Abstract
The molecular classification of the porcine enterovirus (PEV) groups II and III was investigated. The sequence of the almost complete PEV-8 (group II) genome reveals that this virus has unique L and 2A gene regions. A reclassification of this group into a new picornavirus genus is suggested. PEV group III viruses are typical enteroviruses. They differ from other enteroviruses by a prolonged stem-loop D of the 5′-cloverleaf structure.
Porcine enteroviruses (PEVs), which are the causative agents of neurological disorders, fertility disorders, and dermal lesions of swine (5, 6, 13, 26), were previously divided into three groups according to the physicochemical properties of the virions, the type of cytopathic effect, and their growth properties in certain host cell lines (14, 31). Sequencing of almost the complete genomes of all group I prototype strains revealed a phylogenetically distinct virus group (4, 9, 30). Consequently, PEV-1 was reclassified as porcine teschovirus 1 (PTV-1) (species Porcine teschovirus, genus Teschovirus), and the remaining serotypes of PEV group I were proposed as tentative members of the same species within this new genus (12, 30). Available sequence information from Peng and coworkers (unpublished data; GenBank accession no. Y14459 and AJ001391), Lin and coworkers (unpublished data; accession no. AJ250675), and Kaku et al. (10) indicates that the viruses of group III belong to the Enterovirus genus, while PEV-8 (group II) does not. However, the lack of comprehensive sequence data—which are necessary for a molecular classification—prompted us to determine the sequence of the complete genome of the PEV-10 prototype strain LP54 and the almost complete genome of the PEV-8 prototype strain V13. Partial sequences of the 5′ nontranslated regions (5′-NTRs) and the L-encoding gene region of the PEV-8 field isolates 16-SX, 26-TXII, 39-VII, Sek 1562/98, and partial 5′-NTR sequences of the PEV-9 prototype strain UKG/410/73 and the PEV-9 field isolate UKG/216/80 were added to the data collection to ensure the significance of the sequence data.
The prototype strains V13, UKG/410/73, and LP54 were obtained from the Institute for Animal Health Pirbright Laboratory (Pirbright, United Kingdom). The field isolates UKG/216/80 (Institute for Animal Health Pirbright Laboratory); Sek 1562/98 (Institut für Tierzucht, Tierhaltung und Tiergesundheit, Oldenburg, Germany); and 16-SX, 26-TXII, and 39-VII (Landesveterinär- und Lebensmitteluntersuchungsamt Sachsen-Anhalt, Stendal, Germany) were serologically typed by an indirect immunofluorescence assay by using a set of serotype-specific monoclonal antibodies (3). Viruses were propagated in porcine kidney (PS-EK) cells and porcine embryonic testes cells (EHF-R; CCLV RIE170). Prior to sequencing, RNA of virus-infected cells was prepared, reverse transcribed, and amplified essentially as described previously (30). The 5′ and 3′ ends of the viral genomes were amplified with the 5′/3′-RACE (rapid amplification of cDNA ends) kit of Roche Diagnostics, Mannheim, Germany. Based on cloned RACE fragments and available partial sequences (accession no. Y14459, AJ001391, AF250675, and AB049559), a set of 64 specific oligonucleotides were designed and used to amplify large DNA fragments up to 7 kbp. Both strands of the viral genomes were sequenced. Sequencing was done according to the cycle sequencing protocol with an ABI Inc. (Foster City, Calif.) Prism 310 sequencer. Sequences were submitted to GenBank (accession no. AF406813, AF363453, AF363454, and AF363455) and compared to other picornavirus sequences obtained from the GenBank or the Picornavirus Sequence Database (http://www.iah.bbsrc.ac.uk/virus/Picornaviridae/SequenceDatabase/Index.html). Sequences were aligned with the Clustal W program (25) and optimized manually. Neighbor-joining trees were calculated by the quartet puzzling method (23, 24) with the JTT substitution model for amino acid sequences (8). The reliability of clustering was tested by 10,000 iterations in the quartet puzzling method. For tree construction, maximum-likelihood branch lengths were calculated. RNA secondary predictions and free energy calculations were performed with the mfold program, version 3.0 (32).
