Abstract
Inflammatory cytokines are believed to play an important role in the pathogenesis of human immunodeficiency virus type 1-associated encephalitis. To examine this in the simian immunodeficiency virus (SIV)-infected macaque model of neuroAIDS, inflammatory cytokine gene expression was evaluated in the brains of macaques infected with pathogenic SIVmac251 by reverse transcriptase PCR. Interleukin-1 beta was readily detected in the brains of all animals evaluated, regardless of infection status or duration of infection. Tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) transcripts were undetectable in the brains of uninfected control animals but were upregulated at 7 and 14 days postinoculation. At the terminal stage of infection, TNF-α and IFN-γ transcripts were coexpressed in the brains of four of five animals with SIV encephalitis (SIVE). Within an encephalitic brain, TNF-α and IFN-γ transcripts were detected in six of seven regions with histologic evidence of SIVE, suggesting a direct relationship between neuropathology and altered cytokine gene expression. With combined fluorescent in situ hybridization and immunofluorescence, TNF-α-expressing cells were frequently identified as CD68-positive macrophages within perivascular lesions. These observations provide evidence that cytokines produced by activated inflammatory macrophages are an important element in the pathogenesis of SIVE.
Approximately 25% of human immunodeficiency virus type 1 (HIV-1)-infected adults develop a debilitating neurological disorder termed AIDS dementia complex (ADC) (18, 22, 23). The pathological substrate of ADC, termed HIV encephalitis (HIVE), is characterized by perivascular accumulation of macrophages and multinucleated giant cells in the central nervous system (CNS) and abundant infection and activation of brain macrophages (1, 14, 43). Early reports suggested that the level of virus replication within the CNS was correlated with the presence of ADC (43). However, more recent data have demonstrated that the number of activated inflammatory macrophages in the CNS was more closely correlated with dementia in people with AIDS (8). Since HIV-1 does not productively infect neurons, the causes for CNS dysfunction in people with AIDS remain uncertain. Neurological impairment is thought to result from the release of cytokines and other neurotoxins from activated macrophages/microglia in inflammatory infiltrates, some of which are infected with HIV.
Inflammatory cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), and gamma interferon (IFN-γ), have been shown to induce neuronal apoptosis and death in vitro (7) and also function to mediate the activation of monocytes/macrophages, the primary cell type infected with HIV or simian immunodeficiency virus (SIV) in the CNS (44). These cytokines also activate brain endothelial cells (17), thereby facilitating leukocyte recruitment to the CNS. A number of cytokines, including IL-1β and TNF-α, are dysregulated in encephalitic brains of patients with AIDS (30, 31, 34, 38, 39). It is therefore likely that cytokines produced by inflammatory mononuclear cells (29, 31, 40) play a significant role in the neuropathogenesis of AIDS.
We and others have extensively characterized changes in the CNS of macaques infected with pathogenic SIV. From this body of work, it has been well documented that viral neuroinvasion occurs early following SIV infection. Virus is routinely detected in the CNS by 7 to 14 days after infection (2, 6, 11, 15, 44). Neuroinvasion is correlated with increased numbers of perivascular macrophages, enhanced endothelial expression of vascular cell adhesion molecule 1 (VCAM-1), and evidence of intrathecal immune activation (12, 15, 25, 32, 44). During both acute and terminal infections, most SIV-infected cells are perivascular macrophages (12, 44), suggesting that infected cells enter the CNS from the periphery. During the asymptomatic stage of infection, viral load in the CNS decreases, only to rebound again as immunodeficiency develops (32, 44). Similar to what has been described for HIV infection, approximately 25% of SIV-infected macaques develop encephalitis (21, 41). Encephalitis in SIV-infected rhesus macaques is associated with blood-brain barrier disruption (16), enhanced CNS expression of chemokines (28) and chemokine receptors (42), and increased endothelial expression of VCAM-1 (27). Since many of the changes described above can be modulated by proinflammatory cytokines, we were interested in investigating the expression of CNS cytokines during the acute stage of infection, concurrently with HIV or SIV neuroinvasion, and in animals with AIDS, with and without encephalitis.
