Abstract
A critical step in the turnover of yeast mRNAs is decapping. Two yeast proteins, Dcp1p and Dcp2p, are absolutely required for decapping, although their precise roles in the decapping reaction have not been established. To determine the function of both Dcp1p and Dcp2p in decapping, we purified recombinant versions of these proteins from Escherichia coli and examined their properties. These experiments demonstrate that copurification of Dcp1p and Dcp2p yields active decapping enzyme under a variety of conditions. Moreover, Dcp2p alone can have decapping activity under some biochemical conditions. This suggests that Dcp2p can be a catalytic subunit of the decapping complex, and Dcp1p may function to enhance Dcp2p activity, or as an additional active subunit. In addition, recombinant Dcp1p/Dcp2p prefers long mRNA substrates and is sensitive to inhibition by sequestration of the 5′ end but not the 3′ end of the substrate. This suggests that Dcp1p/Dcp2p contains an additional RNA-binding site spatially distinct from the active site. Finally, using two RNA-binding proteins that enhance decapping in vivo (Edc1p and Edc2p), we can reconstitute the activation of decapping with recombinant proteins. This indicates that the Edc1 and Edc2 proteins act directly on the decapping enzyme.
Keywords: Decapping, turnover, decay, yeast
INTRODUCTION
The process of mRNA degradation is a common way to control gene expression. Multiple pathways exist in eukaryotic cells for the degradation of polyadenylated mRNAs (for review, see Beelman and Parker 1995). A major RNA decay pathway has been described in yeast where both unstable and stable mRNAs are primarily degraded by a 5′ → 3′ mechanism of mRNA turnover. In this pathway, the first step is shortening of the 3′ poly(A) tail (Muhlrad and Parker 1992; Decker and Parker 1993). Once the mRNA has become oligo-adenylated (10–12 As), the mRNA becomes a substrate for a decapping reaction (Muhlrad et al. 1994, 1995). After decapping, the mRNA is rapidly degraded in a 5′ → 3′ direction by the Xrn1p exoribonuclease (Hsu and Stevens 1993). Differential stabilities among diverse mRNAs are due to combinatorial variations in the rates of deadenylation and decapping (Muhlrad et al. 1994, 1995; Cao and Parker 2001). Alternatively, following deadenylation, mRNAs can be degraded in a 3′ to 5′ direction by the cytoplasmic exosome (Jacobs-Anderson and Parker 1998) and the resulting cap structure hydrolyzed by the DcpS scavenger decapping enzyme, which is encoded by the DCS1 gene in yeast (Wang and Kiledjian 2001; Liu et al. 2002).
Decapping is a critical control point in the major decay pathway as it both precedes and permits the degradation of the mRNA body. The decapping reaction in vivo requires two interacting proteins, Dcp1p and Dcp2p (Beelman et al. 1996; Dunckley and Parker 1999). The in vivo activity of this Dcp1p/Dcp2p enzyme is modulated in both positive and negative ways. For example, the efficiency of the decapping reaction is enhanced by the activity of the Pat1p/Lsm1-7p complex and Dhh1p, a member of the DEAD box family of RNA helicases (Bonnerot et al. 2000; Bouveret et al. 2000; Tharun et al. 2000; Coller et al. 2001; Fischer and Weis 2002). In addition, two small RNA-binding proteins, Edc1p and Edc2p, can act to stimulate the rate of decapping both in vivo and in vitro (Dunckley et al. 2001; Schwartz et al. 2003). Although not absolutely required for decapping, all these proteins stimulate the decapping of both stable and unstable mRNAs, suggesting they are general activators of the decapping reaction. In contrast, proteins that are involved in translation initiation are general inhibitors of decapping. For example, the stability of the interaction between the cytoplasmic cap-binding complex and the mRNA cap structure influences how quickly the mRNA is decapped (Schwartz and Parker 1999, 2000; Vilela et al. 2000; Ramirez et al. 2002). Transcript-specific binding proteins that promote or inhibit the decapping reaction can also modulate the decapping rates of individual mRNAs. For example, Puf3p binds to the 3′UTR of the COX17 mRNA and enhances the rates of both deadenylation and decapping of this transcript (Olivas and Parker 2000).
To understand the control of decapping, a mechanistic understanding of the properties and functions of the Dcp1 and Dcp2 proteins is required. Toward this end, we purified these proteins from Escherichia coli and examined their properties. These experiments demonstrated that Dcp2p alone has decapping activity under some biochemical conditions, but that activity is more robust in the presence of Dcp1p. Moreover, Dcp1p/Dcp2p prefers long mRNA substrates, suggesting that Dcp1p/Dcp2p contains an additional RNA-binding site that is distinct from the active site. Finally, the addition of recombinant purified Edc1p or Edc2p stimulates the recombinant decapping enzyme, indicating that the Edc1 and Edc2 proteins act directly on the decapping enzyme.
RESULTS
Purification of Dcp1p, Dcp2p, and Dcp1p+Dcp2p
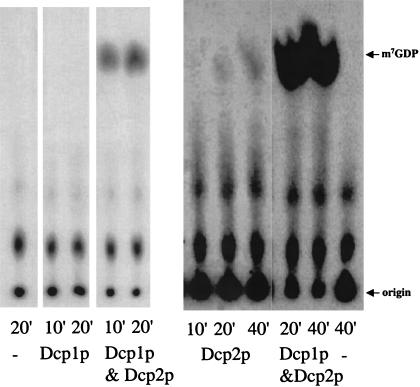
To determine the functional roles of Dcp1p and Dcp2p, we purified the proteins individually or as a complex from E. coli. In these experiments, we used a carboxy-terminal truncated version of Dcp2p, referred to as Dcp2ΔCp. We used this derivative of Dcp2p for two reasons. First, Dcp2ΔCp contains the conserved region of Dcp2p and is sufficient to complement a dcp2Δ strain (Dunckley and Parker 1999). Second, the full-length Dcp2p is particularly prone to proteolysis and difficult to purify (Dunckley and Parker 1999; Dunckley et al. 2001). As reported previously, we were initially unable to obtain any decapping activity from Dcp1p or Dcp2ΔCp under standard decapping conditions when the individual proteins were purified (LaGrandeur and Parker 1998; Dunckley and Parker 1999) (Fig. 1 ▶). We did observe a small amount of activity with Dcp2ΔCp alone. The significance of this activity, however, was unclear as similar low levels of activity were observed with point mutations in Dcp2ΔCp, which inactivate the enzyme in vivo (data not shown).
