Abstract
The NF-κB/Rel family has been implicated in control of transcription of the Bcl-xL gene, a target which mediates cell survival signals. The cytomegalovirus (CMV) immediate-early protein 1 (IE1) was previously shown to induce NF-κB activity. Here, we report that in both vascular smooth muscle cells (SMCs) and NIH 3T3 cells, surprisingly, IE1 failed to induce Bcl-xL promoter activity, although it induced activity of E8-CAT, a reporter construct driven by two copies of the NF-κB element upstream of the c-myc promoter (upstream regulatory element [URE]). Thus, the subunit nature of the NF-κB/Rel factors induced by IE1 was examined using immunofluorescence and immunoblotting. IE1 was found to selectively induce nuclear RelB and p50 in SMCs and NIH 3T3 cells. An increase in RelB protein mediated by IE1 could, in part, be related to an increase in steady-state relB mRNA levels. Consistent with this subunit identification, IE1 was unable to induce E8-CAT activity in relB−/− murine embryonic fibroblast cells. In cotransfection analysis of SMCs and NIH 3T3 cells, RelB and p50 proteins failed to induce Bcl-xL promoter activity while inducing E8-CAT. Furthermore, the NF-κB element of the Bcl-xL promoter only weakly bound RelB-p50 complexes compared to the URE NF-κB element. Overall, these findings demonstrate in SMCs and NIH 3T3 cells that the CMV IE1 protein selectively induces RelB and p50, which fail to activate the Bcl-xL promoter, indicating a strong specificity of binding and activity for the RelB member of the NF-κB family. Furthermore, our results implicate RelB in CMV infection of cells such as vascular SMCs.
NF-κB/Rel is a family of dimeric transcription factors whose DNA binding domains have considerable homology with an ∼300-amino-acid region of the v-Rel oncoprotein and were thus called the Rel homology region (24, 31, 48, 49). Members of the mammalian Rel family include p50 (NFκB1), RelA (p65), RelB, c-Rel, and p52 (10, 26, 27). Classical NF-κB is a heterodimer composed of a 50- (p50) and a 65-kDa (RelA) subunit. The NF-κB binding element consensus sequence is 5′-GGGRNNYYCC-3′ (where R is a purine and Y is a pyrimidine) (26, 27). NF-κB/Rel transcription factors play important roles in the transcriptional regulation of various cellular genes and viral genes (26). NF-κB-regulated cellular genes are involved in control of cell survival, proliferation, adhesion, and immune and inflammatory responses (5, 26, 45, 53). Importantly for survival, the promoter of the antiapoptosis Bcl-xL gene has been shown to contain two NF-κB elements (14, 25).
Transcriptionally active NF-κB is found constitutively in the nuclei of mature B lymphocytes. In most non-B cells, NF-κB complexes are inactive and sequestered in the cytoplasm by direct interaction with specific inhibitor proteins, termed IκBs, which need to be phosphorylated and degraded in order for NF-κB to translocate to the nucleus (11, 59). However, RelB is somewhat unusual in that IκB-α binds only very poorly to this subunit (38), allowing free nuclear movement of RelB-containing complexes, e.g., RelB-p50 (17). Activation of NF-κB can be induced by numerous agents, including tumor necrosis factor alpha, interleukin-1, and oxidative stress (4, 10, 26, 27). In addition, NF-κB can be activated by several viruses, including cytomegalovirus (CMV), human immunodeficiency virus (HIV) type 1, human T-cell lymphotropic virus type 1, hepatitis B virus, and hepatitis C virus (4, 26, 50).
Infection by CMV has been shown to stimulate migration and proliferation of smooth muscle cells (SMCs) in culture (37, 56). CMV has been implicated in the pathogenesis or exacerbation of occlusive vascular diseases, such as atherosclerosis or postangioplasty restenosis (21, 41). The immediate-early protein 1 (IE1), a 491-amino-acid nuclear phosphoprotein, is one of two major products of the ie gene and is a predominant protein expressed during the immediate-early phase of CMV infection (22, 41). Although IE1 is believed to regulate viral gene expression by positive-feedback regulation of the ie promoter (29), other specific functions of this protein have been determined. In particular, IE1 has been found to induce NF-κB activity in fibroblasts and Jurkat T cells (50). Since activation of NF-κB has been shown to prevent virus-induced apoptosis, we tested the ability of IE1 to activate the Bcl-xL promoter. Surprisingly, in both vascular SMCs and NIH 3T3 cells, IE1 failed to activate the Bcl-xL promoter although it activated an NF-κB-element-driven construct. Using immunohistochemistry, Western blotting, electrophoretic mobility shift assays (EMSA), and transfection analysis into relB knockout mouse embryo fibroblasts (MEFs), we demonstrate that IE1 selectively induces RelB-p50 complexes. Furthermore, we show that RelB-p50 complexes are unable to bind to the Bcl-xL NF-κB element or to activate the Bcl-xL promoter. Thus, these results demonstrate selective activation of RelB and p50 by IE1 in SMCs and NIH 3T3 cells and delineate a strong specificity of binding and activity for the RelB member of the NF-κB family. The potential roles of the CMV-induced RelB activity in vascular disease are also discussed.
MATERIALS AND METHODS
Cell culture conditions.
Bovine aortic SMCs were obtained by primary tissue explant from aortae of female calves as previously described (8). Bovine aortic SMCs were maintained in normal growth medium containing Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 1 mM nonessential amino acids, 1 mM sodium pyruvate, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. The cell culture medium was changed every 2 days, and cultures were passaged every 3 to 4 days with trypsin-EDTA. All bovine SMCs used in experiments were within passage 6 from primary explant. NIH 3T3 fibroblasts were maintained, as described above, in growth medium containing 10% fetal bovine serum, 1 mM nonessential amino acids, 5 mM glutamine, 5 mM glucose, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. The cells were passaged prior to reaching confluence. MEFs from relB−/− mice, kindly provided by Falk Weih (Forschungszentrum Karlsruhe, Eggenstein-Leopoldshafen, Germany), were grown as described above for the NIH 3T3 cells.
Plasmids.
The pON2205 construct contains a full-length CMV IE1 protein cDNA, and its expression is driven by the simian virus 40 (SV40) early promoter (kindly provided by Edward Mocarski, Stanford University, Stanford, Calif.) (15). The −298/+22 Bcl-xL-CAT and −298/+22 mkB Bcl-xL-CAT vectors containing the Bcl-xL promoter (−298 to +22) with either wild-type or mutant NF-κB elements driving the chloramphenicol acetyltransferase (CAT) regulatory (14), were kindly provided by C. Gelinas (Robert Wood Johnson Medical School, Piscataway, N.J.). E8-CAT is a two-copy NF-κB upstream reporter element (URE) from upstream of the c-myc promoter-thymidine kinase (TK) promoter-CAT reporter vector constructed as reported previously (20). The RelB expression vector, containing the murine RelB sequence cloned into the pMexNeo backbone (49), was a kind gift of Rodrigo Bravo (Bristol Myers Squibb, Princeton, N.J.). A construct expressing full-length murine RelA was in the pEVRF vector. The SV40 β-galactosidase (β-Gal) reporter vector was as reported previously (2).
Transfection analysis.
Cultures of SMCs or NIH 3T3 cells at 50 to 70% confluence were transiently transfected using FUGENE transfection reagent (Boehringer Mannheim), according to the manufacturer's instructions. After 48 h, the cells were harvested, and cellular extracts were prepared for reporter assays as we have described previously (34).
Indirect immunofluorescence.
Cultures of NIH 3T3 cells or SMCs at 50 to 70% confluence were transfected with 15 μg of either pON2205 IE1 expression plasmid or pCMV empty vector DNA using FUGENE. After 24 h, the cultures were split into eight-well chamber slides, grown overnight, and then fixed with 4% paraformaldehyde for 15 min and permeabilized with 0.5% Triton X-100 for 10 min. For immunofluorescent triple staining, mouse monoclonal anti-IE1 72 antibody (Vancouver Biotech Ltd.) and rabbit antibodies against the NF-κB subunits were used separately and visualized with either anti-mouse immunoglobulin-fluorescein isothiocyanate (FITC) or goat anti-rabbit immunoglobulin-Texas Red (Jackson Immunologicals). The antibodies against the NF-κB p50 (sc-114), RelA (sc-109), c-Rel (sc-372), and RelB (sc-226) subunits were purchased from Santa Cruz Biotechnology. For visualization of nuclei, cells were counterstained with 1 μg of Total 3 DNA stain (T-3604; Molecular Probes)/ml in phosphate-buffered saline for 5 min.