CNS tissues from 22 juvenile or adult rhesus macaques (Macaca mulatta) were evaluated retrospectively in the present study. The 20 SIV-infected animals were inoculated intravenously with uncloned SIVmac251. Virus stocks and doses were the same as those used previously (15, 36, 45). The remaining two animals were uninfected, age-matched controls. The expression of IL-1β, TNF-α, and IFN-γ transcripts was evaluated by reverse transcriptase PCR in the CNS of macaques during both acute and terminal SIV infection essentially as described previously (21). The optimal number of PCR cycles was determined initially by using a variable number of cycles to identify a linear range of amplification for each transcript. When samples were determined to have comparable cDNA based on the intensity of the β-actin PCR product (24), cytokine cDNAs were amplified with published primer sequences (37).
Ten SIV-infected animals were euthanized at timed intervals during the acute stage of infection: five animals were evaluated at 7 days postinoculation (dpi), three animals at 14 dpi, and two animals at approximately 1 month postinoculation (mpi). IL-1β mRNA was readily detected in the brains of all animals evaluated, regardless of infection status or time postinfection. In contrast, TNF-α and IFN-γ could not be detected in the brains of uninfected controls (Table 1). However, by 7 dpi, TNF-α and IFN-γ were present in five of five and four of five infected animals, respectively. Both cytokines were still present in two of three animals evaluated at 14 dpi. However, by 1 mpi, IFN-γ transcripts could no longer be detected in the CNS of either of the two animals evaluated, while TNF-α was detected in the CNS of only one animal.
TABLE 1.
Cytokine changes in the brains of macaques during the acute stage of SIV infection
| Animala | Regionb | Presence (+) or absence (−) of indicated cytokine
|
||
|---|---|---|---|---|
| TNF-α | IFN-γ | IL-1β | ||
| Controls | ||||
| A99-291 | TC | − | − | + |
| A97-128 | FC | − | − | + |
| Infected animals | ||||
| 7 dpi | ||||
| A98-444 | PC | + | + | + |
| A98-212 | FC | + | + | + |
| A98-379 | FC | + | + | + |
| A98-380 | PC | + | + | + |
| A98-443 | PC | + | − | + |
| 14 dpi | ||||
| A98-458 | FC | − | + | + |
| A99-504 | OC | + | + | + |
| A99-513 | FC | + | + | + |
| 1 mpi | ||||
| A99-201 | FC | − | − | + |
| A98-247 | FC | + | − | + |
Animals are designated by their animal identification numbers.\
TC, temporal cortex; FC, frontal cortex; PC, parietal cortex; OC, occipital cortex.
TNF-α and IFN-γ can induce VCAM-1 expression in cultured brain endothelial cells in a variety of species, including rhesus macaques (17). Increased endothelial expression of VCAM-1 in the CNS occurs by 14 dpi with pathogenic SIV, concurrent with viral neuroinvasion (25). Endothelial cell adhesion molecules have been shown to be involved in the adhesion of monocytes to activated brain endothelium in HIV and SIV infections (19, 26). Thus, based on our observations and those of others, we speculate that increased levels of IFN-γ and TNF-α in the CNS of macaques during acute SIV infection play an important role in facilitating the initial entry of mononuclear cells, some of which are infected with SIV, into the CNS via activation of brain endothelium.
Cytokine abnormalities, including increased TNF-α, have been noted in the CNS of HIV-infected patients with ADC (9, 38, 39), suggesting that indirect mechanisms, such as abnormal cytokine expression, may contribute to the pathogenesis of neurological disease. However, it is not clear from these reports whether the observed cytokine changes were directly associated with neuropathology in demented individuals. We therefore sought to investigate the association between cytokine abnormalities and neuropathology by comparing cytokine profiles in the CNS of SIV-infected macaques with and without encephalitis. We evaluated CNS tissues from 10 additional animals that were similarly infected with SIV but were allowed to progress until they were moribund with AIDS. Five animals with AIDS and histologic evidence of SIV encephalitis (SIVE) and five animals with histologically normal brains were selected from a larger group of SIV-infected animals for evaluation of cytokine gene expression. None of the selected animals had histologic evidence of CNS opportunistic infections. The diagnosis of SIVE in animals with AIDS was based on the presence of perivascular accumulations of macrophages and multinucleated giant cells and the presence of SIV demonstrated by in situ hybridization. Riboprobes and methods for SIV localization have been described elsewhere (10, 21). Similar to observations during acute SIV infection, IL-1β was detected in all tissues, regardless of neuropathological status. Both TNF-α and IFN-γ were detected in the brains of four of five animals with SIVE (Table 2), whereas TNF-α or IFN-γ alone was detected in the brains of two of five or one of five animals without encephalitis, respectively (Pz ⩽ 1.25 = 0.89; Z test).
