Abstract
Finger flexor tendon rehabilitation has come a long way, but further advances are possible. Ideally, a healing tendon should move, but under the minimum load necessary to achieve motion. It is possible to design suture repairs that minimize the friction between tendon and sheath while simultaneously maintaining adequate strength to provide a wide margin of safety during therapy. A looped, four-strand modified Kessler repair is a good example of this type of high-strength, low-friction repair. At the same time, rehabilitation methods can also be optimized. A new modified synergistic motion protocol is described in which wrist flexion and finger extension is alternated with wrist and metacarpophalangeal joint extension and finger interphalangeal joint flexion. Based on evidence from basic science studies, the authors hypothesize that this new protocol will deliver more effective proximal tension on the tendon repair than either passive flexion/active extension or synergistic protocols, and may be useful in patients who are not ready for, or are not reliable with, active motion or place and hold protocols. The scientific basis for these new methods is reviewed, and the concept of the “safe zone” for tendon loading, in which tendon motion occurs without gapping of the repair site, is developed.
It has become quite clear that tendon rehabilitation is a critical factor determining the quality of the result after finger flexor tendon repair. Repair technique is important, but it is the way in which the tendon is managed afterwards that determines the outcome. In animal models as well as in humans, it is clear that immobilization results in a soundly healed tendon with unacceptable motion, due to restrictive adhesions, while unrestrained normal motion nearly always results in tendon rupture1-3 In between is an interesting realm of protected motion, in which sound healing can occur in an environment that permits better final motion. The strength of such tendons is even better that that seen in immobilized tendons,3-5 leading some to speculate that tendon loading is beneficial, at some range below that experienced in full activity. However, recent studies suggest strongly that it is motion, rather than loading, that is the key.6,7 Tendons that move as they heal have better final motion and strength than those that don't move, and the difference is not improved by adding loading over and above the amount needed to initiate tendon gliding.
Based on the above understanding, recent research at Mayo Clinic has focused on methods to improve tendon gliding while minimizing the forces involved. These have been of two types: methods that create tendon repairs that are both strong and easy to mobilize, and methods to improve the precise application of minimal forces that assure tendon motion. Both methods are based in turn upon a clear understanding of tendon gliding, and the friction between tendon and tendon sheath. These topics will be explored in turn in the sections that follow.
TENDON FRICTION: WHAT IT IS, WHAT DETERMINES IT, AND HOW IT IS MEASURED
There are no frictionless interfaces. Whenever two objects move past each other while remaining in contact, energy is expended along the interface. In biologic tissues in which such movement occurs, a specialized gliding surface develops and synovial fluid is present. It is becoming clear that, whether the gliding is cartilage on cartilage or tendon on tendon sheath, the basic biologic strategies are very similar. Specifically, the gliding surface in both cases contains a fixed lubricating glycoprotein, called lubricin,8,9 in addition to the high-molecular-weight glycoprotein, aggrecan, which tends to disperse the collagen fibrils and makes the surface structure more resistant to compression10,11 This type of lubrication, called boundary lubrication, is fundamentally different from the lubrication provided by an intervening fluid film, which is called hydrodynamic lubrication9,12-14 Indeed, it may be that the synovial fluid, comprised chiefly of hyaluronan, is more of a high viscosity nutrient delivery vehicle than a lubricant.
Measuring tendon friction is relatively straightforward. If a tendon is sliding under a pulley against a fixed load, then the force on the distal end of the tendon between the load and the pulley is equal to the load, while on the other ( proximal) side of the pulley, the force on the tendon (if it is moving) is equal to the load plus the friction, so is therefore a somewhat higher value. By simply attaching load transducers to a tendon proximal and distal to a pulley or to the entire pulley system, applying a fixed load, and moving the tendon, it is possible to measure the frictional force (Figure 1).15,16
FIGURE 1.

Tendon friction testing device. In this example, the proximal phalanx and its attached A2 pulley are mounted in the center, and the FDP tendon is passing through the pulley at a proximal angle of 30 degrees and a distal angle of 20 degrees. Force transducers are attached to the proximal and distal ends of the tendon. Proximal transducer F2 is attached to a mechanical actuator, which can pull the tendon proximally or release it to move distally, under the influence of a distal load. Distal transducer F1 is attached between the tendon and the load. When the tendon is moved toward the actuator, the force recorded at F1 is equal to the load, and the force at F2 is equal to the load plus the friction force.