Confocal laser scanning microscopy.
Slides were observed under a laser confocal microscope (LSM510; Carl Zeiss). An argon-krypton laser produced excitation bands at 488 nm for FITC and 568 nm for Texas Red and monochromatic light for differential interference contrast images. Fluorescence images were collected with emission filters for 510 to 550 nm for FITC and 585 to 610 nm for Texas Red. Simultaneous images (800 by 600 pixels; 12 bits each) of the FITC label and either differential interference contrast images or Texas Red label were acquired and stored. Digital images were transferred to a computer equipped with Photoshop (Adobe Systems).
Immunoblot analysis.
NIH 3T3 cells and bovine SMCs were collected 48 h after transfection and then incubated for 5 min on ice with cytoplasmic isolation buffer (10 mM HEPES [pH 7.5], 60 mM KCl, 1 mM EDTA, 0.1% Nonidet P-40 [NP-40], 1 mM dithiothreitol, 1 mM phenylmethylsulfonylfluoride [Sigma]). The lysates were centrifuged, and cytoplasmic samples were collected. Nuclei were resuspended in extraction buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% sodium lauryl sarcosine, 1% NP-40, 0.1% sodium dodecyl sulfate, 1 mM EDTA) with homogenization for 30 min. Following centrifugation to remove cell debris, the supernatants were collected and analyzed for protein content using the Bio-Rad protein quantitation kit for detergent lysis, according to the manufacturer's directions. For immunoblots, samples (50 μg of protein) were separated by electrophoresis through a 10% polyacrylamide-sodium dodecyl sulfate gel and transferred to a polyvinylidene difluoride membrane. Rainbow markers (Amersham, Chicago, Ill.) were used to determine the molecular weight. Following incubation for 1 h in goat anti-rabbit horse radish peroxidase-conjugated secondary antibody (1:2,000 in 5% milk), the proteins were visualized by chemiluminescence, as described previously (3). Quantitation by scanning densitometry was performed using a Molecular Dynamics 300A computing densitometer.
EMSA.
Nuclear extracts were prepared from cells essentially as described previously (54). Briefly, cells were resuspended in 1 ml of cold hypotonic RSB buffer (10 mM NaCl, 3 mM MgCl2, 10 mM Tris [pH 7.4]) containing 0.5% NP-40 detergent plus protease inhibitors as described above. Following a 15-min incubation on ice, the cells were Dounce homogenized until cell lysis occurred. Nuclei were resuspended in two packed nuclear volumes of extraction buffer C (420 mM KCl, 20 mM HEPES [pH 7.9], 1.5 mM MgCl2, 0.2 mM EDTA, 20% glycerol) plus protease inhibitors as described above and incubated on ice for 30 min. The protein concentration was determined using the Bio-Rad protein assay, as described above. The sequences of the oligonucleotides containing NF-κB elements upstream of the c-myc (URE) (20) and Bcl-xL (14) promoters are as follows: c-myc, 5′-GATCCAAGTCCGGGTTTTCCCCAACC-3′; Bcl-xL, 5′-AGTGGGGGCGGGGGGGACTGCCCCCTCTCCTT-3′ (the underlined regions indicate the core binding element). The Octomer-1 (Oct-1) oligonucleotide has the following sequence: 5′-TGTCGAATGCAAATCACTAGAA-3′. For the binding reaction, 32P-labeled oligonucleotide (20,000 to 25,000 cpm) was incubated with 5 μg of nuclear extract, 5 μl of sample buffer (10 mM HEPES, 4 mM dithiothreitol, 0.5% Triton X-100, and 2.5% glycerol), and 2.5 μg of poly(dI-dC) as a nonspecific competitor, and the salt concentration was adjusted to 100 mM using buffer C. The reaction was carried out at room temperature for 30 min. DNA-protein complexes were separated as previously described (54). Where indicated, excess unlabeled oligonucleotides were added in the binding reaction.
RNA isolation and analysis.
Total cellular RNA was isolated by the guanidinium isothiocyanate method from control and transfected cultures in P-150 dishes, and samples (30 μg) were subjected to Northern blot analysis, as described previously (51). The 2.1-kb EcoRI insert of the RelB expression vector was used as a probe. Quantitation by scanning densitometry was performed as described above.
RESULTS
IE1 fails to induce the Bcl-xL promoter.
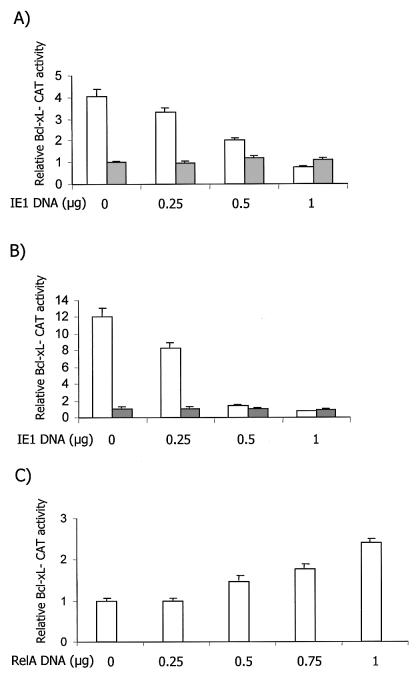
To test the ability of IE1 to induce the Bcl-xL promoter, which is driven by two NF-κB elements, transient-transfection analysis was performed in SMCs. Cultures were cotransfected with either the −298/+22 Bcl-xL-CAT or −298/+22 mkB Bcl-xL-CAT vector, containing the Bcl-xL promoter (−298 to +22) with either wild-type or mutant NF-κB elements, and increasing doses of the pON2205 construct expressing the full-length CMV IE1 protein. Surprisingly, IE1 failed to induce the wild-type Bcl-xL promoter. In fact, a dose-dependent drop in the activity of the wild-type Bcl-xL promoter was seen with IE1 expression, bringing it down to the basal level of the mutant promoter (Fig. 1A). To verify that this was not a cell-type-specific event, a similar cotransfection analysis was performed with NIH 3T3 cells. Again, a dose-dependent reduction in Bcl-xL promoter activity was noted (Fig. 1B). In contrast, cotransfection of p50 with increasing amounts of a RelA expression vector induced the Bcl-xL promoter activity in a dose-dependent fashion in NIH 3T3 cells (Fig. 1C), confirming the observation of Chen and coworkers (14). To verify that IE1 expression can indeed transactivate via NF-κB elements, we coexpressed IE1 with the E8-CAT reporter vector. In this construct, the TK promoter is driven by two copies of the NF-κB regulatory element upstream of the c-myc promoter (URE). IE1 effectively induced NF-κB element-driven promoter activity to a maximum of 10- to 12-fold in SMCs and NIH 3T3 cells, confirming the ability of IE1 to induce NF-κB activity (Fig. 2). Taken together, these results indicate that IE1 fails to induce the Bcl-xL promoter, although, it has the ability to induce an NF-κB element-driven construct. Thus, we next sought to determine the nature of the NF-κB factors induced by IE1.
FIG. 1.
