Abstract
Marek's disease herpesvirus is a vaccine vector of great promise for chickens; however, complete protection against foreign infectious diseases has not been achieved. In this study, two herpesvirus of turkey recombinants (rHVTs) expressing large amounts of infectious bursal disease virus (IBDV) VP2 antigen under the control of a human cytomegalovirus (CMV) promoter or CMV/β-actin chimera promoter (Pec promoter) (rHVT-cmvVP2 and rHVT-pecVP2) were constructed. rHVT-pecVP2, which expressed the VP2 antigen approximately four times more than did rHVT-cmvVP2 in vitro, induced complete protection against a lethal IBDV challenge in chickens, whereas rHVT-cmvVP2 induced 58% protection. All of the chickens vaccinated with rHVT-pecVP2 had a protective level of antibodies to the VP2 antigen at the time of challenge, whereas only 42 and 67% of chickens vaccinated with rHVT-cmvVP2 or the conventional live IBDV vaccine, respectively, had the antibodies. The antibody level of chickens vaccinated with rHVT-pecVP2 increased for 16 weeks, and the peak antibody level persisted throughout the experiment. The serum antibody titer at 30 weeks of age was about 20 or 65 times higher than that of chickens vaccinated with rHVT-cmvVP2 or the conventional live vaccine, respectively. rHVT-pecVP2, isolated consistently for 30 weeks from the vaccinated chickens, expressed the VP2 antigen after cultivation, and neither nucleotide mutations nor deletion in the VP2 gene was found. These results demonstrate that the amount of VP2 antigen expressed in the HVT vector was correlated with the vaccine efficacy against lethal IBDV challenge, and complete protective immunity that is likely to persist for the life of the chickens was induced.
Replication-competent herpesvirus vectors are prospective vaccine vehicles for animals for the following reasons. (i) The vectored vaccines are of the safe subunit type and express multiple antigens. (ii) Both humoral and cellular immune responses against pathogens can be induced in animals. (iii) They have a potential for long-term induction of protective immunity against pathogens in animals.
Marek's disease (MD) virus (MDV) is a cell-associated, lymphotropic alphaherpesvirus of chickens that causes the most-common, highly contagious T-cell lymphoma (6), and all three serotypes of MDV have been completely sequenced (1, 19, 22, 41). The MDV vaccine strains, which are serotypes 1 (MDV1), MDV2, and MDV3 (herpesvirus of turkey [HVT]) (6), have merits as a distinguished vector (7, 15, 24, 30). MDV vaccines can overcome the inhibition of maternal antibodies (28, 35) and might induce long-term protective immunity in chickens. Down-regulation of major histocompatibility complex class I expression is a common mechanism of herpesviruses, including MDV, used to evade cellular immunity and persist in their hosts (17, 20). MDV1 has high vaccine efficacy against MD but grows slowly in cell culture, whereas HVT has a relatively low vaccine efficacy but is highly safe for chickens and grows remarkably well in cell culture. Despite the high potential of the MDV vectors, attempts to elicit complete protection against infections in chickens have not been successful (8, 13, 15, 27, 28, 31-33, 35, 39). The lack of effective MDV1 recombinants is likely due to a variety of factors such as the difficulty in making recombinants without attenuating the virus.
Infectious bursal disease (IBD) virus (IBDV), a member of the Birnaviridae family, causes considerable economic losses in the poultry industry by inducing bursal destruction, immunosuppression, and high mortality in young chickens (21, 23, 42). Although live IBDV vaccines are highly efficacious (18), the vaccine efficacy decreases in the presence of maternal antibodies (23, 38, 42), and some of them cause bursal atrophy (25). Highly efficacious and safe IBD vaccines are needed. The VP2 protein is the conformational protective antigen: VP2 or the neutralizing antibodies can elicit complete protection against a lethal IBDV challenge (2, 4, 9, 10, 14, 23). We previously developed a recombinant MDV (rMDV) expressing VP2 antigen under control of the simian virus 40 (SV40) early promoter and showed that it was safe for chickens. However, the efficacy was partial and did not persist for a long time (39, 40). Recombinant HVT (rHVT) expressing the VP2 antigen under the control of a cytomegalovirus (CMV) promoter also induced partial protection in chickens when one dose was used (8). Further studies are required to improve the vaccine efficacy of MDV-vectored vaccines.
In order to determine the association between the amounts of antigen expressed in an HVT herpesvirus vector and the vaccine efficacy, we developed two rHVTs expressing different amounts of IBDV VP2 antigens under the control of CMV or Pec promoters. The Pec promoter is a new promoter consisting of a CMV enhancer and a β-actin promoter and has promoter activity in chicken embryo fibroblasts (CEFs) approximately three times stronger than that of the CMV promoter (M. Kubomura, A. Fujisawa, T. Okuda, S. Saitoh, and A. Yasuda, unpublished data). The present study indicated that the amount of antigen expressed in the HVT herpesvirus vector was correlated with the vaccine efficacy against IBD. The rHVT expressing larger amounts of VP2 antigen conferred complete protection against the lethal IBDV challenge, which was expected to persist for the lifetime of the chickens.
MATERIALS AND METHODS
Virus and cells.
The HVT FC126 strain was used as a parent virus for construction of rHVTs. The Ehime/91 (E/91) strain of a very virulent IBDV (vvIBDV) (36) was used as the challenge virus. Live IBDV vaccine, IBDV-A, was obtained from The Chemo-Sero Therapeutic Research Institute (Kumamoto, Japan). Both HVT and rHVTs were cultivated in CEFs prepared from 10-day-old specific-pathogen-free (SPF) embryonated chicken eggs of line PDL-1 reared in our institute (12). Dulbecco's minimum essential medium supplemented with 5% fetal calf serum, 10% tryptose phosphate broth, and antibiotics was used as the growth medium.
Construction of plasmids.
Transfer plasmid pNZ45/46VP2 was constructed as follows. A DNA fragment that contained the 3′ end of HVT UL44 and the entire UL45 was obtained by PCR amplification with PFU. DNA polymerase (Stratagene) from the genomic DNA template of the HVT FC126 strain with primers P1 (5′-AAGCTTTCAAGTGATACTGCGTGA-3′, HindIII) and P2 (5′-TTTGGCCAATAAGGCCTATTTACTCATCGCATTAGAGAGG-3′, SfiI) (underlining shows restriction site of indicated restriction enzyme). Another DNA fragment, which covered UL46, was obtained with primers P3 (5′-AATAGGCCTTATTGGCCAAAACACACCTCTAACGGTTAC-3′, SfiI) and P4 (5′-CCCCGAATTCATGGAAGAAATTTCCTCC-3′, EcoRI). These two DNA fragments, overlapping by 20 bp (TTTGGCCAATAAGGCCTATT, SfiI), were used as templates to obtain the 2.9-kbp UL45/46Sfi DNA fragment by PCR amplification with primers P1 and P4. The resulting UL45/46/Sfi DNA fragment contained the SfiI restriction site between UL45 and UL46 and the EcoRI and the HindIII restriction site at each terminal end. The EcoRI-HindIII fragment of UL45/46Sfi DNA was cloned into the pUC18 vector cut with the same restriction enzymes to yield pNZ45/46Sfi. The VP2 gene of the E/91 strain (36), which was a host-protective antigen gene of IBDV, together with a poly(A) signal of MDV UL46 was cut with BglI from pMCSVP2 to yield a DNA fragment of the VP2-poly(A) signal (1.5 kbp), and the 1.5-kbp fragment was then subcloned into the SfiI site of pNZ45/46Sfi to obtain pNZ45/46VP2. These primers were synthesized according to the sequences of GenBank accession numbers M27832, X13371, and D90003.
