Abstract
The flavivirus NS3 protein plays an important role in the cleavage and processing of the viral polyprotein and in the synthesis of the viral RNA. NS3 recruits NS2B and NS5 proteins to form complexes possessing protease and replicase activities through protease and nucleoside triphosphatase/helicase domains. We have found that NS3 also induces apoptosis. Expression of the Langat (LGT) virus NS3 protein resulted in a cleavage of cellular DNA and reduced the viability of cells. Coexpression of NS3 with apoptotic inhibitors (CrmA and P35) and addition of caspase peptide substrates (Z-VAD-FMK and Z-IETD-FMK) to NS3-transfected cells blocked NS3-induced apoptosis. In cotransfection experiments, NS3 bound to caspase-8 and enhanced caspase-8-mediated apoptosis. NS3 and caspase-8 colocalized in the cytoplasm of transfected cells. Deletion analysis demonstrated that at least two regions of NS3 contribute to its apoptotic activities. The protease and helicase domains are each able to bind to caspase-8, while the protease domain alone induces apoptosis. The protease domain and tetrahelix region of the helicase domain are required for NS3 to augment caspase-8-mediated apoptosis. Thus, the LGT virus NS3 protein is a multifunctional protein that binds to caspase-8 and induces apoptosis.
The Flavivirus genus includes mostly arthropod-borne pathogens, many of which are widely distributed throughout the world and cause human diseases that vary in severity. The four serotypes of dengue (DEN) virus, Japanese encephalitis (JE) virus, and yellow fever (YF) virus cause millions of cases of disease annually in the tropics and subtropics. Tick-borne encephalitis (TBE) flaviviruses grouped within the TBE complex (Russian spring-summer encephalitis or TBE, Kyasanur forest disease, Langat [LGT], Louping ill, Negishi, Omsk hemorrhagic fever, and Powassan viruses) are mainly found in the Northern Hemisphere and, except for LGT virus, cause thousands of human cases every year, which can have a mortality as high as 20 to 30%. LGT virus does not naturally infect humans and has been studied as a potential live virus vaccine to protect against encephalitis caused by viruses of the TBE complex (4).
LGT virus, like other flaviviruses, is a small lipid-enveloped, positive-sense RNA virus. During infection, a single polycistronic viral RNA is translated into a polyprotein precursor that is co- and posttranslationally processed by host and viral proteases to produce the virion and replicase components. The structural proteins of flaviviruses, capsid (C), premembrane (preM), and envelope (E), are encoded in the 5′ quarter of the genome, and signal peptidase appears to mediate their cleavages during elongation of the polyprotein precursor through the translocating channel of the rough endoplasmic reticulum. Nonstructural proteins NS2A and -B, NS3, NS4A and -B, and NS5 are cleaved by viral serine protease NS2B-NS3 after a sequence that usually contains two basic residues followed by a short side chain residue (reviewed in reference 19).
NS3 protein exhibits protease, nucleoside triphosphatase (NTPase), helicase, and RNA triphosphatase activities. The serine protease domain is located at the amino terminus of the protein (2, 10). The proteases of DEN, YF, West Nile, Murray Valley, and TBE viruses have all been shown to have activity (5, 20, 23, 26, 33). A catalytic triad of amino acids, His-Asp-Ser, is essential for protease activity in vitro and for viral replication (5, 20, 30, 33). The proteolytic function of the NS3 protein is dependent on the presence of NS2B as a cofactor and is associated with the membrane fraction of infected cells (6).
The replicase activity of the NS3 protein supported by NTPase and helicase domains is associated with the NS3-NS5-RNA complex (reviewed in reference 19). The NTPase domain is located in the middle portion of NS3 slightly overlapping the serine protease domain. An RNA-stimulated NTPase activity has been identified in the NS3 proteins of DEN, YF, West Nile, and JE viruses (7, 17, 18, 27, 32, 34). The domain includes a Walker motif A, involved in binding the β and γ phosphates of nucleoside triphosphate (NTP), and a Walker motif B responsible for chelation of Mg2+ in the Mg-NTP complex. In the absence of substrates, side chains of the Walker motif A and B sequences are bonded to each other and to the conserved segment of NS3, also known as motif III (11), which is involved in transmission of conformational changes accompanying NTP hydrolysis (38).
The helicase domain is located in the carboxy three-fourths of NS3. The flaviviral helicase has been classified as a member of the superfamily 2 helicases (reviewed in reference 15), and helicase activity has been demonstrated for the DEN and JE virus proteins (18, 29). The helicase domain contains at least three clusters of positively charged residues. One cluster, designated as a motif VI (11), is involved in binding RNA backbone phosphates and is associated with an ATPase activity (15). Two other clusters appear to provide additional RNA-protein interaction sites.
The carboxy terminus of NS3 has an RNA triphosphatase activity (35), which modifies the 5′ end of the viral RNA in a complex with guanylyltransferase and methyltransferase to synthesize the m7G-capped structure.
Recently, we reported that infection with LGT virus or expression of the envelope glycoprotein (E) induces apoptosis through a caspase-3 like protease pathway (25). Here we show that the LGT virus NS3 protein binds to caspase-8 and induces apoptosis. Furthermore, we demonstrate that the protease and helicase domains of the protein can bind to caspase-8, while only the protease domain induces apoptosis.
MATERIALS AND METHODS
Cells and media.
The mouse neuroblastoma Neuro-2a cell line was maintained in antibiotic-free minimum essential medium (MEM) with Earle's salts supplemented with 2 mM l-glutamine and 10% fetal bovine serum (BioWhittaker, Walkersville, Md.). Human embryonic kidney 293T cells were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 100 U of penicillin G sodium/ml, and 100 μg of streptomycin sulfate/ml. The monkey kidney Vero cell line was maintained in MEM supplemented with 10% fetal bovine serum, 2 mM l-glutamine, 50 μg of gentamicin/ml, and 25 μg of amphotericin B/ml.
Plasmids.