FIGURE 1.
Purified fractions from cells expressing Dcp1p, Dcp2ΔCp, or Dcp1p and Dcp2ΔCp were assayed for decapping activity. Decapping reactions were carried out as described in Materials and Methods in 10 μL reactions with 1 fmole of MFA2 substrate and ∼20–30 ng of total purified protein. Reaction aliquots were taken and the reaction was stopped at the indicated time points; the reaction products were separated by PEI cellulose TLC. (−) Reaction with no enzyme present.
Coexpression of Dcp1p and Dcp2ΔCp in E. coli yields decapping activity
To determine if the combination of Dcp1p and Dcp2ΔCp was sufficient for producing decapping activity, we coexpressed Flag Dcp1p and His6 Dcp2ΔCp in E. coli and purified over an anti-Flag affinity column. The resulting purified protein fraction contains both Flag Dcp1p and His6 Dcp2ΔCp as shown by both analysis of a silver-stained protein gel and corresponding Western blot analysis (data not shown). The purified fractions were assayed for decapping activity by incubating the protein preparations with cap-labeled MFA2 mRNA and detecting the release of m7GDP by thin layer chromatography. Purified fractions with Dcp1p and Dcp2ΔCp have mRNA decapping activity (Fig. 1 ▶). Similar results are obtained using a Ni2+ affinity column, where both Flag Dcp1p and His6 Dcp2ΔCp copurify and the protein fraction has decapping activity (data not shown). It should be noted that these experiments are done in enzyme excess and are qualitative in nature. These results suggest that the combination of Dcp1p and Dcp2ΔCp is sufficient to produce active decapping enzyme.
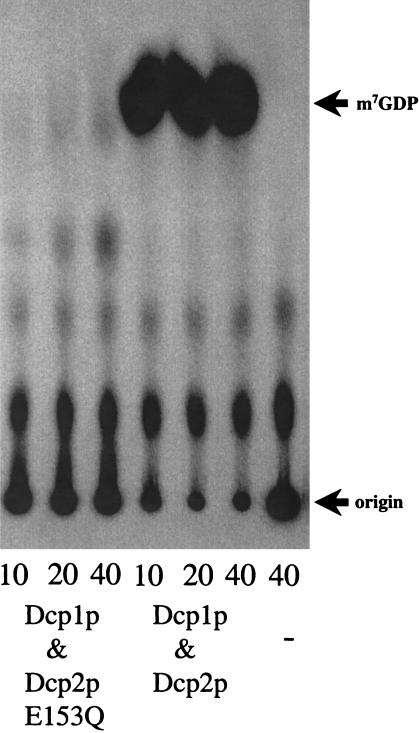
One caveat with the decapping assay used in Figure 1 ▶ is that m7GDP comigrates with inorganic phosphate (Pi) (Wang and Kiledjian 2001). Therefore, we confirmed that the decapping reaction using recombinant enzyme was producing m7GDP by treating the products of the decapping reaction to nucleotide diphosphate kinase (NDPK). This converts m7GDP, but not Pi, to m7GTP, which has a unique migration in the TLC solvent. The product generated by the decapping reaction using the recombinant decapping enzymes is converted to m7GTP by treatment with NDPK, therefore indicating that the recombinant decapping enzyme is producing the expected reaction product, m7GDP (data not shown). In summary, these experiments demonstrated that coexpression of Dcp1p and Dcp2ΔCp was sufficient for producing active recombinant decapping enzyme. To verify that the decapping activity we observed was the same as the actual decapping activity from yeast, we examined the protein requirements for decapping. Dcp2p contains a Nudix, or MutT, motif that is required for decapping in yeast (Dunckley and Parker 1999). Thus, we determined if the Q153E point mutation in the Nudix motif affected the activity of the E. coli-produced enzyme. This point mutation (Q153E) completely blocked mRNA decapping in yeast based on mRNA analysis (Dunckley and Parker 1999). Dcp2ΔCp Q153E still interacts with Dcp1p as the mutant protein copurified with Flag Dcp1p over an anti-Flag column (data not shown). The resulting purified fraction, however, was unable to decap a cap-labeled MFA2 substrate (Fig. 2 ▶). Therefore, similar to the yeast decapping activity, the Nudix motif in Dcp2ΔCp is required for activity when the proteins are purified from E. coli.
FIGURE 2.
Decapping activity requires a normal Nudix motif in Dcp2ΔCp. Decapping assays analyzed the activity of purified protein fractions where Dcp2ΔCp is wild type or has the E153Q mutation. Dcp1p is present and wild type in all these protein preparations. Decapping reactions were carried out with 1 fmole MFA2 substrate in a 10-μL reaction and ∼225 ng of total protein in the purified fractions. Products were separated by PEI cellulose TLC. (−) Reaction with no enzyme present.