The Pec promoter (0.5 kbp) consists of an enhancer domain of the CMV immediate-early promoter and a promoter domain of the β-actin promoter (Kubomura et al., unpublished data [GenBank accession no. AF428265]). The promoter was isolated by digesting the pGIPec+ plasmid with PstI-XbaI restriction enzymes. The digested fragment was cloned into the PstI-XbaI site of pNZ45/46VP2 to obtain pNZ45/46pecVP2. To yield pNZ45/46cmvVP2, the CMV promoter fragment was obtained by digesting pBK-CMV (Stratagene) with EcoT221-BamHI restriction enzymes, and the digested promoter region was inserted into pNZ45/46VP2.
Construction of recombinant virus.
Ten micrograms of high-molecular-weight HVT DNA prepared from HVT-infected CEFs (26) was mixed with 20 μg of purified plasmid DNA of pNZ45/46pecVP2 or pNZ45/46cmvVP2. The DNA mixture was then added to the CEF suspension, and this was followed by electroporation as described previously (39). After 3 days of cultivation of the CEFs in a dish, the CEFs were transferred to two new 96-well plates, and on the following day, one plate was immunologically stained with anti-IBDV rabbit serum. Antigen-positive cells on the other plate were subcultured until most of the cells became positive for the VP2 antigen. The antigen-positive cells were then treated with a sonicator to obtain the cell-free rHVT clone. To determine the insertion of the VP2 gene into the genome of the rHVTs, PCR amplification with primers IBD#1 (5′-ATAAGAATGCGGCCGCATGACAAACCCTGCAAGATCAAACCCA-3′) and IBD#2 (5′-ATAGTTTAGCGGCCGCTTACCTCCTTATAGCCCGGATTATGT-3′) was done. The infective titers of the rHVT and wild-type (wt) HVT were determined by counting the plaques after cocultivation of 10-fold dilutions of infected cells with CEFs for 5 days followed by the immunostaining.
Western blot analysis.
Expression of the VP2 antigen in rHVT-infected CEFs was determined by Western blotting analysis with anti-IBDV rabbit antiserum (39). CEFs infected with rHVT or HVT were harvested when 50% of the CEFs showed cytopathic effects (CPEs), and the cell pellets were stored at −80°C before use. The cell pellets were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by transfer to a nitrocellulose membrane. The membrane was incubated with rabbit anti-IBDV antiserum followed by horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G. A 3,3′-diaminobenzidine substrate was used to detect the VP2 band.
Detection of VP2 antigens.
In order to determine the amounts of VP2 antigen produced by each rHVT in vitro, an enzyme-linked immunosorbent assay (ELISA) was used for titrating the VP2 antigen in the culture supernatant (37). The rHVT-infected CEFs (1 × 105 to 2 × 105) were mixed with normal secondary CEFs (107/ml), and the cell mixture was cultivated for 5 days. The culture supernatant was harvested daily for 5 days and stored at −80°C before the ELISA. The wt HVT was used as the control. Culture supernatants were diluted with the ELISA diluent, and the dilutions were tested by the ELISA. The ELISA absorbance (A) values were measured at 492 nm with a microplate reader (model 550; Bio-Rad, Hercules, Calif.), and A values of more than 0.2 were considered positive.
Detection of antibodies to VP2.
Agar gel precipitation (AGP) tests for the detection and titration of anti-VP2 antibodies were performed. The AGP antibody correlated well with the protection from gross lesions caused by vvIBDV. Four units of AGP antigens prepared from bursa of Fabricius (BF) homogenates obtained from chickens inoculated with vvIBDV E/91 strain were used (39). After 2 days of incubation at room temperature, AGP antibody titers were determined.
Enzyme immunoassay (EIA) was also used to titrate the anti-VP2 antibodies as described previously (39).
Nucleotide sequence analysis of VP2 gene of rHVT.
To determine the stability of the VP2 expression cassette of the herpesvirus vector during latency, the VP2 gene of the rHVTs was sequenced. Each of three clones of rHVT-pecVP2 or rHVT-cmvVP2 was isolated from vaccinated chickens at 24 weeks of age using secondary CEFs. After cultivation of the rHVTs, the high-molecular-weight DNAs of each rHVT were prepared using a QIAamp DNA Blood Kit (catalog no. 51104; QIAGEN, Hilden, Germany). VP2 gene or VP2 gene subfragments were amplified from the high-molecular-weight DNAs by PCR. To amplify the VP2 gene (1.5 kb) from rHVT-cmvVP2 clones, a pair of primers, MD#38 (5′-GCTCGCGCGCCTGCAGGTCG-3′) and MD#39 (5′-GGGCCTGAAATGAGCCTTGG-3′), was used. To amplify the two VP2 subfragments, VP2-a (0.7 kb) and VP2-b (0.9 kb), from two rHVT-pecVP2 primer pairs, MD#36 (5′-CGGCTCTGACTGACCGCGTC-3′) with IBD#8 (5′-GAAGGTCACGGCGTTTATG-3′) and IBD#15 (5′-CCCAGAGTCTACACCATA-3′) with MD#41 (5′-GCGATTATTATGAAGTCTAC-3′), respectively, were used. After purification of the amplified VP2 gene or its gene fragments, the nucleotide sequences were determined with a DNA sequencing kit (catalog no. 402079; Applied Biosystems) followed by analyzing the products with a sequence analyzer (model 310 Genetic Analyzer; Perkin-Elmer Biosystems).
Protection against vvIBDV infection in chickens.