IE1 fails to induce Bcl-xL promoter activity in SMCs and NIH 3T3 cells. (A) Cultures of SMCs at 50 to 70% confluence were transiently transfected, in triplicate, with 0, 0.25, 0.5, or 1 μg of pON2205 vector expressing IE1 protein, 0.5 μg of either the wild-type −298/+22 Bcl-xL-CAT (open bars) or −298/+22 mkB Bcl-xL-CAT (solid bars) Bcl-xL reporter vector, 0.5 μg of SV40 β-Gal expression vector, and enough pBluescript to make a total of 2.5 μg of DNA. After 48 h, the cells were harvested and CAT activity and β-Gal activity were measured. The data presented are the mean of four separate experiments. Baseline untreated mutant Bcl-xL promoter CAT activity was set at 1. The results are expressed as mean plus standard error of the mean. (B) Cultures of NIH 3T3 cells were transfected and processed as described for panel A. (C) Cultures of NIH 3T3 cells were transfected, in triplicate, with 0, 0.25, 0.5, 0.75, or 1 μg of RelA expression vector, 0.5 μg of p50 expression vector, 0.5 μg of wild-type −298/+22 Bcl-xL-CAT, 0.5 μg of β-Gal expression vector, and enough pBluescript to make a total of 2.5 μg of DNA and processed as described for panel A. Baseline untreated wild-type Bcl-xL promoter CAT activity was set at 1.
FIG. 2.
IE1 induces E8-CAT c-myc URE NF-κB element-driven TK promoter activity in SMC and NIH 3T3 cells. Cultures of either SMCs (A) or NIH 3T3 cells (B) were transiently transfected, in triplicate, with the indicated amounts of pON2205 vector expressing IE1 protein, 0.5 μg of E8-CAT vector, 0.5 μg of SV40 β-Gal expression vector, and enough pBluescript to make a total of 2.5 μg of DNA. After 48 h, the cells were harvested, and CAT activity and β-Gal activity were measured. The data presented are the mean of four separate experiments. Baseline untreated E8-CAT activity was set at 1. The results are expressed as mean plus standard error of the mean.
IE1 induces nuclear colocalization of RelB and p50.
To determine the subunit nature of the NF-κB activity induced by IE1, immunohistochemistry was performed. NIH 3T3 cells were selected initially due to their higher level of transfection efficiency compared to the SMCs. Cultures of NIH 3T3 cells were transfected with pON2205 IE1 expression vector or pCMV empty vector DNA and analyzed with antibodies specific for expression of IE1, p50, RelA, RelB, and c-Rel proteins, as described in Materials and Methods. Using a monoclonal IE1 FITC-labeled antibody, intense diffuse staining for IE1 protein was noted over the nucleus, with a much lower level in the cytoplasm, as expected (Fig. 3). When NF-κB subunit expression was examined using Texas Red label, cells positive for IE1 showed nuclear localization of both p50 and RelB (Fig. 3), which can be better seen in the merged panels. When double label for both the anti-IE1 (green) and anti-RelB or anti-p50 (red) antibodies was examined simultaneously, yellow fluorescence was seen in the nuclei, signifying colocalization of these proteins (Fig. 3, right panels). In contrast, only a low level of yellow fluorescence was seen with RelA and c-Rel. These data indicate that IE1 induces nuclear localization of the p50 and RelB proteins, whereas RelA and c-Rel remain predominantly in the cytoplasm. Similar analysis for IE1 and the p50, RelB, and RelA subunits was performed in SMC cultures (Fig. 4). The IE1 protein again predominantly appeared to localize to the nucleus. When the NF-κB subunits were examined, p50 and RelB subunits were detected in the nuclei of IE1-expressing cells. In contrast, RelA displayed a predominant cytoplasmic localization. Thus, immunohistochemical analysis in both NIH 3T3 and SMC cultures indicates that expression of IE1 leads to selective activation of the p50 and RelB subunits of NF-κB.
FIG. 3.
Cytoimmunochemical analysis of the effects of IE1 on nuclear localization of RelB and p50 protein in NIH 3T3 cells. Cultures of NIH 3T3 cells were transfected with either pON2205 IE1 expression plasmid or pCMV empty vector DNA, and split into eight-well chamber slides for analysis, as described in Materials and Methods. For immunofluorescent triple staining, IE1 was visualized with FITC-mouse monoclonal anti-IE1 72 antibody (green), and Texas Red antibodies were used against either the NF-κB p50, RelA, RelB, or c-Rel subunit (red), as indicated. For visualization of nuclei, cells were counterstained with Total 3 DNA stain (blue). The slides were observed under an LSM510 laser confocal microscope using a 40× objective. Overlapping FITC and Texas Red signals are also shown (Merged); a yellow-orange color indicates colocalization.
FIG. 4.
Cytoimmunochemical analysis indicates that IE1 selectively induces nuclear colocalization of RelB and p50 protein in SMCs. Cultures of SMCs were transfected and analyzed as described in the legend to Fig. 3 for immunofluorescent triple staining for IE1 (green); either NF-κB p50, RelA, or RelB subunits (red); and nuclei (blue). Overlapping FITC and Texas Red signals are also shown (Merged); a yellow-orange color indicates colocalization.
IE1 selectively induces nuclear localization of NF-κB RelB and p50 subunits.
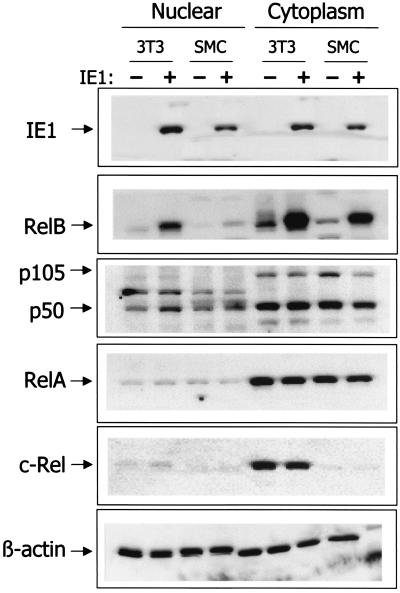
To verify the nuclear translocation of NF-κB subunits induced by IE1 expression, immunoblot analysis was performed. Forty-eight hours following transfection of NIH 3T3 or SMC cultures with pON2205 IE1 expression vector or pCMV empty vector DNA, cultures were harvested and nuclear and cytoplasmic extracts were prepared. Immunoblot analysis of these extracts confirmed expression of IE1 protein only in NIH 3T3 cells and SMCs transfected with pON2205 vector DNA, as expected (Fig. 5). Substantial IE1 expression was seen in the nuclei of both NIH 3T3 fibroblasts and SMCs; however, the level in the NIH 3T3 cells was somewhat higher, probably due to the higher transfection efficiency with the fibroblasts than with the SMCs. In cells transfected with the IE1 expression vector, a significant increase in the amount of RelB protein was detected in the cytoplasm. Upon expression of IE1, the level of RelB was found to increase by 6.1 ± 0.4- and 5.8 ± 0.4-fold in the NIH 3T3 cells and SMCs, respectively. Furthermore, consistent with the immunohistochemistry presented above, substantial amounts of RelB can also be detected in the nuclear preparations. Analysis of p50 and p105 subunit expression indicated that IE1 caused a slight reduction of the precursor p105 protein in the cytoplasm and a commensurate increase in the levels of nuclear p50. Of note, no p105 was detected in the nucleus, confirming the purity of the nuclear preparations. In contrast to the effects on RelB and p50, no substantial change in either the level of expression or localization of the c-Rel or RelA protein was detected (Fig. 5). Equal loading was confirmed by analysis of β-actin levels. Taken together, our results indicate that RelB and p50 are selectively induced by IE1 in both SMCs and NIH 3T3 cells.
FIG. 5.
CMV IE1 protein induces nuclear levels of RelB and p50 proteins. Cultures (P-100 dish) of either SMCs or NIH 3T3 cells were transfected with 10 μg of pON2205 vector expressing IE1 protein (+) or pCMV empty vector DNA (−). After 48 h, cytoplasmic and nuclear lysates were prepared and samples (30 μg) were subjected to immunoblot analysis for IE1, RelB, p50 and p105, RelA, c-Rel,and β-actin, as indicated.
IE1 does not induce NF-κB element-driven reporter activity in relB−/− MEFs.