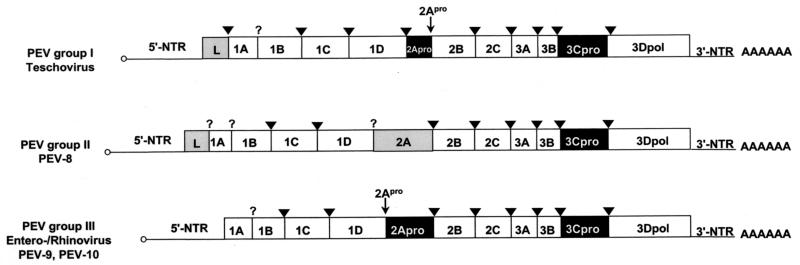
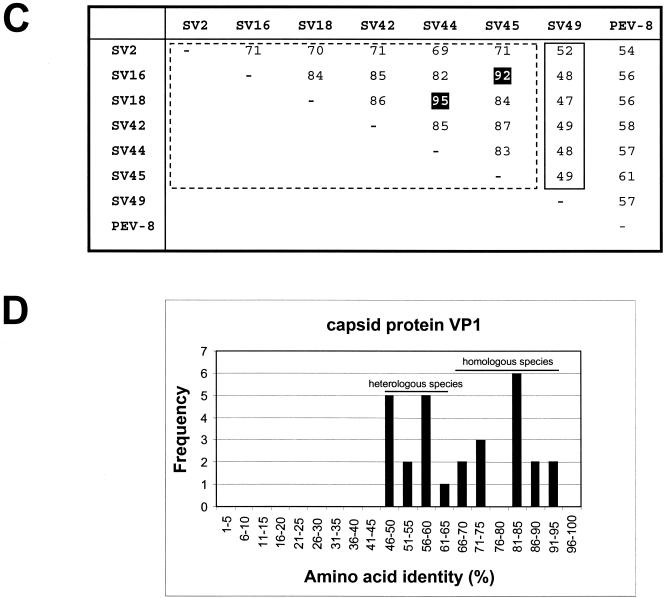
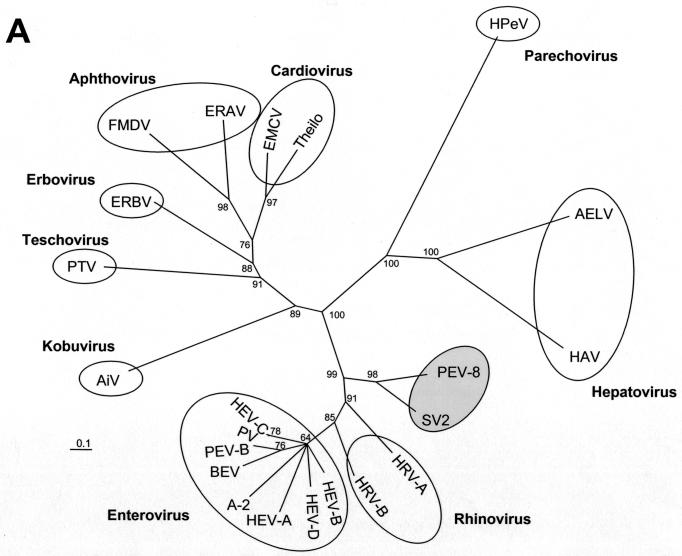
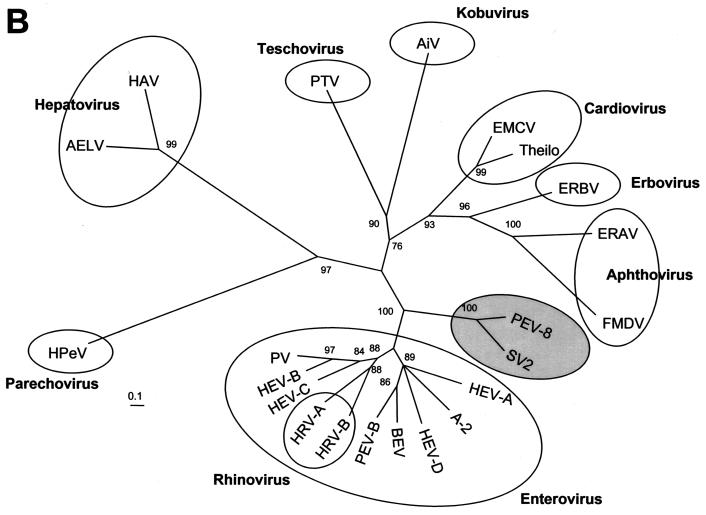
Our sequencing results indicate that the genome organization of the PEV group III (i.e., PEV-9 and -10) corresponds to the typical enterovirus or rhinovirus genome, while PEV group II (PEV-8) differs from the enteroviruses by two deviant features: the polyprotein is preceded by a leader protein, and the 2A protein has an unusual length of about 226 amino acids. Figure 1 compares the genome organizations of the three PEV groups. These differences in the genome organization should be considered as distinctive features of PEV group I (teschoviruses) and group II with respect to the enterovirus genus. According to M. H. V. van Regenmortel, genus demarcation criteria include differences in gene maps and variation in the fine structure of the proteins (27). Sequence alignments and phylogenetic comparisons of the capsid protein precursor (P1) and the 3D polymerase (3Dpol) amino acid sequences with those of each recognized and proposed picornavirus species reveal a close relationship of PEV-8 with the simian picornavirus SV2 and a distant relationship with all picornavirus genera (Fig. 2). Recently, the simian picornaviruses SV2, SV16, SV18, SV42, SV44, SV45, and SV49 were proposed to be members of a new picornavirus genus (18). Although related, PEV-8 and SV2 are clearly distinct from each other, as shown in Table 1. The amino acid identities of the capsid proteins range from 46 to 75% (P1, 61%). Pairwise comparison of the VP1 capsid proteins of these simian picornaviruses with PEV-8 VP1 (Fig. 2C) reveals the existence of three species. While a large cluster comprising SV2, SV16, SV18, SV42, SV44, and SV45 has amino acid identity values ranging from 71 to 95%, the amino acid identity values of SV49 and the large cluster range from 47 to 52%. Comparison of PEV-8 with the simian piocornaviruses results in amino acid identity values ranging from 54 to 61%. A discontinuous frequency distribution of the amino acid identity values (Fig. 2D) strongly supports the hypothesis that these viruses belong to three different species. In previous investigations, this approach was successfully applied to provide evidence for species distinction (19, 28).