TABLE 2.
Cytokine gene expression in encephalitic and nonencephalitic brains of adult macaques terminally infected with SIV
| Animala | Regionb | Presence (+) or absence (−) of indicated cytokine
|
||
|---|---|---|---|---|
| TNF-α | IFN-γ | IL-1β | ||
| Controls | ||||
| A99-291 | TC | − | − | + |
| A97-128 | FC | − | − | + |
| Infected animals | ||||
| SIV without encephalitis | ||||
| A99-676 | TC | − | − | + |
| A99-319 | TC | + | − | + |
| A97-556 | PC | − | − | + |
| A99-338 | TC | − | + | + |
| A99-200 | TC | + | − | + |
| SIVE | ||||
| A99-696 | TC | + | + | + |
| A97-251 | FC | + | + | + |
| A98-209 | FC | − | − | + |
| A97-76 | TC | + | + | + |
| A97-06 | C | + | + | + |
Animals are designated by their animal identification numbers.\
TC, temporal cortex; FC, frontal cortex; PC, parietal cortex; C, cortex.
It has been suggested that CNS expression of cytokines in HIV-infected patients with AIDS may reflect a generalized CNS immune activation with terminal disease (34). We therefore sought to investigate whether the expression of IFN-γ and TNF-α in the CNS of animals with SIVE was generalized or associated with areas of neuropathology. TNF-α and IFN-γ gene expression was evaluated in multiple regions within the brain of an animal with SIVE and then correlated with the presence and severity of lesions within each region. The severity and distribution of lesions were scored as described previously (28) and outlined in Table 3. As with our observations when comparing cytokines in encephalitic versus nonencephalitic brains, there was coordinate expression of both TNF-α and IFN-γ in six of seven CNS regions with histologic evidence of SIVE (Table 3). By evaluating multiple regions within an encephalitic brain, we were able to clearly demonstrate a direct association (Pz ⩽ 5.99 = 0.99; Z test) between altered cytokines and the presence of characteristic lesions within these regions. Thus, we can conclude that the enhanced expression of TNF-α and IFN-γ in the CNS of macaques with SIVE is directly associated with areas of neuropathology and does not reflect a generalized phenomenon of CNS immune activation.
TABLE 3.
Expression of proinflammatory cytokines and presence of SIVE in multiple brain regions from a macaque with encephalitis
| Region | Presence (+) or absence (−) of indicated cytokine
|
Severity of SIVEa | Distribution of SIVEb | |
|---|---|---|---|---|
| TNF-α | IFN-γ | |||
| Globus pallidus | + | + | 2 | 2 |
| Thalamus | + | + | 4 | 3 |
| Putamen | + | + | 4 | 2 |
| Frontal cortex | + | + | 4 | 3 |
| Parietal cortex | + | − | 0 | N/A |
| Occipital gray | − | − | 0 | N/A |
| Occipital white | + | + | 3 | 2 |
| Cerebellum gray | + | + | 4 | 2 |
| Cerebellum white | − | + | 4 | 2 |
Severity scoring system: 0, none; 1, minimal; 2, mild; 3, moderate; 4, severe.\
Distribution scoring system: 1, focal; 2, multifocal; 3, diffuse; N/A, not applicable (no lesions).
Our observation that TNF-α and IFN-γ were coordinately expressed in the CNS of acutely infected macaques and in macaques with SIVE suggests that these two cytokines may be functioning synergistically. In human cell lines, TNF-α and IFN-γ have been shown to synergistically regulate the expression of inflammation-associated genes such as those encoding major histocompatibility complex class I, intercellular adhesion molecule 1, and VCAM-1 (4). TNF-α and IFN-γ have also been shown to synergistically enhance fractalkine expression in cultured astrocytes (46). Fractalkine plays an important role in the recruitment and adhesion of monocytes to human endothelial cells (3) and is markedly upregulated in the brains of pediatric patients with HIVE compared to those without HIVE (33). Thus, it is possible that TNF-α and IFN-γ operate synergistically in the CNS to enhance the recruitment and adhesion of monocytes from the peripheral blood into the brain.