Normally, the friction between a human finger flexor tendon and its tendon sheath in the fingers is very low—about 0.1 N, or 10 g force.16 This is the amount of tension that needs to be applied to get the tendon to move, over and above the tension needed to counter the weight of the moving part (a phalanx, finger, or hand, as the case may be). This frictional force would be somewhat less at lower angles of tendon–pulley contact, and higher at greater angles, because the frictional force is proportional to the load applied to the pulley, which increases as the arc of contact increases. Thus, while the frictional coefficient (a property of the interacting materials–in this case, the tendon and pulley) does not change, the total frictional force is greater in a digit flexed, say 90 degrees at the proximal interphalangeal joint than it is in one flexed 30 degrees. To use scientific terms that can be compared to everyday use, the tendon gliding surface in zone 2 has a coefficient of friction of about 0.03, not very different than metal on ice (0.02), or cartilage on cartilage (roughly 0.01).16 After tendon repair, the force necessary to overcome friction and move the tendon increases, to as much as 6 N, or about 600 g per tendon, depending on the location of the laceration and the type of repair.17-23 Adhesions will increase the resistance to tendon gliding even more.24-26 Gaps in the tendon also increase friction. Small gaps of 1 mm or less have little effect, but gaps of 3 mm or more can completely block tendon motion, beyond the limits of any repair strength.27-29
TENDON REPAIRS: OPTIMIZING STRENGTH AND MOTION
A typical two-strand tendon repair with a 4-0 core suture has a breaking strength of 20 to 30 N, which is to say a load of about 2 or 3 kg, or 5 to 7 pounds.17,21,30 Larger core sutures (3-0 instead of 4-0), locking loops at the suture corners, and multiple strands ( four, six, or eight) can proportionately increase the initial strength of the repair, to as much as 70 N.21,31-33 This still pales in comparison to the breaking strength of an intact tendon, which can exceed 1,000 N (at this point, the tendon usually breaks at the grips of whatever device is holding and stretching it, so we don't know for sure—we have to extrapolate from the strength of partial lacerations).34,35 But breaking strength is not the real measure of the quality of a tendon repair. Before it breaks, the repair will gap. And a gapped repair will trigger and block motion.36,37 Moreover, as noted above, gapped tendons are more likely to have increased friction and thus poor clinical results, especially if the gap exceeds a threshold of 2 mm or so (Figure 2).
FIGURE 2.
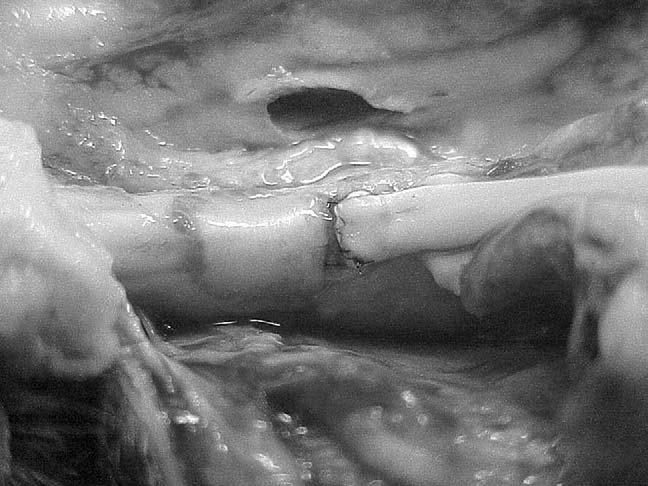
Gapped tendon caught on the pulley edge.
For most repairs, gaps begin to form at roughly two thirds the ultimate failure strength.17,21,38,39 The gap resistance can be increased by a running peripheral “finishing” stitch, especially if it is locked.40,41 Regardless, the point remains the same: the therapist should be aware not only of the ultimate breaking strength of a repair, but also of the strength at which it gaps. One final point about repair strength: the initial strength is as good as it gets for at least a month. In vivo, repair strength at first drops a bit, probably related to ischemia and edema in the repaired tendon. So when rehabilitating a tendon repair, it is important to remember that the “textbook” failure strength needs to be cut by a third to account for gapping, and then dropped maybe 10% or 20% more to account for tendon softening. Suddenly even a repair with a 70 N (15 lb) breaking strength doesn't seem so strong!
What does friction have to do with all of this? For a tendon to move, the force on the tendon must be greater than the sum of the forces opposing tendon motion. For normal active motion, those opposing forces include, more or less in descending order: the weight of the part to be moved (including any load, such as a tool or other object held by or attached to the part), the stiffness of the joints within the moving part, and the friction of the tendon against the surrounding tissues.37 Actually, for a normal finger flexor, only the first force is important; normally, the friction of cartilage on cartilage or tendon on sheath is very low, so that the tendon load is determined by the weight of the finger and anything it might be holding.
In an injured finger, everything changes, except perhaps the weight of the finger, which might increase a little because of edema. However, joint stiffness, whether intrinsic or due to diffuse swelling, becomes a major factor, and significant tendon forces become necessary to overcome it. In addition, there are new sources of resistance to motion within the sheath: damage to the gliding surfaces of tendon and sheath; disruption of the pulley system; the roughness of any tendon repair, including irregularities at the repair site which might lead to triggering; and, later, the effect of any adhesions. Suddenly the equation is much different, and the normal load necessary to actively move a finger in vivo,42-45 usually on the order of 5 N for passive motion and 15 to 30 N for unresisted active motion, suddenly is multiplied several fold. Is it any wonder that even very strong tendon repairs may fail with early active motion?
