Abstract
Herpes simplex virus type 1 (HSV-1) infects a wide range of cells, including dendritic cells. Consequently, HSV-1 vectors may be capable of eliciting strong immune responses to vectored antigens. To test this hypothesis, an HSV-1 amplicon plasmid encoding human immunodeficiency virus type 1 gp120 was constructed, and murine immune responses to helper virus-free amplicon preparations derived from this construct were evaluated. Initial studies revealed that a single intramuscular (i.m.) injection of 106 infectious units (i.u.) of HSV:gp120 amplicon particles (HSV:gp120) elicited Env-specific cellular and humoral immune responses. A potent, CD8+-T-cell-mediated response to an H-2Dd-restricted peptide from gp120 (RGPGRAFVTI) was measured by a gamma interferon ELISPOT and was confirmed by standard cytotoxic-T-lymphocyte assays. Immunoglobulin G enzyme-linked immunosorbent assay analysis showed the induction of a strong, Env-specific antibody response. An i.m. or an intradermal administration of HSV:gp120 at the tail base elicited a more potent cellular immune response than did an intraperitoneal (i.p.) inoculation, although an i.p. introduction generated a stronger humoral response. The immune response to HSV:gp120 was durable, with robust cellular and humoral responses persisting at 171 days after a single 106-i.u. inoculation. The immune response to HSV:gp120 was also found to be dose dependent: as few as 104 i.u. elicited a strong T-cell response. Finally, HSV:gp120 elicited significant Env-specific cellular immune responses even in animals that had been previously infected with wild-type HSV-1. Taken together, these data strongly support the use of helper-free HSV-1 amplicon particles as vaccine delivery vectors.
Genetically engineered herpesviruses have been successfully used for the development of vaccines against important animal diseases, including Aujesky's disease (pseudorabies virus), infectious bovine rhinotracheitis, and swine fever (hog cholera virus) (21, 56, 57). In addition, attenuated herpesviruses have been used for human vaccination (including the Towne strain of human cytomegalovirus and the Oka strain of varicella-zoster virus) (5, 24, 38, 52). Both herpes simplex virus type 1 (HSV-1) and varicella-zoster virus have been used for the expression of foreign genes, since these viruses can accommodate large segments of exogenous DNA with little effect on virus replication (15, 43). Replication-competent and replication-defective gene replacement vectors based on both viruses are being explored as possible human immunodeficiency virus (HIV) vaccine delivery systems (32, 45). The appeal of this approach lies in part in the ability of herpesviruses to (i) elicit strong cytotoxic-T-lymphocyte (CTL) responses; (ii) infect mucosal surfaces; (iii) infect a broad range of cell types, including dendritic cells (1, 23, 30, 40); and (iv) establish a state of persistence in the infected cell. The latter property may conceivably result in more durable immune responses to herpesvirus-based vaccines compared to many other vector approaches.
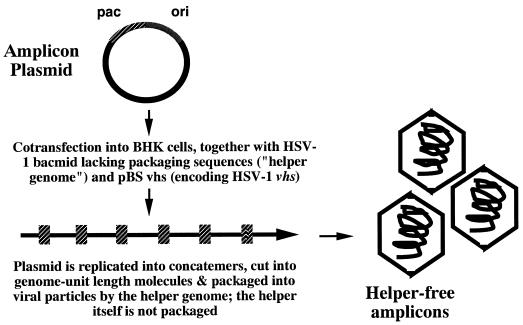
The HSV-1 amplicon is an alternative HSV-1-based gene transfer vector which differs significantly from standard gene replacement vector systems. Most notably, the HSV-1 amplicon is a highly flexible, replication-defective, high-capacity plasmid-based gene transfer vector that contains only the lytic-phase origin of DNA replication and the cleavage and packaging sequences from HSV-1 (13, 46). This plasmid can be replicated and packaged into HSV-1 virions in the presence of either a suitable helper virus or a helper virus genome (13, 46). In this context, ca. 150 kb of concatemeric, replicated amplicon DNA becomes packaged into each virus particle (Fig. 1). Thus, the amplicon particle can contain as many as 15 to 20 copies of the packaged amplicon plasmid. This results in the delivery of multiple gene copies to each individual cell that becomes infected by the amplicon particle.
FIG. 1.
HSV-1 amplicon vector system. The amplicon plasmid contains the HSV-1 origin of DNA replication, the viral cleavage and packaging site, and a mammalian expression cassette encoding the protein of interest (in this case, gp120). This plasmid is cotransfected into BHK cells, along with pBSvhs (which encodes the HSV-1 virion host shutoff protein), and the defective packaging construct (BAC-HSV), which lacks all viral cleavage and packaging elements. Immediately after transfection, the amplicon plasmid undergoes DNA replication (driven by proteins produced from the helper virus genome); this results in the generation of head-to-tail concatemers of the plasmid. These concatemers are cleaved into genome unit-length molecules and then packaged into virus particles, which can be harvested and concentrated and are ready for use.
The amplicon vector system has recently been improved through the development of defective helper-virus genomes which lack viral cleavage and packaging sites of their own (12, 53). These defective helper-virus genomes allow amplicon plasmids to become packaged into HSV-1 particles while failing to become packaged themselves. As a consequence, helper-free amplicon stocks can be readily derived (9, 12, 39, 53). These helper-free amplicon particles express only the exogenous inserted sequence of interest and do not express any open reading frame from HSV-1. Thus, helper-free amplicon particles can be considered to be highly safe and devoid of the ability to express any of the immunomodulatory gene products of HSV-1 itself (e.g., ICP47, which downregulates major histocompatibility complex class I [17, 59]). This may in part explain why helper-free amplicon vectors can infect dendritic cells without inhibiting dendritic-cell maturation or immunostimulatory function (58), unlike either wild-type HSV-1 or recombinant HSV-1 vectors (23, 30, 40).
With the pressing need for an effective HIV-1 vaccine, it seems prudent to explore a wide array of viral delivery systems. In the present study, we constructed an HSV-1 amplicon plasmid that encodes a codon-optimized HIV-1 gp120 insert (4), under the transcriptional control of a strong viral promoter (HSV:gp120). We then packaged this plasmid into HSV-1 particles, which were inoculated into mice to evaluate their ability to elicit immune responses against the encoded gp120. Since CD8+ CTL activity is considered by many to be critical for controlling both HIV infection and infection with the closely related agent simian immunodeficiency virus (SIV) (6-8, 19, 35, 42), we concentrated our analyses on the ability of the HSV-1 amplicon vector to elicit strong CTL responses to gp120. Among the numerous CD8+-CTL epitopes that potentially could be derived from the HIV-1 virion, few are known to be recognized in mice. Among these, an immunodominant H-2Dd-restricted epitope from the V3 loop of HIV-1 gp120 (RGPGRAFVTI) has been described in BALB/c mice (2, 3, 25, 33, 37, 44). Our analyses of Env-specific cellular immune responses to HSV:gp120 amplicon particles therefore focused on this particular epitope.
Overall, the findings reported here show that the HSV:gp120 amplicon vector is capable of eliciting strong Env-specific immune responses after a single inoculation of amplicon particles. The intradermal (i.d.) route of immunization appears to result in the generation of maximal cellular immune responses to gp120, and there is a clear dose effect in terms of the strength of response elicited versus the dose of amplicon delivered. However, even the lowest dose of amplicon that was tested (10,000 infectious particles) was able to generate a strong gp120-specific cellular response. The amplicon-induced immune response was highly durable in mice (lasting >5 months), and strong cellular responses occurred even in animals that had been previously infected with wild-type HSV-1. Overall, these data provide support for further evaluation of this novel approach to HIV-1 vaccine development.
MATERIALS AND METHODS
Cells.
All cells were incubated at 37°C in 5% CO2 under humidified conditions. Culture conditions were as follows: 293 cells were maintained in Dulbecco modified Eagle medium (DMEM; Gibco-BRL, Bethesda, Md.) supplemented with 10% fetal calf serum (FCS; Sigma, St. Louis, Mo.), penicillin, streptomycin, and l-glutamine (Gibco-BRL). P815 (H-2d mastocytoma) cells and EL4 (H-2b thymoma) cells were maintained in RPMI 1640 medium (Gibco-BRL) supplemented with 10% FCS, penicillin, streptomycin, and l-glutamine (Gibco-BRL). Primary splenocytes were prepared and cultured in RPMI 1640 medium supplemented with 10% FCS (HyClone, Logan, Utah), 50 μM β-mercaptoethanol (Gibco-BRL), penicillin, streptomycin, and l-glutamine (Gibco-BRL); this is referred to as CR10-β medium. Baby hamster kidney (BHK) cells were maintained as described previously (27). The NIH 3T3 mouse fibroblast cell line was originally obtained from the American Type Culture Collection and was maintained in DMEM supplemented with 10% FCS, penicillin, and streptomycin.
Amplicon vector construction.
A codon-optimized version of HIV-1 gp120 DNA was kindly provided by Jürgen Haas (Max-von-Pettenkofer Institut, Munich, Germany). This gp120 expression vector was derived from HIV-1 MN, except for the V3 loop, which was derived from HIV-1 LAI and contains an epitope recognized by BALB/c mice in the context of H-2Dd (2, 3, 25, 33, 37, 44). The codon-optimized version of gp120 was selected to allow high-level, Rev-independent expression as described previously (4). The HSV-1 amplicon vector pHSVPrPUC (13) contains the ColE1 plasmid origin, an HSV-1 packaging signal and origin, and a multiple cloning site situated downstream from the HSV-1 immediate-early 4/5 promoter (IE4/5) and upstream from the simian virus 40 polyadenylation signal. A NotI (blunted)-XhoI 1.6-kb fragment encoding the synthetic gp120 sequence was removed from the pSyngp120v3LAI plasmid and cloned directionally into a SalI-cut, BamHI-cut (blunted) pHSVPrPUC vector.