It is believed that neurotoxins produced by inflammatory macrophages contribute to the development of neurologic impairment in ADC. TNF-α is a potent neurotoxin, and its expression has been described in both endothelial cells and macrophages/microglia in tissues from patients with ADC (20, 29, 35, 40). To determine the cellular source of TNF-α and IFN-γ in tissues from macaques with SIVE, we developed a novel fluorescent in situ hybridization technique combined with immunofluorescence. In situ hybridization was performed essentially as described previously (21, 44). Sections were hybridized overnight with antisense cytokine riboprobe. Digoxigenin-labeled riboprobes specific for rhesus macaque TNF-α and IFN-γ genes were synthesized in vitro from T7 RNA polymerase promoter-tailed DNA templates generated by a PCR-based system (5). Bound probes were detected with sheep antidigoxigenin antibodies. Detection was performed with either nitroblue tetrazolium/5-bromo-4-chloro-3-indolylphosphate (NBT/BCIP) for light microscopy or HNPP (2-hydroxy-3-naphthoic acid-2′-phenylanilide phosphate) and Fast Red TR for fluorescent microscopy (Roche Molecular Biochemicals, Indianapolis, Ind.). This was followed by two-color immunofluorescent staining and subsequent confocal imaging as described previously (13, 44). Briefly, tissue sections were sequentially incubated with a polyclonal antibody specific for brain endothelial cells (GLUT-1; Chemicon, Temecula, Calif.) and a monoclonal antimacrophage antibody (CD68, clone KP1; DAKO Corporation, Carpinteria, Calif.), followed by secondary antibodies conjugated either with Alexa Fluor 488 for CD68 detection or with Cy5 for GLUT-1 detection (Molecular Probes, Eugene, Oreg.).
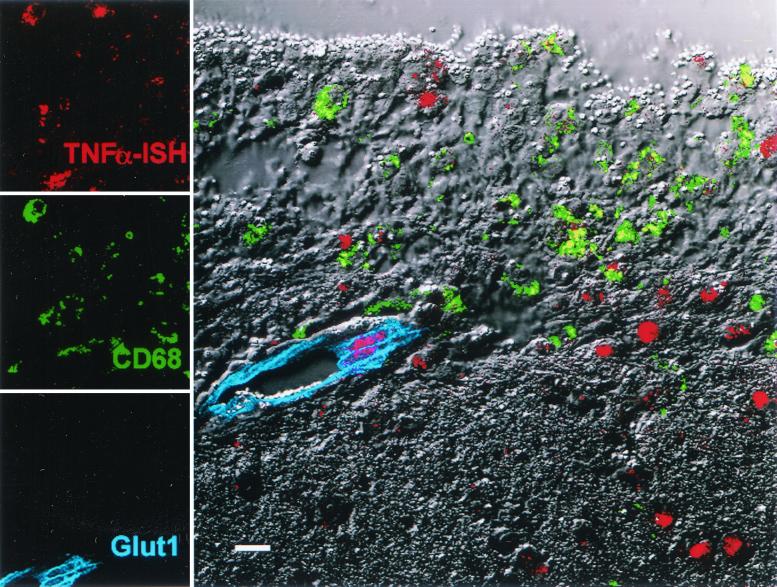
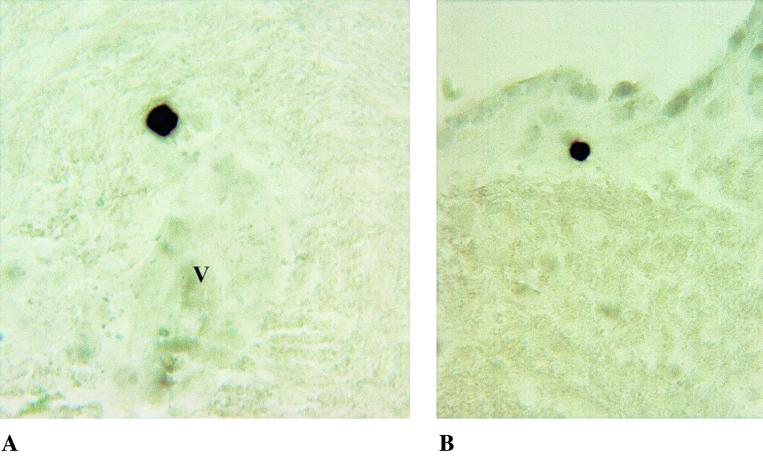
In accordance with data from HIV dementia cases, we demonstrated that CD68+ macrophages within perivascular lesions are a significant source of TNF-α transcripts in the brains of macaques with SIVE (Fig. 1). Other cell types, including brain endothelial cells within lesions (Fig. 1) and ependymal cells (data not shown), were identified as additional sources of TNF-α in encephalitic brain. Other unidentified cells adjacent to lesions also expressed TNF-α. The identification of endothelial cells as a source of TNF-α provides additional evidence for the key role of endothelial activation in the pathogenesis of SIVE. In comparison, IFN-γ transcripts were restricted to isolated mononuclear cells typically adjacent to small blood vessels (Fig. 2). Hybridized cells, which are morphologically compatible with lymphocytes, were intensely positive for IFN-γ. These cells did not appear to be directly associated with inflammatory lesions. Evaluation of the brains of patients with HIVE showed a similar association between macrophage infiltration, increased expression of endothelial adhesion molecules, and increased expression of proinflammatory cytokines (19), suggesting a common role for cytokines in HIV and SIV neuropathogenesis.
FIG. 1.