NS3, Flag epitope-tagged NS3 (F-NS3), and their truncated forms were constructed by amplifying the coding sequence of NS3, using pCINS2B-NS3 (25) as a template and appropriate primers. The 5′ end primers each contained an XbaI site and the Flag epitope sequence, DYKDDDDK, inserted in frame after the first methionine codon. The 3′ end primers contained the stop codon followed by a NotI site that allowed the PCR products to be cloned into the XbaI-NotI site of the pCI expression vector (Promega, Madison, Wis.). One common 3′ end primer (5′-GGTCTTGCGGCCGCTCAG6446CGTCTTCCAGAAGC6460) and four 5′ end primers (5′-GGTGCGAGATCTAGAATGA4598CGGATCTTGTGTTTTC4564, 5′-GGTGCGTCTAGAATGGACTACAAGGACGACGATGACAAAA4598CGGATCTTGTGTTTTC4564, 5′-GGTGCGTCTAGAATGGACTACAAGGACGACGATGACAAAG5711CCAGAGGAGGAGCC5725, and 5′-GGTGCGTCTAGAATGGACTACAAGGACGACGATGACAAAG5981GAAGAGTGGGCAGG5995) were used to construct pCINS3, encoding the NS3 domain, pCIF-NS3, encoding F-NS3, pCIF-NS3372st, encoding the carboxy-terminal 250 amino acids (aa) of NS3 (F-NS3372st), and pCIF-NS3462st, encoding the carboxy-terminal 160 aa of NS3 (F-NS3462st), respectively. The superscript numbers refer to the nucleotide positions within the LGT virus strain TP21 genome (GenBank accession no. P29837). One common 5′ end primer (5′-GGTGCGTCTAGAATGGACTACAAGGACGACGATGACAAAA4598CGGATCTTGTGTTTTC4564) and three 3′ end primers (5′-GGTCTTGCGGCCGCTCAC5980CTGCGCTGTGCAGC5966, 5′-GGTCTTGCGGCCGCTCAA5710ATTGACGGAACGAAC5695, and 5′-GGTCTTGCGGCCGCTCAC5140TGTGGGAGATTTGG5126) were used to construct pCIF-NS3461del, encoding the amino-terminal 461 aa of NS3 (F-NS3461del), pCIF-NS3371del, encoding the amino-terminal 371 aa of NS3 (F-NS3371del), and pCIF-NS3181del, encoding the 181 aa of NS3 (F-NS3181del), respectively. Primers 5′-GGTGCGAGATCTAGAATGA4598CGGATCTTGTGTTTTC4564 and 5′-GGTCTTGCGGCCGCTCAC5980CTGCGCTGTGCAGC5966 were used to construct pCINS3461del, encoding the amino-terminal 461 aa of NS3 (NS3461del).
Plasmids expressing caspase-8 (pCI-Caspase-8), hemagglutinin (HA) epitope-tagged caspase-2 (Casp2-HA) (pCI-Caspase-2-HA) and caspase-8 (Casp8-HA) (pCI-Caspase-8-HA), caspase-9 (pCI-Caspase-9), cowpox virus CrmA (pCI-CrmA), or mutant forms of caspase-2 (Casp2m-HA), caspase-8 (Casp8m-HA), caspase-3 (Casp3m-MYC), and caspase-9 (Casp9m-MYC) were gifts from Charles M. Zacharchuk and Safraz A. Memon (National Cancer Institute, National Institutes of Health [NIH], Bethesda, Md.). Each of the mutant caspases is identical to the wild type except that the catalytic domain has been mutated to prevent caspase activation. Plasmids expressing Flag-epitope tagged FasL (F-FasL) and green fluorescent protein (GFP)-fused mutant caspase-8 (Casp8m-GFP) were gifts from Lilia L. Bi and Richard M. Siegel (National Institute of Allergy and Infectious Diseases, NIH, Bethesda, Md.). pCI-P35 expressing the Autographa californica nuclear polyhedrosis virus p35 was described previously (3).
TUNEL assay.
Vero cells (6 × 105) were seeded in culture dishes containing glass coverslips and transfected with 1 μg of pCI or 1 μg of pCINS3 using LipofectAmine Plus (Invitrogen, Gaithersburg, Md.). At 18 h after transfection, the cells were fixed with 3% paraformaldehyde and labeled by the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) reaction with the Fluorescein in situ Cell Death Detection Kit (Roche Molecular Biochemicals, Indianapolis, Ind.). Cells stained with fluorescein isothiocyanate were examined by fluorescence microscopy.
β-Galactosidase transient-expression assay for apoptosis.
Neuro-2a and Vero cells (3 × 105) were transfected with 1 μg of pCINS3 or plasmids expressing NS3 mutants and 0.125 μg of pCMVβ-gal (Stratagene, La Jolla, Calif.) by using LipofectAmine Plus (Invitrogen). In some transfections, plasmids expressing caspases, pCI-Caspase-9 (0.4 μg), pCI-Caspase-8 (0.1 μg), pCI-Caspase-8-HA (0.1 μg), and pCI-Caspase-2-HA (0.05 μg), or apoptosis inhibitors, pCI-CrmA (1.5 μg) and pCI-P35 (1.5 μg), were added. Each transfection mixture also contained pCI to maintain a constant amount of plasmid DNA. In inhibition experiments, caspase substrates (50 μM) Z-IETD-FMK, Z-LEHD-FMK, and Z-VAD-FMK (R&D Systems, Minneapolis, Minn.) were added immediately after transfection and 24 h later to the culture medium to prevent apoptosis. At various times, cells were fixed and stained, and the percentage of apoptotic cells relative to vector control (pCI)-transfected cells, set at 0%, was calculated as described previously (24, 25). Each data point represents the results of three independent experiments, and error bars indicate the standard deviations.
Coimmunoprecipitation and Western blot analysis.
Plasmids expressing HA.11 or MYC epitope-tagged caspases with point mutations were used to prevent apoptosis in transfected cells. 293T cells (0.5 × 106) were transfected with 1 μg of plasmid DNA by using FuGENE6 (Roche Molecular Biochemicals). At 18 h after transfection, the cells were lysed in 300 μl of radioimmunoprecipitation assay (RIPA) buffer (10 mM Tris-HCl [pH 8.0], 100 mM NaCl, 1 mM EDTA, 1% NP-40, 0.5% deoxycholate, 0.5% sodium dodecyl sulfate [SDS]) on ice for 30 min. The lysate was centrifuged at 12,000 × g for 10 min, and an aliquot of the supernatant was used directly for immunoblotting to verify protein expression. Approximately one-half of the remaining supernatant was incubated for 1 h at 4°C with murine anti-HA.11 or anti-MYC monoclonal antibodies (MAb) followed by goat anti-mouse immunoglobulin G (IgG) Dynabeads M-450 (Dynal, Lake Success, N.Y.). In the converse coimmunoprecipitation assay, the remainder of the supernatant was incubated with anti-Flag M2 affinity resin for 1 h at 4°C and the resin was washed four times in RIPA buffer and washed twice in RIPA buffer with 0.8 M NaCl. Boiled immunoprecipitates and cell lysates were fractionated on SDS-polyacrylamide gels. Proteins were transferred to membranes, incubated with antibodies to Flag, HA.11, or MYC epitopes or caspase-3, -8, or -9 followed by horseradish peroxidase-conjugated secondary antibodies, and visualized by using the ECL Western blotting detection system (Amersham Pharmacia Biothech, Piscataway, N.J.).
Immunofluorescence.
Neuro-2a, Vero, and 293T cells were transfected as described above. At 18 h after transfection, the cells were fixed in methanol, washed with 0.4% Tween 20 in Tris-buffered saline, pH 7.2, and incubated with LGT-specific hyperimmune mouse ascitic fluid or murine anti-Flag M2 MAb followed by rhodamine-conjugated goat anti-mouse IgG (for F-NS3 and F-NS3 mutants). DAPI (4′,6′-diamidino-2-phenylindole; 1 μg/ml) was added to the antibodies to localize nuclei. Cells were visualized by fluorescence microscopy with the appropriate filters at ×1,000 magnification.