FIG. 1.
Genome organization of the three PEV groups. The open reading frames are flanked on either side by NTRs. Gene regions (not drawn to scale) are presented. Gene regions encoding proteases are highlighted in black, and those encoding L proteins and the PEV-8 2A gene region are shaded in gray. Viruses of PEV group I, which were recently reclassified as teschoviruses, are characterized by a foot-and-mouth disease virus-like 2A peptidase and a leader protein of unknown function. PEV-8, the only known member of PEV group II, has a leader protein and a 2A protein of unknown function. A 90-nucleotide stretch of the 5′-NTR has a striking homology to the teschovirus 5′-NTR, while the 3′-NTR shares similarities with the PEV group III viruses. PEV-9 and -10 (PEV group III) are typical enteroviruses. Their genome organization is identical to that of entero- and rhinoviruses. The genome-linked 3B peptides at the 5′ end (small circles) and the poly(A) tails at the 3′ ends are indicated. The processing sites of 3C protease (arrowheads) and 2A protease (small arrows) are also shown. A question mark symbolizes unknown proteolytic activities responsible for the maturation cleavage of the 1AB precursor and the release of the PEV-8 leader protein.
FIG. 2.
Phylogenetic relationships of PEV-8 to other picornaviruses. Unrooted neighbor-joining trees of the 3D polymerase (A) and the P1 capsid proteins (B) of 22 picornavirus species. Amino acid sequences were aligned with the Clustal W program. Maximum-likelihood branch lengths were calculated by the quartet puzzling method. Branch lengths are proportional to genetic divergence. The scale bar indicates the number of amino acid substitutions per site. Circles indicate picornavirus genera. The proposed genus containing PEV-8 and SV2 is indicated by a shaded circle. Numbers at nodes represent percentages of bipartitions in intermediate trees that have been generated in 10,000 puzzling steps. Note that capsid protein sequences (B) are not suited to differentiate the enterovirus and rhinovirus genera. (C) Pairwise comparison of VP1 sequences of PEV-8 and seven simian picornaviruses. Percent amino acid identities are given. The dotted box indicates amino acid identity values of a major cluster of serotypes that likely represent a new species. The comparison of two strains of the same serotype results in high amino acid identity values, which are highlighted. The small box marks pairwise comparisons of SV49, with the major serotype cluster indicating that SV49 may belong to a different species. (D) Frequency distribution of pairwise amino acid identity scores of the VP1 capsid proteins. The amino acid identity scores of up to 65% are characteristic of comparisons of heterologous species, while amino acid identity scores above 66% indicate comparisons of (i) different strains of homologous serotypes or (ii) heterologous serotypes of homologous species. A-2, A-2 plaque virus; AELV, avian encephalomyelitis-like virus; AiV, aichivirus; BEV, bovine enterovirus; EMCV, encephalomyocarditis virus; ERAV, equine rhinitis A virus (formerly equine rhinovirus 1); ERBV, equine rhinitis B virus (formerly equine rhinovirus 2); FMDV, foot-and-mouth disease virus; HAV, hepatitis A virus; HEV, human enterovirus; HPeV, human parechovirus (formerly echoviruses 22 and 23); HRV, human rhinovirus; PV, poliovirus; SV, simian virus; Theilo, theilovirus.
TABLE 1.