Demonstration of TNF-α transcripts in paraffin-embedded sections of brain from an SIV-infected macaque with SIVE. Many TNF-α+ cells are CD68+ macrophages (colocalized as yellow) located within a focus of inflammation. TNF-α transcripts are also evident within endothelial cells adjacent to the lesion and other cells within the neuropil. Bar = 10 μm.
FIG. 2.
Demonstration of IFN-γ transcripts by in situ hybridization on paraffin-embedded sections of brain from an SIV-infected macaque with SIVE. Isolated, intensely positive mononuclear cells, consistent with lymphocytes, are evident adjacent to small blood vessels (indicated by the letter V) (A) and within the subependyma (B).
Acknowledgments
We thank the pathologists and staff within the Division of Comparative Pathology at the New England Regional Primate Research Center for performing necropsies and histology. We also thank Robin Rodriguez and Pyone Pyone Aye at the Tulane Regional Primate Research Center for graphical and statistical assistance, respectively.
This work was supported by Public Health Service grants NS35732, NS30769, MH61192, RR000164, and RR000168. A. A. Lackner is the recipient of an Elizabeth Glaser Scientist award.
REFERENCES
- 1.Bell, J. E. 1998. The neuropathology of adult HIV infection. Rev. Neurol. 154:816-829. [PubMed] [Google Scholar]
- 2.Chakrabarti, L., M. Hurtrel, M. A. Maire, R. Vazeux, D. Dormont, L. Montagnier, and B. Hurtrel. 1991. Early viral replication in the brain of SIV-infected rhesus monkeys. Am. J. Pathol. 139:1273-1280. [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman, G. A., K. E. Moores, J. Gohil, T. A. Berkhout, L. Patel, P. Green, C. H. Macphee, and B. R. Stewart. 2000. The role of fractalkine in the recruitment of monocytes to the endothelium. Eur. J. Pharmacol. 392:189-195. [DOI] [PubMed] [Google Scholar]
- 4.Cheshire, J. L., and A. S. Baldwin, Jr. 1997. Synergistic activation of NF-κB by tumor necrosis factor alpha and gamma interferon via enhanced IκBα degradation and de novo IκBβ degradation. Mol. Cell. Biol. 17:6746-6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cone, R. W., and E. Schlaepfer. 1997. Improved in situ hybridization to HIV with RNA probes derived from PCR products. J. Histochem. Cytochem. 45:721-727. [DOI] [PubMed] [Google Scholar]
- 6.Demuth, M., S. Czub, U. Sauer, E. Koutsilieri, P. Haaft, J. Heeney, C. Stahl-Hennig, V. ter Meulen, and S. Sopper. 2000. Relationship between viral load in blood, cerebrospinal fluid, brain tissue and isolated microglia with neurological disease in macaques infected with different strains of SIV. J. Neurovirol. 6:187-201. [DOI] [PubMed] [Google Scholar]
- 7.Downen, M., T. D. Amaral, L. L. Hua, M. L. Zhao, and S. C. Lee. 1999. Neuronal death in cytokine-activated primary human brain cell culture: role of tumor necrosis factor-alpha. Glia 28:114-127. [PubMed] [Google Scholar]
- 8.Glass, J. D., H. Fedor, S. L. Wesselingh, and J. C. McArthur. 1995. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann. Neurol. 38:755-762. [DOI] [PubMed] [Google Scholar]
