Abstract
The more severe form of dengue virus infection, dengue hemorrhagic fever, is characterized by plasma leakage and derangements in hemostasis. As elevated interleukin-8 (IL-8) levels have been observed in sera from patients with more severe disease manifestations, a study was initiated to look at the effect of dengue virus infection in vitro on proinflammatory cytokine secretion and expression. A significant increase in IL-8 levels in the culture supernatant of primary human monocytes infected with dengue 2 virus (D2V) New Guinea C (NGC) was found by enzyme-linked immunosorbent assay. Additionally, by reverse transcriptase PCR, the mRNA was also augmented. Among the proinflammatory cytokines and their mRNAs measured (IL-6, IL-1β, IL-8, and tumor necrosis factor alpha), IL-8 showed the greatest change following D2V infection. Similarly, two cell lines, 293T (a human epithelial cell line) and ECV304 (an endothelial cell line), were permissive to D2V NGC and responded to the infection by increasing the synthesis of IL-8. Nuclear factor kappa B (NF-κB) and nuclear factor IL-6 (NFIL-6) are primary mediators of IL-8 expression. We studied the transcriptional regulation of IL-8 in the ECV304 and 293T cell lines and found that the induction of IL-8 gene expression involved the activation of NF-κB (P = 0.001) and, to a lesser extent, the activation of NFIL-6 in ECV304 cells only. We next observed by the chromatin immunoprecipitation procedure in vivo acetylation of core histones bound to the IL-8 promoter after D2V infection. IL-8 produced by infected monocytes and also IL-8 that may be produced by endothelial or other epithelial cells is associated with the hyperacetylation of histones bound to the IL-8 promoter in addition to the activation of transcription by NF-κB. We hypothesize that the overall increase in IL-8 synthesis observed in this in vitro study may play a role in the pathogenesis of the plasma leakage seen in dengue hemorrhagic fever and dengue shock syndrome.
Dengue virus infection has reemerged as a more severe illness in humans than it was in the past, and its immunopathology has been recently reviewed (30). Severe dengue virus infections can cause dengue hemorrhagic fever (DHF) or dengue shock syndrome, a potentially fatal plasma leakage syndrome. One of the intriguing events during inflammation is the occurrence of vascular leakage. Capillary leakage has been linked to mediators that are secreted by cells in the circulation (13).
Interleukin-8 (IL-8) is a small, secreted molecule with chemoattracting functions containing four characteristic cysteines (C) with the general arrangement CXC. IL-8 is secreted from a variety of cell types and has numerous roles, including inflammation, cell recruitment, lymphoid trafficking, wound healing, angiogenesis, and metastasis. Among the diverse biological functions of IL-8 are the recruitment of eosinophils, neutrophils (26), and naïve T cells to the surface of the endothelium.
The neutrophilic leukocytes synthesize de novo up to 30 times more IL-8 during their migration (31). Activation by β2 integrin (LFA-1/CD11a) for binding of leukocytes (10, 11) and up-regulation of adhesion molecules to increase extravasation of monocytes and capillary leakage (15) are among the functions of the IL-8 protein.
In vitro, endothelial cells can be infected by dengue virus (5), and infected endothelial cells produce IL-8 (17), activate complement, and may undergo apoptosis (2). Dengue virus-infected monocytes have been shown to activate endothelial cells in vitro, and such activation involves tumor necrosis factor alpha (TNF-α) and IL-1β (1).
We demonstrate that infection of primary human monocytes by dengue 2 virus (D2V) New Guinea C (NGC) induces the secretion of cytokines, primarily IL-8, and that inactivated viral antigens can induce IL-8 transcription in endothelial cell lines. We also show that the control of IL-8 expression at the transcriptional level by NF-κB (8) is tightly linked to virus titers and, to a lesser extent, viral antigens. Additionally, the hyperacetylated histones H3 and H4 were bound to the IL-8 promoter, indicating that chromatin remodeling may modulate IL-8 expression in D2V-infected cells. In dengue virus infections, IL-8 has been associated with disease severity (18, 27). The secretion of IL-8 by dengue virus-infected or antigen-stimulated epithelial or endothelial cells could contribute to local inflammatory responses at specific sites of capillary damage.
MATERIALS AND METHODS
Cell lines and cultured primary cells.
The simian virus 40-transformed human cell line 293T (renal epithelial cells) was grown in minimum essential medium (MEM; Life Technologies, Rockville, Md.) supplemented with 10% fetal calf serum (FCS) and 100 U of penicillin/ml plus 100 μg of streptomycin/ml (P/S; Life Technologies, Gaithersburg, Md.). ECV304 cells (human transformed large-vessel umbilical cord endothelial cells) were grown in M199 medium (Life Sciences) supplemented with 10% FCS and P/S. Both cell lines were obtained from The American Type Culture Collection (ATCC; Manassas, Va.).
Monocyte isolation.
Blood (120 ml) was collected in the presence of heparin from healthy human volunteers living in Massachusetts with no known history of exposure to dengue or yellow fever virus. Peripheral blood mononuclear cell (PBMC) isolation was performed using Ficoll-Hypaque (Sigma Chemical Co., St. Louis, Mo.). The plasma obtained was saved for monocyte isolation (see below). Monocytes/macrophages were prepared from PBMC as described elsewhere (21). Briefly, 175-cm2 culture plates were coated with a sterile aqueous solution of 3% gelatin for 1 h, and then excess gelatin was removed and the flasks were dried overnight at 50°C. Plasma (10 ml) obtained from the PBMC preparation was used to coat the plates at 37°C in a CO2 incubator for 1 h. The plasma was then aspirated, and the flasks were washed with phosphate-buffered saline (PBS). Next, the washed PBMCs were resuspended in 15 ml of RPMI medium supplemented with 10% FCS and P/S. The cells were incubated at 37°C for 90 min in a CO2 incubator, and attached monocytes/macrophages were washed gently with RPMI medium three times. The flasks were incubated for 5 min at 4°C with cold PBS (no Mg2+ or Ca2+) to detach the cells. The cells were collected by centrifugation at 700 × g for 10 min and used immediately by resuspending them in RPMI containing 2% FCS. For viral infections, 0.5 × 106 to 2 × 106 cells/ml in six-well culture plates were used. The purity of the monocyte/macrophage isolation was confirmed by flow cytometry (fluorescence-activated cell sorter [FACS]) analysis using anti-CD14-fluorescein isothiocyanate (FITC), with an average of 90% CD14-positive cells.
Each donor was used for independent D2V NGC infections. Five subjects were utilized for characterization of D2V NGC infection by immunofluorescence.
Viral infections.
Viral stocks of D2V strain NGC were prepared as described elsewhere (20). High-viral-titer stocks (106 to 107 PFU/ml) were obtained from insect cell C6/36 (ATCC) supernatants. Monocytes/macrophages were infected for 16 h at 1 to 5 PFU/cell in RPMI medium containing 2% FCS at 37°C and 5% CO2. After overnight infection, the plates were centrifuged at 700 × g for 10 min at 15°C in a Sorval RT6000B refrigerated centrifuge, and the medium was replaced with RPMI plus 10% FCS with P/S for 48 h. Primary monocytes were infected with D2V NGC for 2, 6, 7.5, 17, and 18 h for time course experiments. The control C6/36 supernatants were obtained from cultures of C6/36 insect cells prepared in the same way the virus was harvested. Aliquots of the supernatant were kept at −80°C until use.
