Abstract
Retroviral Gag polyproteins contain regions that promote the separation of virus particles from the plasma membrane and from each other. These Gag regions are often referred to as late assembly (L) domains. The L domain of human immunodeficiency virus type 1 (HIV-1) is in the C-terminal p6gag domain and harbors an essential P(T/S)APP motif, whereas the L domains of oncoretroviruses are in the N-terminal half of the Gag precursor and have a PPXY core motif. We recently observed that L domains induce the ubiquitination of a minimal HIV-1 Gag construct and that point mutations which abolish L domain activity prevent Gag ubiquitination. In that study, a peptide from the Ebola virus L domain with overlapping P(T/S)APP and PPXY motifs showed exceptional activity in promoting Gag ubiquitination and the release of virus-like particles. We now show that a substitution which disrupts the PPXY motif but leaves the P(T/S)APP motif intact abolishes L domain activity in the minimal Gag context, but not in the context of a near full-length HIV-1 Gag precursor. Our results reveal that the P(T/S)APP motif does not function autonomously and indicate that the HIV-1 nucleocapsid-p1 region, which is proximal to p6gag, can cooperate with the conserved L domain core motif. We have also examined the effects of ubiquitin mutants on virus-like particle production, and the results indicate that residues required for the endocytosis function of ubiquitin are also involved in virus budding.
Retroviral Gag polyproteins have specific regions that facilitate the final separation of assembled virus particles from the cell surface and from each other (9, 15, 21, 24, 36-40). These regions, which are known as late assembly (L) domains, harbor short, highly conserved proline-rich motifs that are thought to constitute interaction sites for cellular factors. The first L domain was identified in human immunodeficiency virus type 1 (HIV-1), where it is located in p6gag, the C-terminal domain of the Gag precursor (9). Within p6gag, a P(T/S)APP motif near the N terminus of the domain is crucial for virus release, while other regions of p6gag appear to be largely dispensable, at least in the context of a full-length Gag precursor (9, 15). Although p6gag is the most variable of the Gag domains of primate immunodeficiency viruses, the P(T/S)APP motif is absolutely conserved among these viruses and is also found at an equivalent location in other lentiviruses, even though these viruses do not possess a p6gag domain. The only exception is equine infectious anemia virus (EIAV), which uses a YXXL motif in its unique C-terminal Gag domain for virus release (24).
While the L domains of lentiviruses occupy a C-terminal position within Gag, those of oncoretroviruses are in the N-terminal half of the Gag polyprotein. The best-studied oncoretroviral L domain, that of Rous sarcoma virus (RSV), maps to the 11-amino-acid p2b region, which is located between the matrix (MA) and capsid (CA) domains of the Gag precursor (36, 37). More recently, it was shown that Mason-Pfizer monkey virus and Moloney murine leukemia virus (Mo-MuLV) harbor L domains at an equivalent location (38, 40). In all three viruses, the essential core of the L domain has the sequence PPPY, which is highly conserved among oncoretroviruses but absent from the HIV-1 Gag precursor. Interestingly, conserved PPXY motifs, often in combination with a P(T/S)AP motif, are also found in the matrix proteins of certain enveloped negative-strand RNA viruses (4, 11, 32). These appear to have a role similar to that of the corresponding retroviral motifs, since mutations in the PPXY motifs of vesicular stomatitis virus and Ebola virus inhibited budding from the plasma membrane (10, 16).
Retroviruses have long been known to contain small amounts of ubiquitin (26), a highly conserved 76-amino-acid polypeptide that can be covalently attached to lysine residues in other proteins. In HIV-1 and Mo-MuLV, lysine residues in the vicinity of the L domain are monoubiquitinated in a small fraction of virion-associated Gag molecules (19, 20). While the enzymatic machinery involved remains unknown, it has recently been reported that the P(T/S)APP motif in HIV-1 p6gag interacts with the putative ubiquitin regulator Tsg101 (35). Several studies now indicate that L domains engage the cellular ubiquitination machinery to promote virus release (22, 27, 32). These studies show that compounds which block proteasomal degradation inhibit the budding of different retroviruses at a late stage. It is thought that proteasome inhibitors interfere with budding solely because they deplete the intracellular levels of free ubiquitin. Consistent with this interpretation, RSV budding could be restored in the presence of a proteasome inhibitor by overexpressing ubiquitin, or by fusing ubiquitin directly to Gag (22). Independent evidence for a role of ubiquitin in retroviral budding is provided by our recent observation that L domains induce the ubiquitination of a minimal HIV-1 Gag molecule (32). Importantly, the ubiquitination of Gag was induced by unrelated L domains and was prevented by mutations that blocked L domain function. These results indicated that the recruitment of a component of the cellular ubiquitination machinery plays a crucial role in L domain function.
A peptide from the candidate Ebola virus L domain with combined P(T/S)APP and PPXY motifs was exceptionally active in stimulating virus-like particle (VLP) production by a minimal HIV-1 Gag molecule that lacked most of MA, about two-thirds of CA, and all of nucleocapsid (NC) and p6gag (1, 32). In the present study, we find that in this minimal Gag context the PPXY motif is required for the induction of Gag ubiquitination and VLP production, implying that the P(T/S)APP L domain core motif by itself is insufficient. In marked contrast, a mutant peptide which retained only the P(T/S)APP motif was fully active as an L domain when fused to a nearly full-length HIV-1 Gag polyprotein that lacked only p6gag. Our results reveal a context-dependent activity of the P(T/S)APP motif and indicate that HIV-1 Gag regions outside of p6gag can cooperate with the conserved L domain core motif to enhance Gag ubiquitination and to promote virus release. To elucidate the role of ubiquitin in L domain function, we also examined whether VLP production in our system is affected by the coexpression of mutant ubiquitins. The results of these experiments suggest a link between the endocytosis function of ubiquitin and its involvement in virus exocytosis.
MATERIALS AND METHODS
Gag constructs.