Amino acid sequence relationships between PEV-8 and the other picornavirus species
| Genus | Species/serotypeg | % Amino acid identity
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L | 1AB | 1C | 1D | 2A | 2B | 2C | 3AB | 3C | 3D | ||
| Proposed new genusa | SV2 | 21 | 75 | 62 | 46 | 30 | 40 | 51 | 39 | 59 | 66 |
| Enterovirus | PV/PV1 | NDe | 42 | 35 | 21 | 14 | 10 | 37 | 27 | 47 | 53 |
| HEV-A/CVA16 | ND | 44 | 39 | 24 | 15 | 20 | 36 | 29 | 50 | 51 | |
| HEV-B/CVB3 | ND | 44 | 36 | 25 | 19 | 16 | 32 | 26 | 51 | 53 | |
| HEV-C/CVA21 | ND | 44 | 36 | 26 | 14 | 19 | 34 | 27 | 46 | 53 | |
| HEV-D/EV70 | ND | 43 | 38 | 21 | 15 | 11 | 35 | 26 | 51 | 54 | |
| BEV/BEV1 | ND | 44 | 39 | 19 | 14 | 22 | 34 | 25 | 45 | 51 | |
| PEV-B/PEV10 | ND | 43 | 40 | 18 | 19 | 14 | 33 | 31 | 51 | 53 | |
| A-2b | ND | 42 | 37 | 22 | 14 | 22 | 39 | 21 | 48 | 50 | |
| SV6c | NAf | NA | NA | 20 | NA | NA | NA | NA | NA | NA | |
| N203d | NA | NA | NA | 25 | NA | NA | NA | NA | NA | NA | |
| Rhinovirus | HRV-A/HRV1b | ND | 42 | 40 | 19 | 15 | 10 | 36 | 27 | 43 | 53 |
| HRV-B/HRV14 | ND | 44 | 31 | 20 | 11 | 17 | 37 | 21 | 48 | 53 | |
| Aphthovirus | FMDV/FMDV-A10 | 5 | 22 | 34 | 19 | ND | 7 | 25 | 7 | 16 | 32 |
| ERAV/ERAV | 1 | 26 | 31 | 7 | ND | 3 | 26 | 10 | 18 | 30 | |
| Cardiovirus | EMCV/EMCV-Mengo | 2 | 32 | 33 | 10 | 11 | 11 | 22 | 15 | 12 | 32 |
| Theilovirus/TMEV-DA | 2 | 30 | 36 | 12 | 10 | 15 | 23 | 16 | 15 | 31 | |
| Erbovirus | ERBV/ERBV | 8 | 29 | 33 | 15 | ND | 3 | 22 | 12 | 17 | 34 |
| Hepatovirus | HAV/HAV-LA | ND | 15 | 14 | 5 | 4 | 10 | 20 | 12 | 18 | 25 |
| AEV/AEV | ND | 18 | 21 | 9 | 4 | 14 | 21 | 3 | 17 | 29 | |
| Kobuvirus | Aichivirus/AiV | 4 | 24 | 22 | 8 | 5 | 13 | 17 | 6 | 16 | 32 |
| Parechovirus | HPeV/HPeV1 | ND | 9 | 13 | 16 | 4 | 7 | 19 | 5 | 16 | 25 |
| Teschovirus | PTV/PTV-1 | 2 | 28 | 25 | 9 | ND | 2 | 24 | 15 | 19 | 35 |
Proposed new genus that includes SV2, SV16, SV18, SV42, SV44, SV45, and SV49 (18).\
A-2 plaque virus is a member of a proposed new species that also includes SV4 and SV28.\
Proposed new species.\
N203 is member of a proposed new species that also includes N125.\
ND, not done.\
NA, sequences not available.\
Species abbreviations are defined in the legend to Fig. 2.
Some of the nonstructural proteins show a surprisingly low degree of sequence conservation: pairwise comparisons of the leader protein, 2A protein, and 3A protein of PEV-8 and SV2 result in amino acid identity values of 21, 30, and 32%, respectively. Among the most conserved nonstructural proteins are 3B (VPg) and the 3Dpol, with amino acid identity values of 68 and 66%, respectively. The alignment of the 2C+3CD nonstructural protein reveals 60% amino acid identity. Several sequence motifs are conserved: (i) the myristoylation motif (GxxxS) at the N terminus of the capsid protein VP4, (ii) the GxxGxGKS motif for nucleoside triphosphate (NTP) binding sites of 2C, the catalytic triad of the 3C protease (PEV-8, H40-E70-C146; PEV-10, H40-E71-C147), an RNA-binding motif of the 3C protease (KFRDI), and the SGxxxTxxxNSx29YGDD motif of RNA-dependent RNA polymerases. The comparison of PEV-8 to other picornavirus species reveals amino acid identity values ranging from 25% (parechoviruses and hepatoviruses) to 54% (enteroviruses and rhinoviruses) for the 3Dpol sequence (Table 1). Taken together, the differences in (i) genome organization, (ii) fine structure of the proteins, (iii) host range, and (iv) cell type specificity indicate that PEV-8, SV49, and SV2/SV16/SV18/SV42/SV44/SV45 may be considered as members of distinct species within a new picornavirus genus.