Virus plaque assay.
Monolayers of Vero cells were cultured in MEM at 50% confluence the day before infection. Infection was performed for 2 h in a CO2 incubator at 37°C. The cells were washed gently with MEM, and then a layer of methylcellulose at 1.85% in RPMI medium was added. The plates were allowed to incubate for 7 to 10 days and were stained with a 10% ethanol solution of 0.2% crystal violet.
Fluorescence detection of infected primary human monocytes/macrophages.
D2V-infected primary human monocytes were analyzed by FACS using intracellular labeling (Cytofix/Cytoperm Plus kit; Pharmingen/Becton Dickinson, San Diego, Calif.) with mouse polyclonal D2V antibody, purified from hyperimmune mouse ascitic fluid (ATCC), diluted 1:500 in PBS and a secondary antibody against mouse immunoglobulin G (IgG) coupled with FITC (Sigma). For membrane labeling of primary monocytes, we used a murine IgG1 monoclonal antibody to CD14 coupled to FITC (Becton Dickinson, Franklin Lakes, N.J.).
Preparation of Vero and Vero-infected antigens.
Uninfected or dengue virus-infected Vero cells were grown in MEM supplemented with 2% FCS until 50% of the cells showed cytopathic effect. As described elsewhere (19), for each 75-ml flask (Becton Dickinson), 15 ml of medium was added and used to scrape off the cells. The cells were pelleted, washed three times with cold PBS, and fixed with 0.025% (final concentration) glutaraldehyde in PBS for 15 min at 4°C. The fixed cells were then washed three times with PBS and one time with RPMI medium. RPMI was added to a final volume of 1 ml, sonicated, and stored at −80°C until use. The quality of antigen preparations was confirmed by T-cell proliferation assays as described previously (25).
Cytokine assays.
IL-8, IL-6, TNF-α, IL-1-β, and IL-10 were measured in cell culture supernatants by enzyme-linked immunosorbent assay (Quantikine; R&D Systems). The minimum detectable concentrations were 10 pg/ml for IL-8, 3.9 pg/ml for IL-10, 4.4 pg/ml for TNF-α, 1 pg/ml for IL-1-β, and 0.7 pg/ml for IL-6. The results obtained were expressed as picograms per 106 cells.
Measurement of IL-8 mRNA by RT-PCR.
Semiquantitative reverse transcriptase PCR (RT-PCR) was used to measure levels of mRNAs of IL-8, IL-1-β, and IL-6, as well as the control genes L35a (ribosomal protein) and CXCR2. The primer pairs used were as follows: IL-8 sense, 5-AAG AGA GCT CTG TCT GGA CC, and antisense, 5-GAT ATT CTC TTG GCC CTT GG; IL-6 sense, 5-TTC GGT CCA GTT GCC TCT C, and antisense, 5-TGG CAT TTG TGG TTG GGT CA; IL-1β sense, 5-AAG CTT GGT GAT GTC TGG, and antisense, TGA GAG GTG CTG ATG TAC CA; CXCR2 sense, 5-CCG GGC GTG GTG GTG AG, and antisense, 5-TCT GCC TTT TGG GTC TTG TGA ATA; and L35a sense, 5-CTT CTC TTA CCG CCA TCT TC, and antisense, 5-TCC TTG AGG GGT ACA GCA TC.
Multiplex RT-PCR was performed with a kit from BioSource International (Human Sepsis-Related Cytokines set 1) to detect the expression of human TNF-α, IL-1β, IL-6, IL-12, IL-8, and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) genes simultaneously. The PCR primers were designed to generate products of 921 (GAPDH), 680 (TNF-α), 555 (IL-1β), 450 (IL-12), 360 (IL-6), and 300 (IL-8) bp. Two hundred nanograms of RNA was used to synthesize cDNA using Sensiscript RT (Qiagen). Total RNA was measured by absorbance at 260 nm in a Biophotometer (Eppendorf). The PCR conditions were 96°C for 1 min and 55°C for 4 min for 2 cycles; 94°C for 1 min and 55°C for 2.5 min for 28 cycles; and 70°C for 1 min, followed by a soak at 4°C until it was run on a 1% agarose Tris-borate-EDTA (TBE) gel and scanned by GelDoc System 2000 using Quantity One software (Bio-Rad). Semiquantitative fold induction was calculated using Image version 1.62 software from the National Institutes of Health.
Quantitative RT-PCR for IL-8.
We used the 5700 Sequence Detection System and (TaqMan IL-8 and β-actin probe and primers Applied Biosystems). Protocols for RT-PCR were done according to the recommendations of the manufacturer. The PCR protocol was 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 30 s, followed by annealing and extension at 60°C for 1 min.
mRNA was obtained from monocytes/macrophages infected with D2V NGC for 48 h at 1 multiplicity of infection (MOI) or exposed to insect cell supernatant or culture medium alone for the same amount of time. Total RNA from ECV304 cells infected as described above was obtained, and RNA from each cell type and condition was quantified by absorbance at 260 nm. RT-PCR containing 100 ng of total RNA, in triplicate, was used for amplification of IL-8 mRNA and endogenous control (β-actin) mRNA, which was used to normalize the relative increase in IL-8 mRNA. For quantitative analysis, the mean of the triplicate samples was obtained using a standard curve from 200 fg to 2 ng of control DNA and Taqman primers and probes. The ratios of infected versus uninfected cells treated with C6/36 insect cell supernatant were calculated. The mean value of the relative increase was reported.
Transfection and promoter-reporter system plasmids.
Promoter activity was assayed 48 h postinfection and 72 h posttransfection. Luciferase reporter plasmids for the IL-8 promoter region (−1370 to +82) were a kind present from W. Reed, Environmental Protection Agency, Chapel Hill, N.C. Three plasmids were used. One contained wild-type (WT) binding sites for both NF-κB and NFIL-6 (TGCAAATGTGGAATTTCC), and the other two contained mutant binding sites for NF-κB (TTGCAAATGAATAATTTCC) (ΔNFκB) or NFIL-6 (TTACGAGTGTGGAATTTCC) (ΔNFIL-6). (Boldface indicates bases that participate in binding sites, while underlining indicates sites of mutations from the wild-type sequence.) The sequence containing the WT binding sites, as well as each of the mutated PCR products, was generated by PCR and cloned into a firefly luciferase expression plasmid, PGL2-basic. A Qiagen plasmid isolation kit was used to obtain DNA for mammalian transfection. For transfection studies, ECV304, 293T, and other control cell lines were grown to 50% confluence in 35-mm-diameter tissue culture dishes. The cells were transfected using 6 μl of Fugene6 (Roche Diagnostic Corp., Indianapolis, Ind.) and 2 μg of plasmid DNA per plate. The cells were incubated overnight, trypsinized, and harvested for infection with D2V NGC virus (MOI = 1) or for antigen stimulation in a six-well plate format containing M199 medium as described above in the procedure for infection of cells. The stimulations consisted of 1 ml of insect cell medium (C6/36), uninfected or infected Vero cell antigen preparation (50 μl/well), and culture medium alone. Following a 48-h incubation, the cells were scraped off the plates and resuspended using lysis buffer (Promega, Madison, Wis.). Cell extracts were prepared by freezing and thawing the cells three times, alternating between a 37°C water bath and a dry ice-ethanol bath. Unbroken cells and debris were spun down at 10,000 rpm for 2 min at 4°C. The extracts were stored at −70°C after an aliquot was withdrawn to measure luciferase activity in a Monolith Luminometer 2016 (Analytical Luminescence Laboratory) using the luciferase reporter assay system (Promega). Luciferase activity values were normalized with protein values after quantification (Bio-Rad). The substrate for luminescence was added at the moment the samples were read.