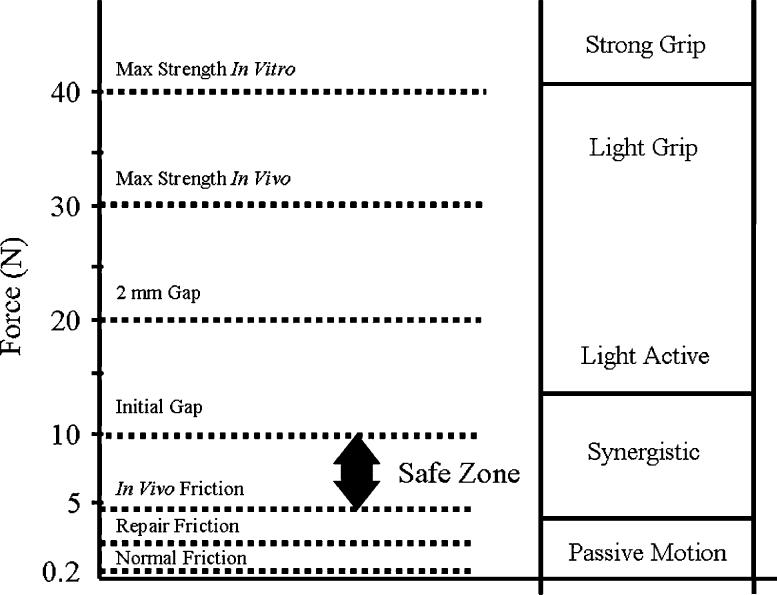
An understanding of the concepts of gliding resistance, gapping and tendon repair mechanics leads to an appreciation of the “safe zone”—the range of applied tendon loads large enough to induce tendon motion, yet small enough to avoid creation of a repair site gap or tendon rupture (Figure 3). For weak repairs with high friction, there may be no safe zone—the load needed to induce motion exceeds the gap resistance of the repair. For others, the zone may be quite narrow- a small range of acceptable loads within which the tendon moves, but beyond which gapping or failure of the repair occurs.
FIGURE 3.
The safe zone for a typical four-strand repair with a 40-N (9-lb) breaking strength is actually within a much smaller range, perhaps between 5 and 10 N (1-2 lb).
What can be done to improve the situation? Surgeons have several factors under their control. Careful tissue handling and accurate repair of joint and soft-tissue injury can minimize edema and the limitations due to joint irregularity. These will reduce the load needed to get the tendon moving. More importantly, a tendon repair can be chosen not only for its strength, but also for its gliding ability (Figure 4).17,19,21 This, too, can have a large effect on the load needed to initiate tendon motion.
FIGURE 4.
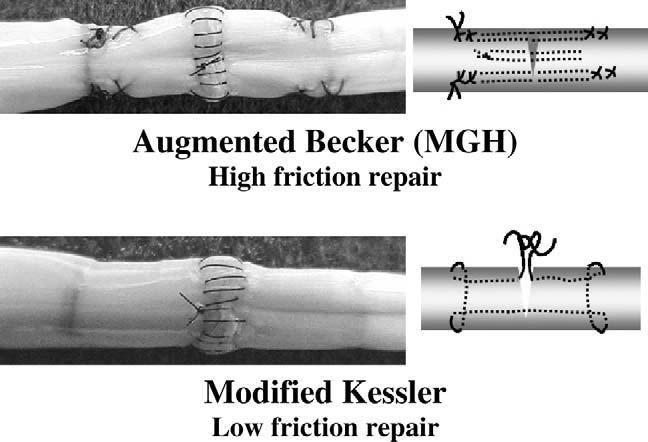
Examples of repairs resulting high and low friction between adjacent surfaces.
It is now clear that tendon repairs can be strong or weak, rough or smooth, and those aspects can be separately controlled. Not only that; they can be taught, by providing feedback on friction and breaking strength in cadaver repairs, so that even a new trainee's clinical repairs can be as technically perfect as a senior consultant's (we are currently using this teaching method at the Mayo Clinic). Factors that affect a tendon repair's strength relate to the suture caliber (3-0 is better than 4-0, for example), the number of suture strands crossing the repair site ( four is better than two), and the type of suture loops used to hold the tendon on either side (locking is best).17,46 Factors that affect a tendon repair's smoothness relate to the roughness of the suture material (monofilament [Nylon, Prolene] and coated [Ethibond, Ticron] sutures are better than braided [Mersilene, Vicryl] ones) and the roughness of repair ( proportional to the number and size of knots and suture loops on the tendon surface).23 By designing a repair with larger, smoother suture, and buried locking loops and knots, a strong repair can be created with friction minimized. We have found in our laboratory that a doubled modified Kessler repair with locking loops fits this bill nicely.17 Other repairs, such as the Savage, Lee, double Tsuge, and MGH, are similarly strong, but extract a higher price in terms of friction. And this is not just a theoretical advantage: in vivo animal studies have shown that higher-friction repairs result in more adhesions, and are more likely to abrade the undersurface of the pulley than lower-friction repairs.25
One final issue regarding repair deserves some comment. A repair should be strong and smooth; it should also, at a minimum, not interfere with the healing process (repairs that might actually augment healing will be discussed later). Repairs made of resorbable suture or allogeneic or xenogeneic substances can induce an inflammatory foreign body reaction that adversely affects the tendon healing process. Repairs comprised of or augmented by rigid implants also have the potential to interfere with tendon healing or tendon gliding, if they are large enough to occlude the contact surface between the cut tendon ends, or physically block tendon motion.
TENDON REHABILITATION: CAN WE FINE TUNE OUR PROTOCOLS ANY BETTER?
The goal of tendon repair and rehabilitation is to achieve a normally gliding and functioning tendon. This in turn has two interrelated requirements: a repair strong enough not to break during rehabilitation, and a rehabilitation method that achieves tendon motion while respecting the mechanical limits of the chosen repair technique. As noted above, the in vitro breaking strength of a repair is not the key strength measure; instead, the most important parameter is the load at which gaps appear in vivo, usually about two thirds of the in vitro breaking strength, or even less in some repair configurations. Rehabilitation methods must respect this upper limit of load, and yet devise a way of achieving tendon motion. All this must be considered in the context of a damaged tendon sheath and the fact that a postinjury, postoperative digit is likely to be stiffer than a normal one, especially in the critical first few days after surgery; therefore, the tension requirements to induce tendon motion are likely to be much higher than normal. In essence, in the early postoperative period, the safe zone narrows dramatically, with both a rising of the lower threshold, gliding resistance, and a lowering of the upper threshold, effective repair strength.