One-day-old SPF White Leghorn chickens of line M (Nippon Institute of Biological Science, Tokyo, Japan) were assigned to four groups of 12 chickens each. Each group of chickens was reared in a negative-pressure isolator set in a negative-pressure chicken house. Chickens of groups 1 and 2 were vaccinated subcutaneously with 103 or 104 infective doses (ID) of rHVT-pecVP2 at 1 day of age, respectively. Those of group 3 were vaccinated with 104 ID of rHVT-cmvVP2 on that day. Those of group 4 were vaccinated orally with one dose of a commercial live vaccine, IBDV-A, at 1 week of age. Antigenicity of IBDV-A is almost identical to that of the vvIBDV E/91 strain. A normal control group was also assigned. At 4 weeks of age, sera were taken from these chickens, and both vaccinated or unvaccinated control chickens were then challenged orally with the vvIBDV E/91 strain (105 50% embryo ID/0.1 ml/chicken). After clinical signs and mortality had been observed for 7 days, both dead and surviving chickens, which were treated with chloroform, were subjected to examinations of BF gross lesions.
Persistent infection with rHVTs in chickens.
Each of five chickens in four groups described above was used to determine the viral titers during latency. Peripheral blood lymphocytes (PBL) were taken at 2, 3, 4, 8, 12, 16, 20, and 24 weeks of age from these chickens. The PBL prepared from 1 ml of heparinized blood (approximately 2 × 107 to 3 × 107) at each sampling time were cocultivated with 8 × 106 primary CEFs and then cultured in a 96-well microplate. After 4 days' cultivation, the cultured cells were transferred to a new microplate to subculture for another 5 days. We used one tip for each well to prevent the cross-contamination among wells in this cell passage. The titers were expressed as the numbers of wells showing CPEs (infective units/milliliter). The virus titers were semiquantitative, because virus titers of more than 100 infective units were not countable in this method.
Persistence of serum antibody responses in chickens.
Each of five 1-day-old SPF chicks of line M was separately reared in each negative-pressure isolator throughout this experiment. Groups 1, 2, or 3 were vaccinated subcutaneously with 103 ID of rHVT-pecVP2, rHVT-cmvVP2, or wt HVT at 1 day of age, respectively. Group 4 was vaccinated orally with one dose of commercial live vaccine IBDV-A at 1 week of age. Sera were taken at 2, 4, 8, 12, 16, 20, 24, 28, and 30 weeks of age from these chickens and stored at −30°C before the titration of antibodies to VP2 by AGP tests.
To determine whether chickens were resistant to the vvIBDV challenge at 30 weeks of age, the chickens were challenged with the E/91 strain (105 50% embryo ID/0.1 ml/chicken). After clinical signs had been observed, sera were taken at 32 weeks to determine the antibody responses to the VP2 antigens. Both AGP tests and EIA (38) were used for titration of the antibodies to VP2 antigens.
Association of virus replication efficiency and antibody response levels.
In order to determine the correlation between rHVT replication efficiency in chickens and the serum antibody responses to VP2 antigen, the average titers of rHVT-pecVP2 or rHVT-cmvVP2 (infectious units/milliliter) in each chicken from 12 to 24 weeks of age were calculated, as well as those of the AGP antibodies to the VP2 antigen. The pairs of titers of individual chickens were plotted, and the correlation coefficient of each rHVT was estimated.
RESULTS
Characterization of rHVT.
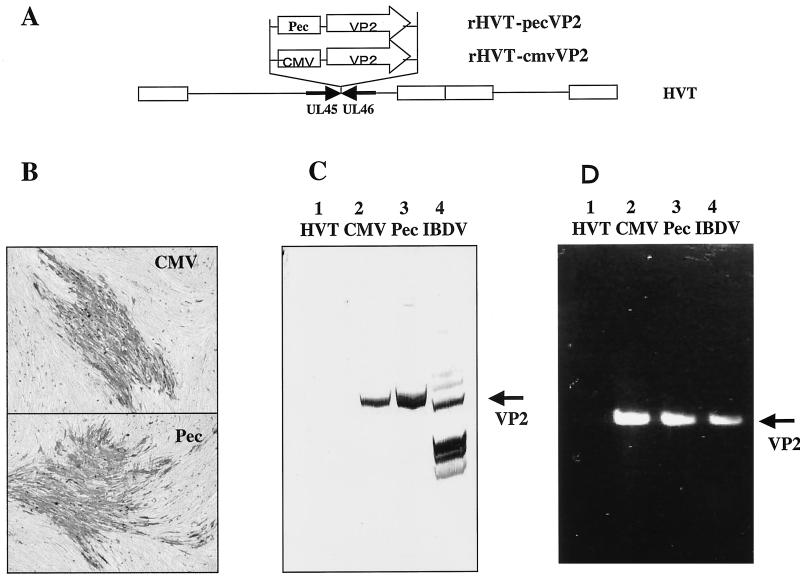
To determine the construction of rHVTs, we first analyzed the rHVTs for the presence of the IBDV VP2 gene by PCR with primers IBD#1 and IBD#2. The VP2 gene was amplified from DNAs prepared from CEFs infected with rHVT-pecVP2 or rHVT-cmvVP2 but not from the wt HVT (Fig. 1D), indicating that the VP2 gene was integrated into the HVT genome in both rHVTs.
FIG. 1.
Characterization of rHVTs. (A) Schematic representation of rHVT-pecVP2 (Pec) and rHVT-cmvVP2 (CMV). (B) Immunological staining of CEFs infected with either rHVT-pecVP2 or rHVT-cmvVP2 with anti-IBDV rabbit antiserum followed by horseradish peroxidase-labeled goat anti-rabbit immunoglobulin G. (C) CEFs infected with rHVT-cmvVP2 (CMV), rHVT-pecVP2 (Pec), HVT, or IBDV J1 strains were analyzed by immunoblotting for VP2 antigen expression with rabbit anti-IBDV antiserum. (D) PCR analysis was performed on cellular DNA of CEFs infected with rHVT-cmvVP2 (CMV), rHVT-pecVP2 (Pec), or HVT for the presence of the VP2 gene. Reverse transcription was performed on genomic double-stranded RNA of IBDV E/91 strain followed by PCR for amplification of VP2 gene.
In order to determine the VP2 protein expression in the rHVTs, CEFs infected with the rHVT were immunologically stained with rabbit anti-IBDV serum. Plaques of rHVT-cmvVP2 or rHVT-pecVP2 were stained with the antibody to VP2, but those of wt HVT were not (Fig. 1B). The expected size (42 kDa) of the VP2 protein was detected by Western blotting analysis in cell lysates prepared from CEFs infected with rHVT-pecVP2 or rHVT-cmvVP2 but not with wt HVT (Fig. 1C). These results confirmed that both rHVTs expressed the entire VP2 antigen.
Production of VP2 antigen by rHVT in vitro.
