing requires the presence of cycloheximide, (ii) avian cells expressing TVB and chronically infected by ALV-B and ALV-E are killed in the presence of cycloheximide (7), (iii) downregulation of NF-κB by TD-IκB renders TVB-expressing cells susceptible to ALV-B or -E envelope protein-mediated cell death, and (iv) chemical inhibitors of NF-κB enhance the cytopathic potential of ALV-B.
We propose that this NF-κB-mediated protective pathway keeps cells alive during the initial round of infection and during the chronic phase of infection. NF-κB activation is able to trigger apoptosis in some cell lines by inducing proapoptotic factors. However, it is unlikely that DF1 cells are killed by high NF-κB levels, since transient NF-κB induction in ALV-B-infected cells did not have any cytotoxic effects. The presence of an antiapoptotic pathway could explain why ALV-B is only minimally cytopathic during the initial phase of infection. ALV-B-induced cytopathic effects that are seen after several rounds of infection might be triggered by a shift in TVB signaling at the onset of cell killing. In mammalian cells, mitochondria play a central role in regulating apoptosis induction and are known to release a set of proapoptotic proteins such as cytochrome c, Apaf-1 (41), apoptosis inducing factor (16, 31), and Smac/DIABLO (9, 30, 35). Antiapoptotic factors could be blocked by pro-apoptotic mitochondrial proteins that are released from mitochondria. It remains to be shown whether mitochondria are activated and release factors at the onset of ALV-B-mediated cell killing. It is also conceivable that protective factors such as cIAP, A20, and BclXL are induced during the early phase of infection and blocked at the onset of cell killing by proapoptotic factors. The regulation and activities of factors that mediate cell killing by ALV-B remain to be determined.
Acknowledgments
We thank Steve Porcelli, Mathew Scharff, and David Fidock for critical reading of the manuscript.
This work was supported in part by NIH grant CA62000 (to J. A. T. Young).
REFERENCES
- 1.Adkins, H. B., J. Brojatsch, J. Naughton, M. M. Rolls, J. M. Pesola, and J. A. Young. 1997. Identification of a cellular receptor for subgroup E avian leukosis virus. Proc. Natl. Acad. Sci. USA 94:11617-11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adkins, H. B., J. Brojatsch, and J. A. Young. 2000. Identification and characterization of a shared TNFR-related receptor for subgroup B, D, and E avian leukosis viruses reveal cysteine residues required specifically for subgroup E viral entry. J. Virol. 74:3572-3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashkenazi, A., and V. M. Dixit. 1998. Death receptors: signaling and modulation. Science 281:1305-1308. [DOI] [PubMed] [Google Scholar]
- 4.Bates, P., J. A. Young, and H. E. Varmus. 1993. A receptor for subgroup A Rous sarcoma virus is related to the low density lipoprotein receptor. Cell 74:1043-1051. [DOI] [PubMed] [Google Scholar]
- 5.Beg, A. A., and D. Baltimore. 1996. An essential role for NF-κB in preventing TNF-alpha-induced cell death. Science 274:782-784. [DOI] [PubMed] [Google Scholar]
- 6.Bowers, W. J., L. A. Baglia, and A. Ruddel. 1996. Regulation of avian leukosis virus long terminal repeat-enhanced transcription by C/EBP-Rel interactions. J. Virol. 70:3051-3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brojatsch, J., J. Naughton, H. B. Adkins, and J. A. Young. 2000. TVB receptors for cytopathic and noncytopathic subgroups of avian leukosis viruses are functional death receptors. J. Virol. 74:11490-11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brojatsch, J., J. Naughton, M. M. Rolls, K. Zingler, and J. A. Young. 1996. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell 87:845-855. [DOI] [PubMed] [Google Scholar]