Recombinant decapping activity prefers longer mRNA substrates and requires an exposed cap
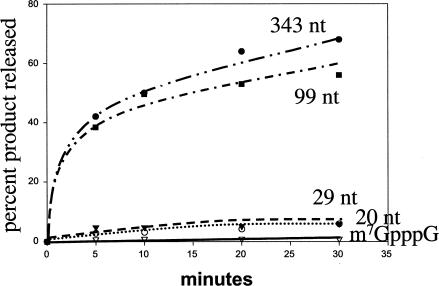
The ability to produce active decapping enzyme in E. coli allowed us to examine various aspects of the decapping reaction. For example, the yeast-purified mRNA decapping activity was found to prefer substrates longer than 29 nucleotides (LaGrandeur and Parker 1998). Using the recombinant decapping activity we re-analyzed the size-dependence of the decapping reaction to determine if the requirement is an intrinsic property of the enzyme itself or a feature conveyed by other yeast proteins that copurify. As shown in Figure 3 ▶ the decapping activity is maximal with longer mRNA substrates (>60% of the 99- and 343-nucleotide-long substrate was decapped in 30′ reaction) and is markedly less with shorter substrates (up to 6% of the 20- and 29-nucleotide-long substrate was decapped) and very small amounts of activity were detected with cap analog substrates (7meGpppG) (1% of the substrate was decapped). Therefore, the requirement for longer, capped mRNA substrates is an inherent requirement of the Dcp1p/Dcp2p decapping enzyme.
FIGURE 3.
Decapping enzyme prefers longer substrates. A graphical representation of the activity observed with recombinant Dcp1p/Dcp2ΔCp decapping activity and different-sized MFA2 substrates. The sizes are indicated on the graph: 343 nucleotides (solid circles); 99 nucleotides (solid squares); 29 nucleotides (solid triangles); 20 nucleotides (open circles); and 1 nucleotide (cap analog, m7GpppG) (open triangles). Reactions were carried out as described in Materials and Methods with 1 fmole of the indicated MFA2 substrate, 10-μL reaction and 20 ng total protein. Products were separated by PEI cellulose TLC and quantitated with a Molecular Dynamics PhosphorImager.
There are two possible explanations for why a longer RNA substrate is preferred by the decapping enzyme. First, it could be that the enzyme contains an RNA-binding site separate from the active site for decapping and a longer substrate can more easily interact with both sites. Alternatively, it could be that the 3′ end of the RNA is required for binding or catalysis, and a longer RNA substrate allows enough flexibility in the substrate for both ends to interact with the enzyme simultaneously.
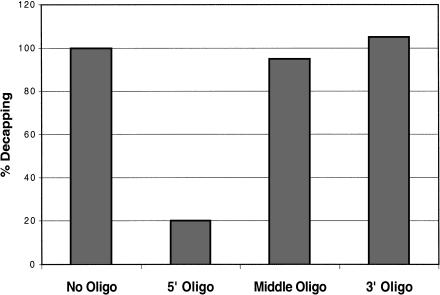
Two experiments were done to examine the role of the 3′ end in decapping. First, a 26-base DNA oligonucleotide with a 3′ methyl group was ligated onto the 3′ end of the mRNA, thereby replacing the normal RNA 3′ end with DNA sequences. The substrate with a DNA 3′ end was decapped as efficiently as an mRNA with a normal RNA 3′ end (data not shown). In a second experiment, the requirement for different portions of the substrate was tested by the addition of DNA oligonucleotides complementary to the 25 nucleotides at the 5′ end of the substrate, 26 nucleotides in the middle, or the 30 nucleotides at the 3′ end of the substrate. Only the oligonucleotide annealed at the 5′ end of the cap-labeled MFA2 substrate caused a significant decrease in decapping activity with the average inhibition by the 5′ oligo being fivefold (Fig. 4 ▶). This argues that sequestration of the 5′ end of the mRNA can inhibit decapping, and raises the possibility that the decapping enzyme must interact with nucleotides near the 5′ end. We interpret these experiments to indicate that the 3′ end is not required for efficient decapping and therefore that the length-dependence is likely to represent an additional RNA-binding site on the decapping enzyme.
FIGURE 4.
Oligonucleotide Inhibition of decapping activity. The effects of adding oligonucleotides complementary to the substrate at different positions are shown following a decapping reaction (see Materials and Methods for sequences). The activity measurements indicated in the graph are normalized to the amount of activity in reactions with no oligo.
The Edc proteins enhance the recombinant decapping activity
The ability to produce recombinant decapping enzyme allows for analysis of the activity of other proteins implicated from yeast genetic screens for their ability to alter the decapping activity directly. Two small proteins known to enhance decapping are Edc1p and Edc2p. These proteins were first identified as high copy suppressors of dcp1 and dcp2 point mutations (Dunckley et al. 2001). In addition, Edc1p and Edc2p purified from E. coli bind RNA, stimulate the activity of decapping enzyme purified from yeast, but do not show decapping activity themselves (Schwartz et al. 2003). Given this, we used the recombinant Dcp1/Dcp2ΔCp decapping enzyme to determine if Edc1p and Edc2p act directly on the decapping enzyme.