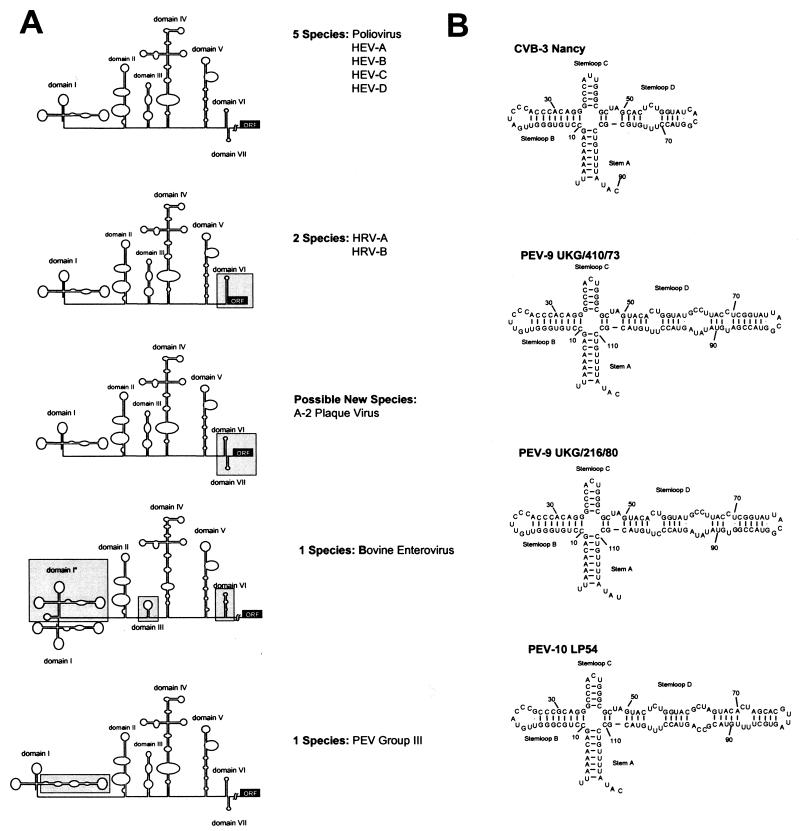
Enteroviruses and rhinoviruses are characterized by highly conserved RNA secondary structures, which serve in the initiation of replication and translation (22). This feature is commonly used for the group-specific detection of human enteroviruses (e.g., reference 11) and PEVs (29). However, the 5′-NTRs of the PEV group II and III viruses contain unique sequences. While the overall RNA folding pattern of the 5′-NTR is conserved for the sequenced PEV-9 and -10 strains, the 5′-cloverleaf of these viruses has a unique insertion leading to a prolongation of subdomain D of the cloverleaf (Fig. 3). Although the biologic function of the insertion is still unknown, this observation is of special interest, because subdomain D of poliovirus and rhinovirus 14 was previously identified to bind the 3C/3CD proteinase (1, 15). The resulting ribonucleoprotein complex is a prerequisite for the initiation of positive-strand RNA synthesis (2). This could hint at a modified mechanism of replication initiation or translation initiation.
FIG. 3.
Analysis of enteroviral and rhinoviral 5′-NTRs. (A) Five folding patterns of the 5′-NTRs of the enteroviral and rhinoviral species are schematically illustrated. With respect to the human enteroviruses, the rhinoviruses and the animal enteroviruses (including A-2 plaque virus as a possible new enterovirus species) show significant differences, which are boxed. (B) Comparison of the 5′ cloverleaf of a typical human enterovirus (CVB3) and sequenced virus strains of PEV group III. The stem-loop D of the PEVs is significantly prolonged.
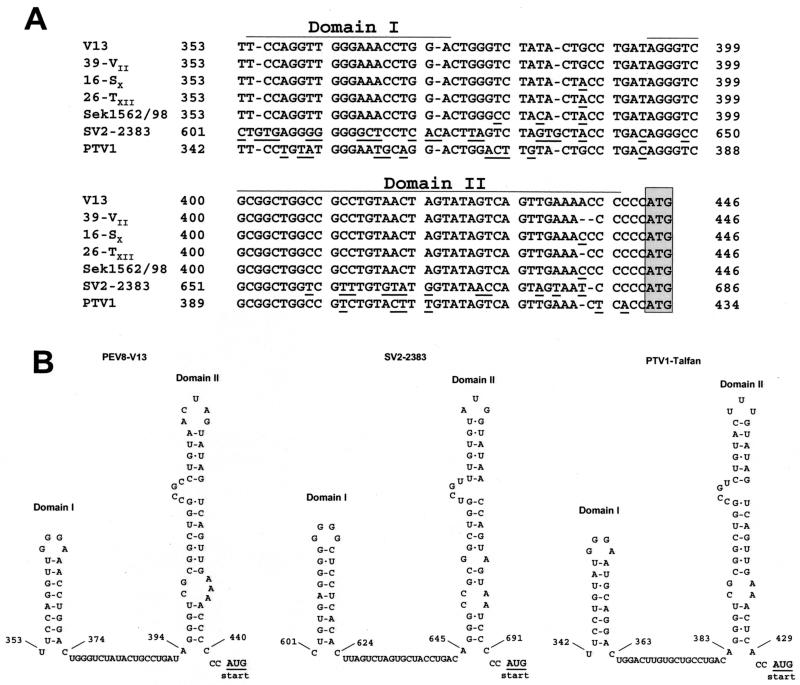
The 5′-NTR sequence of the PEV group II is still incomplete, because several attempts to determine its very 5′ end failed. However, the present sequence data of the 5′-NTR (partial sequence of 446 nucleotides) indicate that these viruses share no sequence homology to the enteroviruses. This explains the failure of enterovirus-specific PCR protocols to detect PEV group II viruses (29). Instead, a stretch of 90 nucleotides exhibits a striking homology to the PTVs. This genome region precedes the AUG start codon and allows the formation of two putative RNA secondary structures (Fig. 4). A covariance analysis including all sequenced PTV strains and four PEV-8 field isolates indicates that these putative secondary structures are conserved. The sequence homology of PEV-8 and PTV could reflect a convergent evolution in the attempt of the virus to achieve optimal adaptation to a common host. Another explanation could be a recombination event during the evolution of these viruses. The latter appears to be less likely, because, so far, recombination has only been observed for very closely related viruses.