Paired t tests were use for statistical analysis of the difference between groups.
Electrophoretic mobility gel shift assays.
Nuclear extracts from 107 cells were prepared as previously described with modifications (4, 9). After the ECV304 or 293T cells were infected at an MOI of 1 with D2V NGC or stimulated with 50 μl of antigen preparation, the monolayers were washed with PBS and the cells were collected by scraping them into 5 ml of PBS. The cells were centrifuged for 5 min at 4°C and 700 × g, and the pellet obtained was resuspended in cytoplasmic extraction buffer A (10 mM HEPES [pH 7.9], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol [DTT], and 0.5 mM phenylmethylsulfonyl fluoride [PMSF]) and incubated on ice inside a glass Dounce homogenizer. The lysates were passed 30 times through the Dounce homogenizer and centrifuged in an Eppendorf microcentrifuge at 10,000 rpm to obtain the nuclear extract as a pellet. Buffer C (20 mM HEPES [pH 7.9], 25% glycerol, 0.42% NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM DTT, and 0.5 mM PMSF) was added to the volume of pellet generated in a 1/1 ratio (100 μl) and incubated for 1 h at 4°C with rotation. After the extracted nuclear proteins were obtained, the soluble fraction was separated by centrifugation (15,000 rpm at 4°C for 15 min in an Eppendorf microcentrifuge) and dialyzed against a 20-fold volume of buffer D (20 mM HEPES [pH 7.9], 20% glycerol, 0.1 M KCl, 0.2 mM EDTA, 0.5 mM DTT, and 0.5 mM PMSF) using a microdialyzer (QuixSep Membrane Filtration Products, San Antonio, Tex.). To make the probe, complementary primers were annealed in vitro in 200 mM Tris-HCl [pH 8.0]-100 mM MgCl2-250 mM NaCl at a concentration of 1.25 μg/μl of primer. Probes for the WT (GTGGATTTC) and for the mutant (GAATAATTTCC) were made. T4 kinase labeling (Life Technologies) was performed according to the manufacturer's protocol with purification by size exclusion chromatography using Sephadex G25 QuickSpin columns (Roche). The binding conditions were 30,000 cpm, 0.02 pmol of labeled probe, 1 μg of poly(dI-dC)/μl, 10 mM Tris-HCl (pH 7.5), 100 mM KCl, 1 mM DTT, 10% glycerol, and 5 μg of nuclear extract. The reaction mixtures were incubated at room temperature for 35 min. The protein-DNA complexes were resolved in a nondenaturing 7%, 0.5× TBE acrylamide-glycerol gel at 200 V for 2.5 h, dried, and viewed by autoradiography after 24 h. The probe binding specificity was demonstrated by competition assays using up to a 100-fold excess of unlabeled WT or unlabeled mutated probe. A labeled probe of an SP1 promoter region (5′GACTCGGGTCAAAACTCCTT3′) was used as a negative control.
ChIPs.
Chromatin immunoprecipitations (ChIPs; Upstate NewYork Biosystems) were performed using nuclear extracts prepared as recommended by the manufacturer. Briefly, two 20-mm-diameter plates of ECV304 cells in M199 medium plus 2% FCS were grown to confluence and were infected using 1 MOI of D2V NGC for 48 h. Other conditions tested were incubation with 1 ml of insect cell supernatant (C6/36) and 1 h of treatment with 100 ng of recombinant human TNF-α (R&D Systems)/ml as positive controls for chromatin rearrangement. To cross-link histones to DNA, formaldehyde diluted to a final concentration of 1% (20 ml of medium and 540 μl of 37% formaldehyde) was incubated for 10 min at 37°C in a CO2 incubator. Culture vessels were sealed with parafilm (American National Can, Neenah, Wis.) and placed in the incubator. After fixation, the cells were washed once in cold PBS containing 1 mM PMSF and then scraped off the plate into the same medium. The cells were then centrifuged for 5 min at 700 × g and 4°C, resuspended in 1 ml of sodium dodecyl sulfate (SDS) lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl, pH 8.1), and incubated for 10 min on ice.
To reduce the DNA length to between 200 and 1,000 bp, the lysates were sonicated on ice at maximum capacity with a 3-mm-diameter stainless steel probe (Pirtek sonic dismembrator model 150) three times for 30 s each time. Then, a fraction of the lysate was run in a 1% TBE agarose gel to test the DNA fragment size.
The rest of the lysate was diluted fivefold in ChIP dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris [pH 8.1], 16.7 mM NaCl, and protease inhibitors [1 μM each PMSF, leupeptin, and aprotinin A]). A portion of the chromatin preparation (10%) was saved to calculate input DNA, which represents the amount of total DNA present in the samples before immunoprecipitations.
Immunoprecipitation.
To reduce nonspecific background, the chromatin was precleared with a solution containing 80 μl of salmon sperm DNA-protein A agarose for 30 min at 37°C with agitation. After incubation, the supernatant fraction was collected. Next, 5 μl of anti-acetyl histone 3 (H3) and histone 4 (H4), ChIPs grade, was added to 1 ml of precleared chromatin solution and then incubated overnight at 4°C with rotation. To collect the immune complexes, 60 μl of salmon sperm DNA-protein A agarose was added the next day for 1 h at 4°C with rotation.
Beads were pelleted by centrifugation and then washed five times for 3 to 5 min each time with 1 ml each of the following sequence of buffers: (i) 0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl (pH 8.1), 150 mM NaCl; (ii) 0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM Tris-HCl (pH 8.1), 500 mM NaCl; (iii) 0.25 M LiCl, 1% NP-40, 1% sodium deoxycholate, 1 mM EDTA, 10 mM Tris-HCl (pH 8.1); and (iv) TE (pH 8.0) twice.
To elute immune complexes, the pelleted beads were vortexed briefly to mix them and were incubated with 250 μl of 1% SDS in 0.1 M NaHCO3 at room temperature for 15 min with rotation. The supernatant fraction (eluate) was transferred to another tube, and elution of the beads was repeated. Then, 20 μl of 5 M NaCl was added to the combined eluates as well as the control input DNA, and the samples were reverse cross-linked at 65°C for 16 h. The DNA was recovered by Qiagen extraction columns (PCR purification kit) in a total volume of 30 μl; 3 μl (10%) of this material was used in PCR to determine the gene or promoter of interest.