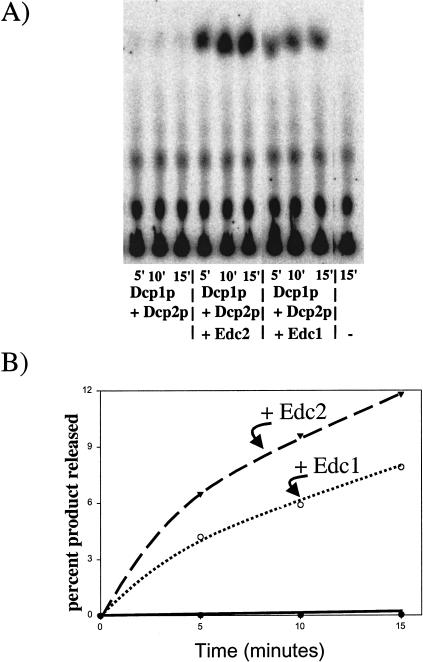
To test the ability of Edc1p and Edc2p to stimulate decapping activity directly, the recombinant Edc1 and Edc2 proteins were added to decapping reactions with the recombinant Dcp1p/Dcp2ΔCp. It should be noted that in these experiments we significantly reduced the amount of decapping enzyme present in the reactions compared with the earlier experiments to allow any stimulation to be more easily observed. An important result is that both Edc1p and Edc2p were independently able to directly stimulate the Dcp1p/ Dcp2ΔCp decapping activity (Fig. 5 ▶). The recombinant Edc1 protein increases the decapping of a 99-nucleotide-long RNA substrate by an average of 18-fold. Edc2 protein increases the decapping of a 99-nucleotide-long substrate by, on average, 32-fold. We interpret these results to indicate that Edc1p and Edc2p can activate decapping of the Dcp1p/Dcp2ΔCp enzyme directly and without the requirement for any additional yeast proteins.
FIGURE 5.
Edc proteins stimulate recombinant decapping activity. (A) Decapping reactions were carried out in a 15-μL reaction with Edc1p (8 fmoles), Edc2p (8 fmoles), or BSA present. In these reactions, a lower concentration of Dcp1p/Dcp2ΔCp was used to allow the stimulation of activity by Edc proteins to be visualized. The first panel is a PEI cellulose analysis of the reaction products generated after the indicated incubation time. (−) Reaction with no enzyme present. (B) A graphical representation of the data reactions with Edc2p (solid triangles), Edc1p (open circles), and no Edc protein but BSA (solid circles) with percent product plotted against time.
Dcp2ΔCp contains intrinsic decapping activity
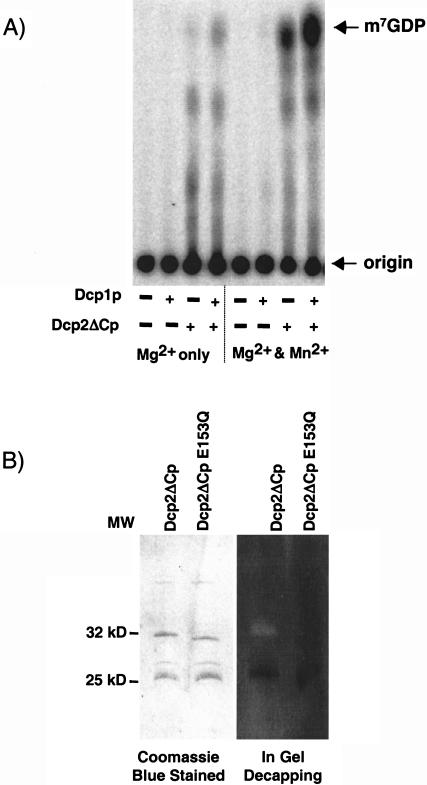
In the course of this work, the human Dcp2p was isolated and shown to be sufficient for decapping activity when produced in E. coli (Wang et al. 2002). Furthermore, more recent data indicate that the use of manganese in the decapping reaction significantly increases the decapping activity of human Dcp2 (C. Piccirillo and M. Kiledjian, in prep.). Given this, we examined the activity of the yeast Dcp1p, Dcp2ΔCp, and the Dcp1p/Dcp2ΔCp complex, formed by mixing individually purified Dcp1p and Dcp2ΔCp, with Mg2+ or Mg2+ and Mn2+ as cofactors using the buffer conditions where human Dcp2p gave activity. An important result was that Dcp2ΔCp was clearly active in the presence of Mn2+ (Fig. 6A ▶, lane 7). Consistent with Dcp2ΔCp being capable of decapping, low level of decapping can be seen in the presence of Mg2+ under these biochemical conditions. These results suggest that yeast Dcp2ΔCp has intrinsic decapping activity. This conclusion was verified by an in-gel assay where Dcp2ΔCp was resolved on an SDS-polyacrylamide gel containing cap-labeled RNA. The proteins were renatured as described in Materials and Methods and tested for decapping activity in the standard decapping buffer containing 2 mM Mn2+. As seen in Figure 6B ▶, Dcp2ΔCp was able to decap the gel-imbedded RNA as indicated by the zone of radioactive clearing around the protein. Further confirmation that the detected activity is a function of Dcp2ΔCp is demonstrated by the lack of decapping activity (and clearing) by the Nudix mutant protein Dcp2ΔCp Q153E . These data demonstrate that Dcp2ΔCp contains decapping activity and is able to function independent of any other protein. However, as seen in Figures 1, 2, and 6A ▶ ▶ ▶, Dcp1p augments this activity.
FIGURE 6.
Mn2+ stimulates recombinant yeast decapping activity. (A) Decapping reactions were carried out by incubating 300 ng of the indicated histidine-tagged Dcp1p, Dcp2ΔCp, or Dcp2ΔCpQ153E proteins with cap-labeled pcP-G16RNA for 30 min at 37°C in our standard reaction buffer (Mg2+ only) or buffer supplemented with 2 mM MnCl2(Mg2+ and Mn2+). Standards were simultaneously developed and their positions are denoted on the right of the panel. (B) Gel-isolated Dcp2p is enzymatically active. Three micrograms of His-Dcp2 ΔC and Dcp2ΔC Q153E3 were resolved by a standard SDS/PAGE and visualized by Coomassie blue staining (left panel) or resolved in an identical gel containing cap-labeled pcP-G16 RNA and renatured and assayed for decapping activity (right panel) (see Materials and Methods). Protein size markers are indicated between the gels.