Groth47 has outlined a very useful hierarchy of tendon rehabilitation that takes into consideration the progressive strengthening of a tendon repair over time (i.e., a raising of the upper limit of the safe zone), and strives to match the rehabilitation method to the specific phase of tendon healing. The result is a graded program of passive to active therapy that is both logical and effective. Shortly after tendon repair, there is no tendon healing, so the surgical repair must stand on its own in keeping the tendons ends approximated. At the same time, the digit is likely to be stiff and swollen. Active motion would require strong loading, to overcome the digit stiffness at a time when the repair is at its weakest. Therefore, the principle here is to use passive motion to overcome the stiffness in the first few days after tendon repair, with place and hold tendon loading being added once full passive motion is achieved, to achieve tendon gliding. Active exercise is deferred until some tendon healing has occurred. Factors affecting the success of such a program include the initial strength of the repair (even light active motion involves significant loading) and the ability of the patient to understand and cooperate with the program (the amount of muscle contraction applied to the holding phase). This latter factor has another interesting dimension: De Jong et al.48 have shown that a change in the central neuromotor control of finger motion occurs very early in the tendon healing process, a change that may make active exercise less predictable in the postoperative setting than it would be in the absence of tendon injury.
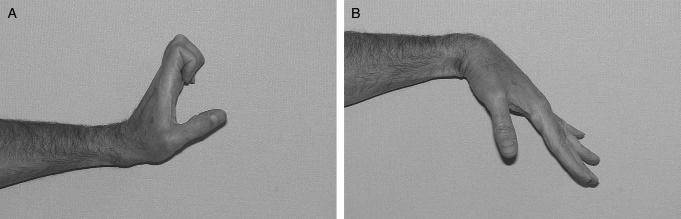
Passive motion is useful not only because it mobilizes joints, but also because it has the potential to mobilize the tendon. It is quite clear that passive extension of the finger and wrist can pull a flexor tendon to its most distal position, and that this effect can be graded by the amount of extension provided. This phenomenon is incorporated into every tendon rehabilitation program, whether of the fully passive Duran type, of the active extension/passive flexion Kleinert type, or of fully active protocols. What is less clear is to what extent passive flexion can achieve that the tendon is moved to its most proximal position by such measures. Recent work suggests that the proximally directed tendon force applied with simple passive finger flexion with the wrist flexed is minimal,49 and that it is not increased by adding synergistic wrist extension.50 In normal tendons, with a normally lubricated sheath, this minimal load can overcome their very low gliding resistance, so that tendon motion occurs. In essence, a low load strategy is likely to fit nicely in the safe zone, above the minimum threshold of gliding resistance, so motion occurs, and well below any load that would risk tendon injury. In the scenario of an injured tendon and sheath, with the added friction of the laceration and tendon repair, and in the face of an injured sheath, the safe zone is altered, and it is unlikely that the new, higher threshold of friction can be overcome by simple passive finger flexion. There is, however, a middle ground between passive exercise, which is likely to fail to cross the threshold for tendon gliding, and active exercise, which may exceed the threshold of repair gap strength. If passive metacarpophalangeal joint extension is combined with passive wrist extension and passive interphalangeal joint flexion, roughly 100 to 150 g proximally directed force can be generated. This should be enough to overcome the higher gliding resistance of most tendon repairs, while remaining well below the gap threshold. We are currently incorporating this modified synergistic protocol in our tendon rehabilitation programs at Mayo Clinic, as a bridge between passive flexion and place and hold exercise (Figure 5).
FIGURE 5.
Modified synergistic early motion protocol. A: extension phase; B: flexion phase.
When should tendon rehabilitation begin? While there has been a trend for an earlier and earlier start to passive mobilization after tendon repair, the question arises as to whether early motion can ever be too early. Several studies have shown that this is indeed the case, at least in animal models.18,51 Starting passive motion therapy the day after surgery certainly does not help, and may in fact hurt the final result. In the first few days after surgery, the gliding resistance is high. Very early motion, starting the day after surgery, can be associated with an increased risk of adhesion formation. This is probably because such therapy can provoke fresh bleeding within the already traumatized digit. In contrast, if the digit is immobilized for a few days after surgery, the digit stiffness and the friction between tendon and sheath fall in the first five days after surgery. In essence, by waiting, the safe zone widens as the upper limit of repair breaking strength remains unchanged, while the lower threshold of gliding resistance drops. If immobilization continues, the gliding resistance rises again and the safe zone once again narrows, suggesting that waiting beyond this period may also promote the development of adhesions. It seems better, therefore, to wait several days (three to five), but not as long as a week, for the postoperative thrombus to mature and for the gliding environment to improve before starting rehabilitation.
WHAT THE FUTURE HOLDS: ENHANCED HEALING, ENGINEERED REPAIRS AND GLIDING SURFACES
It is clear that we have come a long way in tendon surgery and rehabilitation in the last 50 years. When Sterling Bunnel wrote the first edition of his landmark text, Surgery of the Hand, in 1943, the outlook for a patient with a lacerated flexor tendon in zone 2, then commonly called “no man's land,” was not good. At best, about half of normal motion might be restored by a delayed primary graft, which might oblige the patient to spend six months or more in rehabilitation. At worst, the resulting stiff finger might be amputated to improve function of the remaining uninjured digit. Today, we can predictably restore 80% or more of normal motion with a primary repair, with rehabilitation usually completed in two or three months. But we did not quite move from no man's land to the promised land. The final result is still rarely a restoration of normal motion of the finger and function of the hand, and failures still occur, more likely due to ruptures rather than to adhesions. We are probably close to the end of the road when it comes to further improvements in repair techniques and rehabilitation methods. What might the next breakthrough be?