Helper virus-free HSV amplicon packaging.
Packaging of virus vectors was performed as described previously (9). Briefly, on the day prior to transfection, 2 × 107 BHK cells were seeded in a T-150 flask and incubated overnight at 37°C in 5% CO2. On the day of transfection, 1.8 ml of OptiMEM (Gibco-BRL), 25 μg of pBAC-V2 DNA (53), 7 μg of pBSKS (vhs) (9), and 7 μg of amplicon vector DNA were combined in a sterile polypropylene tube. Then, 70 μl of Lipofectamine Plus reagent (Gibco-BRL) was added over a period of 30 s to the DNA mix, and the mixture was incubated at 22°C for 20 min. In a separate tube, 100 μl of Lipofectamine (Gibco-BRL) was mixed with 1.8 ml of OptiMEM, and this mixture was also incubated at 22°C for 20 min. After the incubations, the contents of the two tubes were combined over a period of 30 s and incubated for an additional 20 min at 22°C. During this second incubation, the medium in the seeded T-150 flask was removed and replaced with 14 ml of OptiMEM. The transfection mix was added to the flask and allowed to incubate at 37°C for 5 h. The transfection mix was diluted with an equal volume of DMEM plus 20% FBS, 2% penicillin-streptomycin, and 2 mM hexamethylene bisacetamide (HMBA) and then incubated overnight at 34°C. The following day the medium was removed and replaced with DMEM plus 10% FBS, 1% penicillin-streptomycin, and 2 mM HMBA. The packaging flask was incubated an additional 3 days before the virus was harvested and stored at −80°C until purification. Viral preparations were subsequently thawed, sonicated, clarified by centrifugation, and concentrated by ultracentrifugation through a 30% sucrose cushion. Viral pellets were resuspended in 100 μl of phosphate-buffered saline (PBS) and stored at −80°C until use. Titers of vectors were determined as described previously by using expression and transduction titering methods (10). Virus was diluted in sterile Dulbecco PBS (D-PBS; Gibco-BRL) to the appropriate concentration prior to injection. In addition, two separate irrelevant amplicons were prepared as controls by using the same vector system described above; one contained the gene for Escherichia coli β-galactosidase (LacZ), and one contained the gene for murine intestinal alkaline phosphatase (MIAP).
Verification of gp120 expression.
To verify gp120 expression, 293 cells were first seeded in 12-well plates and allowed to adhere overnight at 37°C in 5% CO2. Cells were then either infected with HSV:gp120 amplicon particles or with HSV:MIAP amplicon particles as a negative control. On the day of infection, medium was removed, and cells were infected at a multiplicity of infection (MOI) of 0.5 in a volume of 0.5 ml at 37°C in 5% CO2. After 1 h, 1.5 ml of supplemented DMEM was added to the wells, and the cells were incubated for 48 h. Medium was collected from the wells and concentrated by using the Microcon microconcentrator (M-50; Millipore, Bedford, Mass.). Concentrated supernatants were combined with an equal volume of Laemmli buffer and were separated on 4 to 15% gradient sodium dodecyl sulfate-polyacrylamide gels (Bio-Rad, Hercules, Calif.). Full-range rainbow molecular weight markers (Amersham/Pharmacia, Piscataway, N.J.) were added as a standard. The gels were transferred to nitrocellulose and were blocked in PBST buffer (1× PBS, 0.1% Tween 20) containing 5% nonfat milk. The blots were incubated with the AD3 anti-gp120 monoclonal antibody (AIDS Repository, Rockville, Md.) for 1 h at room temperature. Blots were extensively washed with PBST. The blots were then incubated with sheep anti-mouse horseradish peroxidase secondary antibody (Amersham). Blots were again washed extensively with PBST. Enhanced chemiluminescence (Amersham) was performed for 1 min, and the blots were exposed to X-ray film (Kodak, Rochester, N.Y.).
Peptide.
The H-2Dd-restricted 10-mer epitope peptide from the V3 loop (RGPGRAFVTI) was synthesized by Alpha Diagnostics (San Antonio, Tex.), purified by high-pressure liquid chromatography and analyzed by mass spectroscopy.
Mice.
Female BALB/c mice aged 5 to 6 weeks were obtained from Taconic Laboratories (Germantown, N.Y.) and maintained according to University of Rochester guidelines. Prior to all injections, mice were bled orbitally, and sera were stored at −80°C until testing. For allogeneic CTL cultures, female C57BL/6 mice, aged 5 to 6 weeks, were utilized and handled as indicated above. Mice were injected by using a 28G 0.5-in. 1-ml insulin syringe (Becton Dickinson, Franklin Lakes, N.J.). Injections were given intramuscularly (i.m.) in the right hind hamstring muscle, i.d. at the base of the tail, or intraperitoneally (i.p.) in the body cavity. After inoculation, mice were incubated for 21 to 22 days prior to sacrifice and analysis.
At euthanization, sera were obtained by cardiac puncture, and spleens were removed by using sterile technique with sterile forceps and tweezers and transferred to 60-mm tissue culture dishes containing 5 ml of RPMI 1640 medium (Gibco-BRL). The spleens were aseptically transferred to 100-mm tissue culture dishes containing 10 ml of CR10-β. Autoclaved frosted glass slides (VWR, Media, Pa.) were used to gently grind the spleens to obtain a single cell suspension. Splenocytes were centrifuged, and the cell pellets were resuspended in 10 ml of CR10-β, counted, and diluted to a concentration of 4 × 107 cells/ml.
Two to three days before the CTL cultures were prepared, lipopolysaccharide (LPS) blasts were prepared as stimulator cells for the CTL cultures. Syngeneic BALB/c mice were euthanized, and their spleens were processed as described above. Naive splenocytes were cultured in T-75 flasks at a concentration of 4 × 107 cells/flask in 30 ml of CR10-β supplemented with 150 μl of LPS (1 mg/ml in sterile H2O, from E. coli serotype O55:B5) and 30 μl of dextran sulfate (7 mg/ml in sterile PBS). Flasks were incubated at 37°C in 5% CO2. After the incubation period, flasks were examined to look for blast-like cells. LPS blasts were washed extensively with RPMI 1640 medium and then pulsed with the RGPGRAFVTI peptide for 60 min in a 37°C water bath. Blasts were irradiated in a cesium-137 source for 10 min (ca. 3,000 rads) and then diluted to a concentration of 107 cells/ml prior to use.
As an allogeneic control for CTL expansion and killing assessment, one C57BL/6 mouse was euthanized per experiment (as described above), and its spleen processed as described above. The splenocytes were diluted to a concentration of 2 × 107 cells/ml and irradiated along with the LPS blasts.
ELISPOT assay.
The rat anti-mouse gamma interferon monoclonal antibody AN18 was produced from the corresponding hybridoma, which was kindly provided by Edith Lord (University of Rochester Medical Center). Hybridoma supernatants were concentrated by using the Cellmax system (Spectrum Laboratories, Laguna Hills, Calif.) according to the manufacturer's instructions. Ninety six-well membrane plates (Millipore) were coated with the AN18 monoclonal antibody diluted in 1× D-PBS (Gibco-BRL), and plates were washed with RPMI 1640 medium (Gibco) and blocked for at least 1 h at room temperature with CR10-β. Splenocytes from immunized animals were diluted in a background of syngeneic splenocytes to allow each enzyme-linked immunospot (ELISPOT) well to contain a fixed cell concentration of 106 cells per well. Splenocytes were cultured either in 2 μM (final concentration) of the V3 peptide diluted in CR10-β or in CR10-β alone for 18 h at 37°C in 5% CO2. Plates were washed extensively with PBST. Biotinylated rat anti-mouse gamma interferon was added to the wells (clone XMG1.2; PharMingen, San Diego, Calif.) and incubated for 2 h at room temperature. Streptavidin-alkaline phosphatase was added (Jackson Immunoresearch, West Grove, Pa.) to the well, and the mixture was allowed to incubate 1 h at room temperature. Plates were washed as described above. Vector Blue substrate (Vector Laboratories, Burlingame, Calif.) was prepared according to the manufacturer's instructions, added to the plates, and incubated for 10 min at room temperature in the dark. Plates were then washed extensively with distilled H2O, allowed to dry overnight, and then counted and analyzed by Zellnet Consulting, Inc. (New York, N.Y.).
CTL cultures.
To expand the CTL population from the inoculated animals to a level that could be observed by CTL assay, CTL cultures were prepared. Multiple wells were set up per animal to be tested. A 1-ml portion of CR10-β was combined with 0.1 ml of experimental splenocytes (4 × 106 cells) and either 0.1 ml of irradiated, V3-peptide-pulsed LPS blasts (106 cells) to determine the peptide-specific response or 0.1 ml of irradiated C57BL/6 cells (2 × 106 cells) to test for the allogeneic response. Cultures were incubated for 5 days at 37°C in 5% CO2.
The cytotoxic activity of gp120-specific CTLs was determined by JAM assay (29). Briefly, P815 and EL4 cells were seeded at 2 × 105 cells/ml on the day prior to the assay. On the day of the assay, the cells were centrifuged and resuspended in RPMI 1640 medium supplemented with 10% FCS. The cells were labeled with [3H]thymidine (5 μCi/ml; New England Nuclear, Boston, Mass.) for 3 h at 37°C in 5% CO2, washed with RPMI 1640 medium, and diluted to a concentration of 105 cells/ml in CR10-β. CTL effectors were washed extensively with RPMI 1640 medium and resuspended in a fixed volume of CR10-β. The CTLs were added to 96-well U-bottom plates (Falcon) in triplicate, to give responder/target ratios from 100:1 to 1.6:1. Ten thousand radiolabeled targets were then added either in the presence or in the absence of the V3 peptide. Plates were centrifuged briefly to pellet CTL effectors and targets and then incubated at 37°C in 5% CO2. After 3 h, plates were harvested onto glass filters (Perkin-Elmer Wallac, Gaithersburg, Md.) by using a 96-well automated harvester (Tomtec, Hamden, Conn.). After they were dried, the filters were subjected to scintillation counting analysis by using the Microbeta 1450 Trilux counter (Perkin-Elmer Wallac). Cytotoxicity was determined according to the following formula: percent cytotoxicity = [(S − E) ÷ S] × 100, where E is the experimentally retained DNA in the presence of killers (in counts per minute) and S is the retained DNA in the absence of killers (spontaneous) as described previously (29).