The primers used for the IL-8 promoter region were as follows: sense, 5-GTG TGA TGA CTC AGG TTT GCC C-3 at position −97, and antisense, 5-GTG TGA TGA CTC AGG TTT GCC C-3 at position −34. The chemokine receptor CCR1 and granulocyte-macrophage colony-stimulating factor (GM-CSF) promoters were also amplified by PCR as controls, using a 4800 Cycler (Applied Biosystems). The PCR conditions were 94°C for 3 min; 35 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min; and a termination step of 72°C for 5 min. The PCR contained 2.0 mM MgCl2, 200 μM deoxynucleoside triphosphates, 100 nM each primer, and 2.5 U of AmpliTaq (Applied Biosystems). The intensities under UV light of ethidium bromide-stained bands from immune precipitations were normalized to the intensities of bands from the input material to determine the relative increase of the immunoprecipitated bands. The 2% agarose Tris-acetate-EDTA gels were scanned by GelDoc System 2000 using Quantity One software (Bio-Rad). Fold induction was calculated using Image version 1.62 (National Institutes of Health).
The primer sequences for GM-CSF were as follows: sense, 5-CTG ACC ACC TAG GGA AAA GGC-3, and antisense, 5-CAG CCA CAT CCT CCT CCA GAG AAC-3.
RESULTS
Isolation of primary human monocytes and infection with D2V NGC.
Monocytes were isolated from PBMCs by gelatin plate absorption, the purity was demonstrated to be 90% by flow cytometry analysis using CD14 (data not shown), and they were infected with D2V NGC as described in Materials and Methods. The rate of infection of monocytes varied from donor to donor from 2 to 33% (Fig. 1). Forty-eight hours after infection, infected 293T or ECV304 cells were stained for intracellular D2V particles; the level of infection in both cell lines approximated 100% for an MOI of 1. At the same MOI, the levels of infection remained constant for different experiments (data not shown).
FIG. 1.
(A) Representative experiment showing FACS analysis of primary human monocytes uninfected (black lines) and infected (gray lines). Cells were stained with mouse polyclonal D2V antibody diluted 1:500 in PBS, purified from hyperimmune mouse ascitic fluid (ATCC), and a secondary antibody against mouse IgG coupled with FITC. (B) Representative experiment from those in panel A viewed by fluorescence microscopy at ×400 amplification. The top panel shows the uninfected primary monocytes after indirect staining of dengue antigens. The bottom panel shows 48-h-infected primary monocytes that have undergone the same antibody staining procedure.
IL-8 levels in supernatants from dengue virus-infected monocytes and 293T and ECV304 cells.
Baseline cytokine levels were determined for uninfected monocytes over 96 h in the presence of C6/36 cell supernatant. The amount of IL-8 in the culture medium was variable and donor dependent. IL-8 secretion per million cells accumulated at high levels (300,000 pg/ml) compared to IL-6 (5,000 pg/ml) in one donor. Lower levels of IL-1β, TNF-α, and IL-10 were detected. Basal levels of IL-8 secretion for 293T and ECV304 cells were significantly lower than for monocytes (<500 pg/ml), as well as more constant between infection rates. In primary human monocytes at day 1 postinfection, no differences were observed between dengue virus-infected and uninfected monocytes/macrophages exposed to insect cell medium (C6/36 cell supernatant). However, at 48 h of infection, a significant increase in IL-8 was observed in the dengue virus-infected monocytes.
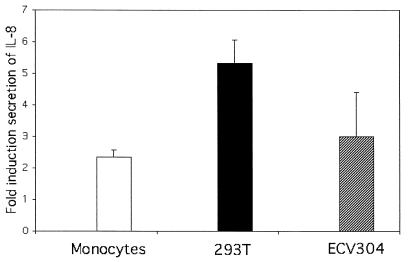
The absolute values of accumulated IL-8 in monocytes infected with D2V NGC in the first 48 h were on average 468,390 ± 24,300 pg/ml; those for C6/36 supernatant treatment were 199,000 ± 18,000 pg/ml (2.35-fold increase; P = 0.008; n = 3 [Fig. 2]), and those for medium alone were 72,000 pg/ml. For dengue virus-infected 293T cells (5.3-fold increase; P = 0.01; n = 3 [Fig. 2]), the mean values were 2,180 ± 571 and 403.76 ± 51.30 pg/ml for infected and C6/36 supernatants, respectively. For ECV304 (2.65-fold increase; P = 0.01; n = 3), the values were 80.0 ± 0.10 pg/ml for D2V NGC infection and 30.2 ± 3.90 pg/ml for C6/36 supernatant.
FIG. 2.
IL-8 enzyme-linked immunosorbent assay of cell culture supernantants. The results show the average (and standard deviation) fold induction in the production of IL-8 from three independent experiments (n = 3) 48 h postinfection.
Detection of IL-8 mRNA in monocytes and ECV304 cells by semiquantitative and quantitative RT-PCR.
The linear PCR amplification method using various cDNA amounts for RT-PCR of uninfected and infected cells was used for IL-8, IL-6, and the L35a housekeeping gene. The increase in IL-8 was calculated by integrating the area under the lane, using imaging data analysis on the ethidium bromide-stained gel normalized by the signal obtained from the L35a amplicon. Both, IL-8 and IL-6 transcripts increased. IL-8 was increased 5.4-fold relative to loading controls in D2V-infected monocytes/macrophages compared with uninfected C6/36 treatment (Fig. 3A, 1 μl of cDNA). For ECV304, the fold increase of IL-8 mRNA was greater (14-fold) than for monocytes, due to the very low levels of IL-8 expression in insect cell medium-treated cells (Fig. 3B, 3 μl of cDNA). Time course studies following D2V NGC infection of monocytes demonstrated that IL-8 expression was detectable between 2 and 6 h postinfection in monocytes (data not shown). A similar increase in IL-1β mRNA expression was detected in monocytes, with a smaller increase for ECV304 cells (data not shown).
FIG. 3.
RT-PCR of IL-8, IL-6, and ribosomal protein L35a using two concentrations of cDNAs. For each PCR, the first two lanes represent the control medium (C6/36; insect cell supernatant) and the third and fourth lanes represent D2V NGC-infected cells at an MOI of 1. The amplicon corresponding to IL-8 from endothelial cells had a higher molecular weight due to an alternative spliced mRNA. The PCR conditions were the same except for the number of cycles; for monocytes, the total number of cycles was 25, and for ECV304 cells, it was 35.
The semiquantitative data obtained by image analysis of the ethidium-bromide-stained gels were confirmed using TaqMan. Two independent donors were used for isolation and infection of primary monocytes, with 100 ng of RNA per RT reaction, and the absolute values for IL-8 and β-actin mRNA levels were obtained (r = −0.996; slope = −3.32; intercept = 36.2). IL-8 mRNA was induced 4.8 and 5.75 times, respectively. For ECV304 mRNA preparations, IL-8 was induced 12.9-fold in D2V NGC-infected cells.