Our findings led to the prediction that IE1 would not be able to induce the NF-κB element-driven E8-CAT reporter construct in cells that lack the relB gene. To test this hypothesis, relB−/− MEFs were cotransfected with 0.5 μg of E8-CAT/ml in the absence or presence of 0.25, 0.5, or 0.75 μg of pON2205 construct (Fig. 6A). Expression of IE1 failed to induce E8-CAT activity in the relB−/− MEFs in contrast to its effects in NIH 3T3 cells or SMCs, where a dose-dependent increase was observed (Fig. 2). In fact, IE1 caused a decrease in E8-CAT activity in MEFs lacking RelB expression, which is likely due to the ability of IE1 to induce the p50 NF-κB subunit, which binds very avidly but cannot transactivate via the URE and has been shown to repress its activity (35). To confirm that E8-CAT vector activity can be induced by NF-κB in the relB−/− MEFs, we performed a similar cotransfection experiment with expression vectors for RelA and p50 (Fig. 6B). Increasing RelA expression induced E8-CAT in the relB−/− MEFs, consistent with our previous observations (34, 35), demonstrating the ability of the E8-CAT vector to be activated by NF-κB. These findings indicate that the functional NF-κB activity induced by IE1 contains a RelB subunit. Furthermore, they suggest that IE1 does not induce other transactivating NF-κB subunits.
FIG. 6.
CMV IE1 fails to induce NF-κB activity in relB−/− MEFs. Cultures of relB−/− MEFs at 50 to 70% confluence were transiently transfected, in triplicate, with 0, 0.25, 0.5, or 0.75 μg of either pON2205 vector expressing IE1 protein (A) or RelA expression vector plus 0.5 μg of p50 expression vector (B) and 0.5 μg of E8-CAT reporter vector, 0.5 μg of SV40 β-Gal expression vector, and enough pBluescript to make a total of 2.5 μg of DNA. After 48 h, the cells were harvested, and CAT activity and β-Gal activity were measured. The data presented are the mean of two separate experiments. Baseline untreated E8-CAT activity was set at 1. The results are expressed as the mean plus standard error of the mean.
IE1 increases relB mRNA levels.
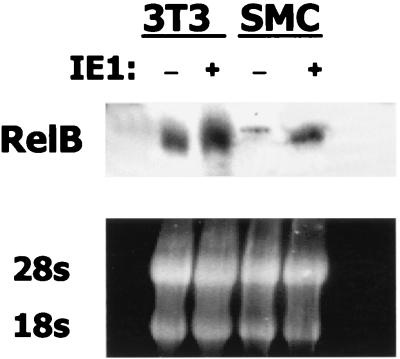
An increase in RelB protein was noted above (Fig. 5) in the immunoblot analysis of cytoplasmic extracts of IE1-transfected cells. To assess whether IE1 caused a commensurate increase in relB mRNA levels, Northern blot analysis was performed. RNA was isolated 48 h following transfection of NIH 3T3 and SMC cultures with pON2205 IE1 expression vector or from control, untransfected cultures. Northern blot analysis indicated relB mRNA levels were substantially increased upon expression of IE1 (Fig. 7). Equal loading was confirmed by ethidium bromide staining of the 18S and 28S rRNAs (Fig. 7). In three separate experiments, average fold increases of 2.82 ± 0.04 and 4.05 ± 0.08 were noted in the NIH 3T3 and SMC cultures, respectively. Thus, the elevated levels of RelB protein seen upon IE1 expression are due, in part, to increased expression of relB mRNA.
FIG. 7.
CMV IE1 protein induces relB mRNA levels. Cultures of SMCs at 50 to 70% confluence were transiently transfected with 10 μg of pON2205 vector expressing IE1. After 48 h, RNA was isolated from untransfected (−) or transfected (+) cultures, and samples (30 μg) were subjected to Northern blot analysis for relB mRNA (upper panel). Equal loading and RNA integrity were confirmed by ethidium bromide staining of the 28S and 18S rRNA (lower panel).
RelB-p50 fails to transactivate the Bcl-xL promoter.
The failure of IE1 to induce the Bcl-xL promoter suggested that RelB-p50 complexes are unable to transactivate this promoter, in contrast to the observed transactivation by RelA and c-Rel (14). To test this hypothesis, cultures of either SMCs (Fig. 8A) or NIH 3T3 cells (Fig. 8C) were transfected with the wild-type Bcl-xL promoter construct in the presence of 0.5 μg of p50 expression vector and increasing amounts of RelB expression vector ranging from 0.25 to 1.0 μg. A dose-dependent decrease in Bcl-xL reporter activity was observed in both SMCs (Fig. 8A) and NIH 3T3 cells (Fig. 8C). Thus, RelB-p50 complexes are unable to transactivate this promoter. As a control, the SMC or NIH 3T3 cultures were similarly cotransfected with E8-CAT and p50 and RelB expression vectors (Fig. 8B and D). Cotransfection of p50 and RelB was able to transactivate the E8-CAT NF-κB element-driven construct in both cell lines, as expected. Thus, RelB-p50 heterodimeric complexes appear unable to activate the Bcl-xL promoter, consistent with the inability of IE1 to induce the activity of this promoter.
FIG. 8.
RelB-p50 fails to induce the Bcl-xL promoter in either SMCs or NIH 3T3 cells. (A and B) Cultures of SMCs at 50 to 70% confluence were transiently transfected, in triplicate, with 0, 0.25, 0.5, 0.75, or 1 μg of RelB expression vector as indicated; 0.5 μg of either the wild-type −298/+22 Bcl-xL-CAT (A) or E8-CAT (B) reporter vector; 0.5 μg of p50 expression vector; 0.5 μg of SV40 β-Gal expression vector; and enough pBluescript to make a total of 2.5 μg of DNA. After 48 h, the cells were harvested, and CAT activity and β-Gal activity were measured. The data presented are the mean of two separate experiments. Baseline untreated Bcl-xL and E8-CAT activitywas set at 1, and the relative (Rel.) activities are presented. The results are expressed as mean plus standard error of the mean. (C and D) Cultures of NIH 3T3 cells were transfected as described above with either the wild-type −298/+22 Bcl-xL-CAT (C) or E8-CAT (D) reporter vector, and the relative activities are presented as in panels A and B. (E) Cultures of NIH 3T3 cells were transfected (+) as described above with 0.5 μg of wild-type −298/+22 Bcl-xL-CAT and 0.5 μg of both RelA and p50 expression vectors in the absence or presence of 0.5 μg of CMV IE1 or RelB expression vector plus 0.5 μg of SV40 β-Gal expression vector and enough pBluescript to make a total of 2.5 μg of DNA. After 48 h, the cells were harvested, and CAT activity and β-Gal activity were measured. The data presented are the mean of two separate experiments. Baseline untreated Bcl-xL CAT activity was set at 1, and the relative activities are presented.
Since expression of RelB reduced the activity of the Bcl-xL promoter, we next tested its ability to affect transactivation by RelA. NIH 3T3 cells were cotransfected with RelA and p50 expression vectors in the absence or presence of vectors expressing either RelB or IE1 (Fig. 8E). The ∼3-fold activation of the Bcl-xL promoter seen upon expression of RelA-p50 was completely inhibited upon coexpression of either RelB directly or CMV IE1, which induces synthesis of RelB and p50. These results indicate that RelB can inhibit transactivation of the Bcl-xL promoter by RelA-p50.
RelB-p50 complexes fail to bind to the Bcl-xL NF-κB element.