The previously described Δ-ZWT minimal Gag construct is based on the vpu-positive HXBH10 variant of HIV-1HXB2 (1). The Δ-ZWT gag gene lacks codons 8 through 277 and has the NC-p1-p6 region replaced by the GCN4 leucine zipper domain (1). Δ-ZWT-Eb (32) is a variant of Δ-ZWT that has the peptide ILPTAPPEYMEA from the Ebola virus matrix protein (Eb peptide) fused to the C terminus of the Δ-ZWT Gag molecule. The Eb peptide, which harbors overlapping P(T/S)APP and PPXY L domain core motifs, is immediately followed by a stop codon. A vpu-negative version of Δ-ZWT-Eb was obtained by replacing a SalI-NheI fragment (nucleotides 5789 to 7263 of HXBH10) with the corresponding fragment from HXB2, which harbors a defective vpu gene. The (Y/G)Eb variant of Δ-ZWT-Eb has codon 9 (TAC) of the Eb peptide replaced by GGC, which disrupts the PPXY motif but leaves the P(T/S)APP L domain core motif intact. The Δ-ZWT-p6 minimal Gag construct (1) has the 52-amino-acid p6gag domain of HIV-1HXB2 fused to the C terminus of Δ-ZWT.
To replace the p6gag domain of the full-length HIV-1 Gag precursor with a heterologous L domain, a synthetic sequence encoding the Eb peptide, followed by a stop codon, was inserted 3′ of codon 1 of p6gag. Because the resulting MA-CA-p2-NC-p1-Eb Gag construct retained the frameshift signal required for the synthesis of Gag-pol, the codon for Asp25 of the HIV-1 protease was changed to a codon specifying glutamic acid to prevent Gag processing. The Y/G variant of MA-CA-p2-NC-p1-Eb has codon 9 of the Eb peptide replaced by GGC to disrupt the PPXY motif in the heterologous L domain. For control purposes, we also generated a variant that harbors a stop codon directly after the coding sequence for MA-CA-p2-NC-p1. The ZWT-Eb and ZWT-(Y/G)Eb Gag constructs were obtained by restoring HIV-1 gag codons 8 through 277 in Δ-ZWT-Eb and Δ-ZWT-(Y/G)Eb by using standard cloning methods.
Ubiquitin expression vectors.
A previously described pBJ5-based mammalian expression vector for an octameric ubiquitin precursor with a hemagglutinin (HA) tag at the N terminus of each unit (32) was cut with XbaI and religated. This manipulation yielded an expression vector for monomeric HA-tagged ubiquitin with a C-terminal extension that is rapidly removed by ubiquitin C-terminal hydrolases (data not shown). To obtain an expression vector for nontagged monomeric ubiquitin, DNA encoding a KOZAK sequence and amino acids 1 to 76 of ubiquitin plus a random C-terminal extension was PCR amplified and cloned into the NotI and EcoRI sites of pBJ5 (obtained from Richard Bram). The ubiquitin gene in this mammalian expression vector is under the control of the highly active SRα promoter (33). Point mutations in the ubiquitin coding sequence were generated by PCR mutagenesis by using a QuickChange mutagenesis kit (Stratagene). Each construct was confirmed by DNA sequencing.
Transfection and analysis of VLP formation.
HeLa cells (0.8 × 106) or 293T cells (6 × 106) were seeded into 80-cm2 tissue culture flasks 24 h before transfection. HeLa cultures were transfected by a calcium phosphate precipitation technique with 15 μg of each proviral Gag construct, except in cotransfection experiments, where 10 μg of each plasmid were used. After metabolic labeling with [35S]methionine (50 μCi/ml) from 48 to 60 h posttransfection, the culture supernatants were collected and the cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (140 mM NaCl, 8 mM Na2HPO4, 2 mM NaH2PO4, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.05% sodium dodecyl sulfate [SDS]). 293T cells were transfected with 15 μg of DNA by using the LipofectAMINE 2000 reagent (Life Technologies). The cells were incubated with serum-free transfection mixture for 4 h, and fetal calf serum (FCS) was then added to a final concentration of 10%. The transfection mixture was removed 1 h later, and the cells were starved for 30 min in serum-free medium lacking methionine, followed by metabolic labeling with [35S]methionine (100 μCi/ml) for 1 h in the presence of 5% FCS. After a 2-h chase in medium containing 10% FCS, supernatants were harvested and the cells were lysed in RIPA buffer.
Supernatants were clarified by low-speed centrifugation and passaged through 0.45-μm-pore-size filters. VLP released during the labeling or chase periods were spun through 20% sucrose cushions (in phosphate-buffered saline) for 2 h at 4°C and 27,000 rpm in a Beckman SW28 rotor. Pelleted VLP were lysed in RIPA buffer, and viral proteins were directly analyzed by SDS-polyacrylamide gel electrophoresis (PAGE). To examine the intracellular Gag expression levels, the cell lysates were immunoprecipitated with rabbit anti-CA polyclonal antiserum (Advanced Biotechnologies) or with serum from a patient infected with HIV-1.
For immunoblot analysis, aliquots of lysed VLP were resolved by SDS-PAGE and electroblotted onto a Hybond-C Extra membrane (Amersham Pharmacia). The membrane was then autoclaved in deionized water for 30 min as described previously (17) to expose latent antigenic sites on ubiquitin. Ubiquitin conjugates were detected with a rabbit anti-ubiquitin antiserum (Sigma) and enhanced chemiluminescence reagents (Amersham Pharmacia).
RESULTS
The P(T/S)APP L domain core motif is inactive in a minimal Gag context.