- 9.Glass, J. D., S. L. Wesselingh, O. A. Selnes, and J. C. McArthur. 1993. Clinical-neuropathologic correlation in HIV-associated dementia. Neurology 43:2230-2237. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch, V., D. Adger-Johnson, B. Campbell, S. Goldstein, C. Brown, W. R. Elkins, and D. C. Montefiori. 1997. A molecularly cloned, pathogenic, neutralization-resistant simian immunodeficiency virus, SIVsmE543-3. J. Virol. 71:1608-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hurtrel, B., L. Chakrabarti, M. Hurtrel, M. A. Maire, D. Dormont, and L. Montagnier. 1991. Early SIV encephalopathy. J. Med. Primatol. 20:159-166. [PubMed] [Google Scholar]
- 12.Hurtrel, B., L. Chakrabarti, M. Hurtrel, and L. Montagnier. 1993. Target cells during early SIV encephalopathy. Res. Virol. 144:41-46. [DOI] [PubMed] [Google Scholar]
- 13.Klein, R. S., K. C. Williams, X. Alvarez-Hernandez, S. Westmoreland, T. Force, A. A. Lackner, and A. D. Luster. 1999. Chemokine receptor expression and signaling in macaque and human fetal neurons and astrocytes: implications for the neuropathogenesis of AIDS. J. Immunol. 163:1636-1646. [PubMed] [Google Scholar]
- 14.Lackner, A. A., M. O. Smith, R. J. Munn, D. J. Martfeld, M. B. Gardner, P. A. Marx, and S. Dandekar. 1991. Localization of simian immunodeficiency virus in the central nervous system of rhesus monkeys. Am. J. Pathol. 139:609-621. [PMC free article] [PubMed] [Google Scholar]
- 15.Lane, J. H., V. G. Sasseville, M. O. Smith, P. Vogel, D. R. Pauley, M. P. Heyes, and A. A. Lackner. 1996. Neuroinvasion by simian immunodeficiency virus coincides with increased numbers of perivascular macrophages/microglia and intrathecal immune activation. J. Neurovirol. 2:423-432. [DOI] [PubMed] [Google Scholar]
- 16.Luabeya, M. K., L. M. Dallasta, C. L. Achim, C. D. Pauza, and R. L. Hamilton. 2000. Blood-brain barrier disruption in simian immunodeficiency virus encephalitis. Neuropathol. Appl. Neurobiol. 26:454-462. [DOI] [PubMed] [Google Scholar]
- 17.MacLean, A. G., M. S. Orandle, X. Alvarez, K. C. Williams, and A. A. Lackner. 2001. Rhesus macaque brain microvessel endothelial cells behave in a manner phenotypically distinct from umbilical vein endothelial cells. J. Neuroimmunol. 118:223-232. [DOI] [PubMed] [Google Scholar]
- 18.Navia, B. A., B. D. Jordan, and R. W. Price. 1986. The AIDS dementia complex. I. Clinical features. Ann. Neurol. 19:517-524. [DOI] [PubMed] [Google Scholar]
- 19.Nottet, H. S., Y. Persidsky, V. G. Sasseville, A. N. Nukuna, P. Bock, Q. H. Zhai, L. R. Sharer, R. D. McComb, S. Swindells, C. Soderland, and H. E. Gendelman. 1996. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J. Immunol. 156:1284-1295. [PubMed] [Google Scholar]
- 20.Nuovo, G. J., and M. L. Alfieri. 1996. AIDS dementia is associated with massive, activated HIV-1 infection and concomitant expression of several cytokines. Mol. Med. 2:358-366. [PMC free article] [PubMed] [Google Scholar]
- 21.Orandle, M. S., K. C. Williams, A. G. MacLean, S. V. Westmoreland, and A. A. Lackner. 2001. Macaques with rapid disease progression and simian immunodeficiency virus encephalitis have a unique cytokine profile in peripheral lymphoid tissues. J. Virol. 75:4448-4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price, R. W. 1988. Dementia associated with AIDS. Trans. Assoc. Life Insur. Med. Dir. Am. 71:235-240. [PubMed] [Google Scholar]