Transcriptional control of IL-8 in 293T and ECV304 cells.
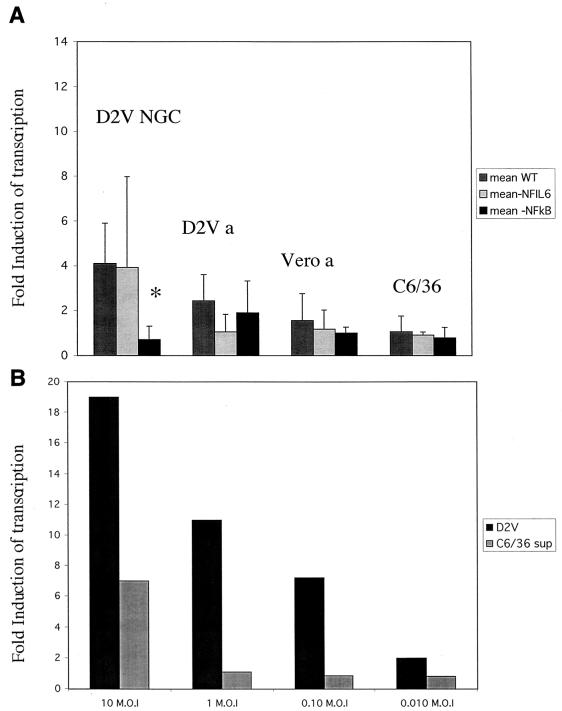
The role of NF-κB in IL-8 expression was examined in 293T epithelial and ECV304 endothelial cells. To examine transcriptional regulation of IL-8, a reporter plasmid containing luciferase under the control of the IL-8 promoter was utilized. WT and mutant IL-8 promoters were compared. As NF-κB and NFIL-6 can operate either independently or cooperatively to control IL-8 expression, mutants for both binding sites were tested. The results of the luciferase expression studies are shown in Fig. 4A and 5A. Dengue virus (D2V NGC) exposure was controlled by C6/36 cell supernatant exposure, while dengue virus antigen was controlled by Vero cell extract. IL-8 transcription was induced 7.54- ± 4.25-fold (P = 0.04; n = 3) by D2V NGC infection and 3.9- ± 1.37-fold (P = 0.04; n = 3) by exogenous D2V antigen stimulation in ECV304 cells. For 293T cells, IL-8 transcription was induced 4.4- ± 2.54-fold (P = 0.07; n = 3) by D2V and 2.44- ± 1.16-fold (not significant) after exogenous antigen stimulation. Expression of luciferase was significantly reduced (P = 0.001) in the NF-κB mutant transfectants for both cell types (Fig. 4A and 5A). For 293T cells, luciferase was expressed at the same level for the WT and NFIL-6 mutant plasmids, indicating that NF-κB and not NFIL-6 appears to be the primary mediator of the activation of the IL-8 promoter in 293T cells, whereas in ECV304 cells the NFIL-6 mutant had a significantly lower luciferase expression. Also, dengue virus antigen was effective in stimulating IL-8 promoter activity in ECV304 cells whereas it did not have the same effect in 293T cells. The specificity of the effect of dengue virus on IL-8 promoter activity is further shown by the relationship between the multiplicity of viral infection and promoter activation. For both 293T and ECV304 cells, there was a clear dose-response of transcription activation (Fig. 4B and 5B).
FIG. 4.
(A) Induction of luciferase expression by WT, NFIL-6 mutant, and NF-κB mutant IL-8 promoters in transfected 293T cells stimulated with D2V NGC, C6/36 cell supernatant, inactivated dengue virus antigen (D2V a), and control Vero cell antigen (Vero a) 48 h after transfection. The MOI was 1. The asterisk indicates the statistically significant difference (P < 0.001) between the WT and the NF-κB mutant. The error bars indicate standard deviations. (B) Transcription induction of luciferase by increasing amounts of virus at 48 h posttransfection of WT plasmid in 293T cells at MOIs from 10 to 0.01. Each bar represents the average of duplicate readings. D2V, virus-infected WT-transfected cells; C6/36 sup, WT-transfected cells treated with control insect cell supernatant.
FIG. 5.
(A) Induction of luciferase by WT, NFIL-6 mutant, and NF-κB mutant IL-8 promoters in ECV304 cells stimulated with D2V NGC, C6/36 cell supernatant, dengue virus antigen (D2V a), and Vero cell extract or antigen (Vero a) preparations for 48 h after transfection. The MOI was 1. The asterisks indicate the statistically significant differences (P < 0.001) between the WT and the NF-κB and NFIL-6 mutants. For the D2V antigen-treated cells, the WT and both of the mutant IL-8 promoters had significant differences (P < 0.05). The error bars indicate standard deviations. (B) Transcription induction of luciferase by increasing amounts of virus at 48 h posttransfection of WT plasmid in ECV304 cells at MOIs from 1 to 0.001. Each bar represents the average of duplicate readings. D2V, virus-infected WT-transfected cells; C6/36 sup, WT-transfected cells treated with control insect cell supernatant. Note the difference in MOI with 293T cells (Fig. 4B).
Nuclear extracts from infected or uninfected cells were prepared and then incubated with a labeled NF-κB oligonucleotide probe, both in the presence and absence of an unlabeled competitor oligonucleotide (Fig. 6). Extracts were prepared 3 h postinfection, which was important for demonstration of NF-κB binding, because at later time points (48 and 72 h) nuclear extracts did not show labeled probe binding activity. This may be related to the cytopathic effects observed at these time points (data not shown). However, 3 h postinfection, specific binding of NF-κB was observed only in the presence of virus in ECV304 cells (Fig. 6, second lane from left). Binding could be successfully blocked with an excess of unlabeled NF-κB probe but not with a control SP1 probe (Fig. 6, second, third, and eighth lanes from left). In a separate experiment, a mutant probe for NF-κB (the same point mutations were present as in the luciferase mutant plasmid) showed a lower-intensity binding than the WT probe (Fig. 6, three right lanes). When gel shift assays were performed with 293T nuclear extracts 3 h postinfection, results similar to those using ECV304 cells were obtained (data not shown).
FIG. 6.
Gel shift assay showing specificity of NF-κB 32P-labeled probe binding in D2V NGC-infected ECV304 cells. The last (right-hand) four lanes represent a separate experiment. The NF-κB mutant probe described in Materials and Methods shows limited binding to the nuclear extracts from infected ECV304 cells (last [far-right] lane) compared to the next-to-last lane.
ChIP.