DISCUSSION
Dcp1p and Dcp2p constitute the decapping holoenzyme
Several lines of evidence now suggest that the yeast decapping enzyme can be considered an enzyme complex of Dcp1p and Dcp2p. First, both of these proteins are absolutely required for decapping in vivo (Beelman et al. 1996; Dunckley and Parker 1999). Second, Dcp1p and Dcp2p show numerous two-hybrid interactions and copurify in several genomic screens (Uetz et al. 2000; Gavin et al. 2002). In addition, direct biochemical purification of either Dcp1p or Dcp2p expressed in yeast, or E. coli, copurifies the other partner (Dunckley and Parker 1999; Dunckley et al. 2001; data not shown). Similarly, recent results indicate that the mammalian homologs of Dcp1p and Dcp2p also coimmunoprecipitate, indicating that this interaction is conserved (Lykke-Andersen 2002).
An important issue is the specific roles of the yeast Dcp1p and Dcp2p in the process of mRNA decapping. Several observations indicate that Dcp2p is a catalytic subunit capable of cleaving the pyrophosphate cap linkage. This was first suggested by the Nudix motif in Dcp2p, which is required for decapping function, and constitutes a specific protein structure capable of cleaving different substrates of the class XpppY, producing Xpp and pY (Bessman et al. 1996). More importantly, we have now demonstrated that under specific biochemical conditions, yDcp2p is capable of decapping an mRNA (Fig. 6A ▶). In addition, yDcp2p can be renatured in an SDS gel to yield decapping activity (Fig. 6B ▶). These results are consistent with work on the hDcp2p, which is sufficient for decapping activity by itself (Wang et al. 2002). Because the Nudix motif is required for decapping in vivo and in vitro, the simplest interpretation is that the Dcp2p catalyzed decapping reaction is the major catalytic event in decapping.
The conclusion that Dcp2p can catalyze the mRNA decapping alone raises the question of how does Dcp1p function in mRNA decapping. Two observations suggest that Dcp1p enhances the activity of Dcp2p. First, Dcp1p is required for all decapping in vivo (Beelman et al. 1996). This indicates that Dcp2p cannot function without Dcp1p in vivo. Second, under conditions where Dcp2p is not sufficient for decapping in vitro, the Dcp1p/Dcp2p complex shows robust decapping activity (Fig. 1 ▶). Moreover, as this activity is dependent on the Nudix motif in Dcp2p (Fig. 2 ▶), we interpret this reaction to be Dcp2p-catalyzed.
In addition to enhancing Dcp2p activity, several pieces of evidence suggest that Dcp1p may also have a catalytic activity. First, decapping activity colocalizes with Dcp1p renatured in gels (LaGrandeur and Parker 1998). Second, separation of Dcp1p and Dcp2p by high salt washes yielded preparations of Dcp1p with decapping activity and no detectable Dcp2p (Dunckley et al. 2001). However, neither experiment can rule out the possibility that an undetected fragment of Dcp2p is present and responsible for the observed decapping activity. In addition, recombinant Dcp1p has been reported to have decapping activity (Vilela et al. 2000). However, we have been unable to obtain active recombinant Dcp1p under a variety of conditions (Fig. 1 ▶; data not shown). Given these results, and the clear decapping activity of Dcp2p, we cannot rule out the possibility that the Dcp1p/Dcp2p complex contains two different decapping proteins.
The possibility that the decapping complex may contain two catalytic subunits is intriguing because other enzymes involved in eukaryotic mRNA decay exist in complexes of multiple proteins with similar enzymatic activities. The most dramatic example of this phenomenon is the exosome, which contains 10 different potential 3′ to 5′ exonucleases (Mitchell et al. 1997; Allmang et al. 1999). In addition, in the major yeast deadenylase complex, consisting of Ccr4p, Pop2p, and Not1p–Not5p, both Ccr4p and Pop2p contain exonuclease motifs. In this case, Ccr4p, and perhaps Pop2p, can function as an mRNA deadenylase in vitro (Daugeron et al. 2001; Tucker et al. 2001, 2002; Chen et al. 2002). These observations raise the question of why eukaryotic mRNA decay enzymes are clustered in these complexes. One possibility is that the association of nucleases with similar activity allows for the control of substrate specificity. Specifically, these complexes may allow the cell to limit multiple nucleases simultaneously from acting on inappropriate substrates as well as coordinate their activity with correct substrates.
Modulation of decapping activity
One of the interesting properties of the Dcp1/Dcp2p decapping enzyme is that is shows greater activity on longer mRNA substrates. This was first reported with the enzyme purified from yeast (LaGrandeur and Parker 1998) and has now been extended to recombinantly produced enzyme (Fig. 4 ▶). This indicates the length preference is a property of the enzyme and cannot be attributed to any copurifying proteins. Consistent with this view, the recombinant human Dcp2p decapping activity also prefers longer substrates (Wang et al. 2002). The preference for a longer substrate implies that the Dcp2p also contains a site for binding RNA distinct from the active site. By filling this site, the activity of decapping would be enhanced, either by more efficient interaction of the substrate and the enzyme, or by inducing conformational changes that activate the decapping enzyme. Such a mechanism might be biologically relevant by dictating a requirement for substantial exposed RNA, which might correspond to an mRNP state targeted for decay, before decapping.
Edc1p and Edc2p also activate decapping (Dunckley et al. 2001; Schwartz et al. 2003). We have now shown that this effect is directly on the decapping enzyme and can be reconstituted with recombinant proteins (Fig. 5 ▶). In principle, the Edc proteins could enhance decapping by one of two likely mechanisms. First, because Edc1 and Edc2p are RNA-binding proteins, the Edc proteins might stimulate decapping by binding both the mRNA substrate and the Dcp1p/Dcp2p decapping enzyme, thereby enhancing the interaction of the enzyme with the substrate and/or the catalytic rate of the enzyme. Alternatively, and not mutually exclusive, Edc1p or Edc2p could act as allosteric activators of the decapping enzyme independent of any Edc–RNA interaction. In either case, the production of recombinant proteins should allow the clear resolution of the mechanisms and control by these proteins and other activators or inhibitors of decapping.