RESULTS
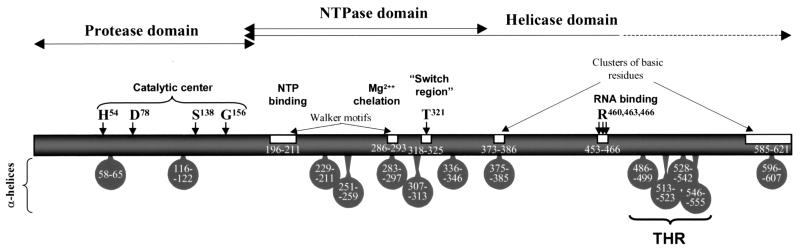
We performed a computer search using the LGT virus polyprotein to identify homologs of cellular proteins involved in apoptosis. One region of the LGT virus polyprotein (residues 1981 to 2071, accession no. P29837), which is located in the helicase domain of NS3 and has a predicted tetrahelical structure (Fig. 1), had a weak homology with the caspase recruitment domain of adapter proteins, such as DEFCAP-L (accession no. NP_055737) and Apaf-1 (accession no. o14727), that are known to mediate apoptosis (12, 21). Therefore, we examined the ability of the LGT virus NS3 protein to modulate apoptosis and interact with caspases.
FIG. 1.
Diagram showing the domains, conserved motifs, and predicted α-helical regions of LGT flavivirus NS3 protein. Numbers refer to amino acid positions in NS3 protein (GenBank accession no. P29837). The predicted tetrahelix region (THR) is indicated.
LGT virus NS3 induces apoptosis in mammalian cells.
The LGT virus NS3 gene was cloned into a mammalian expression vector under the control of the human cytomegalovirus IE promoter. Neuro-2a, Vero, and 293T cells were transfected with the NS3 plasmid, and protein expression was confirmed by immunofluorescence using LGT virus-specific hyperimmune mouse ascitic fluid. The protein was visualized 18 h after transfection in the cytoplasm of the cells and had a diffuse staining pattern as previously described for the NS3 and NS2B-NS3 proteins (25, 36). Furthermore, the identical electrophoretic mobilities of the NS3 proteins expressed in both the NS3-transfected and the LGT virus-infected Neuro-2a cells were confirmed by Western blot analysis with anti-NS3 MAb (data not shown).
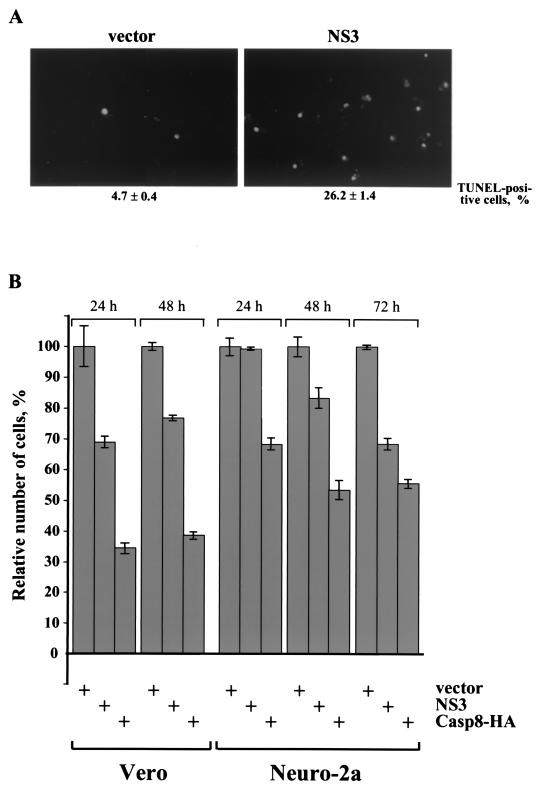
To determine if cellular DNA cleavage, a hallmark of apoptosis, occurs in NS3-transfected cells, the cells were subjected to a TUNEL assay. Eighteen hours after transfection, approximately 26% of Vero cells transfected with a plasmid encoding NS3 were TUNEL positive. In contrast, only 5% of TUNEL-positive cells were observed in an experiment with the empty vector (Fig. 2A).
FIG. 2.
LGT virus NS3 protein induces apoptosis. (A) Expression of NS3 in Vero cells results in cleavage of cellular DNA. Vero cells transfected with empty vector or plasmid expressing NS3 were fixed 18 h after transfection and processed for TUNEL assay. TUNEL-positive cells were visualized by fluorescence microscopy (magnification, ×200). (B) NS3 expression results in loss of viable cells. Vero and Neuro-2a cells were cotransfected with plasmids expressing β-galactosidase and NS3, Casp8-HA, or the empty vector. Cells were fixed and stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside), and the number of viable, blue-stained flat cells was determined at 24, 48, and 72 h after transfection relative to vector-transfected cells at the same times, set at 100%.
To examine if NS3 expression affects the viability of cells, Vero and Neuro-2a cells were cotransfected with a plasmid expressing β-galactosidase (to visualize transfected cells) and empty vector DNA, a plasmid expressing NS3, or a plasmid expressing caspase-8 (a positive control for apoptosis). Since dying cells detach from the surface of the culture plate and are removed prior to fixation, the loss of β-galactosidase-containing cells is a quantitative parameter of viable cells (13). The number of viable (flat blue) cells was reduced in both Vero and Neuro-2a cells transfected with the NS3 gene or the caspase-8-HA gene, compared to cells transfected with vector DNA (Fig. 2B). At 24 h after transfection of Vero cells with the NS3 gene, there was a 30% reduction in cell viability. However, there was no reduction in the number of viable NS3-expressing Neuro-2a cells at this time. The number of viable, NS3-transfected Neuro-2a cells decreased at 48 h, and there was a 30% reduction in cell viability at 72 h (Fig. 2B).
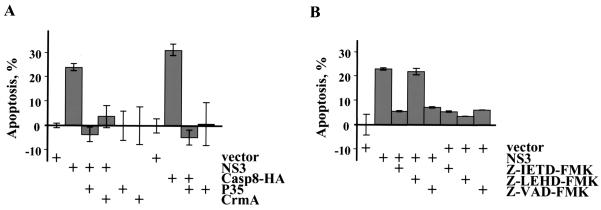
To verify that the reduction in cell viability was due to apoptosis and not due to death by another pathway, the LGT virus NS3 protein was coexpressed with two inhibitors of apoptosis, CrmA and P35. Cowpox virus protein CrmA prevents apoptosis triggered by caspases 1, 8, and 10. Baculovirus protein P35 blocks caspases 1, 3, 6, 8, and 10. Both inhibitors are cleaved by caspases, and the cleaved products trap caspases in a covalent complex. Coexpression of CrmA or P35 with NS3 blocked cell death in Neuro-2a cells (Fig. 3A). As a positive control, CrmA or P35 also blocked caspase-8-induced cell death. Furthermore, NS3-transfected Neuro-2a cells were treated with peptide inhibitors. Both Z-VAD-FMK and Z-IETD-FMK, pan-caspase and caspase-8-specific inhibitors, respectively, completely blocked apoptosis, while Z-LEHD-FMK, a caspase-9-specific inhibitor, had no effect (Fig. 3B). Together these data confirm that the LGT virus NS3 protein induces apoptosis in mammalian cells.