In some experiments, CD8+-cell depletion was accomplished with the use of anti-CD8a Ly-2 magnetic beads (Miltenyi-Biotec), according to the manufacturer's instructions. To verify successful elimination of the CD8+ cells, splenocytes were stained with fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD8 (Ly-2; BD PharMingen) and were examined by using a FACScan cytometer (Becton Dickinson).
Tetramer analysis.
H-2Dd streptavidin-allophycocyanin tetramers containing the V3 peptide were prepared by the NIH Tetramer Facility (Emory University, Atlanta, Ga.). Tetramer staining proceeded as follows: 1 × 106 to 2 × 106 freshly isolated splenocytes were washed once and resuspended in 50 μl of ice-cold fluorescence-activated cell sorting (FACS) buffer (1× PBS [pH 7.2] containing 0.09% sodium azide and 2% fetal bovine serum). For staining, 50 μl of FACS buffer containing 0.1 μg of FITC-conjugated anti-mouse CD8 (Ly-2) and 0.5 μg of tetramer were added to the cells, mixed well, and kept on ice for 1 h in the dark. The cells were subsequently washed with FACS buffer twice, resuspended in 400 μl of FACS buffer, and analyzed on a FACSCalibur (BD PharMingen) by using CellQuest software. A total of 100,000 events were collected, and the histograms shown represent cells gated initially by forward scatter and side scatter.
Anti-gp160 ELISA.
ELISA (enzyme-linked immunosorbent assay) plates were coated overnight at 4°C with recombinant soluble HIV-1 MN gp160 (Protein Sciences, Meridien, Conn.), diluted in coating buffer (0.1 M NaHCO3 [pH 8.6]). Plates were washed with PBS-T buffer and then blocked for 2 h at 37°C by using PBS-T containing 3% bovine serum albumin (Sigma). Plates were washed again in PBS-T, and sera were diluted, added to the ELISA plates in duplicate, and incubated for 1 h at 37°C. Plates were washed as described above. Anti-mouse immunoglobulin G (IgG) Fc-specific secondary antibody (Sigma) was added to the plates, and they were incubated for 1 h at room temperature. After washing, ABTS [2,2′azinobis(3-ethylbenzthiazolinesulfonic acid)] substrate (Sigma) was added to the plates and, after enough time for color development, the absorbance was measured at 405 nm. Endpoint titers were ascertained by using a scatter plot with optical density (OD) values on the y axis and dilution−1 on the x axis, for which the x-axis scale was logarithmic. After the data were plotted, a logarithmic curve fit was applied to each individual dilution series, and the point where the curve fit intersects the positive-negative cutoff value was determined. This cutoff value was calculated as the mean OD of all preimmune sera plus four standard deviations. For the experiment involving the preinfection of mice with wild-type HSV-1, the cutoff value was calculated slightly differently due to the fact that the HSV-1-preinfected mice exhibited an elevation in their background level of serologic reactivity to HIV-1 gp120. The cutoff value was calculated as the mean OD plus four standard deviations of all sera collected from either preinfected or naive mice.
Anti-HSV ELISA.
Sera were tested for anti-HSV IgG antibodies by ELISA as described previously (20). Briefly, ELISA plates were coated with UV-inactivated HSV overnight at 4°C. Plates were washed with PBST and blocked with PBS-0.1% bovine serum albumin for 1 h at 37°C. Plates were again washed with PBST. Sera were diluted and incubated in duplicate on the HSV-coated plates overnight at 4°C. Plates were washed with PBST and treated with alkaline phosphatase-conjugated goat anti-IgG (Cappel, Worthington, Pa.) for 3 h at 37°C. Plates were washed with PBST and incubated with p-nitrophenyl phosphate substrate (Sigma), and the absorbance was read at 405 nm.
Virus neutralization assays.
Sera from mice subjected to preinfection with HSV-1 (Patton) strain were analyzed for the presence of virus-neutralizing antibodies as follows. Briefly, sera obtained either prior to or 5 weeks after HSV-1 (Patton) infection were diluted 1:20 and 1:100 in PBS and then combined with an equal volume (1,000 infectious units [i.u.], 100 μl) of HSV:lacZ amplicon particles. After incubation at 37°C for 135 min, this preparation was then added to Vero cells in 24-well tissue culture plates for 60 min at 37°C. DMEM medium containing 5% FCS was then added to each well (1.0 ml), and cells were allowed to incubate at 37°C in 5% CO2 for 48 h. At the end of the given time period, the cells were fixed with 1% glutaraldehyde, washed with 1× PBS, and analyzed for β-galactosidase activity by a histochemical staining method with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) as the substrate. For each serum and dilution tested, the percent reduction in LacZ-positive cells was calculated relative to cultures that were exposed to untreated HSV:lacZ amplicon particles.
RESULTS
Generation of helper virus-free amplicons.
Figure 1 depicts the generation of the helper virus-free amplicons. Briefly, BHK cells were cotransfected with three plasmids: (i) the amplicon plasmid encoding the gp120 expression cassette, as well as critical cis-acting elements necessary for plasmid replication and packaging by the helper virus (i.e., the HSV-1 lytic-phase origin of DNA replication and the viral cleavage/packaging motif); (ii) the defective helper virus genome (an HSV BACmid lacking all viral cleavage and packaging motifs); and (iii) a plasmid encoding the HSV vhs gene, which allows for the generation of higher-titer amplicon stocks (9). This procedure produces helper-free, replication-defective HSV-1 particles that contain 15 to 20 copies of the amplicon plasmid, arranged in a concatemeric configuration; the particles are devoid of helper virus genomes (<1 helper virus genome per 106 i.u. of amplicon particles; W. J. Bowers and H. J. Federoff, unpublished data) and are incapable of directing the synthesis of any HSV-1 gene products since they lack any coding sequence from HSV-1 (9, 12, 39, 53).
gp120 is expressed and secreted from cells infected with HSV:gp120 amplicon particles.
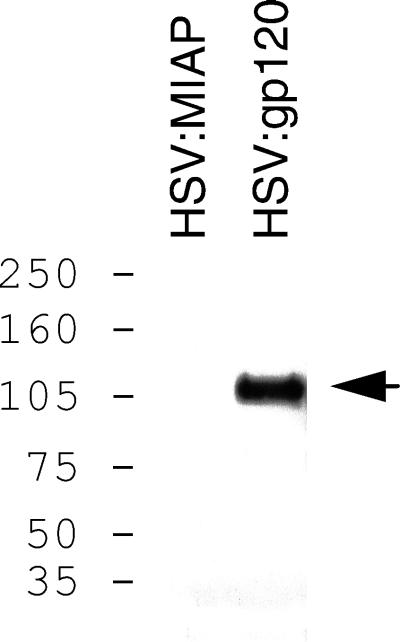
To verify that the amplicon utilized in our immunization studies could lead to transcription and translation of gp120 in infected cells, we tested the purified amplicon particles in vitro. 293 cells were infected with either HSV:gp120 amplicon particles or with HSV:MIAP (as a negative control) at an MOI of 0.5; cells were then incubated for 48 h. At the end of this time, culture supernatant proteins were concentrated, resolved on a polyacrylamide gel, and immunoblotted with an anti-gp120 monoclonal antibody (AD3). As shown in Fig. 2, a band of ∼120 kDa was detected in culture supernatants from cells infected with the HSV:gp120 amplicon particles but not in supernatants from cells exposed to the HSV:MIAP amplicon particles.
FIG. 2.
Amplicon-directed expression of HIV gp120 in 293 cells. 293 cells were infected with either HSV:gp120 amplicon particles or with amplicon particles encoding MIAP (HSV:MIAP) at an MOI of 0.5. At 48 h after infection, cell culture supernatants were collected, concentrated, subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and analyzed by immunoblot with the gp120-specific monoclonal antibody AD3 (the position of gp120 is indicated by the arrow). The numbers on the left denote the positions of the prestained molecular mass markers (Amersham-Pharmacia) which were included on the gel; sizes are in kilodaltons.
Inoculation with HSV:gp120 amplicon particles results in strong Env-specific cellular and humoral immune responses.