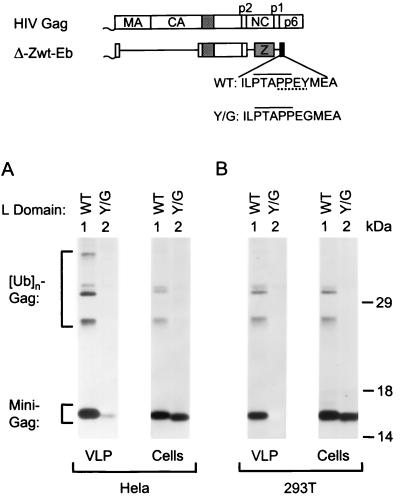
We previously used the minimal HIV-1 Gag construct Δ-ZWT to compare the ability of viral sequences to promote VLP release (1, 32). Unexpectedly, the N-terminal 14 amino acids of HIV-1 p6gag, which harbor the conserved P(T/S)APP L domain core motif, showed little activity when fused to the C terminus of Δ-ZWT (1). In marked contrast, the 12-amino-acid peptide ILPTAPPEYMEA from the Ebola virus L domain (Eb peptide) proved to be exceptionally active in stimulating VLP production by the Δ-ZWT construct (32). The Eb peptide harbors entwined P(T/S)APP and PPXY motifs in the sequence PTAPPEY, which is also found at the core of the HIV-1 L domain, except that the latter has a Glu in place of the Tyr residue. To examine the contribution of the Tyr in the Eb peptide, we changed this residue to Gly in the context of the Δ-ZWT-Eb minimal Gag construct, yielding Δ-ZWT-(Y/G)Eb. This single-amino-acid change disrupted the PPXY motif in the Eb peptide but left the P(T/S)APP L domain core motif intact.
Transfection of the minimal Gag constructs into HeLa cells revealed that the Y/G substitution in the Eb peptide reduced VLP production ∼10-fold as quantitated by PhosphorImager analysis (Fig. 1A). As previously reported (32), VLP produced by the parental Δ-ZWT-Eb construct contained the 16-kDa minimal Gag molecule and slower-migrating Gag-ubiquitin conjugates. The Y/G substitution did not significantly affect the intracellular levels of the 16-kDa minimal Gag molecule as determined by immunoprecipitation with anti-CA serum (Fig. 1A). Interestingly, mono- and diubiquitinated Gag species were clearly detectable in the lysate of cells transfected with the parental Δ-ZWT-Eb construct, indicating that these cell-associated ubiquitin conjugates were not rapidly degraded. In the presence of the Y/G substitution, the monoubiquitinated species appeared significantly reduced and the diubiquitinated form was no longer visible, suggesting that the substitution interfered with the ability of the L domain to induce Gag ubiquitination.
FIG. 1.
A substitution that disrupts the PPXY motif but not the overlapping P(T/S)APP motif in the Ebola virus-derived peptide ILPTAPPEYMEA abolishes L domain activity in a minimal Gag context. HeLa (A) or 293T (B) cells were transfected with variants of the highly L domain-dependent Δ-ZWT minimal HIV-1 Gag construct that have either the WT peptide or the Y/G mutant peptide at the C terminus. To compare the levels of VLP formation, particulate material released into the medium during 12 h of metabolic labeling (A) or during a 2-h chase period (B) was pelleted through sucrose and analyzed directly by SDS-PAGE (left panels). Cell-associated Gag protein was detected by immunoprecipitation with anti-CA antiserum (right panels). The positions of migration of molecular weight markers (in kilodaltons) are indicated.
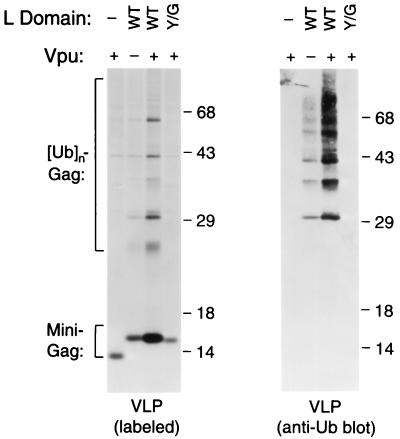
To verify the effect of the Y/G substitution on Gag ubiquitination, we cotransfected an expression vector for simian virus 40 large T antigen to boost expression from the Δ-ZWT-(Y/G)Eb construct, which harbors a T antigen-dependent replication origin. This strategy was based on our observation that the requirement for an L domain can be mitigated by increasing the expression levels of Gag (data not shown). In an attempt to adjust VLP production by the parental Δ-ZWT-Eb construct to levels comparable to those obtained with Δ-ZWT-(Y/G)Eb, we used a vpu-negative version of Δ-ZWT-Eb. Vpu is a small auxiliary protein encoded by HIV-1 that significantly enhances the release of VLP in HeLa cells (8). As shown in Fig. 2, disrupting vpu in the Δ-ZWT-Eb construct reduced VLP production to levels comparable to those obtained with Δ-ZWT or Δ-ZWT-(Y/G)Eb, which express Vpu but lack a functional L domain. However, while VLP produced by the vpu-negative Δ-ZWT-Eb construct contained ubiquitin conjugates that were readily detectable by immunoblotting with anti-ubiquitin serum, VLP produced by Δ-ZWT or Δ-ZWT-(Y/G)Eb lacked such conjugates. These observations confirm that the Y/G substitution interfered with L domain-induced Gag ubiquitination. Furthermore, since disrupting vpu in Δ-ZWT-Eb did not affect the levels of Gag-ubiquitin conjugates in VLP relative to those of unmodified Gag, we conclude that the induction of Gag ubiquitination by viral L domains is independent of Vpu.
FIG. 2.
The presence of a P(T/S)APP motif is insufficient to induce the appearance of Gag-ubiquitin conjugates in VLP produced by a minimal Gag construct. The Δ-ZWT minimal HIV-1 Gag construct, or variants that contain the ILPTAPPEYMEA (WT) peptide or the Y/G mutant peptide at the C terminus, were transfected into HeLa cells, together with a vector that provided simian virus 40 T antigen. A vpu-negative version of the Gag construct was used to suppress the levels of VLP production in the presence of the WT peptide to levels obtained with the parental Δ-ZWT construct, which is vpu-positive but lacks an L domain. VLP released during metabolic labeling were pelleted through sucrose and directly analyzed by SDS-PAGE (left panel). To confirm the presence of ubiquitin conjugates, an aliquot of each VLP preparation was analyzed by immunoblotting with anti-ubiquitin antiserum (right panel). For unknown reasons, the antiserum reacted poorly with monoubiquitinated Gag but readily detected conjugates that contained two or more ubiquitin moieties.