FIG. 4.
Comparison of partial 5′-NTR sequences of PEV-8, SV2, and PTV-1. (A) Sequence alignment of five PEV-8 strains (V13, 39-VII, 16-SX, 26-TXII, and Sek1562/98), SV2, and PTV-1 Talfan. A homology of this part of the 5′-NTR is obvious. Deviant nucleotides are underlined. The proposed AUG start codon is boxed. (B) The highly conserved nucleotides allow the formation of two putative RNA secondary structures, which are likely to play a role in a specific mechanism of translation initiation of these viruses.
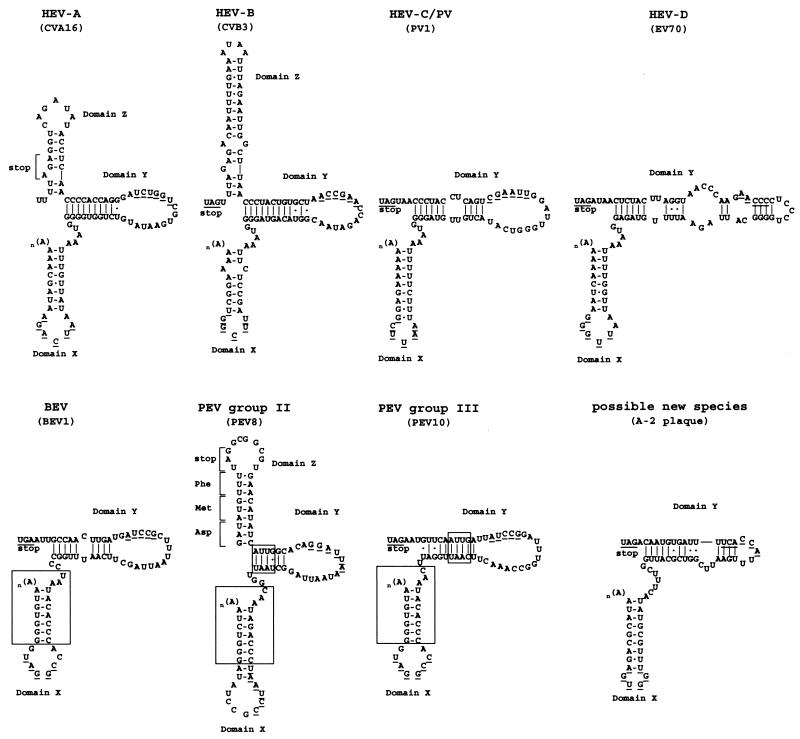
The 3′-NTRs of PEV group III and the bovine enteroviruses are considerably similar (Fig. 5). There is also a similarity of short sequence stretches between PEV groups II and III (boxed in Fig. 5). However, PEV-8 has a third putative stem-loop (designated stem-loop Z) that PEV-9 and -10 lack. Our proposal for the PEV-8 3′-NTR differs from the previously published proposal of Kaku et al. (10). The consideration of several nucleotides of the coding region of PEV-8 results in a folding pattern with a significantly higher free energy value (ΔG = −23.5 kcal/mol versus approximately −16.5 kcal/mol as previously suggested [10]). Recently, kissing interactions between domains X and Y of the 3′-NTR of poliovirus, coxsackievirus B3, and coxsackievirus A9 were suggested (16, 17, 20, 21). Likewise, the putative secondary structures of PEV-8, -9, and -10 allow the formation of a similar pseudoknot-like element, which appears to be conserved among all human and animal enteroviruses (Fig. 5).
FIG. 5.
Comparison of the putative 3′-NTR secondary structures of PEV groups II and III with those of other enterovirus species. HEV, human enterovirus. Each species is represented by a typical member (in parentheses). Large boxes indicate conserved sequences of bovine enterovirus (BEV) and PEV species, and small boxes emphasize additional similarities between PEV groups II and III. The putative domains are designated X, Y, and Z starting from the 3′ end. Nucleotides likely to be involved in the formation of a pseudoknot-like element are underlined.