Stimulation of tendon repair has been a topic of research already for many years. The possibility of using growth factors to speed healing is a common theme applied to all types of wound healing. Some preliminary research looked at the possibility of using gene therapy to administer growth factors to the healing tendon.52-57 Such methods may someday shorten the time from injury to the recovery of full tensile strength of the tendon, and permit an earlier return to work for patients after surgical repairs of tendon injuries.
Growth factors are not likely to work fast enough, though, to allow more vigorous rehabilitation in the critical fist days after tendon repair. It may be possible, however, to use tissue-engineering techniques to reinforce the strength of repairs in the critical early days. For example, the grasp of a suture in a tendon can be augmented by engineering the stiffness of the suture–tendon interface, with agents that increase the crosslinking of collagen specifically for those fibers in contact with the suture. Of course, the reaction must be carefully controlled (otherwise, there is the risk of turning a rope into a pencil!), but theoretically at least it seems possible to strengthen the suture–tendon interface. Perhaps more interesting is the possibility of engineering the tendon gliding surface to minimize the force requirements for tendon gliding and optimize the interface surface by minimizing the development of adhesions. If a repaired tendon could have a gliding resistance similar to that of a normal tendon, then even simple passive therapy would reliably induce good tendon gliding. A fully functional gliding surface would also be likely to be resistant to the development of adhesions, which typically form in areas where the gliding surface has been damaged. Preliminary studies have shown that it is possible to transfect tendon cells with the gene hyaluronan synthase, and that such cells do synthesize hyaluronic acid. In addition, it is possible to use tissue engineering techniques to affix hyaluronic acid, and, potentially, lubricants such as lubricin to the tendon surface.58 In the past, clinicians and researchers have tried simply bathing repaired tendons in lubricating solutions,59-62 but the results have not been consistent. It is likely that free lubricant is rapidly catabolized. Fixing lubricant to the tendon surface not only replicates the normal condition of boundary lubrication, but is also more likely to inhibit enzymatic breakdown or mechanical dispersion of the lubricant during the important early days of tendon rehabilitation. Experiments to study the effect of tissue engineering the surface of experimental tendon grafts are already underway, and show that gliding can be improved and adhesions reduced during the healing period by this approach. Studies of a tissue-engineered experimental tendon repair will begin shortly.
CONCLUSION
The friction between tendon and sheath is an important factor affecting the risk of tendon rupture and the development of tendon adhesions after tendon injury and repair. An increase in surface friction will increase the load placed on the repair site of the tendon during motion, and may also increase the formation of adhesions. Currently, it is possible to devise methods of tendon suture and rehabilitation that minimize friction and maximize gliding within a “safe load zone” allowing tendon motion while avoiding gapping or outright failure of the repair. This concept of a safe zone is useful to consider when approaching the injured tendon, as the surgeon and therapist each can use methods to lower the lower limit of the safe zone by minimizing gliding resistance, which raises the upper limit, ensuring a strong repair. The safe zone is a dynamic range; it will narrow and widen as tendon healing proceeds. In the future, biologic solutions, including gene therapy, may speed tendon healing, thus further raising the upper threshold of the safe zone, while tissue-engineering strategies may offer new possibilities to improve the gliding surface and thereby further lower the lower threshold (Figure 6).
FIGURE 6.
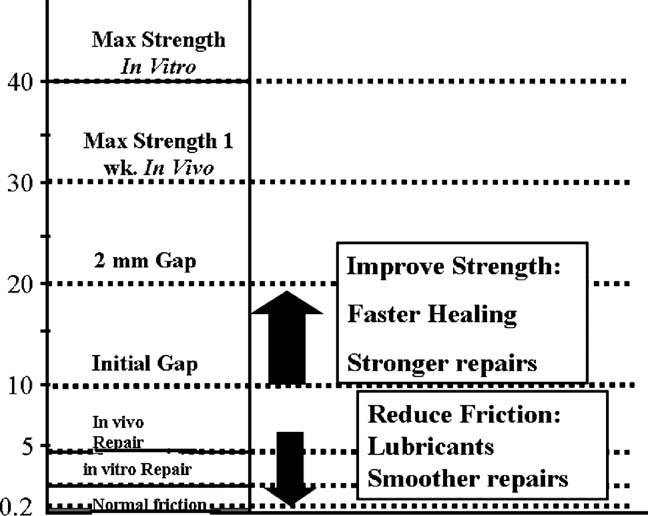
An expanded safe zone.
Footnotes
This research was funded by the National Institute of Arthritis and Muscular and Skin Diseases, National Institutes of Health (Grant AR44391).