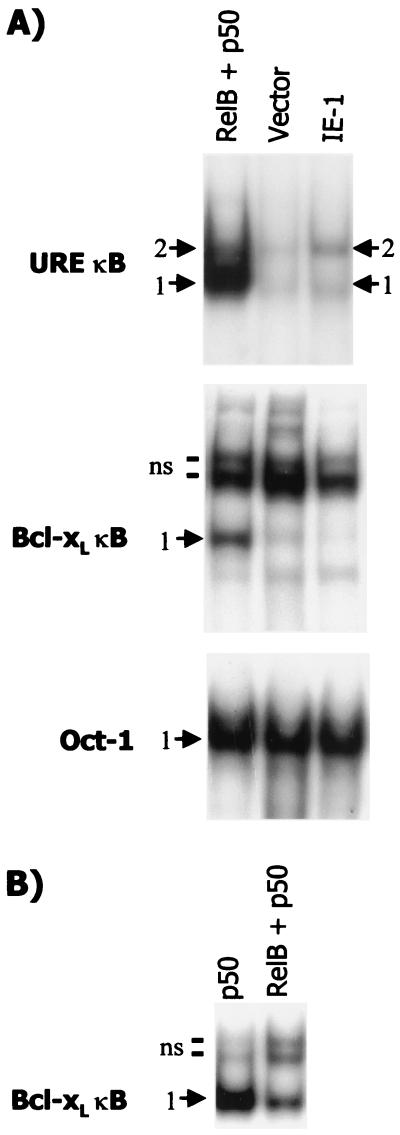
We next sought to determine whether RelB-p50 complexes can bind to the Bcl-xL NF-κB promoter element. Nuclear extracts were prepared from NIH 3T3 cells transfected with either 10 μg of RelB and 5 μg of p50 expression vectors or 10 μg of pON2205 IE1 vector DNA plus 5 μg of pBluescript empty vector DNA or 15 μg of pBluescript empty vector DNA as a control. EMSA was performed using as a probe the NF-κB element upstream of either the c-myc promoter (which is present in the E8-CAT vector) or the Bcl-xL promoter (Fig. 9A). Extracts from cells coexpressing RelB and p50 subunits show induction of two major complexes with the c-myc NF-κB URE as a probe. Antibody supershift analysis indicated the upper complex (band 2) is composed of RelB-p50 heterodimers, while the lower complex (band 1) contains p50 homodimers, as expected (data not shown). Expression of IE1 resulted in a similar pattern, although a lower level of binding was seen, particularly with respect to band 1. EMSA for Oct-1 binding indicated essentially equal loading in the two extracts (Fig. 9A). These findings are consistent with the increase in E8-CAT activity observed upon expression of either RelB-p50 or IE1 in NIH 3T3 cells and SMCs.
FIG. 9.
RelB-p50 complexes bind less effectively to the Bcl-xL than to the c-myc NF-κB element. (A) Cultures (P-150 dish) of NIH 3T3 cells were transfected with either 10 μg of vector expressing RelB plus 5 μg of vector expressing p50 protein (RelB + p50), 10 μg of pON2205 vector expressing IE1 protein plus 5 μg of pBluescript (IE-1), or 15 μg of pBluescript control vector (Vector). After 48 h, nuclear extracts were prepared, and samples (5 μg) were subjected to EMSA using the indicated oligonucleotides containing either the URE NF-κB element from the c-myc gene (URE κB), the Bcl-xL NF-κB element, or the Oct-1 element as a loading control. The positions of the specific complexes are indicated as bands 1 and 2. Several nonspecific complexes (ns) were observed with the Bcl-xL NF-κB oligonucleotide; the two major ones are marked. (B) Cultures (P-150 dish) of NIH 3T3 cells were transfected with either 10 μg of vector expressing RelB plus 5 μg of vector expressing p50 protein (RelB + p50) or 10 μg of vector expressing p50 protein plus 5 μg of pBluescript (p50), and extracts were subjected to EMSA, as described for panel A, using the Bcl-xL NF-κB element. (The higher level of binding seen with extracts from cells transfected with p50 alone versus p50 plus RelB expression vectors can be attributed to the higher level of p50 expression vector used [10 versus 5 μg].)
In contrast, when the Bcl-xL NF-κB element was used as a probe, the extracts from RelB plus p50-transfected cells demonstrated an increase in binding of only one complex (band 1) (Fig. 9A). The upper two complexes were nonspecific, as judged by a competition assay (data not shown). The migration of this complex suggested it consisted of p50 homodimers. To confirm this assessment, NIH 3T3 cells were transfected with 10 μg of p50 expression vector plus 5 μg of pBluescript empty vector DNA. A complex that comigrates with band 1 was observed (Fig. 9B). Thus, the Bcl-xL NF-κB element does not appear to effectively bind RelB-p50 complexes compared to the c-myc NF-κB URE.
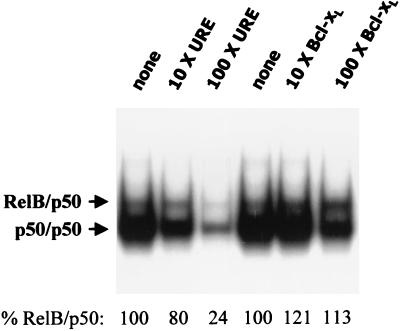
Since an inability of RelB-p50 to bind to the Bcl-xL NF-κB regulatory element could clearly explain the observed failure to induce the promoter, we used competition analysis to directly compare RelB-p50 binding to the two NF-κB elements. The URE was used as a probe. Extracts from the cells expressing RelB-p50 proteins were used in EMSA in the absence or presence of 10- or 100-fold molar excess unlabeled oligonucleotides containing either the URE or Bcl-xL NF-κB elements (Fig. 10). The URE competed very effectively against itself for formation of both complexes. An increasing inhibition of formation of both bands was seen with 10- versus 100-fold molar excess URE. In particular, at 100-fold molar excess, only 24% of the RelB-p50 complex is still present. In contrast, 100-fold molar excess of the Bcl-xL element had no detectable effect against RelB-p50 binding to the NF-κB URE (Fig. 10) and even competed much less effectively for formation of band 1, the p50 homodimer complex, consistent with the relative p50 homodimer binding seen in Fig. 9 with the same extract. Similar results were seen with extracts from cells transfected with the IE1 expression vector (data not shown). Together, these results indicate that the Bcl-xL NF-κB promoter element does not effectively bind RelB-p50 complexes.
FIG. 10.
The Bcl-xL NF-κB element competes very poorly for RelB-p50 binding to the URE. Cultures (P-150 dish) of NIH 3T3 cells were transfected with 10 μg of vector expressing RelB plus 5 μg of vector expressing p50 protein. After 48 h, nuclear extracts were prepared, and samples (5 μg) were subjected to EMSA, using the URE NF-κB element as a probe, in the absence (none) or presence of 10 or 100× molar excess of the indicated unlabeled oligonucleotide as competitor DNA. The resulting autoradiogram was subjected to densitometry, and the RelB-p50 complexes present are given below as percents relative to the control.
DISCUSSION
Here, we observed that the CMV IE1 protein, which is known to elevate NF-κB element-driven activity, failed to induce transactivation of the Bcl-xL promoter in either SMCs or NIH 3T3 cells. Using immunohistochemistry, immunoblotting, and transfection analyses, we have shown that IE1 selectively activates RelB-p50 NF-κB heterodimers, which are unable to transactivate the Bcl-xL promoter. Consistent with these observations, IE1 expression led to transactivation of E8-CAT, a c-myc URE NF-κB element-driven construct, in SMC and NIH 3T3 cells but not in relB−/− MEFs lacking the RelB subunit. RelB is unique among the NF-κB family in that it cannot function as a homodimer and only acts as a heterodimer (49). Unlike RelA or c-Rel, we found that RelB-p50 complexes were unable to bind to the NF-κB element that controls activity of the Bcl-xL promoter (14). (In addition, we observed that a second Bcl-xL NF-κB element further upstream of the start site, identified by Glasgow et al. [25], was also unable to bind to RelB-p50 complexes [data not shown].) Thus, the inactivity of RelB-p50 complexes can be explained by their failure to bind to the specific Bcl-xL promoter NF-κB elements. In contrast, RelB-p50 complexes were able to bind to and transactivate via the NF-κB element upstream of the c-myc promoter, which explains the ability of IE1 to lead to activation of E8-CAT. The reason for the observed repression of the Bcl-xL promoter activity remains to be determined. Since RelB-p50 does not appear able to bind effectively to the Bcl-xL promoter, an indirect effect mediated via another component of the transcription machinery is likely. The increase in RelB protein induced by IE1 could be related, in part, to an increase in relB mRNA levels. Previously, RelB was shown to interact only weakly with IκB-α (18, 38). Thus, the resulting increase in cytoplasmic RelB protein likely leads directly to the observed increase in nuclear levels. Overall, these results indicate the CMV IE1 protein selectively induces RelB-p50 complexes in vascular SMCs and NIH 3T3 cells, and these complexes demonstrate a strong sequence specificity of action.