Accumulation of picornavirus sequence data reveals that certain genome regions of the picornaviruses evolved independently from the other genome regions. Among these genome regions, the leader protein-encoding sequences and the 2A-encoding gene region are most striking. PEV-8, SV2, the teschoviruses, the cardioviruses, and aichivirus have unrelated leader proteins, each of unknown function (Table 1). Recently, the diversity of the picornavirus 2A proteins was described (7). In that study, picornaviruses were divided into four groups in terms of 2A function and sequence homology. In an extension of the proposal by Hughes and Stanway (7), the 2A protein of PEV-8 and SV2 would constitute a fifth type of 2A.
The recent proposal by the Picornavirus Study Group (12) that considers PEV-8 a member of the enterovirus species “PEV-A” should be revised. The data presented here strongly support the establishment of a new picornavirus genus. This reclassification should be done on the basis of a different genome map, unique L and 2A sequences, and a considerable degree of genetic distance from all recognized and proposed picornavirus genera. This new genus should contain three species—PEV-8, SV49, and SV2—together with the related simian picornaviruses SV16, SV18, SV42, SV44, and SV45. Our suggestion would extend a previous proposal for reclassification of simian picornaviruses (18). For consistency, PEV-9 and -10 should not be addressed as enterovirus species “PEV-B,” but as “PEV.”
Acknowledgments
We thank Nigel Ferris (Institute for Animal Health Pirbright Laboratory), Marlies Klopris (Institut für Tierzucht, Tierhaltung und Tiergesundheit, Oldenburg, Germany), and Wolfgang Peter (Landesveterinär- und Lebensmitteluntersuchungsamt Sachsen-Anhalt, Stendal, Germany) for the gifts of virus strains. We are indebted to Steven Oberste and Mark Pallansch (Centers for Disease Control and Prevention, Atlanta, Ga.) for the communication of the SV2 sequence prior to publication. The excellent technical assistance of Veronika Güntzschel and Sabine Wachsmuth is acknowledged.
REFERENCES
- 1.Andino, R., G. E. Rieckhoff, and D. Baltimore. 1990. A functional ribonucleoprotein complex forms around the 5′ end of poliovirus RNA. Cell 63:369-380. [DOI] [PubMed] [Google Scholar]
- 2.Andino, R., G. E. Rieckhoff, P. L. Achacoso, and D. Baltimore. 1993. Poliovirus RNA synthesis utilizes an RNP complex formed around the 5′-end of viral RNA. EMBO J. 12:3587-3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dauber, M. 1999. Identification of group I porcine enteroviruses by monoclonal antibodies in cell culture. Vet. Microbiol. 67:1-12. [DOI] [PubMed] [Google Scholar]
- 4.Doherty, M., D. Todd, N. McFerran, and E. M. Hoey. 1999. Sequence analysis of a porcine enterovirus serotype 1 isolate: relationships with other picornaviruses. J. Gen. Virol. 80:1929-1941. [DOI] [PubMed] [Google Scholar]
- 5.Dunne, H. W., J. L. Gobble, J. F. Hokanson, D. C. Kradel, and G. R. Bubash. 1965. Porcine reproductive failure associated with a newly identified “SMEDI” group of picornavirus. Am. J. Vet. Res. 26:1284-1297. [PubMed] [Google Scholar]
- 6.Harding, J. D. J., J. T. Done, and G. F. Kershaw. 1957. A transmissible polio-encephalomyelitis of pigs (Talfan disease). Vet. Rec. 69:824-832. [Google Scholar]
- 7.Hughes, P., and G. Stanway. 2000. The 2A proteins of three diverse picornaviruses are related to each other and to the H-rev107 family of proteins involved in the control of cell proliferation. J. Gen. Virol. 81:201-207. [DOI] [PubMed] [Google Scholar]
- 8.Jones, D. T., W. R. Taylor, and J. M. Thornton. 1992. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biol. Sci. 8:275-282. [DOI] [PubMed] [Google Scholar]
- 9.Kaku, Y., S. Yamada, and Y. Murakami. 1999. Sequence determination and phylogenetic analysis of RNA-dependent RNA polymerase (RdRp) of the porcine enterovirus 1 (PEV-1) Talfan strain. Arch. Virol. 144:1845-1852. [DOI] [PubMed] [Google Scholar]
- 10.Kaku, Y., A. Sarai, and Y. Murakami. 2001. Genetic reclassification of porcine enteroviruses. J. Gen. Virol. 82:417-424. [DOI] [PubMed] [Google Scholar]
- 11.Kämmerer, U., B. Kunkel, and K. Korn. 1994. Nested PCR for specific detection and rapid identification of human picornaviruses. J. Clin. Microbiol. 32:285-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King, A. M. Q., F. Brown, P. Christian, T. Hovi, T. Hyypiä, N. J. Knowles, S. M. Lemon, P. D. Minor, A. C. Palmenberg, T. Skern, and G. Stanway. 2000. Picornaviridae, p. 657-673. In M. H. V. van Regenmortel, C. M. Fauquet, D. H. L. Bishop, C. H. Calisher, E. B. Carsten, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy. Seventh Report of the International Committee for the Taxonomy of Viruses. Academic Press, New York, N.Y.