REFERENCES
- 1.Mason M, Allen H. The rate of healing of tendons: an experimental study of tensile strength. Ann Surg. 1941;113:424–59. doi: 10.1097/00000658-194103000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Potenza AD. Flexor tendon injuries. Orthop Clin N Am. 1970;1:355–73. [PubMed] [Google Scholar]
- 3.Gelberman RH, Amifl D, Gonsalves M, et al. The influence of protected passive mobilization on the healing of flexor tendons: a biochemical and microangiographic study. Hand. 1981;13:120–8. doi: 10.1016/s0072-968x(81)80051-4. [DOI] [PubMed] [Google Scholar]
- 4.Gelberman RH, Nunley JA, II, Osterman AL, et al. Influences of the protected passive mobilization interval on flexor tendon healing: a prospective randomized clinical study. Clin Orthop. 1991;264:189–96. [PubMed] [Google Scholar]
- 5.Takai S, Woo SL, Horibe S, et al. The effects of frequency and duration of controlled passive mobilization on tendon healing. J Orthop Res. 1991;9:705–13. doi: 10.1002/jor.1100090510. [DOI] [PubMed] [Google Scholar]
- 6.Boyer MI, Gelberman RH, Burns ME, et al. Intrasynovial flexor tendon repair: an experimental study comparing low and high levels of in vivo force during rehabilitation in canines. J Bone Joint Surg [Am] 2001;83:891–9. [PubMed] [Google Scholar]
- 7.Nessler JP, Amadio PC, Berglund LJ, et al. Healing of canine tendon in zones subjected to different mechanical forces. J Hand Surg [Br] 1992;17:561–8. doi: 10.1016/s0266-7681(05)80242-6. [DOI] [PubMed] [Google Scholar]
- 8.Rees SG, Davies JR, Tudor D, et al. Immunolocalisation and expression of proteoglycan 4 (cartilage superficial zone proteoglycan) in tendon. Matrix. Biol. 2002;21:593–602. doi: 10.1016/s0945-053x(02)00056-2. [DOI] [PubMed] [Google Scholar]
- 9.Jay GD, Haberstroh K, Cha CJ. Comparison of the boundary-lubricating ability of bovine synovial fluid, lubricin, and healon. J Biomed Mater Res. 1998;40:414–8. doi: 10.1002/(sici)1097-4636(19980605)40:3<414::aid-jbm11>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 10.Vogel KG, Sandy JD, Pogany G, et al. Aggrecan in bovine tendon. Matrix Biol. 1994;14:171–9. doi: 10.1016/0945-053x(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 11.Brown DC, Vogel KG. Characteristics of the in vitro interaction of a small proteoglycan (pg ii) of bovine tendon with type I collagen. Matrix. 1989;9:468–78. doi: 10.1016/s0934-8832(11)80016-8. [DOI] [PubMed] [Google Scholar]
- 12.Hills BA. Boundary lubrication in vivo. Proc Inst Mech Eng [H] 2000;214:83–94. doi: 10.1243/0954411001535264. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz IM, Hills BA. Surface-active phospholipid as the lubricating component of lubricin. Br J Rheum. 1998;37:21–6. doi: 10.1093/rheumatology/37.1.21. [DOI] [PubMed] [Google Scholar]
- 14.Uchiyama S, Amadio PC, Ishikawa J, et al. Boundary lubrication between the tendon and the pulley in the finger. J Bone Joint Surg [Am] 1997;79:213–8. [PubMed] [Google Scholar]
- 15.An KN, Berglund L, Uchiyama S, et al. Measurement of friction between pulley and flexor tendon. Biomed Sci Instrum. 1993;29:1–7. [PubMed] [Google Scholar]
- 16.Uchiyama S, Coert JH, Berglund L, et al. Method for the measurement of friction between tendon and pulley. J Orthop Res. 1995;13:83–9. doi: 10.1002/jor.1100130113. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka T, Amadio PC, Zhao C, et al. Gliding characteristics and gap formation for locking and grasping tendon repairs: A biomechanical study in a human cadaver model. J Hand Surg [Am] 2004;29:6–14. doi: 10.1016/j.jhsa.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 18.Zhao C, Amadio PC, Paillard P, et al. Digital resistance and tendon strength during the first week after flexor digitorum profundus tendon repair in a canine model in vivo. J Bone Joint Surg [Am] 2004;86:320–7. doi: 10.2106/00004623-200402000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Tanaka T, Amadio PC, Zhao C, et al. Gliding resistance versus work of flexion: two methods to assess flexor tendon repair. J Orthop Res. 2003;21:813–8. doi: 10.1016/S0736-0266(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 20.Paillard PJ, Amadio PC, Zhao C, et al. Gliding resistance after FDP and FDS tendon repair in zone ii: an in vitro study. Acta Orthop Scand. 2002;73:465–70. doi: 10.1080/00016470216323. [DOI] [PubMed] [Google Scholar]
- 21.Momose T, Amadio PC, Zhao C, et al. Suture techniques with high breaking strength and low gliding resistance: experiments in the dog flexor digitorum profundus tendon. Acta Orthop Scand. 2001;72:635–41. doi: 10.1080/000164701317269085. [DOI] [PubMed] [Google Scholar]
- 22.Zhao C, Amadio PC, Zobitz ME, et al. Gliding resistance after repair of partially lacerated human flexor digitorum profundus tendon in vitro. Clin Biomech (Bristol, Avon) 2001;16:696–701. doi: 10.1016/s0268-0033(01)00056-0. [DOI] [PubMed] [Google Scholar]