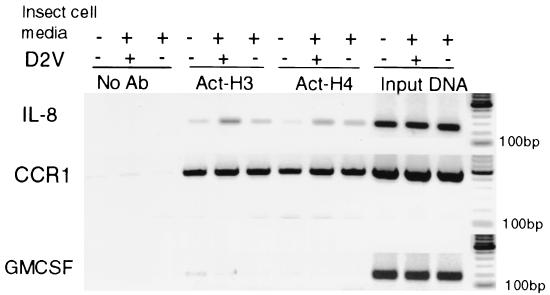
ChIP was used to assess in vivo transcription regulation of IL-8 in primary monocytes. ChIP was performed to examine the association of acetylated core histones with the IL-8 promoter upon D2V infection of primary human monocytes and ECV304. Increased association of the hyperacetylated histones H3 and H4 was observed upon D2V infection of monocytes, as shown in Fig. 7 (fourth and ninth lanes from left). A small increase in histone acetylation at the IL-8 promoter was observed in cells treated with the control supernatant, but this was clearly less than that caused by dengue virus infection. In the case of the GM-CSF promoter, no change was observed in H3 or H4 acetylation patterns. Note that the input DNA signal for IL-8 is similar to the one for CCR1; therefore, the hyperacetylation of H3, only 1.6-fold greater than for C6/36 supernatant and 4-fold greater than for the medium control, is likely to be biologically significant. The hyperacetylation of H4 after infection was marginal, between those for infected and C6/36-conditioned media (1.2-fold).
FIG. 7.
ChIPs of primary human monocytes 48 h postinfection with D2V NGC at an MOI of 1 using no antibodies (No Ab) or antibodies against acetylated H3 and acetylated H4 (Act-H3 and -H4). Input DNA was used to amplify the same promoter region of IL-8 as a loading control for each lane. The chemokine receptor CCR1 promoter was used as a positive control for acetylated H3 and H4. There was no difference in the acetylation of CCR1 between infected and uninfected monocytes (two right lanes for Act-H3 and -H4). An increase in histone acetylation in the dengue virus-infected samples was observed for the IL-8 (middle lanes for Act-H3 and -H4). GM-CSF was used as a negative-control promoter. No significant hyperacetylation was observed (Act-H3 and two left lanes for Act-H4). +, infected; −, uninfected.
DISCUSSION
We have shown an increase in the production of IL-8 in primary human monocytes as a result of dengue virus infection in vitro (Fig. 2 to 5). IL-8 was the most prominent cytokine secreted in the supernatant of cultured monocytes, and the secretion of IL-8 by dengue virus-infected cells was twice that of mock-infected cells. For the three types of cells tested, the control culture supernatants from uninfected C6/36 cells elicited a stimulatory response; however, this was clearly less than the response to virus infection. This may warrant further study. The mRNA quantification by RT-PCR using a sequence detection system showed induction of IL-8 mRNA in dengue virus-infected monocytes and ECV304 cells equivalent to that shown by semiquantitative analysis (4- to 5-fold and 12- to 17-fold, respectively). Using semiquantitative RT-PCR, the amplicon corresponding to IL-8 mRNA from monocytes had a lower molecular weight in monocytes, due to an alternative spliced form of IL-8 mRNA in ECV304 cells (Fig. 3B). The amount of accumulated IL-8 in the culture medium correlated with the amount of mRNA detected by RT-PCR. Monocytes were more prominent producers of IL-8 mRNA than endothelial cells; baseline levels and induced levels were both higher for monocytes than for the other cell types. This is in agreement with monocytes being a specialized cell for the secretion of chemokines.
The induction of transcription of IL-8 was triggered by viral infection as well as by inactivated viral antigens in ECV304 cells. Using a reporter gene to assess transcription from the IL-8 promoter, we found that the transfected reporter gene expression required at least 48 h of infection for luciferase activity to be detected. In ECV304 cells, coordinated control by two binding sites for transcription factors contributed to the control of RNA synthesis, whereas in 293T epithelial cells, NF-κB was the major controller of luciferase reporter gene activity (Fig. 4A).
Interestingly, dengue virus antigens may transduce signals through cell membrane interactions in endothelial cells and trigger IL-8 promoter activation. However, only infectious viral particles significantly change IL-8 promoter activation in epithelial cells. The dose-response observed in both cell lines tested by luciferase reporter activity indicates that IL-8 transcription correlates with dengue virus infection.
A correlation between serum IL-8 levels and infection was originally proposed by Raghupathy et al. in dengue virus-infected individuals. DHF patients had higher levels of IL-8 than dengue fever (DF) patients (27). The high levels of IL-8 were between 200 and 5,568 pg/ml, and RT-PCR of PBMCs of infected individuals showed positive mRNA IL-8 signal. In another study, the levels of circulating IL-8 in patients were between 977 and 20,000 pg/ml (2).
IL-8 has chemoattractant activity for neutrophils, and degranulation of neutrophils was demonstrated in patients with DF and DHF (18). The authors found that elastase, a product of the degranulating activity, as well as plasma IL-8 levels were elevated in patients with dengue shock syndrome in comparison with patients with DF or healthy controls.
Our data support the findings of these clinical studies, in which levels of circulating IL-8 are higher in DHF patients than in patients with less severe dengue virus infections or healthy controls. Furthermore, the cells that are most likely responsible for the increase in circulating IL-8 levels are the monocytes/macrophages.
The differences observed between ECV304 and 293T cells in NF-κB and NFIL-6 control of IL-8 promoter activation reflect the specificity of transcriptional control of IL-8 in each cell type. Activation of transcription by viral antigens may be dependent on specific cell surface receptors present in some cells but not others. Likewise, the binding and the type of nuclear factors present in different cells can vary. Due to the differences between epithelial and endothelial cells in the potential expression of membrane receptors and cellular proteins implicated in the signaling pathways, the control of transcription activation of IL-8 may also differ. In epithelial cells of the bronchia, IL-8 release is enhanced by the presence of C5a and C5a receptor (33) and is dependent on protein kinase C activation.
The pathway by which IL-8 production is regulated in infected ECV304 cells has not been elucidated. Utilizing the same cell line, the binding of the complement component C3a to the constitutively expressed C3a receptor resulted in a time- and dose-dependent response in IL-8 production. Pretreatment with pertussis toxin inhibited this response, indicating that the action of C3a was mediated by a G protein-coupled pathway (24). Also, in ECV304 cells it was shown that vitamin C inhibits the synthesis of IL-8. The role of p38 in the activation of NF-κB was shown when the specific p38 inhibitor SB203580 reversed the inhibitory effect of vitamin C on IκB kinase (IKK) activity, IκBα phosphorylation, and NF-κB activation. The results identify p38 as an intracellular target for high-dose vitamin C (6). The mechanism by which IL-8 is actively transcribed may differ among the three different cell lines tested here and deserves further study. Similarly, the bacterial human pathogen Helicobacter pylori induces IL-8 in mononuclear cells, as well as in gastric epithelial cells, yet the mechanism for IL-8 production differs between cell types (7). The chemotaxis of neutrophils in the intestinal epithelium occurs via activation of p38 and p44/42 MAPK by IL-8 (34). Different agents vary in their pathways for activation of inflammation or cell infiltration. In the case of viral respiratory pneumonia caused by adenovirus 7, the mechanism of IL-8 induction is through the Ras/Raf/MEK/Erk pathway, which is different from that used by H. pylori (34).
Infections by other viruses, such as respiratory syncytial virus, induce IL-8 production (22), as do infections by bacteria or parasites, including Mycobacterium tuberculosis (35). The transcriptional regulation of IL-8 has also been shown in a range of inflammatory responses, including cancer (16), atherosclerosis (28), and arthritis (23).