To determine whether the requirement for an intact PPXY motif in the minimal Gag context reflected a cell-type-specific phenomenon, we also examined the effect of the Y/G substitution in 293T cells. In initial experiments, in which VLP production was measured 2 days after transfection, the Y/G change had only a modest effect (data not shown). Because the high levels of cell-associated Gag that had accumulated by this time may have compensated for a defect in VLP production, 293T cells were subsequently pulse-labeled as early as 4.5 h posttransfection, and VLP production was then measured during a 2-h chase period. Even at this early time point, large amounts of particulate Gag were detected in the culture supernatant if the parental Δ-ZWT-Eb construct was transfected (Fig. 1B). Remarkably, the Y/G substitution in the Eb peptide completely abolished VLP production in this setting, whereas the cell-associated Gag signal obtained by immunoprecipitation with anti-CA serum was reduced less than twofold (Fig. 1B). Immunoprecipitation from the cell lysates also showed that the Y/G substitution prevented the appearance of bands that comigrated with mono- and diubiquitinated Gag. Since the Y/G substitution left the P(T/S)APP L domain core motif in the Eb peptide intact, we infer that the presence of this motif was not sufficient to induce detectable ubiquitination of the minimal Gag molecule nor was it sufficient to stimulate VLP production.
A role for HIV-1 NC-p1 in Gag ubiquitination and L domain activity.
The finding that the presence of a P(T/S)APP motif was insufficient to provide L domain activity in the minimal HIV-1 Gag context was unexpected in the light of previous studies. First, we previously showed that the N-terminal 16 residues of p6gag promote virus release in the context of an otherwise full-length HIV-1 Gag polyprotein (9). Second, the N-terminal 12 residues of HIV-1 p6gag, which contain the P(T/S)APP L domain core motif, were sufficient to rescue VLP production by a RSV L domain mutant (21). Similarly, a Mo-MuLV L domain mutant could be rescued by the first 18 amino acids of HIV-1 p6gag (39). A potential explanation that would reconcile these observations with our current results is that the Δ-ZWT minimal Gag construct lacks determinants that cooperate with the P(T/S)APP L domain core motif in the context of a full-length Gag polyprotein.
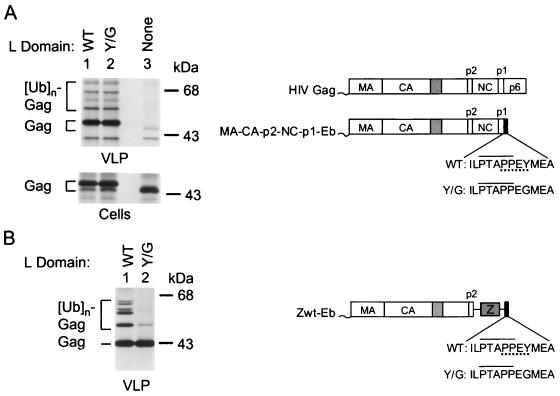
To test this hypothesis, we fused either the wild-type (WT) or the Y/G Eb peptide to the C terminus of HIV-1 MA-CA-p2-NC-p1, which corresponds to a Gag precursor that lacks p6gag but is otherwise full-length. As described above, the WT Eb peptide, which harbored both a P(T/S)APP and a PPXY motif, was highly active as an L domain in the Δ-ZWT minimal Gag context, whereas the Y/G Eb peptide, which retained only the P(T/S)APP motif, was inactive. In contrast, when added to the nearly full-length HIV-1 Gag precursor, the WT and Y/G Eb peptides were similarly active, both in inducing Gag ubiquitination and in promoting VLP production in transfected HeLa cells (Fig. 3A). Quantitation by PhosphorImager analysis and normalization for the levels of cell-associated Gag (Fig. 3A) revealed that the WT and Y/G Eb peptides increased VLP production 16- and 12-fold, respectively. These results indicate that in its natural context the P(T/S)APP motif suffices to provide L domain activity, a finding consistent with the fact that HIV-1 p6gag, and indeed all of HIV-1 Gag, lacks a PPXY motif.
FIG. 3.
NC-p1-dependent induction of Gag ubiquitination by the P(T/S)APP L domain core motif. (A) HeLa cells were transfected with the nearly full-length HIV-1 Gag constructs MA-CA-p2-NC-p1-Eb (lane 1), MA-CA-p2-NC-p1-(Y/G)Eb (lane 2), and MA-CA-p2-NC-p1 (lane 3). [35S]methionine-labeled VLP produced by the transfected cells were spun through sucrose and directly analyzed by SDS-PAGE. Cell-associated Gag protein was detected by immunoprecipitation with anti-CA antiserum. (B) HeLa cells were transfected with the Gag constructs ZWT-Eb (lane 1) and ZWT-(Y/G)Eb (lane 2), which harbor a leucine zipper domain in place of NC-p1. VLP released during metabolic labeling with [35S]methionine was directly analyzed by SDS-PAGE.
The Δ-ZWT minimal Gag molecule lacks several Gag regions present in MA-CA-p2-NC-p1, including NC-p1, which is replaced by a leucine zipper domain (1). To examine whether the presence of NC-p1 was required for the induction of Gag ubiquitination by the P(T/S)APP L domain core motif, we made use of the previously described ZWT Gag molecule (1). The ZWT Gag precursor has the domain organization MA-CA-p2-Z (Z symbolizes the GCN4 leucine zipper) and differs from MA-CA-p2-NC-p1 only by the presence of a leucine zipper domain in place of NC-p1. We and others have shown that leucine zipper domains in this position can rescue VLP production in the absence of NC and of p6gag, at least when Gag expression levels are relatively high (1, 32, 41).