The Pec promoter is stronger than the CMV promoter or the SV40 early promoter on CEFs. To assess the significance of the strong promoter to be used in the HVT herpesvirus vector in vitro, culture supernatants were harvested daily from the rHVT-infected CEF cultures, and the amounts of VP2 antigens produced by each rHVT were determined by ELISA for detecting the VP2 antigen (37). In this experiment, rHVT viral titers of each inoculum were adjusted, and dishes showing almost identical intensity of the CPE caused by each HVT were selected in this experiment. As shown in Fig. 2, rHVT-pecVP2 produced the VP2 antigen more quickly and to a greater extent than did rHVT-cmvVP2. The rHVT-pecVP2 synthesized the VP2 antigen four times more abundantly than the rHVT-cmvVP2 in vitro. No VP2 antigen was detected in the culture supernatant of wt HVT-infected CEF cultures. These results indicated that a strong Pec promoter could produce large amounts of antigens by the HVT herpesvirus vector system in vitro.
FIG. 2.
Kinetics of VP2 antigen expression in CEFs infected with rHVT-pecVP2 (•), rHVT-cmvVP2 (▪), or wt HVT (▵). CEFs were inoculated with each virus, and the culture supernatants were harvested daily. Serial dilutions of the culture supernatants were tested by ELISA for the detection of the IBDV VP2 antigen. Antigen titers were expressed as a reciprocal of the sample dilution which showed a positive reaction.
Vaccine efficacy of rHVTs against lethal vvIBDV infection.
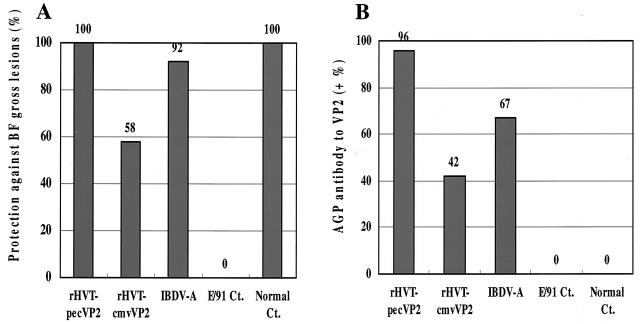
In order to determine the efficacy of the rHVTs against a lethal IBDV challenge, SPF chickens vaccinated with each recombinant vaccine or the conventional live vaccine were challenged with vvIBDV at 4 weeks postvaccination. As a result, neither clinical signs nor mortality was observed in chickens vaccinated with rHVT-pecVP2, rHVT-cmvVP2, or the live vaccine IBDV-A after the lethal challenge with IBDV. Chickens of the unvaccinated challenge control group showed severe clinical signs (100%) and high mortality (43%), whereas those of a healthy control group did not. All of the chickens vaccinated with the rHVT-pecVP2 (10 of 10 and 12 of 12) did not have any gross lesions, whereas only 58% (7 of 12) and 92% (11 of 12) of chickens vaccinated with rHVT-cmvVP2 or IBDV-A did not have the lesions (Fig. 3A). The gross lesions of the conventional live vaccine group were nonacute bursal atrophy, which might be due to the live vaccine itself as shown previously (38). All of the chickens in the challenge control group had severe gross lesions (seven of seven), although no bursal lesions were observed in the healthy control group (zero of six). These results demonstrated that the rHVT-pecVP2 vaccination conferred complete protection against the lethal vvIBDV challenge at 4 weeks postvaccination.
FIG. 3.
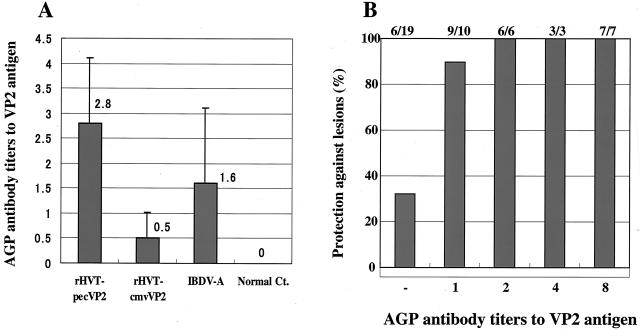
Protective efficacy of rHVTs against lethal IBDV challenge. (A) Chickens were vaccinated with each rHVT or conventional live vaccine IBDV-A and challenged with a vvIBDV at 4 weeks of age. One hundred percent (22 of 22), 58% (7 of 12), and 92% (11 of 12) of chickens vaccinated with rHVT-pecVP2, rHVT-cmvVP2, or IBDV-A, respectively, were protected against IBDV gross lesions. (B) Sera were tested for the presence of anti-VP2 antibodies detected by AGP tests. Ninety-six percent (21 of 22), 42% (5 of 12), and 67% (8 of 12) of chickens vaccinated with rHVT-pecVP2, rHVT-cmvVP2, or IBDV-A, respectively, had the AGP antibodies to the VP2 antigen.
Antibody responses to VP2 antigens after the vaccination with rHVTs.
To assess each rHVT vaccine to induce the serum antibodies to the VP2 antigen in chickens, serum antibody titers to the VP2 antigen were determined by the AGP tests. The AGP antibodies to VP2 were highly correlated with protection against the gross lesions caused by vvIBDV challenge (40). As shown in Fig. 3B, most of the chickens vaccinated with rHVT-pecVP2 (96%, 21 of 22) had the AGP antibodies to the VP2 antigen at 4 weeks postvaccination, whereas 42% (5 of 12) and 67% (8 of 12) of chickens vaccinated with rHVT-cmvVP2 or IBDV-A, respectively, had the AGP antibodies. The average AGP antibody titer of the rHVT-pecVP2 group (2.8) was higher than that of the rHVT-cmvVP2 (0.5) or IBDV-A (1.6) vaccine groups (Fig. 4A). Healthy control chickens had no AGP antibodies. As shown in Fig. 4B, there is a high correlation (96%, 25 of 26) between the presence of AGP antibodies to VP2 and protection against lethal vvIBDV challenge. These results indicated that the HVT herpesvirus vector vaccine employing a strong Pec promoter to express foreign antigens could elicit high levels of serum antibody responses in vivo.
FIG. 4.
Serum immune responses to IBDV VP2 antigen at 4 weeks of age of chickens vaccinated with rHVTs. (A) Serial dilutions of the sera were used for the titration of anti-VP2 antibodies by AGP tests. Error bars, standard deviations. (B) Correlation between titers of AGP antibody to VP2 antigen and protection against BF gross lesions caused by vvIBDV infection were determined.
Persistent infection with rHVT in vaccinated chickens during latency.