MATERIALS AND METHODS
Construction of Flag–Dcp1p and His6 Dcp2p overexpression vector
The plasmid expressing a His–Dcp1p in E. coli (pRP785) was constructed by amplifying Dcp1p with PCR primers oRP247 (5′-CAGACGGGATCCTCAGCAAAAGAATCTTTTGG-3′) and oRP248 (5′-CAGAGAGGCGCCATGACCGAGCAAC-3′) and inserting the NarI/BamHI cut fragment into the NarI/BamHI sites of pPROEX-1 (GIBCO/BRL).
The plasmid for overproducing both DCP1 and DCP2 in E. coli, pRP 1071, was constructed as follows. His6 DCP2ΔC gene was created by PCR amplifying DCP2 from pRP 925 with the oligonucleotides oRP1126 (CAGAGAGAGCTCGATGTCACTGCCGC TACGACAC) and oRP 1127 (CAGACGGGATCCTCACTCTTG GCTCGAGGGTACCTG) and inserting the SacI/BamHI-cut PCR product into the SacI/BamHI sites of pPROEX-1 (GIBCO-BRL). FLAG DCP1 gene was obtained from a BglII and BamHI digestion of a pET 5a vector with FLAG DCP1 inserted into the NdeI–BamHI sites (a gift from John McCarthy). The BglII and BamHI fragment with FLAG DCP1 was ligated into the BamHI site of pPROEX-1 vector to create pRP1080.
The Q153E mutation was introduced into DCP2 by using the QuikChange site-directed mutagenesis kit (Stratagene) and the oligonucleotides GCATTAGAGAAGTGAAAGAACAAATTG GTTTCGATTTGACGG (oRP 1131) and CCGTCAAATCGAAAC CAATTTGTTCTTTCACTTCTCTAATGC (oRP 1132).
Expression and purification of Flag–Dcp1p and His6Dcp2ΔCp
Expression of both genes is controlled from the pPROEX-1 lacZ promoter in DH5α cells. Cells containing pRP1071 or pRP1080 are grown in 750 mL of LB media containing ampicillin at 37°C and cells were induced once the OD 600 nm reached 0.4–0.6 with 0.6 mM IPTG (isopropyl-b-D-thiogalactopyranoside) for 3 h at 37°C. Induced cells were collected by centrifugation at 5000 rpm for 15 min at 4°C, washed with water, recollected, and frozen.
Frozen cell pellets were thawed on ice, resuspended in ∼4 mL of sonication buffer (10 mM Tris-HCl, pH 7.5, 1 M KCl, 2mM magnesium acetate, 1 mM DTT, 1 mg/mL lysozyme) in the presence of Mini complete-EDTA free protease inhibitors (La Roche), incubated for 30 min on ice, then sonicated for four, 15-sec rounds, with 1 min on ice incubation between rounds. The cell debris was pelleted by centrifugation in a tabletop centrifuge at 11K for 10 min. The supernatant was removed and used in the subsequent steps.
The clarified cell lysate was diluted with no salt sonication buffer to a final concentration of 100 mM KCl, it was then bound to anti-Flag affinity resin by mixing 1 h at 4°C. Approximately 1 mL of resin was used for a 750 mL culture of cells grown, induced, and lysed. The resin was spun down at 2000 rpm for 5 min at 4°C; the supernatant was saved as the flow-thru, the resin was poured into a solid support column, and washed with 50 column volumes of wash buffer (10 mM Tris-HCl, pH 7.5, 100 mM KCl, 2mM magnesium acetate, 1 mM DTT). The column was then washed with 10 column volumes of high-salt wash buffer (same as wash buffer but with 700 mM KCl) followed by washing with 10 column volumes of wash buffer. Proteins were eluted with wash buffer containing 200 μg/mL FLAG peptide and dialyzed overnight at 4°C into 50% glycerol, 50 mM Tris-HCl, pH 7.5, 1 mM DTT, and stored at −20°C. Purified protein fractions were analyzed by standard SDS polyacrylamide gel electrophoresis methods on 12% polyacrylamide gels.
Decapping reactions and analysis on TLC plates
Cap-labeled MFA2 mRNA substrates of various nucleotide lengths were prepared as previously described (LaGrandeur and Parker 1998). Except the short substrates, 29-, 20-, and 1- (cap analog m7GpppG) nucleotides-long were generated by capping a 99-nucleotide-long MFA2 transcript, annealing an oligonucleotide, cleaving the RNA with RNase H for 1 h at 30°C, treating the DNA with DNase I, RNase free (La Roche), and purifying the RNAs by polyacrylamide electrophoresis. The oligonucleotide used for generating the 29-nucleotide MFA2 was the oligonucleotide GATC GGTTGCATTAAGGTTGGTA (oRP 1136), for the 20-nucleotide and cap analog MFA2 mRNA substrates the oligonucleotide was GCATTAAGGTTGGTAGTTATTGTT (oRP 1135). Cap-labeled pcP-G16 RNA corresponding to the pcDNA3 polylinker with a G-track of 16 guanosines was prepared as previously described (Wang and Kiledjian 2001).