To obtain versions of ZWT that harbor either combined P(T/S)APP and PPXY motifs, or only the P(T/S)APP motif, we attached either the WT or the Y/G Eb peptide to the C terminus of ZWT. VLP production by the ZWT-Eb and ZWT-(Y/G)Eb constructs after transfection into HeLa cells was comparable (Fig. 3B), a finding consistent with our previous observation that the ZWT construct does not significantly benefit from the presence of an L domain under the conditions used (1). However, whereas VLP produced by ZWT-Eb contained a ladder of prominent Gag-ubiquitin conjugates, only a single faint band that migrated at the position of monoubiquitinated Gag was visible in ZWT-(Y/G)Eb VLP. Thus, if the NC-p1 domain of the HIV-1 Gag precursor was replaced by a heterologous leucine zipper domain, the (Y/G)Eb peptide, in contrast to the WT Eb peptide, essentially lost its ability to induce the ubiquitination of Gag. These observations provide evidence that the P(T/S)APP L domain core motif in the (Y/G) Eb peptide cooperated with determinants in NC-p1 in the induction of Gag ubiquitination.
Inhibition of VLP production by overexpression of mutant ubiquitin.
Several recent studies implicate ubiquitination in viral budding (10, 22, 27, 32), but the exact role of this modification remains to be determined. Ubiquitin is a modifying group that functions in diverse cellular processes. For instance, polyubiquitin chains that are linked through Lys48 mark proteins for proteasomal degradation (3, 6, 13, 23). In contrast, chains that are linked through ubiquitin residue Lys63 appear to have regulatory roles that do not involve the proteasome (5, 14, 23, 30), including the ubiquitin-dependent endocytosis of certain plasma membrane proteins (7, 31). A hydrophobic patch on the surface of ubiquitin that includes Ile44 is required both for recognition by the proteasome and for endocytosis (2, 18, 29); on the other hand, a second hydrophobic patch that centers around Phe4 is specifically required for endocytosis (12, 28, 29).
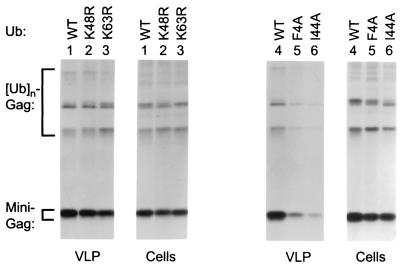
In an effort to determine whether any of these ubiquitin residues are involved in virus budding, we performed a mutagenic analysis in which Lys48 and Lys63 were individually replaced with Arg, and Phe4 and Ile44 were each changed to Ala. Because ubiquitin is normally expressed as a head-to-tail multimer and subsequently processed by hydrolases, we previously expressed HA-ubiquitin as an octameric precursor (32). However, to facilitate the mutagenesis of ubiquitin, we made a construct that expresses HA-ubiquitin as a monomer. Coexpression with HIV-1 Gag constructs showed that this modified vector in fact yielded higher levels of Gag/HA-ubiquitin conjugates than the original version (data not shown). The single-amino-acid substitutions were initially made in the context of HA-tagged ubiquitin to verify that the mutations did not affect ubiquitin expression levels. However, to assess effects on VLP production, the mutations were subsequently introduced into nontagged ubiquitin to avoid possible interference by the epitope tag.
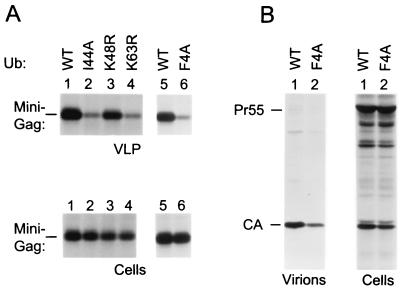
Since we anticipated that the relative abundance of endogenous ubiquitin would complicate efforts to observe phenotypes associated with mutant ubiquitin expression, we attempted to maximize the sensitivity of the assay by using Δ-ZWT-Eb, which exhibits unusually pronounced L domain-induced ubiquitination (32). Also, perhaps because Gag-Gag contacts are minimal, Δ-ZWT-Eb is exquisitely dependent on its L domain for VLP production (see Fig. 1). The Δ-ZWT-Eb construct was cotransfected with expression vectors for WT or mutant ubiquitin, and the effects on VLP production were compared relative to those observed on the expression levels of Gag. As shown in Fig. 4 (left panel), K48R ubiquitin expression reduced the VLP- and cell-associated Gag levels to a similar extent, indicating that the effect on VLP production was merely a consequence of reduced Gag expression or stability. K63R ubiquitin reproducibly impaired VLP production about twofold more than K48R ubiquitin, whereas the cell-associated Gag levels were slightly less affected than in the presence of K48R ubiquitin. Thus, K63R ubiquitin appeared to have a specific, although quite modest, effect on VLP production. A more pronounced specific reduction in VLP production was observed with F4A or I44A ubiquitin (Fig. 4, right panel). Normalized for the levels of cell-associated Gag, the expression of I44A ubiquitin reproducibly led to about a fivefold decline in VLP production by Δ-ZWT-Eb as determined by PhosphorImager analysis.
FIG. 4.
Effects of ubiquitin mutants on VLP production by a minimal HIV-1 Gag construct carrying the Ebola virus L domain. HeLa cells were cotransfected with Δ-ZWT-Eb, together with an expression vector for WT or mutant ubiquitin, as indicated. To compare VLP production, the cells were labeled with [35S]methionine, and particulate material released into the culture medium was pelleted through sucrose and analyzed by SDS-PAGE. Cell-associated Gag protein was detected by immunoprecipitation with anti-CA antiserum.
A fivefold reduction in VLP production was also seen when I44A ubiquitin was coexpressed with the Δ-ZWT-p6 minimal Gag construct (Fig. 5A, lanes 1 and 2), which harbors HIV-1 p6gag instead of the Ebola virus L domain. In the presence of the HIV-1 L domain, K63R ubiquitin was as effective as I44A ubiquitin in inhibiting VLP formation (Fig. 5A, lanes 2 and 4), and F4A ubiquitin had an even stronger effect (lanes 5 and 6), reducing VLP production up to 10-fold. In contrast, K48R ubiquitin again had little if any specific effect on VLP production when the intracellular Gag levels were taken into account (lane 3). As shown in Fig. 5B, F4A ubiquitin reduced particle production by a full-length molecular clone of HIV-1 by about threefold relative to the cell-associated Gag levels (Fig. 5B). Thus, while its effect on minimal Gag constructs was somewhat more pronounced, the mutant ubiquitin also interfered with the function of authentic HIV-1 Gag.