In order to demonstrate the persistent infection of rHVT in chickens, virus isolation tests were performed by cocultivation of PBL with secondary CEF feeder cells. PBL were prepared from 1 ml of heparinized blood collected from chickens vaccinated with rHVT or wt HVT and cocultivated with secondary CEFs. The virus samples were cultivated for a total of 9 days with one subculture passage. Average virus titers of four vaccine groups were obtained from each of two vaccinated chickens at 2, 3, 4, and 8 weeks of age and each of five vaccinated chickens at 12, 16, 20, and 24 weeks of age. The virus titer of rHVT-pecVP2 was maintained at a low level throughout the experiment, with a peak titer at 4 weeks of age (Fig. 5A). The virus titer then gradually decreased up to 20 weeks of age. The rHVT-pecVP2 was not recovered from two of the five chickens at 20 and 24 weeks of age in the first trial but was recovered in the second trial. Low virus titers of rHVT-pecVP2 at 2 to 3 weeks of age may be due to the variation in virus titers among chickens vaccinated with the rHVT-pecVP2, because we observed that the rHVT-pecVP2 virus titer was between 10 to 40 infectious viruses/ml of blood at 1 week of age in another experiment (data not shown). In contrast, the virus titers of rHVT-cmvVP2 were higher than that of rHVT-pecVP2 and almost comparable to that of wt HVT with the virus peak at 2 weeks of age. Both rHVT-cmvVP2 and wt HVT were recovered from all of the chickens at every point. No HVT was isolated from chickens vaccinated with live vaccine IBDV-A.
FIG. 5.
Persistence of virus infection and serum immune responses in chickens. (A) Kinetics of virus infection in peripheral blood lymphocytes in 1 ml of blood from chickens vaccinated with rHVT-pecVP2 (•), rHVT-cmvVP2 (▪), HVT (▵), or IBDV-A (○). Two samples were used for the 2-, 3-, 4-, and 8-week time points, and five samples were used for the 12-, 16-, 20-, and 24-week time points. Average virus titers (infectious units/milliliter) are shown. (B) Kinetics of serum antibody titers to VP2 antigen. Five serum samples were used for the 2-, 3-, 4-, 8-, 12-, 16-, 20-, 24-, 28-, 30-, and 32-week time points. The serum antibody titers to VP2 antigen were determined by AGP tests. Error bars indicate standard deviations.
Every clone of tHVT-pecVP2 and rHVT-cmvVP2 isolated from the vaccinated chickens expressed VP2 antigens after cultivation in CEFs for 24 weeks.
In addition, because the growth of rHVT-cmvVP2 in chickens was nearly comparable with that of wt HVT, it was shown that the foreign gene insertion site between UL45 and UL46 of HVT did not affect the HVT replication in vivo.
Persistence of serum antibody responses in chickens.
Herpesvirus vector vaccines have a high potential for the long-term induction of immune responses in animals. In order to evaluate the potential of the HVT vector in chickens, we inoculated each rHVT expressing the VP2 antigen into each of five SPF chickens. Their serum antibody responses to the VP2 antigen were assessed by AGP tests for 30 weeks at an interval of 4 weeks. Average titers of the AGP antibody to the VP2 antigen of each vaccine group are shown in Fig. 5B. The AGP antibody titer of the rHVT-pecVP2 group was the highest among these three vaccine groups throughout the experiment. The titer of antibody to VP2 continuously increased up to 16 weeks of age, and then the antibody peak titer of 1:16 persisted in chickens until the end of the experiment. In contrast, the antibody kinetic curve of the rHVT-cmvVP2 group was maintained at a titer of approximately 1:1; the peak antibody titer of IBDV-A (1:4) was detected at 3 weeks of age, and then the titer gradually decreased and became undetectable at 20 weeks of age.
At 30 weeks of age, in order to determine whether these vaccinated chickens were protected against the vvIBDV challenge, all of the chickens were challenged with vvIBDV. Because chickens of this age do not have BF and are resistant to clinical IBD, susceptibility to IBDV infection was mainly determined by seroconversion. Before the challenge, the average EIA antibody titers against VP2 antigens of chickens vaccinated with rHVT-pecVP2, rHVT-cmvVP2, the conventional live vaccine, or wt HVT were 1:7,760, 1:367, 1:121, and 1: <10, respectively (Table 1). Two weeks after the challenge there was no increase in titer of antibody against the VP2 antigen in all of the chickens vaccinated with the rHVT-pecVP2 or the conventional live IBDV vaccine, whereas some or all of the chickens vaccinated with rHVT-cmvVP2 (three of five) or HVT (five of five) serologically responded after the challenge (Table 1). The seroconversion in some chickens vaccinated with rHVT-cmvVP2 was consistent with partial protection against the BF gross lesions at 4 weeks of age (Fig. 3A). These results indicated that the rHVT-pecVP2 vaccination maintained a protective level of antibody responses in chickens throughout the experiment.
TABLE 1.
Serum antibody responses to IBDV VP2 antigens of chickens vaccinated with recombinant or conventional live vaccines after challenge with vvIBDV at 30 weeks of agea
| Vaccination and chicken no. | EIA antibody titer at wk:
|
|
|---|---|---|
| 30 | 32 | |
| rHVT-pecVP2b | ||
| 1 | 2,560 (8) | 2,560 (8) |
| 2 | 10,240 (32) | 10,240 (32) |
| 3 | 40,960 (64) | 40,960 (64) |
| 4 | 2,560 (4) | 2,560 (4) |
| 5 | 10,240 (16) | 10,240 (16) |
| Avg Ab titer | 7,760 | 7,760 |
| rHVT-cmvVP2c | ||
| 6 | 640 | 640 (2) |
| 7 | 160 | 640 (1) |
| 8 | 640 | 2,560 (4) |
| 9 | 160 | 160 |
| 10 | 640 | 640 |
| Avg Ab titer | 367 | 840 |
| HVTd | ||
| 11 | <10 | 10,240 (32) |
| 12 | <10 | 10,240 (16) |
| 13 | <10 | 40 |
| 14 | <10 | 40 |
| 15 | <10 | 160 |
| Avg Ab titer | <10 | 640 |
| IBDV-Ae | ||
| 16 | 10 | 10 |
| 17 | 640 | 640 |
| 18 | 160 | 160 |
| 19 | 160 | 160 |
| 20 | 160 | 160 |
| Avg Ab titer | 121 | 121 |
The serum antibody (Ab) titers to IBDV VP2 antigens were determined both at the challenge (30 weeks of age) and 2 weeks later (32 weeks of age) by EIA and AGP tests as described in Materials and Methods (results for AGP tests are shown in parentheses).\
Responders, zero of five chickens.\
Responders, three of five chickens.\
Responders, five of five chickens.\
Responders, zero of five chickens.
Correlation between virus replication efficiency in chickens and the humoral immune responses.