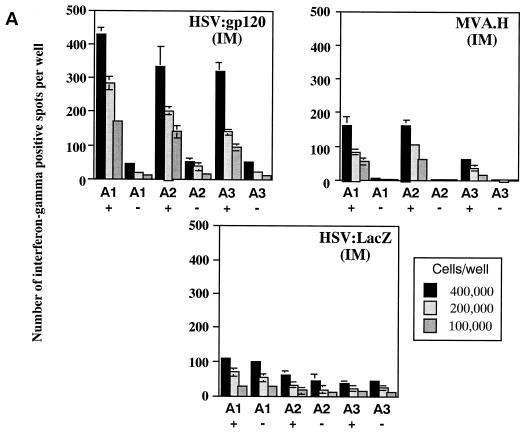
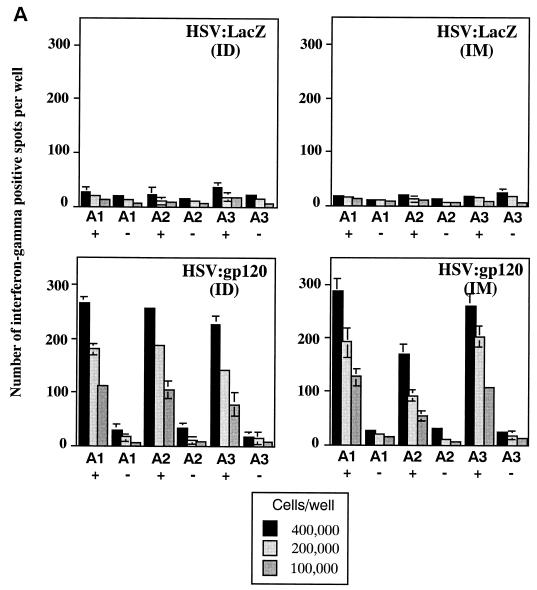
Groups of female BALB/c mice were injected i.m. with 106 i.u. of either HSV:gp120 or HSV:lacZ amplicon particles or 106 PFU of MVA.H; three mice were used per experimental group. At 22 days after injection, mice were sacrificed and bled, and their splenocytes were tested for gamma interferon release by ELISPOT and for cytotoxic function by JAM assay. Figure 3A shows that the frequency of splenocytes secreting gamma interferon in response to V3 peptide-specific stimulation was high in mice immunized with HSV:gp120 (ca. 1,000 spots per 106 splenocytes). In contrast, the frequency of these peptide-specific, gamma interferon-producing cells was considerably less in splenocytes harvested from mice that had been immunized with the MVA.H vector, or with HSV:lacZ (as an irrelevant control). It should be noted that the MVA.H vector expresses the same immunodominant HIV-1 V3 loop peptide (RGPGRAFVTI) that is contained within the HSV:gp120 construct (14); it was therefore included in these initial experiments as a positive control.
FIG. 3.
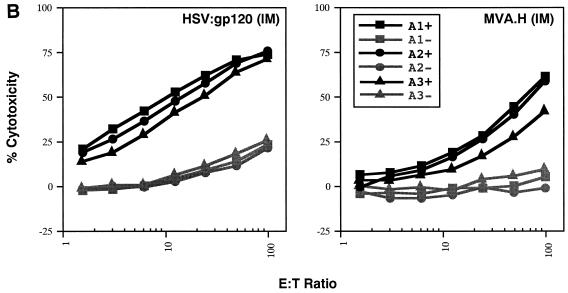
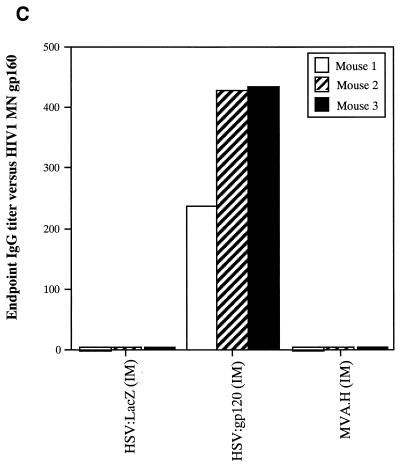
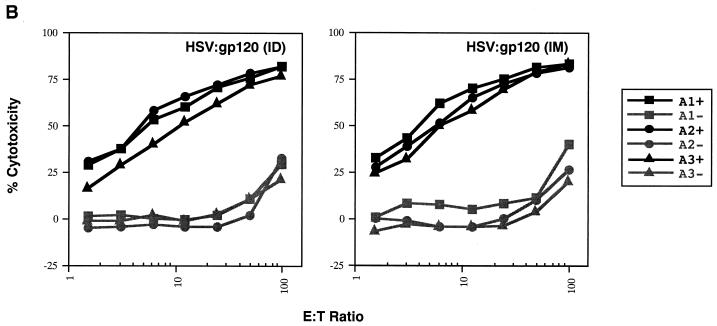
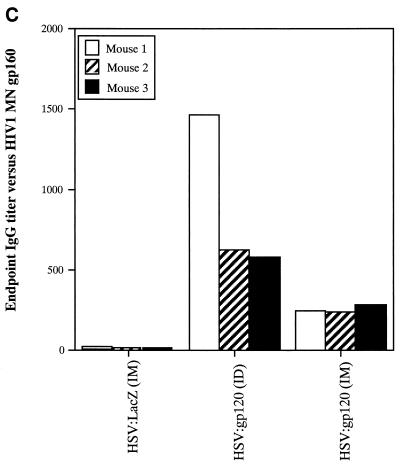
A single inoculation of HSV:gp120 amplicon particles results in potent and cellular immune responses to gp120. (A) Analysis of gamma interferon release by ELISPOT assay. Mice were inoculated with either 10 6 infectious units of HSV:gp120 or HSV:lacZ amplicon particles or 10 6 PFU of MVA.H. Splenocytes were analyzed 22 days postinfection for gamma interferon release by ELISPOT assay. Results are shown for each individual mouse (A1, A2, and A3) in the presence (+) or absence (−) of the immunodominant V3 peptide from HIV-1 (RGPGRAFVTI). In all cases, three different input levels of splenocytes were analyzed in triplicate (400,000, 200,000, or 100,000 cells as indicated). The results shown represent the mean the standard error of the mean for each datum point. This experiment is representative of two experiments that were performed, both of which yielded similar results. In all cases, relatively few gamma interferon-producing cells were detected in the absence of the specific HIV-1 peptide or in animals immunized with an irrelevant vector (HSV:lacZ). (B) Vaccine-elicited T cells possess potent cytolytic activity. Splenocytes were harvested from immunized mice at 22 days postinfection. The cells were then cultured for 5 days in the presence of irradiated syngeneic LPS blasts pulsed with the class I-restricted epitope. Cytolytic function was determined by incubating various amounts of syngeneic P815 target cells with effector cells, generating different effector-to-target (E:T) ratios. The results are shown for each individual mouse (A1, A2, and A3) in the presence (+; solid lines) or absence (−; shaded lines) of the immunodominant V3 peptide from HIV-1 (RGPGRAFVTI). The results represent the mean values from triplicate samples. This experiment is representative of two experiments that were performed, both of which yielded similar results. (C) Inoculation of mice with a single dose of HSV:gp120 amplicon particles results in a substantial Env-specific humoral response. Estimated endpoint titers are shown; this experiment is representative of two experiments that were performed, both of which yielded similar results.
The cytotoxic function of murine splenocytes corresponded well with the ELISPOT data. In particular, splenocytes from the animals inoculated with HSV:gp120 amplicon particles exhibited potent cytotoxic activity, whereas splenocytes from MVA.H-treated mice showed a much lower level of cytotoxicity (Fig. 3B). A strong humoral response was observed only in mice inoculated with the HSV:gp120 amplicon particles, as measured by ELISA (Fig. 3C). The absence of a detectable Env-specific antibody response at a 1:10 dilution in mice immunized with the MVA.H vector was expected, since this vector encodes only a string of CTL epitopes (including the V3 loop peptide) rather than the authentic gp120 protein.
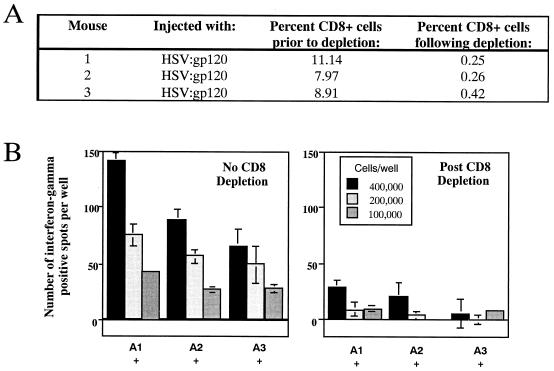
Cells secreting gamma interferon are predominantly CD8+ cells. To verify that the gamma interferon-secreting splenocytes were actually CD8+ cells and not another cell type capable of secreting this cytokine, we analyzed splenocytes from animals inoculated 22 days earlier with the HSV:gp120 amplicon particles both prior to and following treatment with an anti-CD8a monoclonal antibody coupled to magnetic beads. Both treated and untreated cells were subsequently stained with an anti-CD8 FITC-conjugated antibody and analyzed by flow cytometry. Figure 4A indicates that the overwhelming majority of CD8+ cells were eliminated by this treatment. When a gamma interferon ELISPOT assay was performed with these cells, a significant decrease in the frequency of gamma interferon-producing cells was observed in splenocyte cultures that had been depleted of CD8+ cells (Fig. 4B). This finding indicates that the splenocytes which secrete gamma interferon in response to V3-peptide stimulation in this assay are indeed CD8+ cells.
FIG. 4.
Gamma interferon-producing cells detected in the ELISPOT assay correspond to CD8+ T lymphocytes. Splenocytes were collected from immunized animals at 22 days postinfection, and CD8+ T lymphocytes were depleted from this population by using anti-CD8a coupled to magnetic particles. Primary splenocytes were isolated and stained with FITC-conjugated anti-CD8 Ly-2 monoclonal antibody either prior to or after depletion with anti-CD8a magnetic beads. Cells were analyzed by using a FACScan cytometer. (A) Percentage of CD8+ cells in the splenocyte population (before and after immunodepletion) for each individual mouse. (B) Splenocytes were subsequently tested for gamma interferon release by ELISPOT assay. The results are shown for each individual mouse (A1, A2, and A3) in the presence of the immunodominant V3 peptide from HIV-1 (RGPGRAFVTI). In all cases, three different input levels of splenocytes were analyzed in triplicate (400,000, 200,000 or 100,000 cells as indicated). The results shown represent the mean number of spots ± the standard error of the mean for each datum point. Note that in this experiment, the mean number of spots was calculated as follows: mean number of spots in the presence of the V3 peptide minus mean number of spots detected in the absence of the V3 peptide. This subtraction of nonspecific background reactivity was performed to facilitate comparison between results from the total and CD8-depleted splenocyte populations.