FIG. 5.
Effects of ubiquitin mutants on particle production in the presence of the HIV-1 L domain p6gag. HeLa cells were transfected with the minimal HIV-1 Gag construct Δ-ZWT-p6 (A) or with the full-length HXBH10 molecular clone of HIV-1 (B), along with expression vectors for WT or mutant ubiquitin as indicated. VLP (A) or virions (B) were pelleted through sucrose after metabolic labeling and directly analyzed by SDS-PAGE. Cell-associated Gag protein was detected by immunoprecipitation with anti-CA antiserum (A) or with serum from a patient infected with HIV-1 (B). Pr55, Gag precursor.
DISCUSSION
Short peptides from HIV-1 p6gag that include the P(T/S)APP motif can functionally replace the unrelated L domains of oncoretroviruses (21, 39), suggesting that only a few conserved Gag residues are involved in HIV-1 L domain function. Nevertheless, the results presented here indicate that the P(T/S)APP motif does not function autonomously. Our results imply that the P(T/S)APP L domain core motif, in contrast to the PPXY core motif present in oncoretroviral L domains, depends on other Gag regions in order to function.
We recently reported that a highly L domain-dependent minimal HIV-1 Gag molecule is efficiently complemented by full-length HIV-1 p6gag, whereas the first 14 p6gag residues, which include the P(T/S)APP motif, were insufficient to rescue VLP formation (1). In contrast, VLP production by the minimal Gag molecule was potently enhanced by peptides which harbored a PPXY motif, and in particular by a peptide from the Ebola virus L domain which contained overlapping P(T/S)APP and PPXY motifs (1, 32). In spite of the exceptional potency of the Ebola virus L domain in this context, we now find that its ability to rescue the HIV-1 minimal Gag construct is essentially abolished by a single-amino-acid substitution which disrupts the PPXY motif but leaves the P(T/S)APP motif intact. The single-amino-acid substitution also prevented the ubiquitination of the minimal Gag molecule, confirming the relationship between the recruitment of the cellular ubiquitination machinery and L domain function.
In marked contrast to its importance in the minimal Gag construct, the PPXY motif proved to be dispensable when the Ebola virus L domain was appended to the C terminus of an HIV-1 Gag precursor that lacked only p6gag and thus had the domain organization MA-CA-p2-NC-p1. In the latter context, the mutant Ebola virus peptide was about as efficient as the parental peptide in inducing Gag ubiquitination and in enhancing VLP formation. Thus, in its natural location C-terminal of NC-p1, a P(T/S)APP motif appeared sufficient to provide L domain function, and the presence of a PPXY motif conferred no significant advantage, which may explain why authentic HIV-1 Gag lacks such a motif.
The context-dependent activity of the P(T/S)APP motif suggested a need to cooperate with a Gag region that was present in MA-CA-p2-NC-p1 but absent from the minimal Gag construct. Indeed, our results indicate that the ability of the P(T/S)APP L domain core motif to induce Gag ubiquitination depended on NC-p1. When NC-p1 was replaced by a leucine zipper domain, prominent Gag ubiquitination could still be observed in the presence of combined P(T/S)APP and PPXY motifs, but not if the PPXY motif was disrupted and only the P(T/S)APP motif was left intact.
One explanation for these results is that Gag sequences that participate in the recruitment of the cellular ubiquitination machinery are not exclusively confined to p6gag but extend across domain boundaries into the adjacent NC-p1 region of the Gag precursor. Alternatively, the P(T/S)APP L domain core motif, in contrast to the PPXY motif, may recruit an ubiquitinating enzyme that exhibits a preference for particular lysine residues in NC-p1. The latter model would require that the ubiquitination of Gag itself is functionally relevant, as suggested by the finding that RSV budding from cells depleted for ubiquitin could be rescued by fusing ubiquitin directly to Gag (22). In the case of nonprimate lentiviruses, a role of NC in L domain function is strongly suggested by the organization of the Gag precursor. These lentiviruses lack a p6gag domain but, with the exception of EIAV, instead have a P(T/S)APP motif at the very C terminus of the NC domain. In HIV-1, the conserved L domain core at the N terminus of p6gag may be assisted both by NC-p1 and by the remainder of p6gag, because NC-p1 is clearly dispensable for L domain function if the full-length p6gag domain is present (1).
The mechanistic basis for the involvement of ubiquitin in L domain-mediated virus budding remains unknown. L domains appear to induce the addition of only a few ubiquitin moieties to Gag (32), and these are unlikely to constitute strong degradation signals, since a Lys48-linked chain of at least four ubiquitin subunits is required for efficient recognition by the proteasome (34). The view that the proteasome is not directly involved in L domain function is supported by our finding that the coexpression of K48R ubiquitin, which was expected to cap Lys48-linked chains, had no specific effect on VLP formation. In contrast to K48R ubiquitin, the K63R mutant led to a reduction in VLP formation that was more pronounced than a reduction in Gag expression levels that was observed with all of the mutant ubiquitins tested. Interestingly, the expression of K63R ubiquitin also altered the pattern of cell-associated Gag-ubiquitin conjugates. Such conjugates could be relatively easily detected in the presence of the Ebola virus L domain, which generally induced a more pronounced Gag ubiquitination than did HIV-1 p6gag. Specifically, K63R ubiquitin caused a selective reduction in the intensity of a band that migrated at the expected position of diubiquitinated Gag. The simplest explanation for this observation is that the affected Gag species harbored a Lys63-linked diubiquitin chain.