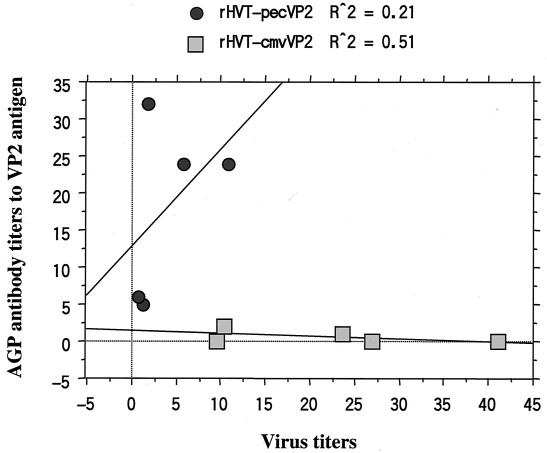
As shown in Fig. 6, there was no significant correlation between the rHVT replication efficiency in chickens and the induced AGP antibody titers with both rHVTs. The EIA antibody titers to the VP2 antigen tested at 30 weeks of age did not correlate with the virus replication efficiency of rHVT-pecVP2 or rHVT-cmvVP2.
FIG. 6.
Association of virus titers in vivo and titers of AGP antibody to IBDV VP2 antigen in each of five chickens vaccinated with rHVT-pecVP2 or rHVT-cmvVP2. Average virus and antibody titers at 12 to 24 weeks of age in each chicken were determined.
Stability of VP2 expression cassette of HVT herpesvirus vectors in chickens.
To determine whether these recovered rHVTs preserve the ability to produce VP2 antigen, culture supernatants were tested for the presence of the VP2 antigen by ELISA. As a result, all of the samples harvested from rHVT-infected CEFs were positive for the VP2 antigen. No VP2 antigen was detected in any samples from the wt HVT group. These results showed that both rHVTs retained the ability to express foreign antigens in chickens for at least 24 weeks.
We sought to determine whether the VP2 gene integrated into the HVT genome was stable during latency. Each of three vaccine isolates of rHVT-pecVP2 or rHVT-cmvVP2 from vaccinated chickens at 24 weeks of age was used to obtain the VP2 gene by PCR amplification. The amplified VP2 DNA fragments were directly sequenced. As a result, in both rHVTs, there was no mutation of the VP2 gene in each of the three isolates, indicating that the VP2 gene integrated into the HVT genome was stable in chickens.
DISCUSSION
The present study demonstrates that the HVT vector vaccine expressing large amounts of antigens under the control of the Pec promoter conferred complete protection against a lethal IBDV challenge (Fig. 3). The rHVT-pecVP2 induced high levels of antibody titer to IBDV VP2, which was approximately 10 times higher than the protection level, one unit of the AGP antibody titer (Fig. 4B). The protection level of the antibody titer would persist for the lifetime of the SPF chickens (Fig. 5B; Table 1). This immunity level against IBDV was much higher than that induced by fowl adenovirus vector (34) fowlpox virus vector (3, 40), MDV vector (39), HVT vector (8), or a herpesvirus-primed and poxvirus-boosted vaccination regimen (40) and may be one of the highest immune responses among those induced by viral vector systems. The HVT expression vector was shown to be stable during infection in chickens and safe for chickens. HVT lacks putative MDV1 virulence genes, including those coding for MEQ, pp24, and pp38 (1). Based on these results, it can be concluded that the HVT herpesvirus vector vaccine for IBD is superior to a conventional live IBDV vaccine in efficacy and safety.
There seem to be several critical points responsible for the high vaccine efficacy of the HVT vector. First, it should be pointed out that the HVT vector has high potency as a vector vaccine; HVT persistently infects PBL during latency and may continuously stimulate host immune systems at lymphoid tissues or feather follicle epithelium (FFE) cells during latency. Continuous stimulation of host immune systems by expressed antigens was demonstrated by the findings that the immune responses to IBDV VP2 increased for 16 weeks in chickens after the vaccination with the rHVT-pecVP2 (Fig. 5B). We have no idea why the immune responses to IBDV VP2 were not enhanced by the rHVT-cmvVP2. Second, the VP2 expression cassette inserted into the site between UL45 and UL46 of an HVT genome did not inactivate any HVT genes. This may help to preserve the viral replication efficiency of HVT in chickens; growth of the rHVT-cmvVP2 in chickens was nearly comparable with that of wt HVT (Fig. 5A). Several nonessential sites in the HVT genome for viral growth are already identified—US7 (8), US10 (27), UL23 (32), and UL40 (8), and two intergenic loci in the BamHI-I and pp38 gene homologue (5). Third, the most critical point is to use a strong promoter in an HVT vector to express large amounts of the antigens. The efficacy of HVT vector vaccines was dependent on the strength of the promoter to express the VP2 antigen. The rHVT-pecVP2 produced VP2 antigens in amounts four times larger than did the rHVT-cmvVP2 in CEFs (Fig. 2), but the virus titer in vivo was inversely lower than that of rHVT-cmvVP2 (Fig. 5A). These results indicate that viral replication is not as important as the amount of antigen expressed to induce strong serum antibody responses. Further studies are required to determine the optimal expression level of the VP2 antigen and replication efficiency in chickens.
Recently, another group showed that an MDV1 vector expressing Newcastle disease virus F glycoprotein under the control of a gB late promoter at the US10 site, rMDV-US10P(F), was more efficacious than an rMDV employing the SV40 late promoter, although the gB promoter was weaker than the SV40 promoter in CEFs (35). This is apparently contrary to our findings. The rMDV-US10P(F) cannot be compared directly with a rHVT-pecVP2 because of the differences in vector strains, gene insertion sites, and promoters. Also, the IBDV VP2 antigen is accumulated in the cytoplasm, whereas the Newcastle disease virus F glycoprotein is expressed on the cell surface (35, 39). Nevertheless, we speculate that this disagreement might be due to the difference in cell types in which the antigens were expressed. MDV infection in chickens is divided into three stages—productive, latent, and transforming infections (6)—and there are two types of productive infection, fully productive infection and productive-restrictive infection. Fully productive infection results in production of infectious viruses in FFE cells, whereas productive-restrictive infection produces nonenveloped, noninfectious viruses in some lymphoid and epithelial cells. Latent infection occurs predominantly in T cells and is nonproductive. Transforming infection is characteristic of T lymphocytes transformed by virulent MDV. The MDV gB late promoter may be active in both productive and productive-restrictive infection stages in the spleen, thymus, or FFE cells as reported for the HVT gB promoter (16). In addition to these infection stages, the Pec promoter might leakily express the antigen in latently infected T cells, although most viral and foreign promoters are suppressed in the activated T cells (6, 29). The Pec promoter is a strong chimera promoter consisting of a CMV enhancer and a β-actin promoter. Interests in the herpesvirus vectors to serve as recombinant vaccines are increasing; further studies are required to understand the expression sites of foreign promoters under the MDV/HVT background in vivo. Also, it is worthwhile to evaluate the potential of the Pec promoter for use in mammalian herpesvirus vectors.