Decapping reactions were carried out as previously described (LaGranduer and Parker 1998) except the reaction buffer consisted of 50 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 50 mM NH4Cl, and 1 mM DTT and each reaction contained ∼1 fmole of the indicated MFA2 substrate. Reactions were stopped by mixing with 1 μL of 0.5 M EDTA to the reaction aliquot and placing on ice. Products were separated on a PEI Cellulose thin layer chromatography plate (Selecto) in 0.45 (NH4)2SO4 and detected with a Molecular Dynamics PhosphorImager. For Figure 6 ▶, the decapping reaction buffer consisted of 10 mM Tris-Hcl (pH7.5), 100 mM K-Ac, 2 mM Mg-Ac, 2 mM DTT, 0.1 mM Spermine, and each reaction contained 0.5 |qx 106 cpm of cap labeled pcP-G16 RNA. Five microliters of the 20 μL reaction products was separated on a PEI Cellulose thin layer chromatography plate (Sigma) in 0.75 LiCl and detected as above.
Decapping reactions with a DNA oligonucleotide inhibitor were performed as described above with the MFA2 mRNA substrate that is 99 nucleotides long and ∼0.6 ngrams of purified decapping protein. The oligonucleotide used to inhibit the 5′ end was GT TGTATGAAGATGATAGCTCGCCC (oRP 1137). The oligonucleotide used to inhibit the 3′ end was CTTTTCAGAGGATT TATCCTTCTGAGTGGC (oRP 1138). The oligonucleotide used to inhibit the middle, complements ∼45 nucleotides from the 5′ end, GTGTGGAAGCAGTGGTGATCGGTTGC (oRP 1130). In each case, the oligonucleotide was used at a variety of concentrations with the results shown in the figure at a concentration of 6 pmoles.
Purification and characterization for the Edc proteins used here are described in Schwartz et al. (2003). Reactions with Edc1p and Edc2p were performed as described above with ∼0.24 ngrams of decapping enzyme; reactions without either Edc protein added had an equal amount of BSA added in the same buffer as the Edc proteins were stored in (20 mM HEPES, pH 7.5 and 50 mM KCl) to keep the amount of protein the same in all reactions.
In-gel decapping assays
In-gel decapping assays were carried out with 3 μg each of Dcp2ΔCp or Dcp2ΔCpQ153E resolved on either a standard 15% SDS-PAGE gel or a 15% SDS-PAGE gel containing cap-labeled pcP-G16 RNA (1 |qx 106 cpm) immobilized in the running gel. The two SDS-PAGE gels were subjected to electrophoresis under identical conditions and proteins in the unlabeled gel were visualized by Coomassie blue staining. Proteins in the cap-labeled RNA-containing gel were renatured at 4°C as described in LaGrandeur and Parker (1998) and assayed for decapping activity at 30°C in the following buffer: (10 mM Tris-Hcl (pH7.5), 100 mM K-Ac, 2 mM Mg-Ac, 2 mM MnCl2, 2 mM DTT, 0.1 mM Spermine). The gel was subsequently dried and exposed to Kodak X-ray film.
Acknowledgments
We thank Carolyn Decker, Ambro van Hoof, and the Parker laboratories for comments on the manuscript. We thank Chris Piccirillo for sharing unpublished observations. A.C-S. was supported by an American Heart Association fellowship. This work was supported by funds from the Howard Hughes Medical Institute (to R.P.) and National Institutes of Health grants GM45553 to R.P. and DK51611 to M.K.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.rnajournal.org/cgi/doi/10.1261/rna.2151403.
REFERENCES
- Allmang, C., Petfalski, E., Podtelejnikov, A., Mann, M., Tollrevey, D., and Mitchell, P. 1999. The yeast exosome and human PM-Scl are related complexes of 3′→5′ exonucleases. Genes & Dev. 13: 2148–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beelman, C.A. and Parker, R. 1995. Degradation of mRNA in eukaryotes. Cell 8: 179–183. [DOI] [PubMed] [Google Scholar]
- Beelman, C.A., Stevens A., Caponigro G., LaGrandeur, T.E., Hatfield L., Fortner, D., and Parker, R. 1996. An essential component of the decapping enzyme required for normal rates of mRNA decay in yeast. Nature 38: 642–646. [DOI] [PubMed] [Google Scholar]
- Bessman, M.J., Frick, D.N, and O’Handley, S.F. 1996. The MutT proteins or “Nudix” hydrolases, a family of versatile, widely distributed, “housecleaning” enzymes. J. Biol. Chem. 271: 25059–25062. [DOI] [PubMed] [Google Scholar]
- Bonnerot, C., Boeck, R., and Lapeyre, B. 2000. The two protein Pat1p (Mrt1p) and Spb8p interact in vivo, are required for mRNA decay, and are functionally linked to Pab1p. Mol. Cell. Biol. 20: 5939–5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouveret, E., Rigaut, G., Shevchenko, A., Wilm, M., and Seraphin, B. 2000. A Sm-like protein complex that participates in mRNA degradation. EMBO J. 19: 1661–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, D. and Parker, R. 2001. Computational modeling of eukaryotic mRNA turnover. RNA 7: 1192–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J., Chiang, Y.C., and Denis, C.L. 2002. CCR4, a 3′-5′ poly(A) RNA and ssDNA exonuclease, is the catalytic component of the cytoplasmic deadenylase. EMBO J. 21: 1414–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller, J.M., Tucker, M., Sheth, U., Valencia-Sanchez, M.A., and Parker, R. 2001. The DEAD box helicase, Dhh1p, functions in mRNA decapping and interacts with both the decapping and deadenylase complexes. RNA 7: 1717–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugeron, M.C., Mauxion, F., and Seraphin, B. 2001. The yeast POP2 gene encodes a nuclease involved in mRNA deadenylation. Nucleic Acids Res. 29: 2448–2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker, C.J. and Parker, R. 1993. A turnover pathway for both stable and unstable mRNAs in yeast: evidence for a requirement for deadenylation. Genes & Dev. 7: 1632–1643. [DOI] [PubMed] [Google Scholar]