- 23.Price, R. W., B. A. Navia, and E. S. Cho. 1986. AIDS encephalopathy. Neurol. Clin. 4:285-301. [PubMed] [Google Scholar]
- 24.Raff, T., M. van der Giet, D. Endemann, T. Wiederholt, and M. Paul. 1997. Design and testing of beta-actin primers for RT-PCR that do not co-amplify processed pseudogenes. BioTechniques 23:456-460. [DOI] [PubMed] [Google Scholar]
- 25.Sasseville, V. G., J. H. Lane, D. Walsh, D. J. Ringler, and A. A. Lackner. 1995. VCAM-1 expression and leukocyte trafficking to the CNS occur early in infection with pathogenic isolates of SIV. J. Med. Primatol. 24:123-131. [DOI] [PubMed] [Google Scholar]
- 26.Sasseville, V. G., W. Newman, S. J. Brodie, P. Hesterberg, D. Pauley, and D. J. Ringler. 1994. Monocyte adhesion to endothelium in simian immunodeficiency virus-induced AIDS encephalitis is mediated by vascular cell adhesion molecule-1/alpha 4 beta 1 integrin interactions. Am. J. Pathol. 144:27-40. [PMC free article] [PubMed] [Google Scholar]
- 27.Sasseville, V. G., W. A. Newman, A. A. Lackner, M. O. Smith, N. C. Lausen, D. Beall, and D. J. Ringler. 1992. Elevated vascular cell adhesion molecule-1 in AIDS encephalitis induced by simian immunodeficiency virus. Am. J. Pathol. 141:1021-1030. [PMC free article] [PubMed] [Google Scholar]
- 28.Sasseville, V. G., M. M. Smith, C. R. Mackay, D. R. Pauley, K. G. Mansfield, D. J. Ringler, and A. A. Lackner. 1996. Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am. J. Pathol. 149:1459-1467. [PMC free article] [PubMed] [Google Scholar]
- 29.Seilhean, D., K. Kobayashi, Y. He, T. Uchihara, O. Rosenblum, C. Katlama, F. Bricaire, C. Duyckaerts, and J. J. Hauw. 1997. Tumor necrosis factor-alpha, microglia and astrocytes in AIDS dementia complex. Acta Neuropathol. 93:508-517. [DOI] [PubMed] [Google Scholar]
- 30.Shapshak, P., I. Nagano, K. Xin, W. Bradley, C. B. McCoy, N. C. Sun, R. V. Stewart, M. Yoshioka, C. Petito, K. Goodkin, et al. 1995. HIV-1 heterogeneity and cytokines. Neuropathogenesis. Adv. Exp. Med. Biol. 373:225-238. [DOI] [PubMed] [Google Scholar]
- 31.Sippy, B. D., F. M. Hofman, D. Wallach, and D. R. Hinton. 1995. Increased expression of tumor necrosis factor-alpha receptors in the brains of patients with AIDS. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10:511-521. [PubMed] [Google Scholar]
- 32.Smith, M. O., M. P. Heyes, and A. A. Lackner. 1995. Early intrathecal events in rhesus macaques (Macaca mulatta) infected with pathogenic or nonpathogenic molecular clones of simian immunodeficiency virus. Lab. Investig. 72:547-558. [PubMed] [Google Scholar]
- 33.Tong, N., S. W. Perry, Q. Zhang, H. J. James, H. Guo, A. Brooks, H. Bal, S. A. Kinnear, S. Fine, L. G. Epstein, D. Dairaghi, T. J. Schall, H. E. Gendelman, S. Dewhurst, L. R. Sharer, and H. A. Gelbard. 2000. Neuronal fractalkine expression in HIV-1 encephalitis: roles for macrophage recruitment and neuroprotection in the central nervous system. J. Immunol. 164:1333-1339. [DOI] [PubMed] [Google Scholar]
- 34.Tyor, W. R., J. D. Glass, J. W. Griffin, P. S. Becker, J. C. McArthur, L. Bezman, and D. E. Griffin. 1992. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann. Neurol. 31:349-360. [DOI] [PubMed] [Google Scholar]