The present study showed that complete protection against IBDV was induced in chickens 4 weeks after vaccination with rHVT-pecVP2 (Fig. 3A) and that the high level of the protective immunity persisted for at least 30 weeks (Fig. 5B; Table 1). We are interested in the immunological mechanisms for quick induction and long-term persistence of the protective immunity in chickens by the HVT herpesvirus vector. Both the amount of the antigen expressed and the persistent infection of the vector in chickens should be critical for inducing a high level of protective immunity for a long time. In addition, the types of cells infected with HVT may also be involved in the high levels of protective immunity. HVT persistently infects CD4+ T helper cells during latency. Antigen expression in lymphoid tissues might efficiently stimulate the host immune system. Immunological characterizations remain to be determined for better understanding of the immune responses elicited by the HVT vector.
Our study showed that the growth of the rHVT-pecVP2 in chickens was much lower than that of the rHVT-cmvVP2 or wt HVT throughout the experiment and decreased further beyond 8 weeks of age (Fig. 5A). Although the mechanisms were not elucidated in this study, one possibility is that the strong protective immunity to VP2 antigens suppresses the vector virus replication in vivo. A similar phenomenon was observed in our previous study, in which strong protective immunity induced by a booster vaccination suppressed the replication of a recombinant herpesvirus in chickens (40). Another possibility is that expression of VP2, an apoptotic inducer, directly induces the apoptosis of infected cells (11). Studies of replication of the herpesvirus vector in vivo are required.
REFERENCES
- 1.Afonso, C. L., E. R. Tulman, Z. Lu, L. Zsak, D. L. Rock, and G. F. Kutish. 2001. The genome of turkey herpesvirus. J. Virol. 75:971-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azad, A. A., N. M. Mckem, I. G. Macreadie, P. Failla, H. G. Heine, A. Chapman, and K. J. Fahey. 1991. Physicochemical and immunological characterization of recombinant host-protective antigen (VP2) of infectious bursal disease virus. Vaccine 9:715-722. [DOI] [PubMed] [Google Scholar]
- 3.Bayliss, C. D., R. W. Peters, J. K. Cook, R. L. Reece, K. Howes, M. M. Binns, and M. E. Boursnell. 1991. A recombinant fowlpox virus that expresses the VP2 antigen of infectious bursal disease virus induces protection against mortality caused by the virus. Arch. Virol. 120:193-205. [DOI] [PubMed] [Google Scholar]
- 4.Becht, H., H. Müller, and H. K. Müller. 1988. Comparative studies on structural and antigenic properties of two serotypes of infectious bursal disease virus. J. Gen. Virol. 69:631-640. [DOI] [PubMed] [Google Scholar]
- 5.Bublot, M., E. Laplace, and J.-C. Audonnet. 1999. Nonessential loci in the BamHI-I and -F fragments of the HVT FC126 genome. Acta Virol. 43:181-185. [PubMed] [Google Scholar]
- 6.Calnek, B. W., and R. L. Witter. 1997. Marek's disease, p. 369-413. In B. W. Calnek, B. W. Barnes, C. W. Beard, L. R. McDougald, and Y. M. Saif (ed.), Diseases of poultry, 10th ed. Iowa State University Press, Ames.
- 7.Cantello, J. L., A. S. Anderson, A. Francesconi, and R. W. Morgan. 1991. Isolation of a Marek's disease virus (MDV) recombinant containing the lacZ gene of Escherichia coli stably inserted within the MDV US2 gene. J. Virol. 65:1583-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darteil, R., M. Bublot, E. Laplace, J.-F. Bouquet, J.-C. Audonnet, and M. Riviere. 1995. Herpesvirus of turkey recombinant viruses expressing infectious bursal disease virus (IBDV) VP2 immunogen induces protection against an IBDV virulent challenge in chickens. Virology 211:481-490. [DOI] [PubMed] [Google Scholar]
- 9.Fahey, K. J., K. Erny, and J. Crooks. 1989. A conformational immunogen on VP-2 of infectious bursal disease virus that induces virus-neutralizing antibodies that passively protect chickens. J. Gen. Virol. 70:1473-1481. [DOI] [PubMed] [Google Scholar]
- 10.Fahey, K. J., P. McWaters, M. A. Brown, K. Erny, V. J. Murphy, and D. R. Hewish. 1991. Virus-neutralizing and passively protective monoclonal antibodies to infectious bursal disease virus of chickens. Avian Dis. 35:365-373. [PubMed] [Google Scholar]
- 11.Fernandez-Arias, A., S. Martinez, and J. F. Rodriguez. 1997. The major antigenic protein of infectious bursal disease virus, VP2, is an apoptotic inducer. J. Virol. 71:8014-8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuta, K., H. Ohashi, J. Obana, and S. Sato. 1980. Performance of 3 successive generations of specific-pathogen-free chickens maintained as a closed flock. Lab. Anim. 14:107-112. [DOI] [PubMed] [Google Scholar]
- 13.Heckert, R. A., J. Riva, J. Cook, J. McMillen, and R. D. Schwartz. 1996. Onset of protective immunity in chicks after vaccination with a recombinant herpesvirus of turkeys vaccine expressing Newcastle disease virus fusion and hemagglutinin-neuraminidase antigens. Avian Dis. 40:770-777. [PubMed] [Google Scholar]
- 14.Heine, H. G., and D. B. Boyle. 1993. Infectious bursal disease virus structural protein VP2 expressed by a fowlpox virus recombinant confers protection against disease in chickens. Arch. Virol. 131:277-292. [DOI] [PubMed] [Google Scholar]
- 15.Hirai, K., and M. Sakaguchi. 2001. Polyvalent recombinant Marek's disease virus vaccine against poultry diseases. Curr. Top. Microbiol. Immunol. 255:261-287. [DOI] [PubMed] [Google Scholar]
- 16.Holland, M. S., C. D. Mackenzie, R. W. Bull, and R. F. Silva. 1998. Latent turkey herpesvirus infection in lymphoid, nervous, and feather tissues of chickens. Avian Dis. 42:292-299. [PubMed] [Google Scholar]
- 17.Hunt, H. D., B. Lupiani, M. M. Miller, I. Gimeno, L. F. Lee, and M. S. Parcells. 2001. Marek's disease virus down-regulates surface expression of MHC (B complex) class I (BF) glycoproteins during active but not latent infection of chicken cells. Virology 282:198-205. [DOI] [PubMed] [Google Scholar]
- 18.Ismail, N. M., and Y. M. Saif. 1991. Immunogenicity of infectious bursal disease viruses in chickens. Avian Dis. 35:460-469. [PubMed] [Google Scholar]
- 19.Izumiya, Y., H. K. Jang, M. Ono, and T. Mikami. 2001. A complete genomic DNA sequence of Marek's disease virus latency. Curr. Top. Microbiol. Immunol. 255:191-221. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, D. S., and A. B. Hill. 1998. Herpesvirus evasion of the immune system. Curr. Top. Microbiol. Immunol. 232:149-177. [DOI] [PubMed] [Google Scholar]
- 21.Kibenge, F. S. B., A. S. Dhillon, and R. G. Russell. 1988. Biochemistry and immunology of infectious bursal disease virus. J. Gen. Virol. 69:1757-1775. [DOI] [PubMed] [Google Scholar]
- 22.Lee, L. F., P. Wu, D. Ren, J. Kamil, H. J. Kung, and R. L. Witter. 2000. The complete unique long sequence and the overall genome organization of the GA strain of Marek's disease virus. Proc. Natl. Acad. Sci. USA 97:6091-6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lukert, P. D., and Y. M. Saif. 1997. Infectious bursal disease, p. 721-738. In B. W. Calnek, B. W. Barnes, C. W. Beard, L. R. McDougald, and Y. M. Saif (ed.), Diseases of poultry, 10th ed. Iowa State University Press, Ames.