- Dunckley, T. and Parker, R. 1999. The DCP2 protein is required for mRNA decapping in Saccharomyces cerevisiae and contains a functional MutT motif. EMBO J. 18: 5411–5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunckley T., Tucker, M., and Parker, R. 2001. Two related proteins, Edc1p and Edc2p, stimulate mRNA decapping in Saccharomyces cerevisiae. Genetics 157: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, N. and Weis, K. 2002. The DEAD box protein Dhh1 stimulates the decapping enzyme Dcp1. EMBO J. 21: 2788–2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin, A.C., Bosche, M., Krause, R., Grandi, P., Marzioch, M., Bauer, A., Schultz, J., Rick, J.M, Michon, A.M., Cruciat, C.M., et al. 2002. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415: 141–147. [DOI] [PubMed] [Google Scholar]
- Hsu, C.L. and Stevens, A. 1993. Yeast cells lacking 5′ to 3′ exoribonuclease I contain mRNA species that are poly(A) deficient and partially lack the 5′ cap structure. Mol. Cell. Biol. 13: 4826–4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs-Anderson, J.S. and Parker, R. 1998. The 3′ to 5′ degradation of yeast mRNAs is a general mechanism for mRNA turnover that requires the SKI2 DEVH box protein and 3′ to 5′ exonucleases of the exosome complex. EMBO J. 17: 1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGrandeur, T.E. and Parker, R. 1998. Isolation and characterization of Dcp1p, the yeast mRNA decapping enzyme. EMBO J. 17: 1487–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, H., Rodgers, N.D., Jiao, X., and Kiledjian, M. 2002. The scavenger mRNA decapping enzyme DcpS is a member of the HIT family of pyrophosphatases. EMBO J. 21: 4699–4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykke-Andersen, J. 2002. Identification of a human decapping complex associated with the hUpf proteins in nonsense-mediated decay. Mol. Cell. Biol. (in press). [DOI] [PMC free article] [PubMed]
- Mitchell, P., Petfalski, E., Shevchenko, A., Mann, M., and Tollervey, D. 1997. The exosome: A conserved eukaryotic RNA processing complex containing multiple 3′&rarr:5′ exoribonucleases. Cell 91: 457–466. [DOI] [PubMed] [Google Scholar]
- Muhlrad, D. and Parker, R. 1992. Mutations affecting stability and deadenylation of the yeast MFA2 transcript. Genes & Dev. 6: 2100–2111. [DOI] [PubMed] [Google Scholar]
- Muhlrad, D., Decker, C., and Parker, R. 1994. Deadenylation of the unstable mRNA encoded by the yeast MFA2 gene leads to decapping followed by 5′ to 3′ degradation of the transcript. Genes & Dev. 8: 855–866. [DOI] [PubMed] [Google Scholar]
- ———. 1995. Turnover mechanisms of the stable PGK1 mRNA in yeast. Mol. Cell. Biol. 1: 2145–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivas, W. and Parker, R. 2000. The Puf3 protein is a transcript-specific regulator of mRNA degradation in yeast. EMBO J. 19: 6602–6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez, C.V., Vilela, C., Berthelot, K., and McCarthy, J.E. 2002. Modulation of eukaryotic mRNA stability via the cap-binding translation complex eIF4F. J. Mol. Biol. 218: 951–962. [DOI] [PubMed] [Google Scholar]
- Schwartz, D. and Parker, R. 1999. Mutations in translation initiation factors lead to increased rates of deadenylation and decapping of yeast mRNAs. Mol. Cell. Biol. 19: 5247–5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 2000. mRNA decapping in yeast requires dissociation of the cap binding protein, eukaryotic translation initiation factor 4E. Mol. Cell. Biol. 20: 7933–7942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, D., Decker, C.J., and Parker, R. 2003. The enhancer of decapping proteins, Edc1p and Edc2p, bind RNA and directly stimulate the activity of the decapping enzyme. RNA (this issue). [DOI] [PMC free article] [PubMed]
- Tharun, S., He, W., Mayes, A.E., Lennertz, P., Beggs, J.D., and Parker, R. 2000. Yeast Sm-like proteins function in mRNA decapping and decay. Nature 404: 515–518. [DOI] [PubMed] [Google Scholar]
- Tucker, M., Valencia-Sanchez, M.A., Staples, R.R., Chen, J., Denis, C.L., and Parker, R. 2001. The transcription factor associated Ccr4 and Caf1 proteins are components of the major cytoplasmic mRNA deadenylase in Saccharomyces cerevisiae. Cell 104: 377–386. [DOI] [PubMed] [Google Scholar]
- Tucker, M., Staples, R.R., Valencia-Sanchez, M.A., Muhlrad, D., and Parker, R. 2002. Ccr4p is the catalytic sub-unit of a Ccr4/Pop2p/Notp mRNA deadenylase complex in Saccharomyces cerevisiae. EMBO J. 21: 1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetz, P., Giot, L., Cagney, G., Mansfield, T.A., Judson, R.S., Knight, J.R., Lockshon, D., Narayan, V., Srinivasan, M., Pochart, P., et al. 2000. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature 403: 601–603. [DOI] [PubMed] [Google Scholar]
- Vilela, C., Velasco, C., Ptushkina, M., and McCarthy, J.E. 2000. The eukaryotic mRNA decapping protein Dcp1 interacts physically and functionally with the eIF4F translation initiation complex. EMBO J. 19: 4372–4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. and Kiledjian, M. 2001. Functional link between the mammalian exosome and mRNA decapping. Cell 107: 751–762 [DOI] [PubMed] [Google Scholar]
- Wang, Z., Jiao, X., Carr-Schmid, A., and Kiledjian, M. 2002. The hDcp2 protein is a mammalian mRNA decapping enzyme. Proc. Natl. Acad. Sci. (in press). [DOI] [PMC free article] [PubMed]