Monoubiquitination and Lys63-linked diubiquitin chains have been shown to play a role in endocytosis (12), a process that involves a budding event from the plasma membrane, as does retrovirus release. A relationship between the endocytosis functon of ubiquitin and its involvement in virus budding is also suggested by our finding that the coexpression of I44A or F4A ubiquitin had a rather pronounced dominant-negative effect on VLP production. Ile44, together with Leu8 and Val70, forms a hydrophobic patch on the surface of ubiquitin that is crucial for monoubiquitin-mediated endocytosis (28). Ile44 appears to be the primary residue required, because its replacement in a recent comprehensive alanine scan had a more pronounced effect on endocytosis than the substitution of any other ubiquitin surface residue (29). While the latter studies were performed in a yeast model system, the importance of Ile44 in ubiquitin-mediated endocytosis has also been demonstrated in human cells (18). Besides its role in endocytosis, Ile44 is required for the targeting of Lys48-linked multiubiquitin chains to the proteasome (2). However, Phe4 is located in a second hydrophobic patch on the ubiquitin surface that is crucial for endocytosis but not for proteasomal degradation (29).
Taken together, our results are compatible with a model in which L domain-induced ubiquitination of Gag or of a cellular factor at the site of virus assembly leads to the recruitment of a component of the endocytic machinery to promote the membrane invagination or fission events required for virus budding. An involvement of the endocytic machinery in retrovirus budding has previously been suggested, based on the observation that the L domain of EIAV recruits the clathrin-associated AP2 adaptor complex to the viral assembly site (25). The L domain of EIAV is unique in that it lacks both a P(T/S)APP and a PPXY motif, and the cellular factor(s) recruited by other L domains are thus likely to be different. Their identification may not only be important to understand the mechanism of enveloped virus release but may also have wider implications for cell biology.
Acknowledgments
We thank Candace Summerford for help with plasmid construction and Dirk Bohmann and Richard Bram for generously providing reagents.
This work was supported by NIH grants AI29873 and AI50466 and by Center for AIDS Research grant AI28691. We also acknowledge the support of the G. Harold and Leila Y. Mathers Charitable Foundation.
REFERENCES
- 1.Accola, M. A., B. Strack, and H. G. Göttlinger. 2000. Efficient particle production by minimal Gag constructs which retain the carboxy-terminal domain of human immunodeficiency virus type 1 capsid-p2 and a late assembly domain. J. Virol. 74:5395-5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beal, R., Q. Deveraux, G. Xia, M. Rechsteiner, and C. Pickart. 1996. Surface hydrophobic residues of multiubiquitin chains essential for proteolytic targeting. Proc. Natl. Acad. Sci. USA 93:861-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chau, V., J. W. Tobias, A. Bachmair, D. Marriott, D. J. Ecker, D. K. Gonda, and A. Varshavsky. 1989. A multiubiquitin chain is confined to specific lysine in a targeted short-lived protein. Science 243:1576-1583. [DOI] [PubMed] [Google Scholar]
- 4.Craven, R. C., R. N. Harty, J. Paragas, P. Palese, and J. W. Wills. 1999. Late domain function identified in the vesicular stomatitis virus M protein by use of rhabdovirus-retrovirus chimeras. J. Virol. 73:3359-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng, L., C. Wang, E. Spencer, L. Yang, A. Braun, J. You, C. Slaughter, C. Pickart, and Z. J. Chen. 2000. Activation of the IκB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103:351-361. [DOI] [PubMed] [Google Scholar]
- 6.Finley, D., S. Sadis, B. P. Monia, P. Boucher, D. J. Ecker, S. T. Crooke, and V. Chau. 1994. Inhibition of proteolysis and cell cycle progression in a multiubiquitination-deficient yeast mutant. Mol. Cell. Biol. 14:5501-5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galan, J., and R. Haguenauer-Tsapis. 1997. Ubiquitin lys63 is involved in ubiquitination of a yeast plasma membrane protein. EMBO J. 16:5847-5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Göttlinger, H. G., T. Dorfman, E. A. Cohen, and W. A. Haseltine. 1993. Vpu protein of human immunodeficiency virus type 1 enhances the release of capsids produced by gag gene constructs of widely divergent retroviruses. Proc. Natl. Acad. Sci. USA 90:7381-7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Göttlinger, H. G., T. Dorfman, J. G. Sodroski, and W. A. Haseltine. 1991. Effect of mutations affecting the p6 gag protein on human immunodeficiency virus particle release. Proc. Natl. Acad. Sci. USA 88:3195-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harty, R. N., M. E. Brown, G. Wang, J. Huibregtse, and F. P. Hayes. 2000. A PPxY motif within the VP40 protein of Ebola virus interacts physically and functionally with a ubiquitin ligase: implications for filovirus budding. Proc. Natl. Acad. Sci. USA 97:13871-13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harty, R. N., J. Paragas, M. Sudol, and P. Palese. 1999. A proline-rich motif within the matrix protein of vesicular stomatitis virus and rabies virus interacts with WW domains of cellular proteins: implications for viral budding. J. Virol. 73:2921-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hicke, L. 2001. Protein regulation by monoubiquitin. Nat. Rev. Mol. Cell. Biol. 2:195-201. [DOI] [PubMed] [Google Scholar]
- 13.Hochstrasser, M. 1996. Ubiquitin-dependent protein degradation. Annu. Rev. Genet. 30:405-439. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann, R. M., and C. M. Pickart. 1999. Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell 96:645-653. [DOI] [PubMed] [Google Scholar]