Immune responses to HSV:gp120 amplicon particles are influenced by the route of vector delivery.
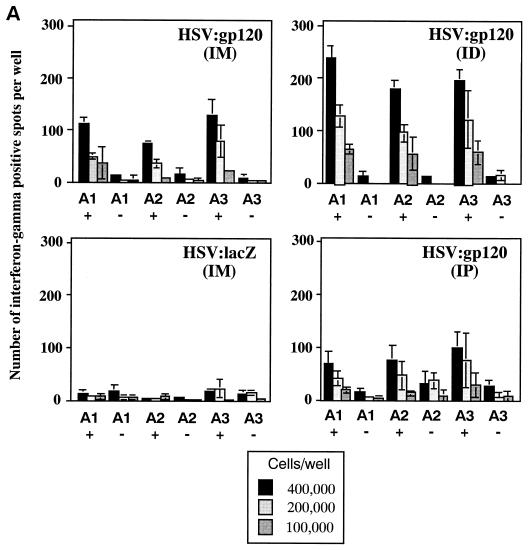
Since various injection sites can lead to differences in immune responses, we inoculated HSV:gp120 amplicon particles (106 i.u.) into groups of 3 mice, by three routes: i.d. at the base of the tail, i.m. in the hamstring muscle, and i.p. One million i.u. of HSV:lacZ was inoculated i.m. in a separate group of three mice as a control. Gamma interferon ELISPOTs and anti-gp160 endpoint serology (IgG ELISA assay) were performed for each mouse 21 days after the vector administration. Figure 5A shows that administration of the HSV:gp120 amplicon particles via the i.d. and i.m. routes resulted in a higher frequency of gamma interferon-producing cells compared to the i.p. route. The data also showed that the i.d. route of immunization resulted in a stronger cellular immune response than the i.m. route, at least when analyzed at this time point (21 days after immunization).
FIG. 5.
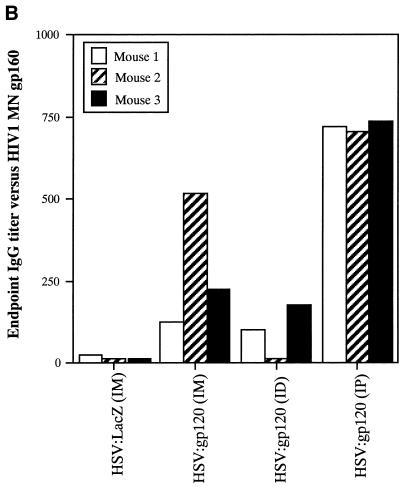
Effect of the route of delivery on immune responses to HSV:gp120 amplicon particles. (A) i.d. inoculation results in higher frequency of gamma interferon-secreting cells. A single inoculation of 10 6 i.u. was introduced in mice via i.d., i.m., or i.p. routes. At 21 days postinoculation, mice were sacrificed, and splenocytes were tested for gamma interferon release by ELISPOT assay. The results are shown for each individual mouse (A1, A2, and A3) in the presence (+) or absence (−) of the V3 peptide. As noted in the legend to Fig. 3A, three different input dilutions of splenocytes were used, and results are presented as the mean of triplicate datum points ± the standard error of the mean. The i.d. and i.m. routes of amplicon delivery resulted in higher frequencies of gamma interferon-secreting cells compared to the i.p. route, with the i.d. route resulting in the highest frequency. (B) i.p. inoculation yields the highest level of anti-gp160 antibodies. Of the three routes of inoculation tested, the i.p. route resulted in a stronger anti-gp160 humoral response compared to either the i.m. or i.d. routes.
Analysis of serologic responses to HIV-1 Env in immunized mice revealed a strikingly different hierarchy among the various routes of administration. In this case, the i.p. route resulted in the highest level of Env-specific IgG production (Fig. 5B).
A single inoculation of HSV:gp120 amplicon particles results in durable cellular and humoral immunity.
Since herpesvirus vectors may have the potential to elicit long-lived immune responses to encoded antigens, we examined the durability of immune responses elicited by a single inoculation of HSV:gp120 amplicon particles. To accomplish this, groups of three mice were inoculated with a single 106 i.u. dose of either HSV:gp120 or HSV:lacZ via either the i.d. or the i.m. route, since these routes gave the strongest levels of cellular immune responses. Approximately 5 months (171 days) after this single inoculation, mice were sacrificed, and their splenocytes were assayed for cytokine production (gamma interferon ELISPOT) and cytolytic function (JAM assay); the level of Env-specific IgG in sera from these mice was also quantitated by using anti-gp160 endpoint serology. This analysis revealed that a single administration of the replication-defective HSV:gp120 amplicon vector resulted in the production of a high level of peptide-specific gamma interferon secreting cells that persisted for >5 months (Fig. 6A). The potent nature of this durable T-cell response was confirmed by analysis of functional cytotoxicity by the JAM assay (Fig. 6B). In addition, endpoint antibody titers calculated from ELISA analysis of sera collected at the 171 day time point also showed the persistence of a strong, Env-specific humoral response (Fig. 6C). The i.d. route of amplicon delivery appeared to elicit higher antibody titers than the i.m. route, although both routes performed similarly in terms of their ability to elicit a cellular immune response to gp120. Overall, it is notable that the long-lived cellular and humoral response to gp120 was induced by a vector that is not capable of replication. It remains unclear whether or not there may be prolonged expression of gp120 in vivo.
FIG. 6.
(A) A single inoculation of HSV:gp120 amplicon particles results in durable cellular immunity, as measured by gamma interferon ELISPOT assay. Mice were inoculated with a single dose of 10 6 i.u. of either HSV:gp120 or HSV:lacZ amplicon particles via either the i.d. or the i.m. routes. Mice were sacrificed, and splenocytes were tested by gamma interferon ELISPOT assay 171 days later. The results are shown for each individual mouse (A1, A2, and A3) in the presence (+) or absence (−) of the V3 peptide. As noted in the legend to Fig. 3A, three different input dilutions of splenocytes were used, and results are presented as the mean of triplicate datum points ± the standard error of the mean. (B) A single inoculation of HSV:gp120 amplicon particles results in durable cellular immunity, as measured by cytotoxic (JAM) assay. Splenocytes were harvested as described above, and cells were cultured in the presence of irradiated syngeneic LPS blasts pulsed with the class I-restricted epitope as indicated. The results are shown across a range of effector-to-target (E:T) cell ratios for each individual mouse (A1, A2, and A3) in the presence (+; dark lines) or absence (−; light lines) of the HIV-1 V3 peptide. (C) A single inoculation of HSV:gp120 amplicon particles elicits long-lasting Env-specific humoral immunity. Mice were injected with 10 6 i.u. of HSV:gp120 or HSV:lacZ amplicon particles. At 171 days after inoculation, mice were bled, and endpoint anti-gp160 titers were determined by ELISA.
Cellular and humoral immune responses are generated in a dose-dependent fashion.
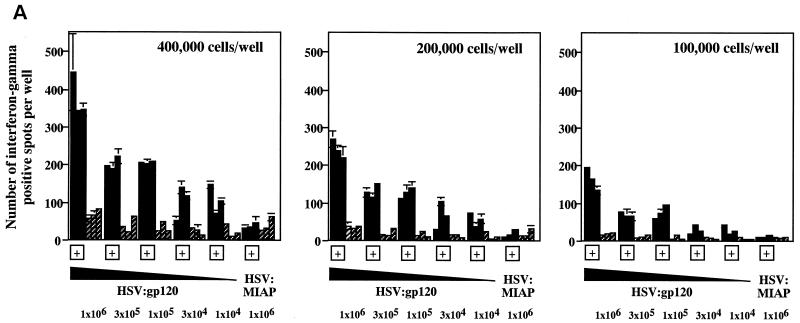
To determine the minimal dose of the HSV:gp120 amplicon that would result in a measurable immune response, we performed a dose-response experiment. Groups of three mice were immunized via the i.d. route with the HSV:gp120 amplicon particles in decreasing dosage levels, which declined in half-log increments from 106 to 104 i.u.; animals were sacrificed and analyzed 21 days later. One group of three mice was inoculated with 106 i.u. of HSV:MIAP amplicon particles as a control. Quantitation of the response was determined by gamma interferon ELISPOT and major histocompatibility complex type I tetramer analyses.