FIG. 3.
Inhibition of NS3-induced apoptosis. (A) Neuro-2a cells were transfected with empty vector or plasmids expressing NS3 or Casp8-HA and inhibitors of apoptosis, baculovirus P35, and cowpox virus CrmA. The number of cells undergoing apoptosis was determined 72 h after transfection. Vector-transfected cells (a negative control for apoptosis) were set at 0%. (B) Neuro-2a cells transfected with empty vector or plasmid expressing NS3 were treated with peptide inhibitors of apoptosis: Z-IETD-FMK, Z-LEHD-FMK, and Z-VAD-FMK. The percentage of apoptotic cells was calculated 48 h after transfection relative to the vector-transfected cells, set at 0%.
LGT virus NS3 binds caspase-8 and enhances caspase-8-mediated apoptosis.
To determine if LGT virus NS3 directly interacts with caspases, immunoprecipitations were performed with NS3 and caspases 2, 3, 8, and 9. To facilitate detection of NS3, a Flag epitope was fused to the amino terminus of NS3 to create F-NS3. F-NS3, like NS3, induced apoptosis in Neuro-2a cells at 48 h but not at 24 h. F-NS3 also did not induce apoptosis in 293T cells at 24 h.
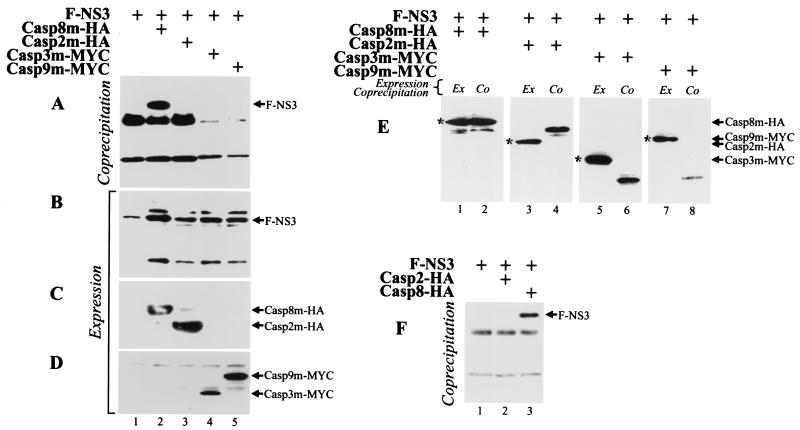
HA.11 epitope- and MYC epitope-tagged caspases 2, 8, 3, and 9 containing point mutations that inactivate the catalytic site (Casp2m-HA, Casp8m-HA, Casp3m-MYC, and Casp9m-MYC, respectively) were coexpressed with F-NS3 in 293T cells. Lysates of cells harvested 18 h after transfection were immunoprecipitated with mouse anti-HA.11 or anti-MYC antibodies and anti-mouse antibody (IgG)-conjugated Sepharose. The coprecipitating Flag epitope-tagged NS3 protein was detected by Western blot analysis using an anti-Flag MAb. F-NS3 interacted with Casp8m-HA (Fig. 4A, lane 2) but not with Casp2m-HA, Casp3m-MYC, or Casp9m-MYC (Fig. 4A, lanes 3 to 5). F-NS3 was not precipitated when incubated with anti-mouse IgG-conjugated Sepharose alone (Fig. 4A, lane 1). All of the proteins were expressed in the lysates (Fig. 4B to D). In the converse experiment, lysates were immunoprecipitated with anti-Flag MAb Sepharose; anti-caspase-8, anti-HA.11, anti-caspase-3, and anti-caspase-9 antibodies were used for detection of caspases that coimmunoprecipitated with F-NS3. Casp8m-HA, but not Casp2m-HA, Casp3m-MYC, or Casp9m-MYC, coprecipitated with NS3 (Fig. 4E).
FIG. 4.
LGT virus NS3 protein binds to human caspase-8. 293T cells were transfected with plasmids expressing F-NS3 and Casp8m-HA, Casp2m-HA, Casp3m-MYC, or Casp9m-MYC, as indicated. At 18 h after transfection, cell lysates were incubated with mouse anti-HA.11 (lanes 1 to 3) or anti-MYC (lanes 4 and 5) MAb and immunoprecipitated by anti-mouse IgG-conjugated Sepharose beads. Bound proteins were separated by SDS-polyacrylamide gel electrophoresis, transferred to a membrane, and probed with anti-Flag MAb to detect coprecipitating F=NS3 (A). Expression of the epitope-tagged proteins in cells was confirmed by immunoblotting aliquots of cell lysates with anti-Flag (B), anti-HA.11 (C), or anti-MYC (D) MAb. (E) 293T cells were transfected with plasmids expressing epitope-tagged proteins, and cell lysates were immunoprecipitated with anti-Flag MAb. Immunoblotting was performed with anti-caspase-8 (lanes 1 and 2), anti-HA.11 (lanes 3 and 4), anti-caspase-3 (lanes 5 and 6), and anti-caspase-9 (lanes 7 and 8) MAb. Caspases are indicated by asterisks. (F) LGT virus NS3 immunoprecipitates with wild-type caspase-8. 293T cells were transfected with plasmids expressing F-NS3 and Casp2-HA or Casp8-HA. Cell lysates were precipitated with the anti-HA.11 antibody, and precipitates were blotted with the mouse anti-Flag antibody.
To verify that NS3 can bind to active caspase-8, 293T cells were cotransfected with Casp8-HA and F-NS3 in the presence of Z-VAD-FMK, an inhibitor of apoptosis. NS3 coimmunoprecipitated with Casp8-HA but not with Casp2-HA (Fig. 4F).