- 15.Huang, M., J. M. Orenstein, M. A. Martin, and E. O. Freed. 1995. p6Gag is required for particle production from full-length human immunodeficiency virus type 1 molecular clones expressing protease. J. Virol. 69:6810-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jayakar, H. R., K. G. Murti, and M. A. Whitt. 2000. Mutations in the PPPY motif of vesicular stomatitis virus matrix protein reduce virus budding by inhibiting a late step in virion release. J. Virol. 74:9818-9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mimnaugh, E. G., P. Bonvini, and L. Neckers. 1999. The measurement of ubiquitin and ubiquitinated proteins. Electrophoresis 20:418-428. [DOI] [PubMed] [Google Scholar]
- 18.Nakatsu, F., M. Sakuma, Y. Matsuo, H. Arase, S. Yamasaki, N. Nakamura, T. Saito, and H. Ohno. 2000. A di-leucine signal in the ubiquitin moiety: possible involvement in ubiquitination-mediated endocytosis. J. Biol. Chem. 275:26213-26219. [DOI] [PubMed] [Google Scholar]
- 19.Ott, D. E., L. V. Coren, E. N. Chertova, T. D. Gagliardi, and U. Schubert. 2000. Ubiquitination of HIV-1 and MuLV Gag. Virology 278:111-121. [DOI] [PubMed] [Google Scholar]
- 20.Ott, D. E., L. V. Coren, T. D. Copeland, B. P. Kane, D. G. Johnson, R. C. Sowder II, Y. Yoshinaka, S. Oroszlan, L. O. Arthur, and L. E. Henderson. 1998. Ubiquitin is covalently attached to the p6Gag proteins of human immunodeficiency virus type 1 and simian immunodeficiency virus and to the p12Gag protein of Moloney murine leukemia virus. J. Virol. 72:2962-2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parent, L. J., R. P. Bennett, R. C. Craven, T. D. Nelle, N. K. Krishna, J. B. Bowzard, C. B. Wilson, B. A. Puffer, R. C. Montelaro, and J. W. Wills. 1995. Positionally independent and exchangeable late budding functions of the Rous sarcoma virus and human immunodeficiency virus Gag proteins. J. Virol. 69:5455-5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patnaik, A., V. Chau, and J. W. Wills. 2000. Ubiquitin is part of the retrovirus budding machinery. Proc. Natl. Acad. Sci. USA 97:13069-13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pickart, C. M. 2000. Ubiquitin in chains. Trends Biochem. Sci. 25:544-548. [DOI] [PubMed] [Google Scholar]
- 24.Puffer, B. A., L. J. Parent, J. W. Wills, and R. C. Montelaro. 1997. Equine infectious anemia virus utilizes a YXXL motif within the late assembly domain of the Gag p9 protein. J. Virol. 71:6541-6546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Puffer, B. A., S. C. Watkins, and R. C. Montelaro. 1998. Equine infectious anemia virus Gag polyprotein late domain specifically recruits cellular AP-2 adapter protein complexes during virion assembly. J. Virol. 72:10218-10221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Putterman, D., R. B. Pepinsky, and V. M. Vogt. 1990. Ubiquitin in avian leukosis virus particles. Virology 176:633-637. [DOI] [PubMed] [Google Scholar]
- 27.Schubert, U., D. E. Ott, E. N. Chertova, R. Welker, U. Tessmer, M. F. Princiotta, J. R. Bennink, H. G. Krausslich, and J. W. Yewdell. 2000. Proteasome inhibition interferes with gag polyprotein processing, release, and maturation of HIV-1 and HIV-2. Proc. Natl. Acad. Sci. USA 97:13057-13062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shih, S. C., K. E. Sloper-Mould, and L. Hicke. 2000. Monoubiquitin carries a novel internalization signal that is appended to activated receptors. EMBO J. 19:187-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sloper-Mould, K. E., J. C. Jemc, C. M. Pickart, and L. Hicke. 2001. Distinct functional surface regions on ubiquitin. J. Biol. Chem. 276:30483-30489. [DOI] [PubMed] [Google Scholar]
- 30.Spence, J., R. R. Gali, G. Dittmar, F. Sherman, M. Karin, and D. Finley. 2000. Cell cycle-regulated modification of the ribosome by a variant multiubiquitin chain. Cell 102:67-76. [DOI] [PubMed] [Google Scholar]
- 31.Springael, J. Y., J. M. Galan, R. Haguenauer-Tsapis, and B. Andre. 1999. NH4+-induced downregulation of the Saccharomyces cerevisiae Gap1p permease involves its ubiquitination with lysine-63-linked chains. J. Cell Sci. 112:1375-1383. [DOI] [PubMed] [Google Scholar]
- 32.Strack, B., A. Calistri, M. A. Accola, G. Palu, and H. G. Göttlinger. 2000. A role for ubiquitin ligase recruitment in retrovirus release. Proc. Natl. Acad. Sci. USA 97:13063-13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takebe, Y., M. Seiki, J.-I. Fujisawa, P. Hoy, K. Yokota, K.-I. Arai, M. Yoshida, and N. Arai. 1988. SRα promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol. Cell. Biol. 8:466-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thrower, J. S., L. Hoffman, M. Rechsteiner, and C. M. Pickart. 2000. Recognition of the polyubiquitin proteolytic signal. EMBO J. 19:94-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.VerPlank, L., F. Bouamr, T. J. LaGrassa, B. Agresta, A. Kikonyogo, J. Leis, and C. A. Carter. 2001. Tsg101, a homologue of ubiquitin-conjugating (E2) enzymes, binds the L domain in HIV type 1 Pr55Gag. Proc. Natl. Acad. Sci. USA 98:7724-7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wills, J. W., C. E. Cameron, C. B. Wilson, Y. Xiang, R. P. Bennett, and J. Leis. 1994. An assembly domain of the Rous sarcoma virus Gag protein required late in budding. J. Virol. 68:6605-6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiang, Y., C. E. Cameron, J. W. Wills, and J. Leis. 1996. Fine mapping and characterization of the Rous sarcoma virus Pr76gag late assembly domain. J. Virol. 70:5695-5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasuda, J., and E. Hunter. 1998. A proline-rich motif (PPPY) in the Gag polyprotein of Mason-Pfizer monkey virus plays a maturation-independent role in virion release. J. Virol. 72:4095-4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan, B., S. Campbell, E. Bacharach, A. Rein, and S. P. Goff. 2000. Infectivity of Moloney murine leukemia virus defective in late assembly events is restored by late assembly domains of other retroviruses. J. Virol. 74:7250-7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yuan, B., X. Li, and S. P. Goff. 1999. Mutations altering the moloney murine leukemia virus p12 Gag protein affect virion production and early events of the virus life cycle. EMBO J. 18:4700-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang, Y., H. Qian, Z. Love, and E. Barklis. 1998. Analysis of the assembly function of the human immunodeficiency virus type 1 Gag protein nucleocapsid domain. J. Virol. 72:1782-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]