Abstract
Recombinant adenoviruses (Ads) are useful tools in gene transfer because they are able to infect a wide variety of tissues and cell types and do not require a replicating target cell. However, transgene expression is only transient due to host innate and acquired immune responses to the virus. Most recombinant Ads have deletions of early region 3 (E3) genes, allowing more space for insertion of the transgene. Although the E3 region is not necessary for infection, it has been observed that these “nonessential” genes have immunomodulatory properties. We demonstrate here that the E3 region of Ad inhibits the activation of NF-κB induced by tumor necrosis factor alpha (TNF-α) and interleukin-1. Ad E3 is able to prevent NF-κB from entering the nucleus, where it is normally active. Ad E3 also appears to function by preventing the activation of the kinase complex, IKK, which is responsible for phosphorylation of IκB that retains NF-κB in the cytoplasm in an inactive state. The prevention of NF-κB activation has been mapped to a complex of two of the seven E3 products, E3-10.4K and E3-14.5K (RIDα/β). These and other studies indicate that, by using Ad vectors containing the E3 region, it may be possible to reduce the harmful proinflammatory effects of TNF-α and other cytokines that compromise the use of Ad gene therapy vectors in vivo.
The ability to infect nondividing cells and express high levels of large transgenes makes adenovirus (Ad) vectors important reagents for gene therapy. First- and second-generation Ad vectors, which are most commonly used in gene therapy applications, have deletions of both the early transcription region 1 (E1) and the early transcription region 3 (E3). The deletion of the E1 region in these vectors prevents the replication of the virus in the human host, whereas E3 genes, which are not required for viral replication in tissue culture, have been removed to allow for insertion of larger transgenes (16, 30). However, the use of these Ad vectors poses significant problems since the viral structural and nonstructural proteins, and probably the transgene itself, activate both the innate and cell-mediated arms of the immune system, leading to a clearance of the transgene within several weeks postinfection (7, 46). Such Ad vectors also stimulate the humoral immune response, which induces neutralizing antibodies and prevents readministration of the virus. Studies have demonstrated that the proinflammatory molecule tumor necrosis factor alpha (TNF-α) plays a central role in several mechanisms of immune-mediated clearance of Ad vectors (12, 25). TNF-α secretion stimulated by Ad infection has been implicated in the induction of adhesion molecules that recruit T cells to the site of infection, as well as in the maturation of dendritic cells that are important in antigen presentation (12, 29). This and the fact that four of the early expressed Ad genes—E1B-19K, E3-14.7K, E3-10.4K, and E3-14.5K—encode products that prevent TNF-α-induced cytolysis of virally infected cells indicate that TNF-α plays an important role in Ad clearance (15). However, the mechanism of TNF-α-mediated Ad clearance is not completely understood.
TNF-α, as well as molecules that are components of infectious agents, and cell products that are induced by virus and bacterial infection (interleukin-1β [IL-1β], double-stranded RNA, and lipopolysaccharide) or cellular stresses (phorbol esters, UV) activate the NF-κB signaling pathway (14, 37). NF-κB is a transcription factor that plays a key role in several important processes, including cell survival, inflammation, and the immune response (1, 2, 37, 38). The prototypical NF-κB molecule consists of a heterodimer of the subunits p65 (RelA) and p50 (14, 28, 37). NF-κB is primarily found in the cytoplasm in an inactive form bound to a member of the IκB family. Upon activation of the resting cells, IκBα is phosphorylated at two key N terminus serines that lead to its ubiquitination and subsequent degradation by the 26S proteasome (6). The free NF-κB is then translocated to the nucleus, where it binds to a variety of promoters that are NF-κB responsive.
When TNF-α binds TNF-α receptor 1 (TNFR1), recruitment of several adapter proteins to the cytoplasmic death domain of TNFR1 occurs. One of these proteins, TRADD (TNFR-associated death domain protein) binds to the death domain of TNFR1 (18). TRADD is able to induce both cell death, in a caspase-dependent manner, and NF-κB activation through the recruitment of receptor interacting protein (RIP) and TNFR-associated factor 2 (TRAF2) proteins (17, 18). The importance of RIP and TRAF2 was determined by overexpression studies showing that they increase NF-κB activity. Furthermore, RIP- or TRAF2-null fibroblast studies have demonstrated RIP to be essential for IκB kinase (IKK) activation and TRAF2 to be essential for IKK recruitment upon stimulation with TNF-α (9).
IKK is a 700- to 900-kDa protein complex consisting of IKKα, IKKβ, and IKKγ (also named NEMO and FIP-3) (23, 34, 45). This complex is found to be responsible for IκB phosphorylation (27, 33, 44, 47). Both IKKα and IKKβ possess kinase activity, although IKKβ appears to play the major role in acute responses to the various stimuli known to activate NF-κB (8). IKKγ/NEMO/FIP-3 does not possess kinase activity but has been shown to play an essential role as a scaffolding protein that interacts with upstream molecules, including RIP, involved in the NF-κB activating pathway (23). We have also demonstrated that FIP-3 binds to the Ad E3-14.7K protein (23).