- 23.Momose T, Amadio PC, Zhao C, et al. The effect of knot location, suture material, and suture size on the gliding resistance of flexor tendons. J Biomed Mater Res. 2000;53:806–11. doi: 10.1002/1097-4636(2000)53:6<806::aid-jbm23>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 24.Strick MJ, Filan SL, Hile M, et al. Adhesion formation after flexor tendon repair: a histologic and biomechanical comparison of 2- and 4-strand repairs in a chicken model. J Hand Surg [Am] 2004;29:15–21. doi: 10.1016/j.jhsa.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Zhao C, Amadio PC, Momose T, et al. The effect of suture technique on adhesion formation after flexor tendon repair for partial lacerations in a canine model. J Trauma-Injury Infect Crit Care. 2001;51:917–21. doi: 10.1097/00005373-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 26.Zhao C, Amadio PC, Momose T, et al. Remodeling of the gliding surface after flexor tendon repair in a canine model in vivo. J Orthop Res. 2002;20:857–62. doi: 10.1016/S0736-0266(01)00168-1. [DOI] [PubMed] [Google Scholar]
- 27.Drape JL, Silbermann-Hoffman O, Houvet P, et al. Complications of flexor tendon repair in the hand: MR imaging assessment. Radiology. 1996;198:219–24. doi: 10.1148/radiology.198.1.8539383. [DOI] [PubMed] [Google Scholar]
- 28.Gelberman RH, Boyer MI, Brodt MD, et al. The effect of gap formation at the repair site on the strength and excursion of intrasynovial flexor tendons: an experimental study on the early stages of tendon-healing in dogs. J Bone Joint Surg [Am] 1999;81:975–82. doi: 10.2106/00004623-199907000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Silfverskiold KL, May EJ, Tornvall AH. Gap formation during controlled motion after flexor tendon repair in zone II: a prospective clinical study. J Hand Surg [Am] 1992;17:539–46. doi: 10.1016/0363-5023(92)90368-y. [DOI] [PubMed] [Google Scholar]
- 30.Thurman RT, Trumble TE, Hanel DP, et al. Two-, four-, and six-strand zone ii flexor tendon repairs: an in situ biomechanical comparison using a cadaver model. J Hand Surg [Am] 1998;23:261–5. doi: 10.1016/s0363-5023(98)80124-x. [DOI] [PubMed] [Google Scholar]
- 31.Winters SC, Gelberman RH, Woo SL, et al. The effects of multiple-strand suture methods on the strength and excursion of repaired intrasynovial flexor tendons: a biomechanical study in dogs. J Hand Surg [Am] 1998;23:97–104. doi: 10.1016/s0363-5023(98)80096-8. [DOI] [PubMed] [Google Scholar]
- 32.Xie RG, Zhang S, Tang JB, et al. Biomechanical studies of 3 different 6-strand flexor tendon repair techniques. J Hand Surg [Am] 2002;27:621–7. doi: 10.1053/jhsu.2002.34311. [DOI] [PubMed] [Google Scholar]
- 33.Angeles JG, Heminger H, Mass DP. Comparative biomechanical performances of 4-strand core suture repairs for zone ii flexor tendon lacerations. J Hand Surg [Am] 2002;27:508–17. doi: 10.1053/jhsu.2002.32619. [DOI] [PubMed] [Google Scholar]
- 34.Erhard L, Zobitz ME, Zhao C, et al. Treatment of partial lacerations in flexor tendons by trimming: a biomechanical in vitro study. J Bone Joint Surg [Am] 2002;84:1006–12. doi: 10.2106/00004623-200206000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Erhard L, Schultz FM, Zobitz ME, et al. Reproducible volar partial lacerations in flexor tendons: a new device for biomechanical studies. J Biomech. 2002;35:999–1002. doi: 10.1016/s0021-9290(02)00038-6. [DOI] [PubMed] [Google Scholar]
- 36.Coert JH, Uchiyama S, Amadio PC, et al. Flexor tendon-pulley interaction after tendon repair: a biomechanical study. J Hand Surg [Br] 1995;20:573–7. doi: 10.1016/s0266-7681(05)80113-5. [DOI] [PubMed] [Google Scholar]
- 37.Zhao C, Amadio PC, Berglund L, et al. A new testing device for measuring gliding resistance and work of flexion in a digit. J Biomech. 2003;36:295–9. doi: 10.1016/s0021-9290(02)00300-7. [DOI] [PubMed] [Google Scholar]
- 38.Dinopoulos HT, Boyer MI, Burns ME, et al. The resistance of a four- and eight-strand suture technique to gap formation during tensile testing: an experimental study of repaired canine flexor tendons after 10 days of in vivo healing. J Hand Surg [Am] 2000;25:489–98. doi: 10.1053/jhsu.2000.6456. [DOI] [PubMed] [Google Scholar]
- 39.Silfverskiold KL, Andersson CH. Two new methods of tendon repair: an in vitro evaluation of tensile strength and gap formation. J Hand Surg [Am] 1993;18:58–65. doi: 10.1016/0363-5023(93)90246-Y. see comment. [DOI] [PubMed] [Google Scholar]
- 40.Lin GT, An KN, Amadio PC, et al. Biomechanical studies of running suture for flexor tendon repair in dogs. J Hand Surg [Am] 1988;13:553–8. doi: 10.1016/s0363-5023(88)80094-7. [DOI] [PubMed] [Google Scholar]