Recently, it has been shown that IL-8 transcription can be regulated by the degree of binding of acetylated core histones. Butyrate, a fermentation product of intestinal bacteria, modifies chromatin structure through histone acetylation, thereby increasing gene transcription of IL-8 in intestinal epithelial cells (12).
Testing of transcription regulation in vivo was done by ChIPs in primary human monocytes and endothelial cells.
We showed a twofold increase in histone acetylation of the IL-8 promoter upon infection with dengue virus in primary monocytes (Fig. 7, fifth and eighth lanes from left). The increase in the transcription of IL-8 is, on one hand, dependent on NF-κB, and on the other, it is also governed by the control of histone acetylation. Increased levels of histone acetylation have been shown to increase IL-8 transcription in intestinal epithelial cells treated with tricostatin A, an inhibitor of histone deacetylases (12). We do not know if dengue virus infection inhibits histone deacetylase activity in monocytes to account for the increase in IL-8 transcription. Basal levels of histone acetylation of the IL-8 promoter were very low and similar to the levels found for GM-CSF (which does not vary between dengue virus-infected and uninfected monocytes). As a positive control, CCR1 acetylation of the promoter was shown to be constant and did not change in the presence of dengue virus (Fig. 7). It is important to point out that the maximum hyperacetylation levels could potentially be reached using alternative promoter regions, as in the case of p21 gene-associated histone acetylation. Richon et al. (29) show that similar levels of hyperacetylation of the p21 promoter (from 1.2- to 5-fold increase) were associated with induction of expression of p21 mRNA and protein.
The status of H3 and H4 acetylation seems to reflect the expression of IL-8, as previously reported for other cell types and systems (32). The control of IL-8 transcription in primary human monocytes (Fig. 7), as well as in ECV304 cells (data not shown), seems to be regulated by chromatin remodeling through H3 and H4 acetylation and correlates with the increase in mRNA levels and secreted protein. In conclusion, in vitro IL-8 transcription increased in infected cells, and the in vivo chromatin arrangements showed increased levels of acetylated H3 and H4 in association with greater IL-8 secretion in infected cells. We show a novel specific acetylation of the IL-8 promoter in response to dengue virus infection. The increase in IL-8 transcription by viral antigen alone in endothelial cells is also a novel observation, since inactivated viral antigens triggering IL-8 expression have not been shown previously. IL-8 expression was controlled by NF-κB binding and promoter activation. The IL-8 promoter also showed greater association with acetylated core histones in infected monocytes and infected endothelial cells. Therefore, at least two levels in the transcriptional control of IL-8 seem to be affected by dengue virus infection. In theory, they could act independently, representing two different levels of transcriptional control. This is novel transcriptional control, not previously described for cytosolic RNA viruses.
There have been several efforts to find markers for disease severity in dengue virus infection (3, 14). We propose that IL-8 may serve as a good marker for infection. Due to the biological importance of IL-8 in vascular leakage, its role in DHF and dengue shock syndrome is an area that should be actively pursued.
Acknowledgments
This work was supported by grant R01 AI30624 from the National Institutes of Health.
The opinions expressed herein are those of the authors and should not be construed as representing the official policies of the National Institutes of Health.
We thank Deb Biswas for his advice on electrophoresis mobility gel shift assays, Jurand Janus for excellent technical expertise, and Sharone Green for critical reading of the manuscript.
REFERENCES
- 1.Anderson, R., S. Wang, C. Osiowy, and A. C. Issekutz. 1997. Activation of endothelial cells via antibody-enhanced dengue virus infection of peripheral blood monocytes. J. Virol. 71:4226-4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avirutnan, P., P. Malasit, B. Seliger, S. Bhakdi, and M. Husmann. 1998. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J. Immunol. 161:6338-6346. [PubMed] [Google Scholar]
- 3.Bethell, D. B., K. Flobbe, X. T. Cao, N. P. Day, T. P. Pham, W. A. Buurman, M. J. Cardosa, N. J. White, and D. Kwiatkowski. 1998. Pathophysiologic and prognostic role of cytokines in dengue hemorrhagic fever. J. Infect. Dis. 177:778-782. [DOI] [PubMed] [Google Scholar]
- 4.Biswas, D. K., A. P. Cruz, E. Gansberger, and A. B. Pardee. 2000. Epidermal growth factor-induced nuclear factor kappa B activation: a major pathway of cell-cycle progression in estrogen-receptor negative breast cancer cells. Proc. Natl. Acad. Sci. USA 97:8542-8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonner, S. M., and M. A. O'Sullivan. 1998. Endothelial cell monolayers as a model system to investigate dengue shock syndrome. J. Virol. Methods 71:159-167. [DOI] [PubMed] [Google Scholar]
- 6.Bowie, A. G., and L. A. O'Neill. 2000. Vitamin C inhibits NF-kappa B activation by TNF via the activation of p38 mitogen-activated protein kinase. J. Immunol. 165:7180-7188. [DOI] [PubMed] [Google Scholar]
- 7.de Jonge, R., J. G. Kusters, M. S. Timmer, V. Gimmel, B. J. Appelmelk, S. Bereswill, A. H. van Vliet, S. G. Meuwissen, M. Kist, C. M. Vandenbroucke-Grauls, and E. J. Kuipers. 2001. The role of Helicobacter pylori virulence factors in interleukin production by monocytic cells. FEMS Microbiol. Lett. 196:235-238. [DOI] [PubMed] [Google Scholar]
- 8.Denk, A., M. Goebeler, S. Schmid, I. Berberich, O. Ritz, D. Lindemann, S. Ludwig, and T. Wirth. 2001. Activation of NF-κB via the IκB kinase complex is both essential and sufficient for proinflammatory gene expression in primary endothelial cells. J. Biol. Chem. 3:3.. [DOI] [PubMed] [Google Scholar]
- 9.Dignam, J. D., R. M. Lebovitz, and R. G. Roeder. 1983. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11:1475-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fibbe, W. E., J. F. Pruijt, Y. van Kooyk, C. G. Figdor, G. Opdenakker, and R. Willemze. 2000. The role of metalloproteinases and adhesion molecules in interleukin-8-induced stem-cell mobilization. Semin. Hematol. 37:19-24. [DOI] [PubMed] [Google Scholar]