- 13.Knowles, N. J. 1988. The association of group III porcine enteroviruses with epithelial tissue. Vet. Rec. 122:441-442. [DOI] [PubMed] [Google Scholar]
- 14.Knowles, N. J., L. S. Buckley, and H. G. Pereira. 1979. Classification of porcine enteroviruses by antigenic analysis and cytopathic effects in tissue culture: description of 3 new serotypes. Arch. Virol. 62:201-208. [DOI] [PubMed] [Google Scholar]
- 15.Leong, L. E.-C., P. A. Walker, and A. G. Porter. 1993. Human rhinovirus-14 protease 3C (3Cpro) binds specifically to the 5′-noncoding region of the viral RNA. J. Biol. Chem. 268:25735-25739. [PubMed] [Google Scholar]
- 16.Melchers, W. J., J. G. J. Hoenderop, H. J. Bruins Slot, C. W. A. Pleij, E. V. Pilipenko, V. I. Agol, and J. M. D. Galama. 1997. Kissing of the two predominant hairpin loops in the coxsackie B virus 3′ untranslated region is the essential structural feature of the origin of replication required for negative-strand RNA synthesis. J. Virol. 71:686-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mirmomeni, M. H., P. J. H. Hughes, and G. Stanway. 1997. A tertiary structure in the 3′ untranslated region of enteroviruses is necessary for efficient replication. J. Virol. 71:2363-2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oberste, M. S., K. Maher, and M. A. Pallansch. 2002. Molecular phylogeny and proposed classification of the simian picornaviruses. J. Virol. 76:1244-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oberste, M. S., K. Maher, D. R. Kilpatrick, and M. A. Pallansch. 1999. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J. Virol. 73:1941-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilipenko, E. V., S. V. Maslova, A. N. Sinyakow, V. I. Agol. 1992. Towards identification of cis-acting elements involved in the replication of enterovirus and rhinovirus RNAs: a proposal for the existence of tRNA-like terminal structures. Nucleic Acids Res. 20:1739-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilipenko, E. V., K. V. Poperechny, S. V. Maslova, W. J. G. Melchers, H. J. Bruins Slot, and V. I. Agol. 1996. cis-element, oriR, involved in the initiation of (−)strand poliovirus RNA: a quasi-globular multi-domain RNA structure maintained by tertiary (kissing) interactions. EMBO J. 15:5428-5436. [PMC free article] [PubMed] [Google Scholar]
- 22.Rueckert, R. R. 1995. Picornaviridae: the viruses and their replication, p. 609-654. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 23.Strimmer, K., and A. von Haeseler. 1996. Quartet puzzling: a quartet maximum likelihood method for reconstructing tree topologies. Mol. Biol. Evol. 13:964-969. [Google Scholar]
- 24.Strimmer, K., N. Goldman, and A. von Haeseler. 1997. Bayesian probabilities and quartet puzzling. Mol. Biol. Evol. 14:210-211. [Google Scholar]
- 25.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trefny, L. 1930. Hromadna onemocnemi vepru na Tesinsku. Zverolek Obz. 23:235-236. [Google Scholar]
- 27.van Regenmortel, M. H. V. 2000. Introduction to the species concept in virus taxonomy, p. 3-16. In M. H. V. van Regenmortel, C. M. Fauquet, D. H. L. Bishop, C. H. Calisher, E. B. Carsten, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy. Seventh Report of the International Committee for the Taxonomy of Viruses. Academic Press, New York, N.Y.
- 28.van Regenmortel, M. H. V., D. H. L. Bishop, C. M. Fauquet, M. A. Mayo, J. Maniloff, and C. H. Calisher. 1997. Guidelines to the demarcation of virus species. Arch. Virol. 142:1505-1518. [PubMed] [Google Scholar]
- 29.Zell, R., A. Krumbholz, A. Henke, E. Birch-Hirschfeld, A. Stelzner, M. Doherty, E. Hoey, M. Dauber, D. Prager, and R. Wurm. 2000. Detection of porcine enteroviruses by nRT-PCR: differentiation of CPE groups I-III with specific primer sets. J. Virol. Methods 88:205-218. [DOI] [PubMed] [Google Scholar]
- 30.Zell, R., M. Dauber, A. Krumbholz, A. Henke, E. Birch-Hirschfeld, A. Stelzner, D. Prager, and R. Wurm. 2001. Porcine teschoviruses comprise at least eleven distinct serotypes: molecular and evolutionary aspects. J. Virol. 75:1620-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zoletto, R. 1965. Caratteristiche differenzialii degli enterovirus suini. Vet. Ital. 6:3-20. [Google Scholar]
- 32.Zuker, M., D. H. Mathews, and D. H. Turner. 1999. Algorithms and thermodynamics for RNA secondary structure prediction: a practical guide, p. 11-43. In J. Barciszewski and B. F. C. Clark (ed.), RNA biochemistry and bio/technology. NATO ASI Series. Kluwer Academic Publishers, Dordrecht, The Netherlands.