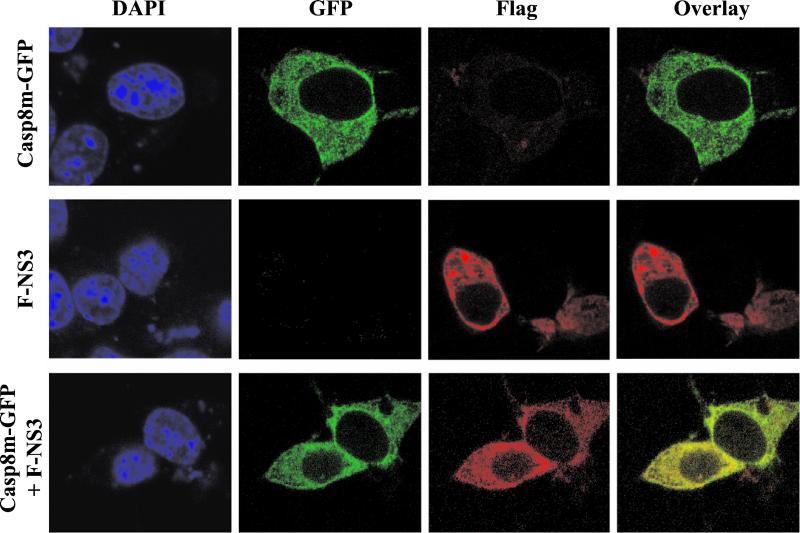
Since LGT virus NS3 binds to caspase-8, these two proteins would be expected to occupy the same subcellular compartment when expressed together. Neuro-2a cells were transfected with plasmids expressing Casp8m-GFP, F-NS3, or both proteins together and analyzed in immunofluorescence assays. F-NS3 was localized in the cytoplasm of cells transfected with F-NS3 alone (Fig. 5), similar to the pattern observed with NS3. In the absence of F-NS3, Casp8m-GFP displayed a diffuse staining pattern in the cytoplasm. When Neuro-2a cells were cotransfected with both genes, the two proteins colocalized in the cytoplasm, as judged by the yellow observed in the overlaid image (Fig. 5).
FIG. 5.
Subcellular colocalization of LGT virus NS3 protein and caspase-8. Neuro-2a cells were transfected with plasmids expressing Casp8m-GFP and F-NS3. At 18 h after transfection, the cells were fixed in methanol and incubated with DAPI to visualize nuclei and a mouse anti-Flag MAb followed by rhodamine-conjugated goat anti-mouse IgG to visualize F-NS3. The same field of cells is shown with confocal microscopy for both DAPI staining and immunofluorescence (GFP, rhodamine, and overlay of GFP and rhodamine).
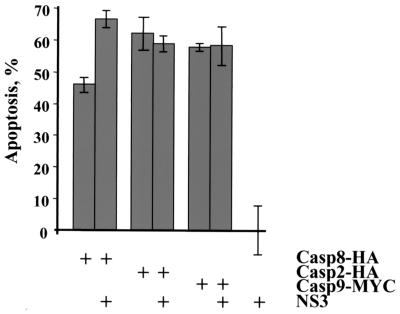
Since caspase-8-binding proteins are known to regulate the activity of caspase-8 (3, 37), it was of interest to determine if NS3 affects caspase-8-mediated apoptosis. The LGT virus NS3 protein was coexpressed with caspase-2, -8, or -9 in Neuro-2a cells. Expression of NS3 alone at 24 h did not induce apoptosis (Fig. 2). However, coexpression of NS3 with Casp8-HA resulted in a 20% increase in the number of apoptotic cells 24 h after transfection, compared to expression of Casp8-HA alone (Fig. 6). NS3 did not augment or inhibit caspase-2- or caspase-9-mediated apoptosis. Thus, NS3 binds to caspase-8 and enhances caspase-8-mediated apoptosis.
FIG. 6.
LGT virus NS3 enhances caspase-8-mediated apoptosis. Neuro-2a cells were cotransfected with plasmids expressing NS3 and Casp8-HA, Casp2-HA, or Casp9-MYC, as indicated. Cells were fixed and stained with X-Gal 24 h after transfection to determine the percentage of apoptotic cells. The data are expressed relative to the level of apoptosis induced by NS3 alone (set at 0%), which is similar to the level for empty vector-transfected cells.
Two regions of NS3 bind to caspase-8.
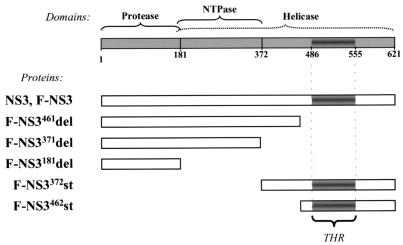
To determine which region(s) of NS3 is involved in binding to caspase-8, five deletion mutants were constructed and examined in immunoprecipitation studies. Three carboxy-terminal truncation mutants, proteins of 461, 371, and 181 aa residues (F-NS3461del, F-NS3371del, and F-NS3181del, respectively), have a deletion of the tetrahelix region (THR) and partial helicase and helicase-NTPase domains, respectively (Fig. 7). Two amino-terminal truncation mutants, proteins of 250 and 160 aa residues (F-NS3372st and F-NS3462st), contain the partial helicase domain and the THR, respectively. All constructs contained amino-terminal Flag epitopes to facilitate their detection. Four of the deletion mutants, F-NS3461del, F-NS3371del, F-NS3372st, and F-NS3462st, were expressed at levels comparable to that at which the wild-type F-NS3 protein was expressed (Fig. 8C, lanes 2 to 4, 6, and 7). F-NS3181del was expressed at a higher level than F-NS3 (Fig. 8C, lanes 2 and 5).
FIG. 7.
Schematic representation of NS3 domains and NS3 mutants. Numbers refer to amino acid locations in the LGT virus NS3, and the THR is indicated.
FIG. 8.
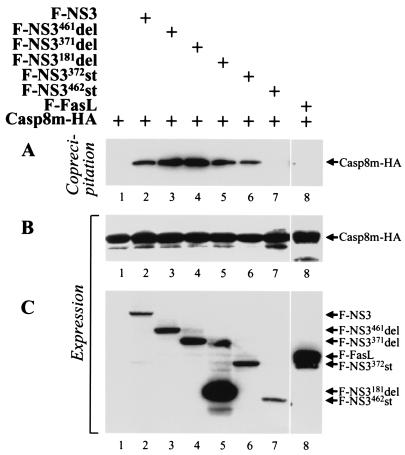
LGT virus NS3 protein contains two caspase-8-binding regions. 293T cells were cotransfected with plasmids expressing Casp8m-HA and F-NS3, Flag-tagged, truncated NS3 mutants, or Flag-tagged Fas ligand. Cell lysates were immunoprecipitated with anti-Flag MAb Sepharose, and bound proteins were separated by SDS-polyacrylamide gel electrophoresis, transferred onto a membrane, and probed with anti-HA.11 MAb to detect coimmunoprecipitating Casp8m-HA (A). Expression of the epitope-tagged proteins in cells was confirmed by immunoblotting aliquots of cell lysates with anti-HA.11 (B) or anti-Flag (C) MAb.
The F-NS3 mutants were coexpressed with Casp8m-HA in 293T cells, and, 18 h after transfection, Flag-tagged proteins were precipitated with anti-Flag M2 MAb Sepharose. Coprecipitating Casp8m-HA was detected by Western blot assay using an anti-HA.11 antibody. Casp8m-HA bound the control protein, F-NS3, as well as four mutants, F-NS3461del, F-NS3371del, F-NS3181del, and F-NS3372st (Fig. 8A, lanes 2 to 6). THR-containing protein F-NS3462st was not coprecipitated with Casp8m-HA (Fig. 8A, lane 7). A Flag-tagged version of FasL (F-FasL) served as a negative control, which was also not coprecipitated with Casp8m-HA. Thus, caspase-8 binds to the protease and helicase domains of NS3.