Previously, IE1 was shown to induce NF-κB activity in human fibroblasts and Jurkat T cells, although at that time the subunit nature of the NF-κB had not been determined (50). Here, we demonstrate that the predominant NF-κB activity induced by IE1 is RelB-p50. HCMV infection has also been shown to lead to a rapid induction of nuclear NF-κB, increased steady-state p50 and RelA mRNA levels, and increased levels of Sp1 (64). The activation of Sp1 was found to mediate induction of the expression of the RelA gene, which contains several Sp1 sites (64, 65). The rapid activation of both Sp1 and NF-κB was subsequently shown to be initiated by engagement of cellular receptors for surface glycoproteins on CMV (65). Together with our findings, these studies lead us to hypothesize the following scenario. Upon HMCV infection, engagement of the receptors for the viral surface glycoproteins activates classical NF-κB and Sp1 (and thereby RelA mRNA levels), which induce the major ie promoter. The consequent expression of IE1, in turn, leads to the sustained activation of RelB-p50 complexes.
The induction of RelB-p50 likely plays a pivotal role in promoting proliferation of vascular SMCs via control of transcription of two proto-oncogenes c-myb and c-myc. RelB has been demonstrated to control elongation of transcription of the c-myb gene (57, 58). As first shown by our laboratory, c-Myb is expressed in bovine vascular SMCs and acts as a progression factor, promoting SMC entry into S phase (12, 40, 46). The role of c-Myb in control of proliferation has been extended to other vascular SMCs in culture and, importantly, has been demonstrated in the in vivo responses of SMCs to vascular injury (28, 33, 39, 52; reviewed in reference 36). We have recently found that the c-myc promoter is transactivated upon cotransfection of RelB in combination with either p50 or p52 (W. Yang and G. E. Sonenshein, unpublished observations), consistent with the URE binding and E8-CAT transfection analyses presented here. Multiple laboratories have demonstrated, using either in vitro or in vivo model systems, that c-myc expression is required for the entry of SMCs into S phase. Our laboratory showed that microinjection of both c-myc and c-myb expression vectors was required for quiescent SMCs to enter S phase in culture (40), while antisense oligonucleotides and ribozyme and dominant-negative-expressing adenoviral vectors have been used in multiple in vivo models of restenosis (52; reviewed in reference 36). Thus, it is likely that the activation of RelB-p50 serves to promote proliferation of vascular SMCs via activation of the c-myc and c-myb genes.
Differential regulation by members of the NF-κB family has been seen previously. Frequently, different subunits of the NF-κB/Rel family have been shown to selectively interact with members of other protein families (6, 16, 23, 30, 42, 44, 55, 62). For example, the RelA subunit has been shown to specifically associate with the glucocorticoid and progesterone receptors (30, 62) and the YB-1 protein (44). We recently reported that the selective interaction of RelA with the arylhydrocarbon receptor promotes transcription of the c-myc gene (32). A similar functional interaction of RelA with the progesterone receptor has been noted (30). In both of these cases, p50 and c-Rel subunits failed to cooperate to affect the transcriptional activity of the target gene. Thus, interaction with other transcription factors is an important mechanism of subunit-specific regulation. In a few cases, differential binding by NF-κB family members has been observed. For example, the HIV long terminal repeat contains two direct repeats of the B site in tandem, which are critical for the initial steps of HIV replication (1, 47). It has been shown that while RelA-p50 complexes up-regulate the HIV promoter through binding to the tandem NF-κB elements in the B site, c-Rel-p50 behaves as a repressor in the context of the HIV long terminal repeat (19). More recently, transactivation of the c-myb gene by RelB-p50 complexes has been shown to be repressed by RelA-p50 via competition for coactivator usage (43). Here, we report the selective inability of the NF-κB RelB family member to transactivate the Bcl-xL promoter due to its inability to bind effectively to the NF-κB regulatory elements upstream of this important survival gene.
A primary effect of IE1 appears to be the induction of relB mRNA. This finding suggests that IE1 likely enhances the rate of relB gene transcription, although other levels of control have not been excluded. In any event, a role for other NF-κB subunits in relB mRNA induction is unlikely, given the failure of IE1 to induce E8-CAT activity in relB−/− MEFs. Furthermore, we have found that coexpression of IκB-α fails to block relB mRNA induction (data not shown). RelB was originally identified as an immediate-early gene product in growth factor-induced fibroblasts (49). RelB is expressed predominantly in lymphoid tissues, where heterodimeric RelB represents the major constitutive κB binding activity (13, 38). Mice with a targeted disruption of relB display inflammatory cell infiltration in several organs, myeloid hyperplasia, and splenomegaly due to extramedullary hematopoiesis. RelB-deficient mice also show abnormal development of thymic medulla and antigen-presenting dendritic cells. In addition to these pathological changes, RelB-deficient mice have multifocal defects in cellular and humoral immune responses (60, 61). Little is known about the full identity of genes regulated by RelB, as most promoters have not been specifically tested with this subunit. Microarray analysis is in progress to identify additional genes regulated by RelB heterodimer complexes.
There is now a large body of evidence demonstrating a role for the NF-κB/Rel family of transcription factors in promoting the protection of cells from apoptosis. Inhibition of constitutively expressed NF-κB leads to apoptosis. Work from our laboratory first showed that inhibition of NF-κB activity is necessary and sufficient to cause the death of murine B-cell lymphomas, hepatocytes, and breast cancer cells (2, 3, 9, 54, 63). RelA-deficient murine embryos die by 14 days of gestation from massive liver degeneration due to apoptosis of liver cells (7). Similarly, inhibition of NF-κB enhances tumor necrosis factor-induced cell death (reviewed in references 5 and 53). Many genes regulated by NF-κB that mediate cell survival have now been identified (reviewed in reference 5). It is thus important to determine the NF-κB complex specificity required for their activation.
Acknowledgments
We thank C. Gelinas, R. Bravo, and E. Mocarski for generously providing cloned DNAs and F. Weih for the relB−/− MEFs. Darin Sloneker is acknowledged for assistance in preparation of the manuscript, and J. Foster is acknowledged for use of the densitometer. We thank J. Shen and R. Romieu-Mourez for their comments on the manuscript.
This work was supported by NIH grants HL13262 and CA36355 (G.E.S.).