- 9.Chai, J., C. Du, J. W. Wu, S. Kyin, X. Wang, and Y. Shi. 2000. Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature 406:855-862. [DOI] [PubMed] [Google Scholar]
- 10.Chu, Z. L., T. A. McKinsey, L. Liu, J. J. Gentry, M. H. Malim, and D. W. Ballard. 1997. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-κB control. Proc. Natl. Acad. Sci. USA 94:10057-10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Degli-Esposti, M. A., W. C. Dougall, P. J. Smolak, J. Y. Waugh, C. A. Smith, and R. G. Goodwin. 1997. The novel receptor TRAIL-R4 induces NF-κB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity 7:813-820. [DOI] [PubMed] [Google Scholar]
- 12.Demarchi, F., M. I. Gutierrez, and M. Giacca. 1999. Human immunodeficiency virus type 1 tat protein activates transcription factor NF-κB through the cellular interferon-inducible, double-stranded RNA-dependent protein kinase, PKR. J. Virol. 73:7080-7086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donahue, P. R., S. L. Quackenbush, M. V. Gallo, C. M. deNoronha, J. Overbaugh, E. A. Hoover, and J. I. Mullins. 1991. Viral genetic determinants of T-cell killing and immunodeficiency disease induction by the feline leukemia virus FeLV-FAIDS. J. Virol. 65:4461-4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dorner, A. J., and J. M. Coffin. 1986. Determinants for receptor interaction and cell killing on the avian retrovirus glycoprotein gp85. Cell 45:365-374. [DOI] [PubMed] [Google Scholar]
- 15.Farrell, P. J. 1998. Signal transduction from the Epstein-Barr virus LMP-1 transforming protein. Trends Microbiol. 6:175-177. [DOI] [PubMed] [Google Scholar]
- 16.Joza, N., S. A. Susin, E. Daugas, W. L. Stanford, S. K. Cho, C. Y. Li, T. Sasaki, A. J. Elia, H. Y. Cheng, L. Ravagnan, K. F. Ferri, N. Zamzami, A. Wakeham, R. Hakem, H. Yoshida, Y. Y. Kong, T. W. Mak, J. C. Zuniga-Pflucker, G. Kroemer, and J. M. Penninger. 2001. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 410:549-554. [DOI] [PubMed] [Google Scholar]
- 17.Kanno, T., K. Brown, and U. Siebenlist. 1995. Evidence in support of a role for human T-cell leukemia virus type I Tax in activating NF-κB via stimulation of signaling pathways. J. Biol. Chem. 270:11745-11748. [DOI] [PubMed] [Google Scholar]
- 18.Lee, E. G., D. L. Boone, S. Chai, S. L. Libby, M. Chien, J. P. Lodolce, and A. Ma. 2000. Failure to regulate TNF-induced NF-κB and cell death responses in A20-deficient mice. Science 289:2350-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.May, M. J., and S. Ghosh. 1998. Signal transduction through NF-κB. Immunol. Today 19:80-88. [DOI] [PubMed] [Google Scholar]
- 20.Paquette, Y., Z. Hanna, P. Savard, R. Brousseau, Y. Robitaille, and P. Jolicoeur. 1989. Retrovirus-induced murine motor neuron disease: mapping the determinant of spongiform degeneration within the envelope gene. Proc. Natl. Acad. Sci. USA 86:3896-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ray, C. A., R. A. Black, S. R. Kronheim, T. A. Greenstreet, P. R. Sleath, G. S. Salvesen, and D. J. Pickup. 1992. Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1β converting enzyme. Cell 69:597-604. [DOI] [PubMed] [Google Scholar]
- 22.Resnick-Roguel, N., H. Burstein, J. Hamburger, A. Panet, A. Eldor, I. Vlodavsky, and M. Kotler. 1989. Cytocidal effect caused by the envelope glycoprotein of a newly isolated avian hemangioma-inducing retrovirus. J. Virol. 63:4325-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riedel, N., E. A. Hoover, R. E. Dornsife, and J. I. Mullins. 1988. Pathogenic and host range determinants of the feline aplastic anemia retrovirus. Proc. Natl. Acad. Sci. USA 85:2758-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenfeld, M. E., L. Prichard, N. Shiojiri, and N. Fausto. 2000. Prevention of hepatic apoptosis and embryonic lethality in RelA/TNFR-1 double knockout mice. Am. J. Pathol. 156:997-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider, P., M. Thome, K. Burns, J. L. Bodmer, K. Hofmann, T. Kataoka, N. Holler, and J. Tschopp. 1997. TRAIL receptors 1 (DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate NF-κB. Immunity 7:831-836. [DOI] [PubMed] [Google Scholar]