- 41.Kubota H, Aoki M, Pruitt DL, et al. Mechanical properties of various circumferential tendon suture techniques. J Hand Surg [Br] 1996;21:474–80. doi: 10.1016/s0266-7681(96)80049-0. [DOI] [PubMed] [Google Scholar]
- 42.Dennerlein JT, Diao E, Mote CD, Jr, et al. In vivo finger flexor tendon force while tapping on a keyswitch. J Orthop Res. 1999;7:178–84. doi: 10.1002/jor.1100170205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dennerlein JT, Diao E, Mote CD, Jr, et al. Tensions of the flexor digitorum superficialis are higher than a current model predicts. J Biomech. 1998;31:295–301. doi: 10.1016/s0021-9290(98)00006-2. [DOI] [PubMed] [Google Scholar]
- 44.Serina ER, Mote CD, Jr, Rempel D. Force response of the fingertip pulp to repeated compression: effects of loading rate, loading angle and anthropometry. J Biomech. 1997;30:1035–40. doi: 10.1016/s0021-9290(97)00065-1. [DOI] [PubMed] [Google Scholar]
- 45.Schuind F, Garcia-Elias M, Cooney WP, III, et al. Flexor tendon forces: in vivo measurements. J Hand Surg [Am] 1992;17:291–8. doi: 10.1016/0363-5023(92)90408-h. [DOI] [PubMed] [Google Scholar]
- 46.Silva MJ, Hollstien SB, Fayazi AH, et al. The effects of multiple-strand suture techniques on the tensile properties of repair of the flexor digitorum profundus tendon to bone. J Bone Joint Surg [Am] 1998;80:1507–14. doi: 10.2106/00004623-199810000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Groth GN. Pyramid of progressive force exercises to the injured flexor tendon. J Hand Ther. 2004;17:31–42. doi: 10.1197/j.jht.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 48.de Jong BM, Coert JH, Stenekes MW, et al. Cerebral reorganisation of human hand movement following dynamic immobilisation. Neuroreport. 2003;14:1693–6. doi: 10.1097/00001756-200309150-00007. [DOI] [PubMed] [Google Scholar]
- 49.Horii E, Lin GT, Cooney WP, et al. Comparative flexor tendon excursion after passive mobilization: an in vitro study. J Hand Surg [Am] 1992;17:559–66. doi: 10.1016/0363-5023(92)90371-u. [DOI] [PubMed] [Google Scholar]
- 50.Zhao C, Amadio PC, Zobitz ME, et al. Effect of synergistic motion on flexor digitorum profundus tendon excursion. Clin Orthop. 2002;396:223–30. doi: 10.1097/00003086-200203000-00033. [DOI] [PubMed] [Google Scholar]
- 51.Halikis MN, Manske PR, Kubota H, et al. Effect of immobilization, immediate mobilization, and delayed mobilization on the resistance to digital flexion using a tendon injury model. J Hand Surg [Am] 1997;22:464–72. doi: 10.1016/S0363-5023(97)80014-7. [DOI] [PubMed] [Google Scholar]
- 52.Hildebrand KA, Frank CB, Hart DA. Gene intervention in ligament and tendon: current status, challenges, future directions. Gene Ther. 2004;11:368–78. doi: 10.1038/sj.gt.3302198. [DOI] [PubMed] [Google Scholar]
- 53.Beredjiklian PK. Biologic aspects of flexor tendon laceration and repair. J Bone Joint Surg [Am] 2003;85:539–50. doi: 10.2106/00004623-200303000-00025. [DOI] [PubMed] [Google Scholar]
- 54.Zhang F, Lineaweaver WC. Growth factors and gene transfer with DNA strand technique in tendon healing. J Long-Term Effects Med Implant. 2002;12:105–12. [PubMed] [Google Scholar]
- 55.Maffulli N, Moller HD, Evans CH. Tendon healing: can it be optimised? Br J Sports Med. 2002;36:315–6. doi: 10.1136/bjsm.36.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lou J, Tu Y, Burns M, et al. Bmp-12 gene transfer augmentation of lacerated tendon repair. J Orthop Res. 2001;19:1199–202. doi: 10.1016/S0736-0266(01)00042-0. [DOI] [PubMed] [Google Scholar]
- 57.Goomer RS, Maris TM, Gelberman R, et al. Nonviral in vivo gene therapy for tissue engineering of articular cartilage and tendon repair. Clin Orthop. 2000;379:189–200. doi: 10.1097/00003086-200010001-00025. [DOI] [PubMed] [Google Scholar]
- 58.Momose T, Amadio PC, Sun YL, et al. Surface modification of extrasynovial tendon by chemically modified hyaluronic acid coating. J Biomed Mater Res. 2002;59:219–24. doi: 10.1002/jbm.1235. [DOI] [PubMed] [Google Scholar]
- 59.Amiel D, Ishizue K, Billings E, Jr, et al. Hyaluronan in flexor tendon repair. J Hand Surg [Am] 1989;14:837–43. doi: 10.1016/s0363-5023(89)80085-1. [DOI] [PubMed] [Google Scholar]
- 60.Meyers SA, Seaber AV, Glisson RR, et al. Effect of hyaluronic acid/chondroitin sulfate on healing of full-thickness tendon lacerations in rabbits. J Orthop Res. 1989;7:683–9. doi: 10.1002/jor.1100070508. [DOI] [PubMed] [Google Scholar]
- 61.Thomas SC, Jones LC, Hungerford DS. Hyaluronic acid and its effect on postoperative adhesions in the rabbit flexor tendon: a preliminary look. Clin Orthop. 1986;206:281–9. [PubMed] [Google Scholar]
- 62.St Onge R, Weiss C, Denlinger JL, et al. A preliminary assessment of Na-hyaluronate injection into “no man's land” for primary flexor tendon repair. Clin Orthop. 1980;46:269–75. [PubMed] [Google Scholar]