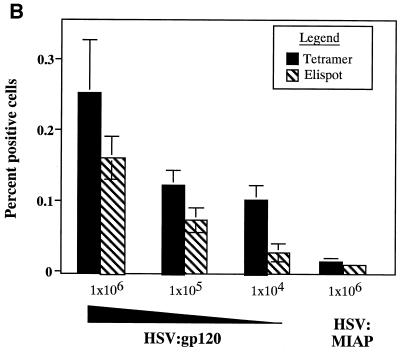
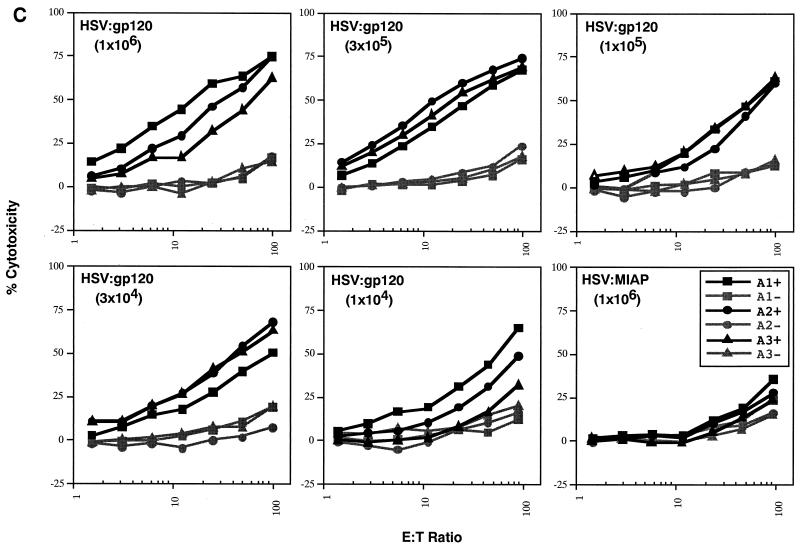
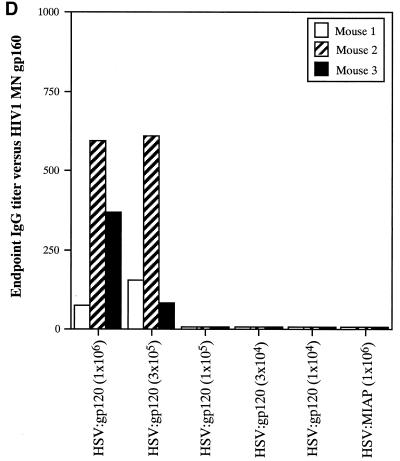
Figure 7A reveals that the frequency of peptide-specific gamma interferon-secreting cells decreases in a continuous fashion as a function of the dose of HSV:gp120 amplicon particles that was administered. Similarly, tetramer staining analysis revealed an association between the dose of vector that was administered and the frequency of H-2Dd peptide-specific CD8+ T lymphocytes (Fig. 7B), as did analysis of cytotoxic activity by splenocytes from the immunized animals (Fig. 7C). In contrast, the humoral response to HSV:gp120 exhibited a more discontinuous relationship with the dose of amplicon particles that was administered. Strong Env-specific antibody responses were detected at the highest dose tested (106 i.u.), but this response became undetectable at doses of 105 i.u. or below (Fig. 7D).
FIG. 7.
The immune response to HSV:gp120 amplicon particles is dose dependent, but strong responses are detected even at very low amplicon doses. (A) Dose dependence of the cellular response to HSV:gp120 amplicon particles, as determined by the gamma interferon ELISPOT assay. Decreasing 0.5-log increments of HSV:gp120 amplicon particles, ranging from 106 to 104 i.u. (as indicated), were inoculated into BALB/c mice via the i.d. route; as a negative control, an additional group of animals received an irrelevant amplicon construct (HSV:MIAP) at the high-dose level (106 i.u.) via the i.d. route. At 21 days postinfection, mice were sacrificed and splenocytes were tested for gamma interferon release by ELISPOT assay. Results are shown for each individual mouse (each bar reflects one mouse), in the presence (+; solid bars) or absence (hatched bars) of the HIV-1 V3 peptide. Three different input levels of splenocytes were analyzed in triplicate (400,000, 200,000, or 100,000 cells as denoted in the three panels). The results shown represent the mean ± the standard error of the mean for each datum point; the experiment shown is representative of two experiments that yielded similar results. (B) Comparison of data from the gamma interferon ELISPOT assay and the tetramer assay. Freshly isolated splenocytes from immunized mice (see above) were also evaluated by staining with an H-2Dd tetramer folded with the V3 peptide (RGPGRAFVTI; see Materials and Methods). The results of tetramer staining (solid bars) and gamma interferon ELISPOT analysis (hatched bars) are expressed as percentages of positive splenocytes; the data show represent mean values from the three mice that were analyzed in each group ± the standard error of the mean. (C) Dose dependence of the cellular response to HSV:gp120 amplicon particles, as determined by functional cytolytic (JAM) assay. Splenocytes were isolated from immunized animals, as described in panel A, and then analyzed for cytolytic activity using the JAM assay (see Materials and Methods) after a 5-day period of in vitro expansion. The results are shown across a range of effector-to-target (E:T) cell ratios for each individual mouse (A1, A2, and A3) in the presence (+; solid lines) or absence (−; shaded lines) of the HIV-1 V3 peptide. (D) Dose dependence of the humoral response to HSV:gp120 amplicon particles. Endpoint antibody titers were calculated by the gp160 IgG ELISA assay.
Prior infection with HSV-1 results in a modest reduction in Env-specific immune responses after administration of HSV:gp120 amplicon particles.
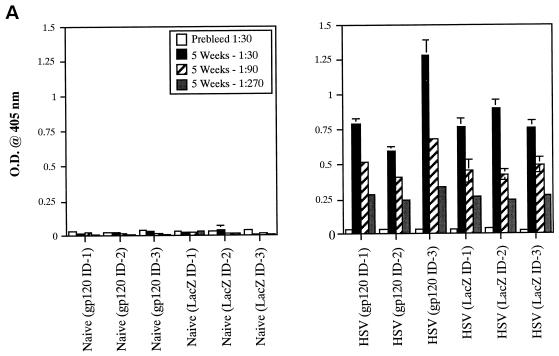
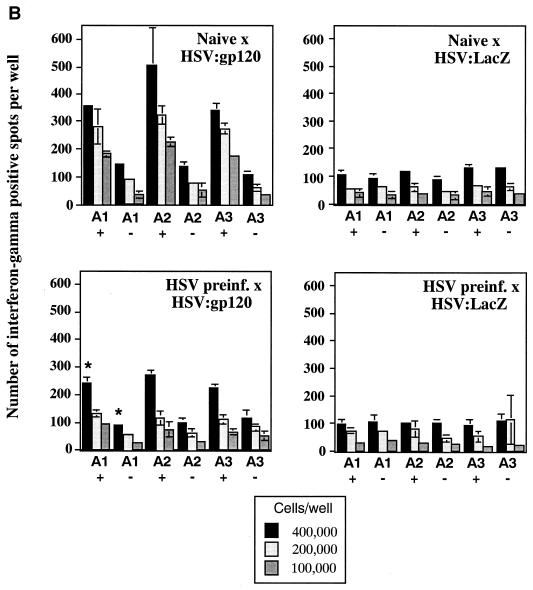
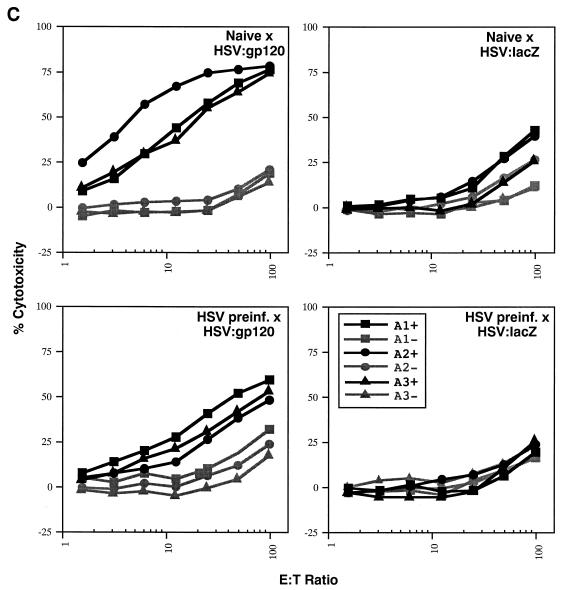
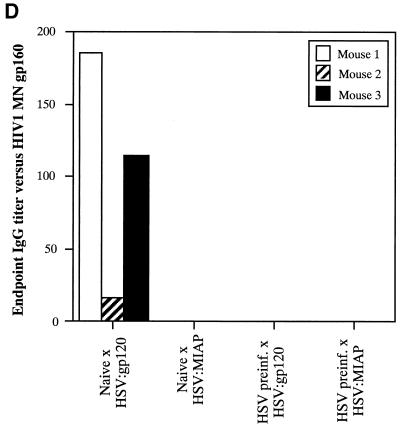
Due to the high level of seroprevalence to HSV-1 in the United States and the possible effect that HSV-specific antibodies might exert on amplicon-elicited immune responses, we conducted an experiment to determine whether prior infection with HSV-1 might reduce immune responses to the vectored HIV-1 gp120 in mice. To accomplish this, mice were either injected with a sublethal dose of the wild-type HSV-1 Patton strain (4 × 107 PFU, administered by the i.p. route) (20) or left untreated (naive). Approximately 5 weeks later, sera were collected from the mice and analyzed for the presence of anti-HSV antibodies by ELISA. Figure 8A splenocytes were analyzed in triplicate (400,000, 200,000, or 100,000 cells), and the results shown represent the mean ± the standard error of the mean for each datum point. Data denoted with an asterisk correspond to mean of only two values, with the corresponding standard deviation; one outlier datum point was discarded. The four panels represent data from naive mice treated with HSV:gp120 or HSV:lacZ (top panels, left and right, respectively) and from HSV-preinfected mice treated with HSV:gp120 or HSV:lacZ (lower panels, left and right, respectively). (C) Mice preinfected with wild-type HSV still mount a strong Env-specific cellular response after inoculation with HSV:gp120 amplicon particles, as determined by functional cytolytic (JAM) assay. Mice were either left untreated (naive) or preinfected with wild-type HSV-1; they were then inoculated with either HSV:gp120 or HSV:lacZ amplicon particles, as outlined above. Splenocytes were harvested at 21 days after immunization with amplicon and were analyzed for functional cytotoxicity by the JAM assay after a 5-day period of in vitro restimulation. The results are shown across a range of effector-to-target (E:T) cell ratios for each individual mouse (A1, A2, and A3) in the presence (+; dark lines) or absence (−; light lines) of the HIV-1 V3 peptide. The four panels represent data from naive mice treated with HSV:gp120 or HSV:lacZ (top panels, left and right, respectively) and from HSV-preinfected mice treated with HSV:gp120 or HSV:lacZ (lower panels, left and right, respectively). (D) Mice preinfected with wild-type HSV mount a weak or undetectable Env-specific humoral response after immunization with HSV:gp120 amplicon particles. Endpoint antibody titers were calculated by a gp160 IgG ELISA. Note that the data shown in this panel are representative of three independent experiments that yielded similar results; this particular data set derives from a different experiment than the datasets shown in panels A to C, as reflected by the use of a different negative control amplicon (HSV:MIAP).