- 26.Schreck, R., B. Meier, D. N. Mannel, W. Droge, and P. A. Baeuerle. 1992. Dithiocarbamates as potent inhibitors of nuclear factor κB activation in intact cells. J. Exp. Med. 175:1181-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schulze-Osthoff, K., D. Ferrari, M. Los, S. Wesselborg, and M. E. Peter. 1998. Apoptosis signaling by death receptors. Eur. J. Biochem. 254:439-459. [DOI] [PubMed] [Google Scholar]
- 28.Siliciano, R. F. 1996. The role of CD4 in HIV envelope-mediated pathogenesis. Curr. Top. Microbiol. Immunol. 205:159-179. [DOI] [PubMed] [Google Scholar]
- 29.Smith, R. E., and E. V. Schmidt. 1982. Induction of anemia by avian leukosis viruses of five subgroups. Virology 117:516-518. [DOI] [PubMed] [Google Scholar]
- 30.Srinivasula, S. M., R. Hegde, A. Saleh, P. Datta, E. Shiozaki, J. Chai, R. A. Lee, P. D. Robbins, T. Fernandes-Alnemri, Y. Shi, and E. S. Alnemri. 2001. A conserved XIAP-interaction motif in caspase-9 and Smac/DIABLO regulates caspase activity and apoptosis. Nature 410:112-116. [DOI] [PubMed] [Google Scholar]
- 31.Susin, S. A., H. K. Lorenzo, N. Zamzami, I. Marzo, B. E. Snow, G. M. Brothers, J. Mangion, E. Jacotot, P. Costantini, M. Loeffler, N. Larochette, D. R. Goodlett, R. Aebersold, D. P. Siderovski, J. M. Penninger, and G. Kroemer. 1999. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 397:441-446. [DOI] [PubMed] [Google Scholar]
- 32.Tartaglia, L. A., T. M. Ayres, G. H. Wong, and D. V. Goeddel. 1993. A novel domain within the 55 kd TNF receptor signals cell death. Cell 74:845-853. [DOI] [PubMed] [Google Scholar]
- 33.Traenckner, E. B., H. L. Pahl, T. Henkel, K. N. Schmidt, S. Wilk, and P. A. Baeuerle. 1995. Phosphorylation of human IκB-alpha on serines 32 and 36 controls IκB-alpha proteolysis and NF-κB activation in response to diverse stimuli. EMBO J. 14:2876-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vaux, D. L., G. Haecker, and A. Strasser. 1994. An evolutionary perspective on apoptosis. Cell 76:777-779. [DOI] [PubMed] [Google Scholar]
- 35.Verhagen, A. M., P. G. Ekert, M. Pakusch, J. Silke, L. M. Connolly, G. E. Reid, R. L. Moritz, R. J. Simpson, and D. L. Vaux. 2000. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 102:43-53. [DOI] [PubMed] [Google Scholar]
- 36.Wang, C. Y., M. W. Mayo, R. G. Korneluk, D. V. Goeddel, and A. S. Baldwin, Jr. 1998. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680-1683. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe-Fukunaga, R., C. I. Brannan, N. G. Copeland, N. A. Jenkins, and S. Nagata. 1992. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature 356:314-317. [DOI] [PubMed] [Google Scholar]
- 38.Weller, S. K., A. E. Joy, and H. M. Temin. 1980. Correlation between cell killing and massive second-round superinfection by members of some subgroups of avian leukosis virus. J. Virol. 33:494-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weller, S. K., and H. M. Temin. 1981. Cell killing by avian leukosis viruses. J. Virol. 39:713-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu, M. X., Z. Ao, K. V. Prasad, R. Wu, and S. F. Schlossman. 1998. IEX-1L, an apoptosis inhibitor involved in NF-κB-mediated cell survival. Science 281:998-1001. [DOI] [PubMed] [Google Scholar]
- 41.Zou, H., W. J. Henzel, X. Liu, A. Lutschg, and X. Wang. 1997. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90:405-413. [DOI] [PubMed] [Google Scholar]