- 11.Fibbe, W. E., J. F. Pruijt, G. A. Velders, G. Opdenakker, Y. van Kooyk, C. G. Figdor, and R. Willemze. 1999. Biology of IL-8-induced stem cell mobilization. Ann. N. Y. Acad. Sci. 872:71-82. [DOI] [PubMed] [Google Scholar]
- 12.Fusunyan, R. D., J. J. Quinn, M. Fujimoto, R. P. MacDermott, and I. R. Sanderson. 1999. Butyrate switches the pattern of chemokine secretion by intestinal epithelial cells through histone acetylation. Mol. Med. 5:631-640. [PMC free article] [PubMed] [Google Scholar]
- 13.Green, S., D. W. Vaughn, S. Kalayanarooj, S. Nimmannitya, S. Suntayakorn, A. Nisalak, R. Lew, B. L. Innis, I. Kurane, A. L. Rothman, and F. A. Ennis. 1999. Early immune activation in acute dengue illness is related to development of plasma leakage and disease severity. J. Infect. Dis. 179:755-762. [DOI] [PubMed] [Google Scholar]
- 14.Green, S., D. W. Vaughn, S. Kalayanarooj, S. Nimmannitya, S. Suntayakorn, A. Nisalak, A. L. Rothman, and F. A. Ennis. 1999. Elevated plasma interleukin-10 levels in acute dengue correlate with disease severity. J. Med. Virol. 59:329-334. [PubMed] [Google Scholar]
- 15.Hazelzet, J. A., R. de Groot, G. van Mierlo, K. F. Joosten, E. van der Voort, A. Eerenberg, M. H. Suur, W. C. Hop, and C. E. Hack. 1998. Complement activation in relation to capillary leakage in children with septic shock and purpura. Infect. Immun. 66:5350-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang, N., J. P. Katz, D. R. Martin, and G. D. Wu. 1997. Inhibition of IL-8 gene expression in Caco-2 cells by compounds which induce histone hyperacetylation. Cytokine 9:27-36. [DOI] [PubMed] [Google Scholar]
- 17.Huang, Y., H. Y. Lei, H. S. Liu, Y. S. Lin, C. C. Liu, and T. M. Yeh. 2000. Dengue virus infects human endothelial cells and induces IL-6 and IL-8 production. Am. J. Trop. Med. Hyg. 63:71-75. [DOI] [PubMed] [Google Scholar]
- 18.Juffrie, M., G. M. van Der Meer, C. E. Hack, K. Haasnoot, Sutaryo, A. J. Veerman, and L. G. Thijs. 2000. Inflammatory mediators in dengue virus infection in children: interleukin-8 and its relationship to neutrophil degranulation. Infect. Immun. 68:702-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kurane, I., B. L. Innis, S. Nimmannitya, A. Nisalak, A. Meager, J. Janus, and F. A. Ennis. 1991. Activation of T lymphocytes in dengue virus infections. High levels of soluble interleukin 2 receptor, soluble CD4, soluble CD8, interleukin 2, and interferon-gamma in sera of children with dengue. J. Clin. Investig. 88:1473-1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurane, I., J. Janus, and F. A. Ennis. 1992. Dengue virus infection of human skin fibroblasts in vitro production of IFN-beta, IL-6 and GM-CSF. Arch. Virol. 124:21-30. [DOI] [PubMed] [Google Scholar]
- 21.Lafrenie, R. M., L. M. Wahl, J. S. Epstein, K. M. Yamada, and S. Dhawan. 1997. Activation of monocytes by HIV-Tat treatment is mediated by cytokine expression. J. Immunol. 159:4077-4083. [PubMed] [Google Scholar]
- 22.Mastronarde, J. G., B. He, M. M. Monick, N. Mukaida, K. Matsushima, and G. W. Hunninghake. 1996. Induction of interleukin (IL)-8 gene expression by respiratory syncytial virus involves activation of nuclear factor (NF)-kappa B and NF-IL-6. J. Infect. Dis. 174:262-267. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki, S., A. Matsukawa, S. Ohkawara, K. Takagi, and M. Yoshinaga. 2000. Neutrophil infiltration as a crucial step for monocyte chemoattractant protein (MCP)-1 to attract monocytes in lipopolysaccharide-induced arthritis in rabbits. Inflamm. Res. 49:673-678. [DOI] [PubMed] [Google Scholar]
- 24.Monsinjon, T., P. Gasque, A. Ischenko, and M. Fontaine. 2001. C3A binds to the seven transmembrane anaphylatoxin receptor expressed by epithelial cells and triggers the production of IL-8. FEBS Lett. 487:339-346. [DOI] [PubMed] [Google Scholar]
- 25.Mori, M., I. Kurane, J. Janus, and F. A. Ennis. 1997. Cytokine production by dengue virus antigen-responsive human T lymphocytes in vitro examined using a double immunocytochemical technique. J. Leukoc. Biol. 61:338-345. [DOI] [PubMed] [Google Scholar]
- 26.Morland, C. M., B. J. Morland, P. J. Darbyshire, and R. A. Stockley. 2000. Migration of CD18-deficient neutrophils in vitro: evidence for a CD18-independent pathway induced by IL-8. Biochim. Biophys. Acta 1500:70-76. [DOI] [PubMed] [Google Scholar]
- 27.Raghupathy, R., U. C. Chaturvedi, H. Al-Sayer, E. A. Elbishbishi, R. Agarwal, R. Nagar, S. Kapoor, A. Misra, A. Mathur, H. Nusrat, F. Azizieh, M. A. Khan, and A. S. Mustafa. 1998. Elevated levels of IL-8 in dengue hemorrhagic fever. J. Med Virol. 56:280-285. [DOI] [PubMed] [Google Scholar]
- 28.Reape, T. J., and P. H. Groot. 1999. Chemokines and atherosclerosis. Atherosclerosis 147:213-225. [DOI] [PubMed] [Google Scholar]
- 29.Richon, V. M., T. W. Sandhoff, R. A. Rifkind, and P. A. Marks. 2000. Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc. Natl. Acad. Sci. USA 97:10014-10019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rothman, A. L., and F. A. Ennis. 2000. Toga/flaviviruses: immunopathology, p. 473-490. In M. W. Cunningham, and R. S. Fujimani (ed.), Effects of microbes on the immune system. Lippincott Williams and Wilkins, Philadelphia, Pa.
- 31.Siddiqui, R., L. Akard, J. Garcia, Y. Cui, and D. English. 1999. Chemotactic migration triggers IL-8 generation in neotrophilic leukocytes. J. Immunol. 162:1077-1083. [PubMed] [Google Scholar]
- 32.Timmermann, S., H. Lehrmann, A. Polesskaya, and A. Harel-Bellan. 2001. Histone acetylation and disease. Cell Mol. Life Sci. 58:728-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wyatt, T., A. Heires, S. D. Sanderson, and A. Floreani. 1999. Protein kinase C activation is required for cigarette smoke-enhanced C5a-mediated release of IL-8 in human bronchial epithelial cells. Am. J. Respir. Cell. Mol. Biol. 21:283-288. [DOI] [PubMed] [Google Scholar]
- 34.Yamada, H., T. Aihara, and S. Okabe. 2001. Mechanism for Helicobacter pylori stimulation of interleukin-8 production in a gastric epithelial cell line (MKN 28): roles of mitogen-activated protein kinase and interleukin-1β. Biochem. Pharmacol. 61:1595-1604. [DOI] [PubMed] [Google Scholar]
- 35.Yamada, Y., A. Nakamura, M. Hosoda, T. Kato, T. Asano, K. Tonegawa, and M. Itoh. 2001. Cytokines in pleural liquid for diagnosis of tuberculous pleurisy. Respir. Med. 95:577-581. [DOI] [PubMed] [Google Scholar]