- 24.Marshall, D. R., J. D. Reilly, X. Liu, and R. F. Silva. 1993. Selection of Marek's disease virus recombinants expressing the Escherichia coli gpt gene. Virology 195:638-648. [DOI] [PubMed] [Google Scholar]
- 25.Mazariegos, L. A., P. D. Lukert, and J. Brown. 1990. Pathogenicity and immunosuppressive properties of infectious bursal disease “intermediate” strain. Avian Dis. 34:203-208. [PubMed] [Google Scholar]
- 26.Morgan, R. W., J. L. Cantello, and C. H. McDermott. 1990. Transfection of chicken embryo fibroblasts with Marek's disease virus DNA. Avian Dis. 34:345-351. [PubMed] [Google Scholar]
- 27.Morgan, R. W., J. Gelb, Jr., C. S. Schreurs, D. Lütticken, J. K. Rosenberger, and P. J. Sondermeijer. 1992. Protection of chickens from Newcastle and Marek's diseases with a recombinant herpesvirus of turkeys vaccine expressing the Newcastle disease virus fusion protein. Avian Dis. 36:858-870. [PubMed] [Google Scholar]
- 28.Morgan, R. W., J. Gelb, Jr., C. R. Pope, and P. J. Sondermeijer. 1993. Efficacy in chickens of a herpesvirus of turkeys recombinant vaccine containing the fusion gene of Newcastle disease virus: onset of protection and effect of maternal antibodies. Avian Dis. 37:1032-1040. [PubMed] [Google Scholar]
- 29.Parcells, M. S., R. L. Dienglewicz, A. S. Anderson, and R. W. Morgan. 1999. Recombinant Marek's disease virus (MDV)-derived lymphoblastoid cell lines: regulation of a marker gene within the context of the MDV genome. J. Virol. 73:1362-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purchase, H. G., and R. L. Witter. 1986. Public health concerns from human exposure to oncogenic avian herpesviruses. J. Am. Vet. Med. Assoc. 189:1430-1436. [PubMed] [Google Scholar]
- 31.Reddy, S. K., J. M. Sharma, J. Ahmad, D. N. Reddy, J. K. McMillen, S. M. Cook, M. A. Wild, and R. D. Schwartz. 1996. Protective efficacy of a recombinant herpesvirus of turkeys as an in ovo vaccine against Newcastle and Marek's diseases in specific-pathogen-free chickens. Vaccine 14:469-477. [DOI] [PubMed] [Google Scholar]
- 32.Ross, L. J., M. M. Binns, P. Tyers, J. Pastorek, V. Zelnik, and S. Scott. 1993. Construction and properties of a turkey herpesvirus recombinant expressing the Marek's disease virus homologue of glycoproteins B of herpes simplex virus. J. Gen. Virol. 74:371-377. [DOI] [PubMed] [Google Scholar]
- 33.Sakaguchi, M., H. Nakamura, K. Sonoda, K. Okamura, H. Yokogawa, K. Matsuo, and K. Hirai. 1998. Protection of chickens with or without maternal antibodies against both Marek's and Newcastle diseases by one-time vaccination with recombinant vaccine of Marek's disease virus type 1. Vaccine 16:472-479. [DOI] [PubMed] [Google Scholar]
- 34.Sheppard, M., W. Werner, E. Tsatas, R. McCoy, S. Prowse, and M. Johnson. 1998. Fowl adenovirus recombinant expressing VP2 of infectious bursal disease virus induces protective immunity against bursal disease. Arch. Virol. 143:915-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonoda, K., M. Sakaguchi, H. Okamura, K. Yokogawa, E. Tokunaga, S. Tokiyoshi, Y. Kawaguchi, and K. Hirai. 2000. Development of an effective polyvalent vaccine against both Marek's and Newcastle diseases based on recombinant Marek's disease virus type 1 in commercial chickens with maternal antibodies. J. Virol. 74:3217-3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsukamoto, K., N. Tanimura, H. Hihara, J. Shirai, K. Imai, K. Nakamura, and M. Maeda. 1992. Isolation of virulent infectious bursal disease virus from field outbreaks with high mortality in Japan. J. Vet. Med. Sci. 54:153-155. [DOI] [PubMed] [Google Scholar]
- 37.Tsukamoto, K., T. Matsumura, M. Mase, and K. Imai. 1995. A highly sensitive, broad-spectrum infectivity assay for infectious bursal disease virus. Avian Dis. 39:575-586. [PubMed] [Google Scholar]
- 38.Tsukamoto, K., N. Tanimura, S. Kakita, K. Ota, M. Mase, K. Imai, and H. Hihara. 1995. Efficacy of three live vaccines against highly virulent infectious bursal disease virus and the optimum vaccination time. Avian Dis. 39:218-229. [PubMed] [Google Scholar]
- 39.Tsukamoto, K., T. Kojima, Y. Komori, N. Tanimura, M. Mase, and S. Yamaguchi. 1999. Protection of chickens against very virulent infectious bursal disease virus (IBDV) and Marek's disease virus (MDV) with a recombinant MDV expressing IBDV VP2. Virology 257:352-362. [DOI] [PubMed] [Google Scholar]
- 40.Tsukamoto, K., T. Sato, S. Saito, N. Tanimura, N. Hamazaki, M. Mase, and S. Yamaguchi. 2000. Dual-viral vector approach induced strong and long-lasting protective immunity against very virulent infectious bursal disease virus. Virology 269:257-267. [DOI] [PubMed] [Google Scholar]
- 41.Tulman, E. R., C. L. Afonso, Z. Lu, L. Zsak, D. L. Rock, and G. F. Kutish. 2000. The genome of a very virulent Marek's disease virus. J. Virol. 74:7980-7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van den Berg, T. P. 2000. Acute infectious bursal disease in poultry: a review. Avian Pathol. 29:175-194. [DOI] [PubMed] [Google Scholar]