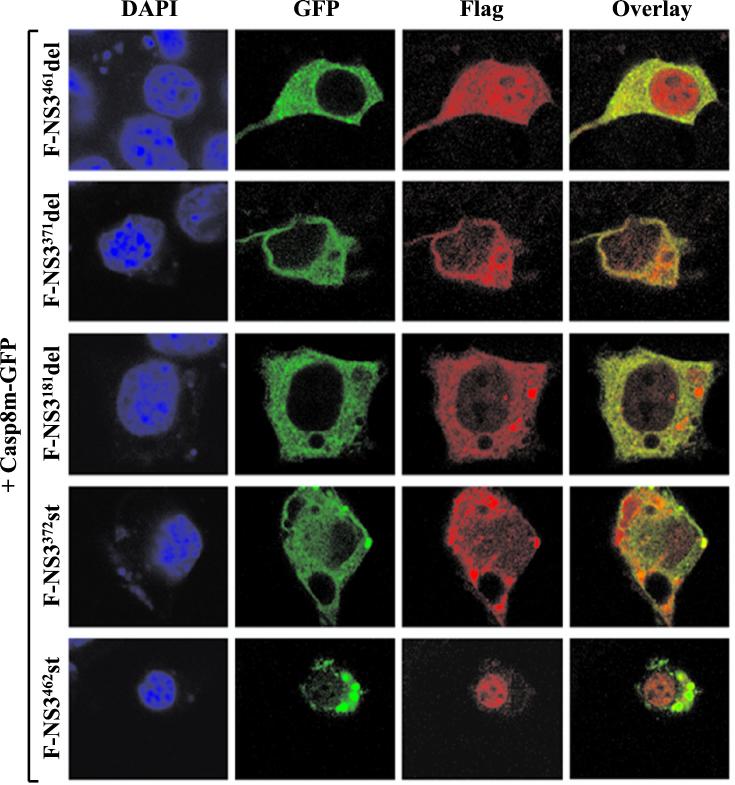
Immunofluorescence assays were performed to determine the subcellular locations of F-NS3 mutants in the presence of caspase-8. F-NS3371del, F-NS3181del, and F-NS3372st had a diffuse cytoplasmic staining pattern similar to that of full-length F-NS3, while F-NS3461del displayed a diffuse staining pattern in the cytoplasm and the nucleus. These four mutants colocalized with Casp8m-GFP in the cytoplasm (Fig. 9). In contrast, F-NS3462st, which did not coprecipitate with caspase-8, was detected in the nuclei of transfected cells and did not colocalize with the Casp8m-GFP protein.
FIG. 9.
Subcellular localization of F-NS3 mutants with Casp8m-GFP. Neuro-2a cells were cotransfected with plasmids expressing Casp8m-GFP and F-NS3461del, F-NS3371del, F-NS3181del, F-NS3372st, or F-NS3462st. At 18 h after transfection, cells were fixed and stained with mouse anti-Flag MAb and rhodamine-conjugated goat anti-mouse IgG to visualize F-NS3 mutants. The same field of cells was examined by confocal microscopy for both DAPI staining and immunofluorescence (GFP, rhodamine, and overlay of GFP and rhodamine).
The protease domain of NS3 induces apoptosis.
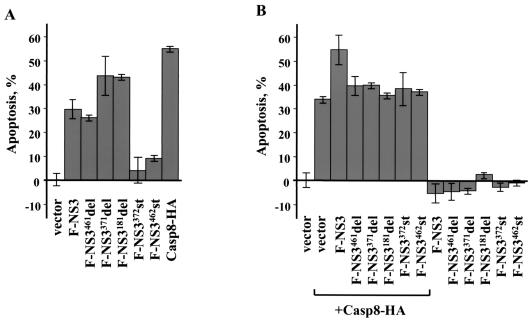
To identify a region(s) in NS3 important for induction of apoptosis, Neuro-2a cells were transfected with plasmids expressing NS3 mutants. Casp8-HA and F-NS3 served as a positive control for apoptosis. At 72 h after transfection, each of the mutants containing the NS3 protease domain induced apoptosis (Fig. 10A). The level of apoptosis induced by F-NS3461del was similar to that observed in the cells expressing F-NS3. Transfection with F-NS3371del or F-NS3181del genes resulted in an approximately 1.5-fold increase in the number of apoptotic cells compared to transfection with F-NS3. There was no induction of apoptosis in cells transfected with F-NS3372st and F-NS3462st genes. In addition, transfection of Neuro-2a cells with a construct expressing a mutant with a carboxy-terminal truncation of the protease domain (aa residues 1 to 99) failed to induce apoptosis of the cells (unpublished data). Thus, the intact protease domain (aa residues 1 to 181) is required for induction of apoptosis by the protein.
FIG. 10.
Induction of apoptotic activities by LGT virus NS3 mutants in Neuro-2a cells. Cells were transfected with plasmids expressing NS3 mutant proteins (A) and/or Casp8-HA (B) and then fixed, stained with X-Gal, and counted to determine the percentage of apoptotic cells at 72 h (A) and 24 h (B) after transfection. Empty vector-transfected cells served as a negative control for apoptosis.
To determine which region(s) of the LGT virus NS3 protein contributes to augmentation of caspase-8-induced apoptosis, Neuro-2a cells were cotransfected with plasmids expressing Casp8-HA and NS3 mutants. No augmentation of caspase-8-induced apoptosis was observed in cells coexpressing any of the mutant proteins with Casp8-HA at 24 h (Fig. 10B), suggesting that multiple regions of NS3 are required for augmentation of apoptosis.
DISCUSSION
In the present study, we show that the LGT virus NS3 protein induces apoptosis and binds to caspase-8. The time course of NS3-induced apoptosis was cell type dependent. In Vero cells, apoptosis was evident at 24 h after transfection, while in Neuro-2a cells, apoptotic cell death was not observed until 48 h after transfection. RNA viruses are thought to induce apoptosis to facilitate release of virions. However, apoptosis needs to be delayed long enough for the virus to complete its replication cycle and to produce sufficient quantities of progeny (reviewed in reference 28). Prior experiments showed that infection of Vero and Neuro-2a cells with the LGT virus resulted in release of infectious virus at 24 h in both cell lines, although LGT virus replicates to peak titers in Vero cells that are about 10-fold higher than those in Neuro-2a cells (25). These differences might be important for LGT virus infection in different cell types in vivo but may be less important for cells in culture.
We found that NS3 coimmunoprecipitated with caspase-8 and localized to the same cellular compartment as caspase-8. NS3 augmented caspase-8-induced apoptosis, and addition of caspase inhibitors to NS3-transfected cells showed that a caspase-8-specific inhibitor but not a caspase-9-specific inhibitor could prevent NS3-induced apoptosis. Caspase-8 contains two death effector domains (DEDs) that bind to the DED of FADD to mediate apoptosis. A number of large DNA viruses, such as molluscum contagiosium virus and several herpesviruses, including Kaposi's sarcoma-associated herpesvirus, equine herpesvirus 2, and bovine herpesvirus 4, encode proteins with DEDs that bind to caspase-8 (3, 13, 31). These viral FLIPs (FLICE-inhibitory proteins) inhibit apoptosis through the interactions of their DEDs with the DEDs in FADD and caspase-8. However, none of the RNA viruses, including LGT virus, is known to contain a DED. Thus, our finding that the LGT virus NS3 protein interacts with caspase-8 suggests a different type of protein-protein interaction than that observed for the DNA virus proteins that bind to caspase-8.