REFERENCES
- 1.Alcami, J. 1995. Absolute dependence on kappa B responsive elements for initiation and Tat-mediated amplification of HIV transcription in blood CD4 T lymphocytes. EMBO J. 14:1552-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arsura, M., M. J. FitzGerald, N. Fausto, and G. E. Sonenshein. 1997. Nuclear factor-κB/Rel blocks transforming growth factor beta1-induced apoptosis of murine hepatocyte cell lines. Cell Growth Differ. 8:1049-1059. [PubMed] [Google Scholar]
- 3.Arsura, M., M. Wu, and G. E. Sonenshein. 1996. TGF beta 1 inhibits NF-κB/Rel activity inducing apoptosis of B cells: transcriptional activation of IκBα. Immunity 5:31-40. [DOI] [PubMed] [Google Scholar]
- 4.Baeuerle, P. A. 1991. The inducible transcription activator NF-κB: regulation by distinct protein subunits. Biochim. Biophys. Acta 1072:63-80. [DOI] [PubMed] [Google Scholar]
- 5.Barkett, M., and T. D. Gilmore. 1999. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene 18:6910-6924. [DOI] [PubMed] [Google Scholar]
- 6.Bassuk, A. G., R. T. Anandappa, and J. M. Leiden. 1997. Physical interactions between Ets and NF-κB/NFAT proteins play an important role in their cooperative activation of the human immunodeficiency virus enhancer in T cells. J. Virol. 71:3563-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beg, A. A., W. C. Sha, R. T. Bronson, S. Ghosh, and D. Baltimore. 1995. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 376:167-170. [DOI] [PubMed] [Google Scholar]
- 8.Beldekas, J. C., L. Gerstenfeld, G. E. Sonenshein, and C. Franzblau. 1982. Cell density and estradiol modulation of procollagen type III in cultured calf smooth muscle cells. J. Biol. Chem. 257:12252-12256. [PubMed] [Google Scholar]
- 9.Bellas, R. E., M. J. FitzGerald, N. Fausto, and G. E. Sonenshein. 1997. Inhibition of NF-κB activity induces apoptosis in murine hepatocytes. Am. J. Pathol. 151:891-896. [PMC free article] [PubMed] [Google Scholar]
- 10.Blank, V., P. Kourilsky, and A. Israel. 1992. NF-κB and related proteins: Rel/dorsal homologies meet ankyrin-like repeats. Trends Biochem. Sci. 17:135-140. [DOI] [PubMed] [Google Scholar]
- 11.Brown, K., S. Gerstberger, L. Carlson, G. Franzoso, and U. Siebenlist. 1995. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science 267:1485-1488. [DOI] [PubMed] [Google Scholar]
- 12.Brown, K. E., M. S. Kindy, and G. E. Sonenshein. 1992. Expression of the c-myb proto-oncogene in bovine vascular smooth muscle cells. J. Biol. Chem. 267:4625-4630. [PubMed] [Google Scholar]
- 13.Carrasco, D., R. P. Ryseck, and R. Bravo. 1993. Expression of relB transcripts during lymphoid organ development: specific expression in dendritic antigen-presenting cells. Development 118:1221-1231. [DOI] [PubMed] [Google Scholar]
- 14.Chen, C., L. C. Edelstein, and C. Gelinas. 2000. The Rel/NF-κB family directly activates expression of the apoptosis inhibitor Bcl-xL. Mol. Cell. Biol. 20:2687-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cherrington, J. M., and E. S. Mocarski. 1989. Human cytomegalovirus ie1 transactivates the alpha promoter-enhancer via an 18-base-pair repeat element. J. Virol. 63:1435-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickinson, L. A., J. W. Trauger, E. E. Baird, P. B. Dervan, B. J. Graves, and J. M. Gottesfeld. 1999. Inhibition of Ets-1 DNA binding and ternary complex formation between Ets-1, NF-κB, and DNA by a designed DNA-binding ligand. J. Biol. Chem. 274:12765-12773. [DOI] [PubMed] [Google Scholar]
- 17.Do, R. K., E. Hatada, H. Lee, M. R. Tourigny, D. Hilbert, and S. Chen-Kiang. 2000. Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J. Exp. Med. 192:953-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobrzanski, P., R. P. Ryseck, and R. Bravo. 1994. Differential interactions of Rel-NF-kappa B complexes with I kappa B alpha determine pools of constitutive and inducible NF-kappa B activity. EMBO J. 13:4608-4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doerre, S., P. Sista, S. C. Sun, D. W. Ballard, and W. C. Greene. 1993. The c-rel protooncogene product represses NF-kappa B p65-mediated transcriptional activation of the long terminal repeat of type 1 human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 90:1023-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duyao, M. P., A. J. Buckler, and G. E. Sonenshein. 1990. Interaction of an NF-kappa B-like factor with a site upstream of the c-myc promoter. Proc. Natl. Acad. Sci. USA 87:4727-4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everett, R. D. 1984. Transactivation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 3:3135-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Everett, R. D., and M. Dunlop. 1984. Trans activation of plasmid-borne promoters by adenovirus and several herpes group viruses. Nucleic Acids Res. 12:5969-5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrier, R., R. Nougarede, S. Doucet, B. Kahn-Perles, J. Imbert, and D. Mathieu-Mahul. 1999. Physical interaction of the bHLH LYL1 protein and NF-κB1 p105. Oncogene 18:995-1005. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh, S., A. M. Gifford, L. R. Riviere, P. Tempst, G. P. Nolan, and D. Baltimore. 1990. Cloning of the p50 DNA binding subunit of NF-κB: homology to rel and dorsal. Cell 62:1019-1029. [DOI] [PubMed] [Google Scholar]
- 25.Glasgow, J. N., T. Wood, and J. R. Perez-Polo. 2000. Identification and characterization of nuclear factor κB binding sites in the murine bcl-x promoter. J. Neurochem. 75:1377-1389. [DOI] [PubMed] [Google Scholar]
- 26.Grilli, M., J. J. Chiu, and M. J. Lenardo. 1993. NF-kappa B and Rel: participants in a multiform transcriptional regulatory system. Int. Rev. Cytol. 143:1-62. [DOI] [PubMed] [Google Scholar]
- 27.Grimm, S., and P. Baeuerle. 1993. The inducible transcription factor NF-kappa B: structure-function relationship of its protein subunits. Biochem. J. 290:297-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jarvis, T. C., L. J. Alby, A. A. Beaudry, F. E. Wincott, L. Beigelman, J. A. McSwiggen, N. Usman, and D. T. Stinchcomb. 1996. Inhibition of vascular smooth muscle cell proliferation by ribozymes that cleave c-myb mRNA. RNA 2:419-428. [PMC free article] [PubMed] [Google Scholar]
- 29.Jenkins, D. E., C. L. Martens, and E. S. Mocarski. 1994. Human cytomegalovirus late protein encoded by ie2: a trans-activator as well as a repressor of gene expression. J. Gen. Virol. 75:2337-2348. [DOI] [PubMed] [Google Scholar]
- 30.Kalkhoven, E., S. Wissink, P. T. van der Saag, and B. van der Burg. 1996. Negative interaction between the RelA (p65) subunit of NF-κB and the progesterone receptor. J. Biol. Chem. 271:6217-6224. [DOI] [PubMed] [Google Scholar]
- 31.Kieran, M., V. Blank, F. Logeat, J. Vandekerckhove, F. Lottspeich, O. Le Bail, M. B. Urban, P. Kourilsky, P. A. Baeuerle, and A. Israel. 1990. The DNA binding subunit of NF-κB is identical to factor KBF1 and homologous to the rel oncogene product. Cell 62:1007-1018. [DOI] [PubMed] [Google Scholar]
- 32.Kim, D. W., L. Gazourian, S. A. Quadri, R. Romieu-Mourez, D. H. Sherr, and G. E. Sonenshein. 2000. The RelA NF-κB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene 19:5498-5506. [DOI] [PubMed] [Google Scholar]
- 33.Lambert, D. L., N. Malik, L. Shepherd, J. Gunn, S. E. Francis, A. King, D. C. Crossman, D. C. Cumberland, and C. M. Holt. 2001. Localization of c-Myb and induction of apoptosis by antisense oligonucleotide c-Myb after angioplasty of porcine coronary arteries. Arterioscler. Thromb. Vasc. Biol. 21:1727-1732. [DOI] [PubMed] [Google Scholar]