FIG> 8.
(A) Inoculation of mice with wild-type HSV-1 Patton elicits a strong humoral response against HSV-1. Mice were infected with 4 × 107 PFU of HSV-1 Patton via the i.p. route, and 5 weeks later, sera were collected and analyzed by anti-HSV-1 IgG ELISA; as a control, HSV-1-naive mice were also analyzed. Results are expressed in terms of OD readings at 405 nm for each individual mouse; the results from naive animals are shown in the left panel, while the results from the Patton strain-infected animals are shown in the right panel. All animals are identified with respect to the experimental group to which they were assigned (for example, mice which subsequently received HSV:gp120 amplicon particles via the i.d. route are denoted as “gp120 ID,” whereas mice which subsequently received HSV:lacZ amplicon particles are denoted as “LacZ ID”). (B) Mice preinfected with wild-type HSV still mount a strong Env-specific cellular response after inoculation with HSV:gp120 amplicon particles, as determined by the ELISPOT assay. Mice were either left untreated (naive) or preinfected with wild-type HSV-1, as outlined above. All mice were then inoculated with either HSV:gp120 or HSV:lacZ amplicon particles (10 6 i.u. via the i.d. route). Splenocytes were harvested at 21 days after inoculation with amplicon particles and were analyzed for gamma interferon release by ELISPOT assay. The results are shown for each individual mouse (each bar reflects one mouse), in the presence (+ peptide) or absence of the HIV-1 V3 peptide. Three different input levels of splenocytes were analyzed in triplicate (400,000, 200,000, or 100,000 cells), and the results shown represent the mean ± the standard error of the mean for each datum point. Data denoted with an asterisk correspond to mean of only two values, with the corresponding standard deviation; one outlier datum point was discarded. The four panels represent data from naive mice treated with HSV:gp120 or HSV:lacZ (top panels, left and right, respectively) and from HSV-preinfected mice treated with HSV:gp120 or HSV:lacZ (lower panels, left and right, respectively). (C) Mice preinfected with wild-type HSV still mount a strong Env-specific cellular response after inoculation with HSV:gp120 amplicon particles, as determined by functional cytolytic (JAM) assay. Mice were either left untreated (naive) or preinfected with wild-type HSV-1; they were then inoculated with either HSV:gp120 or HSV:lacZ amplicon particles, as outlined above. Splenocytes were harvested at 21 days after immunization with amplicon and were analyzed for functional cytotoxicity by the JAM assay after a 5-day period of in vitro restimulation. The results are shown across a range of effector-to-target (E:T) cell ratios for each individual mouse (A1, A2, and A3) in the presence (+; dark lines) or absence (−; light lines) of the HIV-1 V3 peptide. The four panels represent data from naive mice treated with HSV:gp120 or HSV:lacZ (top panels, left and right, respectively) and from HSV-preinfected mice treated with HSV:gp120 or HSV:lacZ (lower panels, left and right, respectively). (D) Mice preinfected with wild-type HSV mount a weak or undetectable Env-specific humoral response after immunization with HSV:gp120 amplicon particles. Endpoint antibody titers were calculated by a gp160 IgG ELISA. Note that the data shown in this panel are representative of three independent experiments that yielded similar results; this particular data set derives from a different experiment than the datasets shown in panels A to C, as reflected by the use of a different negative control amplicon (HSV:MIAP).
Acknowledgments
We thank Sebold Torno from the University of Rochester Animal Tumor Research Facility for assistance with orbital bleeds and injections. We also thank Jan Moynihan for kindly providing the wild-type HSV-1 Patton strain and Sandra Lee for performing the anti-HSV-1 IgG ELISAs. We thank Kim Dreyer-Hocknell for assistance with the flow cytometry analysis. In addition, we thank Darlene Howard and Ann Casey for technical assistance in generating the amplicons. We are grateful to Michael Turner, John Frelinger, and Alexandra Livingstone for helpful discussions and to Jürgen Haas for providing the codon-optimized HIV-1 gp120 construct. The gp120 monoclonal antibody (AD3) HIV-1 reagent was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, from Kenneth Ugen and David Weiner.
This work was supported by National Institutes of Health grants R21 AI46259 to S.D., R21 AI49804 to T.G.E., T32 AI07362 to P.K.H., and P30 AG18254 to H.J.F. and by an AFAR research grant to W.J.B.
REFERENCES
- 1.Abendroth, A., G. Morrow, A. L. Cunningham, and B. Slobedman. 2001. Varicella-zoster virus infection of human dendritic cells and transmission to T cells: implications for virus dissemination in the host. J. Virol. 75:6183-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Achour, A., R. A. Harris, K. Persson, J. Sundback, C. L. Sentman, G. Schneider, Y. Lindqvist, and K. Karre. 1999. Murine class I major histocompatibility complex H-2Dd: expression, refolding and crystallization. Acta Crystallogr. D Biol. Crystallogr. 55:260-262. [DOI] [PubMed] [Google Scholar]
- 3.Achour, A., K. Persson, R. A. Harris, J. Sundback, C. L. Sentman, Y. Lindqvist, G. Schneider, and K. Karre. 1998. The crystal structure of H-2Dd MHC class I complexed with the HIV-1-derived peptide P18-I10 at 2.4 Å resolution: implications for T-cell and NK cell recognition. Immunity 9:199-208. [DOI] [PubMed] [Google Scholar]
- 4.Andre, S., B. Seed, J. Eberle, W. Schraut, A. Bultmann, and J. Haas. 1998. Increased immune response elicited by DNA vaccination with a synthetic gp120 sequence with optimized codon usage. J. Virol. 72:1497-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arvin, A. M. 2001. Varicella vaccine: genesis, efficacy, and attenuation. Virology 284:153-158. [DOI] [PubMed] [Google Scholar]
- 6.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 7.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borrow, P., H. Lewicki, X. Wei, M. S. Horwitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3:205-211. [DOI] [PubMed] [Google Scholar]
- 9.Bowers, W. J., D. F. Howard, A. I. Brooks, M. W. Halterman, and H. J. Federoff. 2001. Expression of vhs and VP16 during HSV-1 helper virus-free amplicon packaging enhances titers. Gene Ther. 8:111-120. [DOI] [PubMed] [Google Scholar]
- 10.Bowers, W. J., D. F. Howard, and H. J. Federoff. 2000. Discordance between expression and genome transfer titering of HSV amplicon vectors: recommendation for standardized enumeration. Mol. Ther. 1:294-299. [DOI] [PubMed] [Google Scholar]
- 11.Chahlavi, A., S. Rabkin, T. Todo, P. Sundaresan, and R. Martuza. 1999. Effect of prior exposure to herpes simplex virus 1 on viral vector-mediated tumor therapy in immunocompetent mice. Gene Ther. 6:1751-1758. [DOI] [PubMed] [Google Scholar]
- 12.Fraefel, C., S. Song, F. Lim, P. Lang, L. Yu, Y. Wang, P. Wild, and A. I. Geller. 1996. Helper virus-free transfer of herpes simplex virus type 1 plasmid vectors into neural cells. J. Virol. 70:7190-7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geller, A. I., and X. O. Breakefield. 1988. A defective HSV-1 vector expresses Escherichia coli β-galactosidase in cultured peripheral neurons. Science 241:1667-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanke, T., T. J. Blanchard, J. Schneider, G. S. Ogg, R. Tan, M. Becker, S. C. Gilbert, A. V. Hill, G. L. Smith, and A. McMichael. 1998. Immunogenicities of intravenous and intramuscular administrations of modified vaccinia virus Ankara-based multi-CTL epitope vaccine for human immunodeficiency virus type 1 in mice. J. Gen. Virol. 79:83-90. [DOI] [PubMed] [Google Scholar]
- 15.Heineman, T. C., B. L. Connelly, N. Bourne, L. R. Stanberry, and J. Cohen. 1995. Immunization with recombinant varicella-zoster virus expressing herpes simplex virus type 2 glycoprotein D reduces the severity of genital herpes in guinea pigs. J. Virol. 69:8109-8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrlinger, U., C. M. Kramm, K. S. Aboody-Guterman, J. S. Silver, K. Ikeda, K. M. Johnston, P. A. Pechan, R. F. Barth, D. Finkelstein, E. A. Chiocca, D. N. Louis, and X. O. Breakefield. 1998. Pre-existing herpes simplex virus 1 (HSV-1) immunity decreases, but does not abolish, gene transfer to experimental brain tumors by a HSV-1 vector. Gene Ther. 5:809-819. [DOI] [PubMed] [Google Scholar]
- 17.Hill, A., P. Jugovic, I. York, G. Russ, J. Bennink, J. Yewdell, H. Ploegh, and D. Johnson. 1995. Herpes simplex virus turns off the TAP to evade host immunity. Nature 375:411-415. [DOI] [PubMed] [Google Scholar]
- 18.Huber, M. T., T. W. Wisner, N. R. Hegde, K. A. Goldsmith, D. A. Rauch, R. J. Roller, C. Krummenacher, R. J. Eisenberg, G. H. Cohen, and D. C. Johnson. 2001. Herpes simplex virus with highly reduced gD levels can efficiently enter and spread between human keratinocytes. J. Virol. 75:10309-10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T-cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karp, J. D., J. A. Moynihan, and R. Ader. 1997. Psychosocial influences on immune responses to HSV-1 infection in BALB/c mice. Brain Behav. Immun. 11:47-62. [DOI] [PubMed] [Google Scholar]
- 21.Kit, S. 1990. Genetically engineered vaccines for control of Aujeszky's disease (pseudorabies). Vaccine 8:420-424. [DOI] [PubMed] [Google Scholar]
- 22.Klein, R. J. 1989. Reinfections and site-specific immunity in herpes simplex virus infections. Vaccine 7:380-381. [DOI] [PubMed] [Google Scholar]
- 23.Kruse, M., O. Rosorius, F. Kratzer, G. Stelz, C. Kuhnt, G. Schuler, J. Hauber, and A. Steinkasserer. 2000. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. J. Virol. 74:7127-7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuter, B. J., R. E. Weibel, H. A. Guess, H. Matthews, D. H. Morton, B. J. Neff, P. J. Provost, B. A. Watson, S. E. Starr, and S. A. Plotkin. 1991. Oka/Merck varicella vaccine in healthy children: final report of a 2-year efficacy study and 7-year follow-up studies. Vaccine 9:643-647. [DOI] [PubMed] [Google Scholar]
- 25.Li, H., K. Natarajan, E. L. Malchiodi, D. H. Margulies, and R. A. Mariuzza. 1998. Three-dimensional structure of H-2Dd complexed with an immunodominant peptide from human immunodeficiency virus envelope glycoprotein 120. J. Mol. Biol. 283:179-191. [DOI] [PubMed] [Google Scholar]
- 26.Logvinoff, C., and A. L. Epstein. 2001. A novel approach for herpes simplex virus type 1 amplicon vector production, using the Cre-loxP recombination system to remove helper virus. Hum. Gene Ther. 12:161-167. [DOI] [PubMed] [Google Scholar]
- 27.Lu, B., and H. J. Federoff. 1995. Herpes simplex virus type 1 amplicon vectors with glucocorticoid-inducible gene expression. Hum. Gene Ther. 6:419-428. [DOI] [PubMed] [Google Scholar]
- 28.Mandell, G. L., J. E. Bennett, and R. Dolin. 1995. Principles and practice of infectious diseases, 4th ed., vol. 2. Churchill Livingstone, Inc., New York, N.Y.