- 35.Tyor, W. R., S. L. Wesselingh, J. W. Griffin, J. C. McArthur, and D. E. Griffin. 1995. Unifying hypothesis for the pathogenesis of HIV-associated dementia complex, vacuolar myelopathy, and sensory neuropathy. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 9:379-388. [PubMed] [Google Scholar]
- 36.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 37.Villinger, F., S. S. Brar, A. Mayne, N. Chikkala, and A. A. Ansari. 1995. Comparative sequence analysis of cytokine genes from human and nonhuman primates. J. Immunol. 155:3946-3954. [PubMed] [Google Scholar]
- 38.Wesselingh, S. L., J. Glass, J. C. McArthur, J. W. Griffin, and D. E. Griffin. 1994. Cytokine dysregulation in HIV-associated neurological disease. Adv. Neuroimmunol. 4:199-206. [DOI] [PubMed] [Google Scholar]
- 39.Wesselingh, S. L., C. Power, J. D. Glass, W. R. Tyor, J. C. McArthur, J. M. Farber, J. W. Griffin, and D. E. Griffin. 1993. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann. Neurol. 33:576-582. [DOI] [PubMed] [Google Scholar]
- 40.Wesselingh, S. L., K. Takahashi, J. D. Glass, J. C. McArthur, J. W. Griffin, and D. E. Griffin. 1997. Cellular localization of tumor necrosis factor mRNA in neurological tissue from HIV-infected patients by combined reverse transcriptase/polymerase chain reaction in situ hybridization and immunohistochemistry. J. Neuroimmunol. 74:1-8. [DOI] [PubMed] [Google Scholar]
- 41.Westmoreland, S. V., E. Halpern, and A. A. Lackner. 1998. Simian immunodeficiency virus encephalitis in rhesus macaques is associated with rapid disease progression. J. Neurovirol. 4:260-268. [DOI] [PubMed] [Google Scholar]
- 42.Westmoreland, S. V., J. B. Rottman, K. C. Williams, A. A. Lackner, and V. G. Sasseville. 1998. Chemokine receptor expression on resident and inflammatory cells in the brain of macaques with simian immunodeficiency virus encephalitis. Am. J. Pathol. 152:659-665. [PMC free article] [PubMed] [Google Scholar]
- 43.Wiley, C. A., and C. Achim. 1994. Human immunodeficiency virus encephalitis is the pathological correlate of dementia in acquired immunodeficiency syndrome. Ann. Neurol. 36:673-676. [DOI] [PubMed] [Google Scholar]
- 44.Williams, K. C., S. Corey, S. V. Westmoreland, D. Pauley, H. Knight, C. deBakker, X. Alvarez, and A. A. Lackner. 2001. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. J. Exp. Med. 193:905-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wykrzykowska, J. J., M. Rosenzweig, R. S. Veazey, M. A. Simon, K. Halvorsen, R. C. Desrosiers, R. P. Johnson, and A. A. Lackner. 1998. Early regeneration of thymic progenitors in rhesus macaques infected with simian immunodeficiency virus. J. Exp. Med. 187:1767-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoshida, H., T. Imaizumi, K. Fujimoto, N. Matsuo, K. Kimura, X. Cui, T. Matsumiya, K. Tanji, T. Shibata, W. Tamo, M. Kumagai, and K. Satoh. 2001. Synergistic stimulation, by tumor necrosis factor-alpha and interferon-gamma, of fractalkine expression in human astrocytes. Neurosci. Lett. 303:132-136. [DOI] [PubMed] [Google Scholar]