- 34.La Rosa, F. A., J. W. Pierce, and G. E. Sonenshein. 1994. Differential regulation of the c-myc oncogene promoter by the NF-κB rel family of transcription factors. Mol. Cell. Biol. 14:1039-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee, H., M. Arsura, M. Wu, M. Duyao, A. J. Buckler, and G. E. Sonenshein. 1995. Role of Rel-related factors in control of c-myc gene transcription in receptor-mediated apoptosis of the murine B cell WEHI 231 line. J. Exp. Med. 181:1169-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, M., A. D. Simon, C. A. Stein, and L. E. Rabbani. 1999. Antisense strategies to inhibit restenosis. Antisense Nucleic Acid Drug Dev. 9:487-492. [DOI] [PubMed] [Google Scholar]
- 37.Lemstrom, K. B., J. H. Bruning, C. A. Bruggeman, I. T. Lautenschlager, and P. J. Hayry. 1993. Cytomegalovirus infection enhances smooth muscle cell proliferation and intimal thickening of rat aortic allografts. J. Clin. Investig. 92:549-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lernbecher, T., U. Muller, and T. Wirth. 1993. Distinct NF-kappa B/Rel transcription factors are responsible for tissue-specific and inducible gene activation. Nature 365:767-770. [DOI] [PubMed] [Google Scholar]
- 39.Macejak, D. G., H. Lin, S. Webb, J. Chase, K. Jensen, T. C. Jarvis, J. M. Leiden, and L. Couture. 1999. Adenovirus-mediated expression of a ribozyme to c-myb mRNA inhibits smooth muscle cell proliferation and neointima formation in vivo. J. Virol. 73:7745-7751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marhamati, D. J., R. E. Bellas, M. Arsura, K. E. Kypreos, and G. E. Sonenshein. 1997. A-myb is expressed in bovine vascular smooth muscle cells during the late G1-to-S phase transition and cooperates with c-myc to mediate progression to S phase. Mol. Cell. Biol. 17:2448-2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mocarski, E. S., M. Bonyhadi, S. Salimi, J. M. McCune, and H. Kaneshima. 1993. Human cytomegalovirus in a SCID-hu mouse: thymic epithelial cells are prominent targets of viral replication. Proc. Natl. Acad. Sci. USA 90:104-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Na, S. Y., B. Y. Kang, S. W. Chung, S. J. Han, X. Ma, G. Trinchieri, S. Y. Im, and J. W. Lee. 1999. Retinoids inhibit interleukin-12 production in macrophages through physical associations of retinoid X receptor and NFκB. J. Biol. Chem. 274:7674-7680. [DOI] [PubMed] [Google Scholar]
- 43.Nicot, C., R. Mahieux, C. Pise-Masison, J. Brady, A. Gessain, S. Yamaoka, and G. Franchini. 2001. Human T-Cell lymphotropic virus type 1 tax represses c-Myb-dependent transcription through activation of the NF-κB pathway and modulation of coactivator usage. Mol. Cell. Biol. 21:7391-7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raj, G. V., M. Safak, G. H. MacDonald, and K. Khalili. 1996. Transcriptional regulation of human polyomavirus JC: evidence for a functional interaction between RelA (p65) and the Y-box-binding protein, YB-1. J. Virol. 70:5944-5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rayet, B., and C. Gelinas. 1999. Aberrant rel/NF-κB genes and activity in human cancer. Oncogene 18:6938-6947. [DOI] [PubMed] [Google Scholar]
- 46.Reilly, C. F., M. S. Kindy, K. E. Brown, R. D. Rosenberg, and G. E. Sonenshein. 1989. Heparin prevents vascular smooth muscle cell progression through the G1 phase of the cell cycle. J. Biol. Chem. 264:6990-6995. [PubMed] [Google Scholar]
- 47.Ross, E. K., A. J. Buckler-White, A. B. Rabson, G. Englund, and M. A. Martin. 1991. Contribution of NF-κB and Sp1 binding motifs to the replicative capacity of human immunodeficiency virus type 1: distinct patterns of viral growth are determined by T-cell types. J. Virol. 65:4350-4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruben, S. M., P. J. Dillon, R. Schreck, T. Henkel, C. H. Chen, M. Maher, P. A. Baeuerle, and C. A. Rosen. 1991. Isolation of a rel-related human cDNA that potentially encodes the 65-kD subunit of NF-κB. Science 251:1490-1493. [DOI] [PubMed] [Google Scholar]
- 49.Ryseck, R. P., P. Bull, M. Takamiya, V. Bours, U. Siebenlist, P. Dobrzanski, and R. Bravo. 1992. RelB, a new Rel family transcription activator that can interact with p50-NF-κB. Mol. Cell. Biol. 12:674-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sambucetti, L. C., J. M. Cherrington, G. W. Wilkinson, and E. S. Mocarski. 1989. NF-kappa B activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J. 8:4251-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schauer, S. L., Z. Wang, G. E. Sonenshein, and T. L. Rothstein. 1996. Maintenance of nuclear factor-kappa B/Rel and c-myc expression during CD40 ligand rescue of WEHI 231 early B cells from receptor-mediated apoptosis through modulation of I kappa B proteins. J. Immunol. 157:81-86. [PubMed] [Google Scholar]
- 52.Schmitt, J. F., M. C. Keogh, U. Dennehy, D. Chen, F. Lupu, K. Weston, D. Taylor, V. V. Kakkar, and N. R. Lemoine. 1999. Tissue-selective expression of dominant-negative proteins for the regulation of vascular smooth muscle cell proliferation. Gene Ther. 6:1184-1191. [DOI] [PubMed] [Google Scholar]
- 53.Sonenshein, G. E. 1997. Rel/NF-kappa B transcription factors and the control of apoptosis. Semin. Cancer Biol. 8:113-119. [DOI] [PubMed] [Google Scholar]
- 54.Sovak, M. A., R. E. Bellas, D. W. Kim, G. J. Zanieski, A. E. Rogers, A. M. Traish, and G. E. Sonenshein. 1997. Aberrant nuclear factor-κB/Rel expression and the pathogenesis of breast cancer. J. Clin. Investig. 100:2952-2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stein, B., and A. S. Baldwin, Jr. 1993. Distinct mechanisms for regulation of the interleukin-8 gene involve synergism and cooperativity between C/EBP and NF-κB. Mol. Cell. Biol. 13:191-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Streblow, D. N., C. Soderberg-Naucler, J. Vieira, P. Smith, E. Wakabayashi, F. Ruchti, K. Mattison, Y. Altschuler, and J. A. Nelson. 1999. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell 99:511-520. [DOI] [PubMed] [Google Scholar]
- 57.Suhasini, M., and R. B. Pilz. 1999. Transcriptional elongation of c-myb is regulated by NF-κB (p50/RelB). Oncogene 18:7360-7369. [DOI] [PubMed] [Google Scholar]
- 58.Suhasini, M., C. D. Reddy, E. P. Reddy, J. A. DiDonato, and R. B. Pilz. 1997. cAMP-induced NF-κB (p50/relB) binding to a c-myb intronic enhancer correlates with c-myb up-regulation and inhibition of erythroleukemia cell differentiation. Oncogene 15:1859-1870. [DOI] [PubMed] [Google Scholar]
- 59.Verma, I. M., J. K. Stevenson, E. M. Schwarz, D. VanAntwerp, and S. Miyamoto. 1995. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 9:2723-2735. [DOI] [PubMed] [Google Scholar]
- 60.Weih, F., D. Carrasco, S. K. Durham, D. S. Barton, C. A. Rizzo, R. P. Ryseck, S. A. Lira, and R. Bravo. 1995. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell 80:331-340. [DOI] [PubMed] [Google Scholar]
- 61.Weih, F., S. K. Durham, D. S. Barton, W. C. Sha, D. Baltimore, and R. Bravo. 1997. p50-NF-κB complexes partially compensate for the absence of RelB: severely increased pathology in p50−/−relB−/− double-knockout mice. J. Exp. Med. 185:1359-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wissink, S., A. van de Stolpe, E. Caldenhoven, L. Koenderman, and, P. T. van der Saag. 1997. NF-kappa B/Rel family members regulating the ICAM-1 promoter in monocytic THP-1 cells. Immunobiology 198:50-64. [DOI] [PubMed] [Google Scholar]
- 63.Wu, M., H. Lee, R. E. Bellas, S. L. Schauer, M. Arsura, D. Katz, M. J. FitzGerald, T. L. Rothstein, D. H. Sherr, and G. E. Sonenshein. 1996. Inhibition of NF-κB/Rel induces apoptosis of murine B cells. EMBO J. 15:4682-4690. [PMC free article] [PubMed] [Google Scholar]
- 64.Yurochko, A. D., T. F. Kowalik, S. M. Huong, and E. S. Huang. 1995. Human cytomegalovirus upregulates NF-κB activity by transactivating the NF-κB p105/p50 and p65 promoters. J. Virol. 69:5391-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yurochko, A. D., M. W. Mayo, E. E. Poma, A. S. Baldwin, Jr., and E. S. Huang. 1997. Induction of the transcription factor Sp1 during human cytomegalovirus infection mediates upregulation of the p65 and p105/p50 NF-κB promoters. J. Virol. 71:4638-4648. [DOI] [PMC free article] [PubMed] [Google Scholar]