Recently, a cellular protein that, like the LGT virus NS3, also binds to caspase-8 but lacks a DED has been isolated (40). This protein is termed the DED-associated factor (DEDAF). As found for the LGT virus NS3, cotransfection of DEDAF with a DED-containing protein enhances the ability of the DED-containing protein to mediate apoptosis.
LGT virus NS3 did not bind to caspase-2 or caspase-9 or augment apoptosis induced by these caspases. Caspases 2 and 9 do not contain DEDs; instead each has a caspase-recruitment domain (CARD) that interacts with the CARD of Apaf-1 to mediate apoptosis. CARD-containing adapter proteins are known to activate caspases and NF-κB (reviewed in reference 21). Since the LGT virus NS3 THR has weak homology to CARDs, we tested the LGT virus NS3 protein for its ability to induce NF-κB activation. Cotransfection of 293T cells with both NS3 and an NF-κB reporter construct failed to transactivate the NF-κB reporter (data not shown).
Previously, we showed that the LGT virus major envelope protein induced apoptosis through a caspase-3-like pathway (25). LGT virus E protein induced apoptosis in both Vero and Neuro-2a cells by 18 h after transfection, while NS3 induced apoptosis in Vero cells more rapidly than in Neuro-2a cells. These results suggest that the LGT virus E protein and NS3 protein induce apoptosis through different pathways. Other RNA viruses encode more than one protein that induce apoptosis. Proteases 2A and 3C of poliovirus, envelope glycoproteins E1 and E2 of Sindbis virus, and four proteins of human immunodeficiency virus, gp120, gp41, Tat, and Vpr, induce apoptosis (1, 8, 9, 14, 16, 22, 39). While it is uncertain why viruses have more than one protein that induce apoptosis, different proteins may provide selective advantage for a virus in different environments.
Earlier we showed that expression of the LGT virus NS2B-NS3 protein did not induce apoptosis in either Vero or Neuro-2a cells (25). Here we show that the NS3 protein binds to caspase-8 and induces apoptosis in these cells. Since the NS3 proteins in other flaviviruses bind to NS2B or NS5 to mediate its proteolytic or NTPase and helicase activities (reviewed in reference 19), the binding of the LGT virus NS3 to other viral or cellular proteins may also be important for regulating its apoptotic activity.
Acknowledgments
G. G. Prikhod'ko and E. A. Prikhod'ko contributed equally to this work.
We are grateful to Charles M. Zacharchuk, Safraz A. Memon, Richard M. Siegel, and Lilia L. Bi for plasmids expressing caspase and FasL genes, to Mir Ali for assistance in constructing truncation mutants of the protease domain, to Owen Schwartz for help with confocal microscopy, and to Alexander E. Gorbalenya for valuable suggestions.
REFERENCES
- 1.Barco, A., E. Feduchi, and L. Carrasco. 2000. Poliovirus protease 3Cpro kills cells by apoptosis. Virology 266:352-360. [DOI] [PubMed] [Google Scholar]
- 2.Bazan, J. F., and R. J. Fletterick. 1989. Detection of a trypsin-like serine protease domain in flaviviruses and pestiviruses. Virology 171:637-639. [DOI] [PubMed] [Google Scholar]
- 3.Bertin, J., R. C. Armstrong, S. Ottilie, D. A. Martin, Y. Wang, S. Banks, G.-H. Wang, T. G. Senkevich, E. S. Alnemri, B. Moss, M. J. Lenardo, K. J. Tomaselli, and J. I. Cohen. 1997. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc. Natl. Acad. Sci. USA 94:1172-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell, M. S., and A. G. Pletnev. 2000. Infectious cDNA clones of Langat tick-borne flavivirus that differ from their parent in peripheral neurovirulence. Virology 269:225-237. [DOI] [PubMed] [Google Scholar]
- 5.Chambers, T. J., R. C. Weir, A. Grakoui, D. W. McCourt, J. F. Bazan, R. J. Fletterick, and C. Rice. 1990. Evidence that the N-terminal domain of nonstructural protein NS3 from yellow fever virus is a serine protease responsible for site-specific cleavages in the viral polyprotein. Proc. Natl. Acad. Sci. USA 87:8898-8902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clum, S., K. E. Ebner, and R. Padmanabhan. 1997. Cotranslational membrane insertion of the serine proteinase precursor NS2B-NS3(Pro) of dengue virus type 2 is required for efficient in vitro processing and is mediated through the hydrophobic regions of NS2B. J. Biol. Chem. 272:30715-30723. [DOI] [PubMed] [Google Scholar]
- 7.Cui, T., R. J. Sugrue, Q. Xu, A. K. Lee, Y. C. Chan, and J. Fu. 1998. Recombinant dengue virus type 1 NS3 protein exhibits specific viral RNA binding and NTPase activity regulated by the NS5 protein. Virology 246:409-417. [DOI] [PubMed] [Google Scholar]
- 8.Gaynor, E. M., and I. S. Y. Chen. 2001. Analysis of apoptosis induced by HIV-1 Vpr and examination of the possible role of the hHR23A protein. Exp. Cell Res. 267:243-257. [DOI] [PubMed] [Google Scholar]
- 9.Goldstaub, D., A. Gradi, Z. Bercovitch, Z. Grosmann, Y. Nophar, S. Luria, N. Sonenberg, and C. Kahana. 2000. Poliovirus 2A protease induces apoptotic cell death. Mol. Cell. Biol. 20:1271-1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorbalenya, A. E., A. P. Donchenko, E. V. Koonin, and V. M. Blinov. 1989. N-terminal domains of putative helicases of flavi- and pestiviruses may be serine proteases. Nucleic Acids Res. 17:3889-3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorbalenya, A. E., E. V. Koonin, A. P. Donchenko, and V. M. Blinov. 1989. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 17:4713-4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hlaing, T., R.-F. Guo, K. A. Dillet, J. M. Loussia, T. A. Morrish, M. M. Shi, C. Vincenz, and P. A. Ward. 2001. Molecular cloning and characterization of DEFCAP-L and -S, two isoforms of a novel member of the mammalian Ced-4 family of apoptosis proteins. J. Biol. Chem. 276:9230-9238. [DOI] [PubMed] [Google Scholar]
- 13.Irmler, M., M. Thome, M. Hahne, P. Schneider, K. Hofmann, V. Steiner, J. L. Bodmer, M. Schroter, K. Burns, C. Mattmann, D. Rimoldi, L. E. French, and J. Tschopp. 1997. Inhibitor of death receptor signals by cellular FLIP. Nature 388:125-126. [DOI] [PubMed] [Google Scholar]
- 14.Joe, A. K., H. H. Foo, L. Kleeman, and B. Levine. 1998. The transmembrane domains of Sindbis virus envelope glycoproteins induce cell death. J. Virol. 72:3935-3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kadare, G., and A.-L. Haenni. 1997. Virus-encoded RNA helicases. J. Virol. 71:2583-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koga, Y., M. Sasaki, H. Yoshida, H. Wigzell, G. Kimura, and K. Nomoto. 1990. Cytopathic effect determined by the amount of CD4 molecules in human cell lines expressing envelope glycoprotein of HIV. J. Immunol. 144:94-102. [PubMed] [Google Scholar]
- 17.Kuo, M. D., C. Chin, S. L. Hsu, J. Y. Shiao, T. M. Wang, and J. H. Lin. 1996. Characterization of the NTPase activity of Japanese encephalitis virus NS3 protein. J. Gen. Virol. 77:2077-2084. [DOI] [PubMed] [Google Scholar]
- 18.Li, H., S. Clum, S. You, K. E. Ebner, and R. Padmanabhan. 1999. The serine protease and RNA-stimulated nucleoside triphosphatase and RNA helicase functional domains of dengue virus type 2 NS3 converge within a region of 20 amino acids. J. Virol. 73:3108-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindenbach, B. D., and C. M. Rice. 2001. Flaviviridae: the viruses and their replication, p. 991-1041. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott, Williams & Wilkins Publishers, Philadelphia, Pa.