- 29.Matzinger, P. 1991. The JAM test. A simple assay for DNA fragmentation and cell death. J. Immunol. Methods 145:185-192. [DOI] [PubMed] [Google Scholar]
- 30.Mikloska, Z., L. Bosnjak, and A. L. Cunningham. 2001. Immature monocyte-derived dendritic cells are productively infected with herpes simplex virus type 1. J. Virol. 75:5958-5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morrison, L. A., and D. M. Knipe. 1996. Mechanisms of immunization with a replication-defective mutant of herpes simplex virus 1. Virology 220:402-413. [DOI] [PubMed] [Google Scholar]
- 32.Murphy, C. G., W. T. Lucas, R. E. Means, S. Czajak, C. L. Hale, J. D. Lifson, A. Kaur, R. P. Johnson, D. M. Knipe, and R. C. Desrosiers. 2000. Vaccine protection against simian immunodeficiency virus by recombinant strains of herpes simplex virus. J. Virol. 74:7745-7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakagawa, Y., T. Takeshita, J. A. Berzofsky, and H. Takahashi. 2000. Analysis of the mechanism for extracellular processing in the presentation of human immunodeficiency virus-1 envelope protein-derived peptide to epitope-specific cytotoxic T lymphocytes. Immunology 101:76-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ng, P., C. Beauchamp, C. Evelegh, R. Parks, and F. L. Graham. 2001. Development of a FLP/frt system for generating helper-dependent adenoviral vectors. Mol. Ther. 3:809-815. [DOI] [PubMed] [Google Scholar]
- 35.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 36.Parks, R. J., L. Chen, M. Anton, U. Sankar, M. A. Rudnicki, and F. L. Graham. 1996. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA 93:13565-13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Plaksin, D., S. Chacko, P. McPhie, A. Bax, E. A. Padlan, and D. H. Margulies. 1996. A T-cell receptor V alpha domain expressed in bacteria: does it dimerize in solution? J. Exp. Med. 184:1251-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plotkin, S. A., S. E. Starr, H. M. Friedman, K. Brayman, S. Harris, S. Jackson, N. B. Tustin, R. Grossman, D. Dafoe, and C. Barker. 1991. Effect of Towne live virus vaccine on cytomegalovirus disease after renal transplant. A controlled trial. Ann. Intern. Med. 114:525-531. [DOI] [PubMed] [Google Scholar]
- 39.Saeki, Y., C. Fraefel, T. Ichikawa, X. O. Breakefield, and E. A. Chiocca. 2001. Improved helper virus-free packaging system for HSV amplicon vectors using an ICP27-deleted, oversized HSV-1 DNA in a bacterial artificial chromosome. Mol. Ther. 3:591-601. [DOI] [PubMed] [Google Scholar]
- 40.Salio, M., M. Cella, M. Suter, and A. Lanzavecchia. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur. J. Immunol. 29:3245-3253. [DOI] [PubMed] [Google Scholar]
- 41.Sandig, V., R. Youil, A. J. Bett, L. L. Franlin, M. Oshima, D. Maione, F. Wang, M. L. Metzker, R. Savino, and C. T. Caskey. 2000. Optimization of the helper-dependent adenovirus system for production and potency in vivo. Proc. Natl. Acad. Sci. USA 97:1002-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 43.Shih, M. F., M. Arsenakis, P. Tiollais, and B. Roizman. 1984. Expression of hepatitis B virus S gene by herpes simplex virus type 1 vectors carrying alpha- and beta-regulated gene chimeras. Proc. Natl. Acad. Sci. USA 81:5867-5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shirai, M., S. Kozlowski, D. H. Margulies, and J. A. Berzofsky. 1997. Degenerate MHC restriction reveals the contribution of class I MHC molecules in determining the fine specificity of CTL recognition of an immunodominant determinant of HIV-1 gp160 V3 loop. J. Immunol. 158:3181-3188. [PubMed] [Google Scholar]
- 45.Shiraki, K., H. Sato, Y. Yoshida, J. I. Yamamura, M. Tsurita, M. Kurokawa, and S. Kageyama. 2001. Construction of Oka varicella vaccine expressing human immunodeficiency virus env antigen. J. Med. Virol. 64:89-95. [DOI] [PubMed] [Google Scholar]
- 46.Spaete, R. R., and N. Frenkel. 1982. The herpes simplex virus amplicon: a new eucaryotic defective-virus cloning-amplifying vector. Cell 30:295-304. [DOI] [PubMed] [Google Scholar]
- 47.Sprecher, E., and Y. Becker. 1987. Herpes simplex virus type 1 pathogenicity in footpad and ear skin of mice depends on Langerhans cell density, mouse genetics, and virus strain. J. Virol. 61:2515-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sprecher, E., and Y. Becker. 1989. Langerhans cell density and activity in mouse skin and lymph nodes affect herpes simplex type 1 (HSV-1) pathogenicity. Arch. Virol. 107:191-205. [DOI] [PubMed] [Google Scholar]
- 49.Sprecher, E., and Y. Becker. 1988. Role of Langerhans cells and Thy. 1+ effector cells in herpes simplex virus-1 infection in the skin of newborn mice. Arch. Virol. 100:285-292. [DOI] [PubMed] [Google Scholar]
- 50.Sprecher, E., and Y. Becker. 1986. Skin Langerhans cells play an essential role in the defense against HSV-1 infection. Arch. Virol. 91:341-349. [DOI] [PubMed] [Google Scholar]
- 51.Sprent, J., and D. F. Tough. 2001. T-cell death and memory. Science 293:245-248. [DOI] [PubMed] [Google Scholar]
- 52.Starr, S. E., H. M. Friedman, and S. A. Plotkin. 1991. The status of cytomegalovirus vaccine. Rev. Infect. Dis. 13:S964-S965. [DOI] [PubMed] [Google Scholar]
- 53.Stavropoulos, T. A., and C. A. Strathdee. 1998. An enhanced packaging system for helper-dependent herpes simplex virus vectors. J. Virol. 72:7137-7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ugen, K. E., Y. Refaeli, U. Ziegner, M. Agadjanyan, M. A. R. Satre, V. Srikantan, B. Wang, A. Sato, W. V. Williams, and D. B. Weiner. 1993. Generation of monoclonal antibodies against the amino terminus of gp120 that elicit antibody-dependent cellular cytotoxicity, p. 215-221. In Vaccines 93. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 55.Umana, P., C. A. Gerdes, D. Stone, J. R. Davis, D. Ward, M. G. Castro, and P. R. Lowenstein. 2001. Efficient FLPe recombinase enables scalable production of helper-dependent adenoviral vectors with negligible helper-virus contamination. Nat. Biotechnol. 19:582-585. [DOI] [PubMed] [Google Scholar]
- 56.van Drunen Littel-van den Hurk, S., S. K. Tikoo, X. Liang, and L. A. Babiuk. 1993. Bovine herpesvirus-1 vaccines. Immunol. Cell Biol. 71:405-420. [DOI] [PubMed] [Google Scholar]
- 57.van Zijl, M., G. Wensvoort, E. de Kluyver, M. Hulst, H. van der Gulden, A. Gielkens, A. Berns, and R. Moormann. 1991. Live attenuated pseudorabies virus expressing envelope glycoprotein E1 of hog cholera virus protects swine against both pseudorabies and hog cholera. J. Virol. 65:2761-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Willis, R. A., W. J. Bowers, M. J. Turner, T. L. Fisher, C. S. Abdul-Alim, D. F. Howard, H. J. Federoff, E. M. Lord, and J. G. Frelinger. 2001. Dendritic cells transduced with hsv-1 amplicons expressing prostate-specific antigen generate antitumor immunity in mice. Hum. Gene Ther. 12:1867-1879. [DOI] [PubMed] [Google Scholar]
- 59.York, I. A., C. Roop, D. W. Andrews, S. R. Riddell, F. L. Graham, and D. C. Johnson. 1994. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell 77:525-535. [DOI] [PubMed] [Google Scholar]