- 20.Lobigs, M. 1992. Proteolytic processing of a Murray Valley encephalitis virus non-structural polyprotein segment containing the viral proteinase: accumulation of a NS3-4A precursor which requires mature NS3 for efficient processing. J. Gen. Virol. 73:2305-2312. [DOI] [PubMed] [Google Scholar]
- 21.Martin, S. J. 2001. Dealing the CARDs between life and death. Trends Cell Biol. 11:188-189. [DOI] [PubMed] [Google Scholar]
- 22.Park, I.-W., C. K. Ullrich, E. Schoenberger, R. K. Ganju, and J. E. Groopman. 2001. HIV-1 Tat induces microvascular endothelial apoptosis through caspase activation. J. Immunol. 167:2766-2771. [DOI] [PubMed] [Google Scholar]
- 23.Preugschat, F., C.-W. Yao, and J. H. Strauss. 1990. In vitro processing of dengue virus type 2 nonstructural proteins NS2A, NS2B, and NS3. J. Virol. 64:4364-4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prikhod'ko, G. G., Y. Wang, E. Freulich, and L. K. Miller. 1999. Baculovirus p33 binds human p53 and enhances p53-mediated apoptosis. J. Virol. 73:1227-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prikhod'ko, G. G., E. A. Prikhod'ko, J. I. Cohen, and A. G. Pletnev. 2001. Infection with Langat flavivirus or expression of the envelope protein induces apoptotic cell death. Virology 286:328-335. [DOI] [PubMed] [Google Scholar]
- 26.Pugachev, K. V., N. Y. Nomokonova, E. Y. Dobrikova, and Y. I. Wolf. 1993. Site-directed mutagenesis of the tick-borne encephalitis virus NS3 gene reveals the putative serine protease domain of the NS3 protein. FEBS Lett. 328:115-118. [DOI] [PubMed] [Google Scholar]
- 27.Takegami, T., D. Sakamuro, and T. Furukawa. 1995. Japanese encephalitis virus nonstructural protein NS3 has RNA binding and ATPase activities. Virus Genes 9:105-112. [DOI] [PubMed] [Google Scholar]
- 28.Teodoro, J. G., and P. E. Branton. 1997. Regulation of apoptosis by viral gene products. J. Virol. 71:1739-1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Utama, A., H. Shimizu, S. Morikawa, F. Hasebe, K. Morita, A. Igarashi, M. Hatsu, K. Takamizawa, and T. Miyamura. 2000. Identification and characterization of the RNA helicase activity of Japanese encephalitis virus NS3 protein. FEBS Lett. 465:74-78. [DOI] [PubMed] [Google Scholar]
- 30.Valle, R. P. C., and B. Falgout. 1998. Mutagenesis of the NS3 protease of dengue virus type 2. J. Virol. 72:624-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, G.-H., J. Bertin, Y. Wang, D. A. Martin, J. Wang, K. J. Tomaselli, R. C. Armstrong, and J. I. Cohen. 1997. Bovine herpesvirus 4 BORFE2 protein inhibits Fas- and tumor necrosis factor receptor 1-induced apoptosis and contains death effector domains shared with other gamma-2 herpesviruses. J. Virol. 71:8928-8932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warrener, P., J. K. Tamura, and M. S. Collett. 1993. RNA-stimulated NTPase activity associated with yellow fever virus NS3 protein expressed in bacteria. J. Virol. 67:989-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wengler, G., G. Czaya, P. M. Färber, and J. H. Hegemann. 1991. In vitro synthesis of West Nile virus proteins indicates that the amino-terminal segment of the NS3 protein contains the active centre of the protease which cleaves the viral polyprotein after multiple basic amino acids. J. Gen. Virol. 72:851-858. [DOI] [PubMed] [Google Scholar]
- 34.Wengler, G., and G. Wengler. 1991. The carboxy-terminal part of the NS3 protein of the West Nile flavivirus can be isolated as a soluble protein after proteolytic cleavage and represents an RNA-stimulated NTPase. Virology 184:707-715. [DOI] [PubMed] [Google Scholar]
- 35.Wengler, G., and G. Wengler. 1993. The NS3 nonstructural protein of flaviviruses contains an RNA triphosphatase activity. Virology 197:265-273. [DOI] [PubMed] [Google Scholar]
- 36.Westaway, E. G., J. M. Mackenzie, M. T. Kenney, M. K. Jones, and A. A. Khromykh. 1997. Ultrastructure of Kunjin virus-infected cells: colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J. Virol. 71:6650-6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang, X., H. Y. Chang, and D. Baltimore. 1998. Autoproteolytic activation of pro-caspases. Mol. Cell 1:319-325. [DOI] [PubMed] [Google Scholar]
- 38.Yao, N., T. Hesson., C. Cable, Z. Hong, A. D. Kwong, H. V. Le, and P. C. Weber. 1997. Structure of the hepatitis C virus RNA helicase domain. Nat. Struct. Biol. 4:463-467. [DOI] [PubMed] [Google Scholar]
- 39.Yao, Q., R. W. Compans, and C. Chen. 2001. HIV envelope proteins differentially utilize CXCR4 and CCR5 coreceptors for induction of apoptosis. Virology 285:128-137. [DOI] [PubMed] [Google Scholar]
- 40.Zheng, L., O. Schickling, M. E. Peter, and M. J. Lenardo. 2001. The death effector domain-associated factor (DEDAF) plays distinct regulatory roles in the nucleus and cytoplasm. J. Biol. Chem. 276:31945-31952. [DOI] [PubMed] [Google Scholar]