As described above, both replication-competent and attenuated E1− and E3− Ads have been observed to upregulate the NF-κB signaling pathway in some cell types very early postinfection, leading to the hypothesis that viral structural proteins are responsible for activating this pathway (25, 29). Furthermore, NF-κB activation as a result of Ad infection has been demonstrated to upregulate interferon-inducible protein 10 (IP-10), which is associated with the Th1 cell-mediated response (5). Many groups have taken a variety of approaches to either block the TNF-α response or, more specifically, the NF-κB pathway to prevent an immune response to the virus and to the subsequent transgene clearance (4, 24). We have utilized overexpressed Ad E3 genes either introduced in viral vectors or as transgenes to successfully abort immune responses in vivo (10, 11, 19, 39). Earlier studies showed that coinsertion of Ad E3 genes with a foreign gene (hBUGT) prolonged gene expression from an Ad viral vector in the Gunn rat model of Crigler-Najjar syndrome and corrected the metabolic deficiency (19, 39). Ad E3 transgenes in the target cells also prevented allogeneic rejection of islets and the onset of autoimmune diabetes in two murine models, including nonobese diabetes mice (11, 41). Based on this knowledge and the finding that the Ad E3-14.7K protein interacts with IKKγ/NEMO/FIP-3, a key molecule on the NF-κB pathway, we initiated studies to determine whether Ad containing E3 genes could modulate the NF-κB signal transduction pathway and which genes might be responsible for this effect. We initially chose a cell line in which Ad infection itself did not induce cytokines, chemokines, or NF-κB. Upon infection of cells with Ad, we saw a significant inhibition of TNF-α-mediated NF-κB activation. This effect mapped to the E3 region of the viral genome and, more specifically, to the E3-10.4K/14.5K complex, also called RIDα/β (for receptor interaction and degradation) because of its effects on the regulation of Fas. Further, studies on the mechanism of this effect have shown that there is a minor effect of 10.4K/14.5K on decreasing the surface expression of the TNFR1. However, the small changes in the levels of the TNFR1 do not seem to explain the inhibitory effects of 10.4K/14.5K on NF-κB activity.
(This research was conducted by J. M. Friedman in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Sue Golding Graduate Division of Medical Sciences, Albert Einstein College of Medicine, Yeshiva University.)
MATERIALS AND METHODS
Cell lines and reagents.
293 and HeLa cells were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum, penicillin (50 U/ml), and streptomycin (50 μg/ml). Suspension HeLa cells were grown in suspension-modified Eagle medium supplemented with 5% fetal bovine serum, penicillin (50 U/ml), and streptomycin (50 μg/ml). Rabbit IκBα antibody was purchased from New England Biolabs, monoclonal IKKα antibody was from PharMingen, and β-tubulin antibody was from Santa Cruz.
Viruses.
The viruses used in these experiments have been described in detail previously and were kind gifts of W. Wold (St. Louis University, St. Louis, Mo.). rec700 is a recombinant Ad5/Ad2 subgenus C virus containing all of the E3 genes but chimeric for the Ad2 10.4K protein (EcoRI-D fragment, map positions 73 to 86) and the Ad5 14.5K and 14.7K proteins (43). All viral E3 deletion mutants were generated in the Wold laboratory from this parent recombinant “wild-type” virus. Ad dl762 has a deletion of 14.7K but expresses wild-type levels of 10.4K/14.5K. Ad dl309 has deletions of 14.7K, 10.4K and 14.5K (20). The virus dl7001 lacks all E3 genes and dl7000 lacks all E3 genes except for 14.7K (31). Ad CMV-GFP (22) contains the gene for the green fluorescent protein (GFP) behind the cytomegalovirus (CMV) promoter, whereas Ad CMV-E3 contains the E3 region from Ad 5 behind the CMV promoter (19). Both CMV-GFP and CMV-E3 were inserted in place of the deleted E1 region in viruses that do not produce any endogenous E3 proteins.
Western blots for IκBα.
A total 106 of HeLa cells, 293 cells, or human umbilical vein endothelial cells (HUVECs) were infected in six-well dishes with the indicated viruses at a multiplicity of infection (MOI) of 2,000 particles/cell for 16 h. Prior to harvest, cells were treated with TNF-α (20 ng/ml) or IL-1 (10 ng/ml). Cells were then lysed with 100 μl of sodium dodecyl sulfate (SDS) loading buffer (50 mM Tris-HCl [pH 6.8], 100 mM dithiothreitol [DTT], 2% SDS, 0.1% bromophenol blue, 10% glycerol) and scraped from the monolayer. One-fifth of the lysate was subjected to SDS-12% polyacrylamide gel electrophoresis (PAGE), and Western blotting with IκBα antibody was performed.
IκBα kinase assay.
To assay for IKK activity, HeLa cells were either not infected (mock infected) or infected with Ad CMV-GFP or Ad CMV-E3 for 16 h at an MOI of 2,000 particles/cell. Cells were then treated with TNF-α (20 ng/ml) for 10 min and lysed by using kinase lysis buffer (20 mM Tris, pH 7.4; 1% Triton X-100; 10% glycerol; 137 mM NaCl; 25 mM β-glycerophosphate; 1 mM Na3VO4; protease inhibitor tablet [Roche]). IKKα was immunoprecipitated by using 2 μg of IKKα antibody (PharMingen). Then, 30 μl of protein A/G beads was added to the immunoprecipitates, followed by incubation for 3 h at 4°C. Immunoprecipitates from the cellular lysates were washed twice in lysis buffer and twice in the kinase reaction buffer (20 mM HEPES, pH 7.5; 10 mM MgCl2; 2 mM DTT; 20 μM ATP; protease inhibitor tablet [Roche]; 1 mM Na3VO4; 2 mM NaF; 20 mM β-glycerophosphate). The assay was performed by adding 2 μg of glutathione S-transferase-IκBα substrate and 10 μCi of [γ-32P]ATP in 30 μl of kinase reaction buffer and incubating the reaction at 30°C for 20 min. Then, 30 μl of 2× SDS-PAGE loading buffer was added, and the samples were boiled. The reactions were subjected to SDS-12% PAGE and visualized by radiography.
Electrophoretic mobility shift assay (EMSA).
Cells were washed once with phosphate-buffered saline solution and allowed to swell in 1.2 ml of 10 mM HEPES [pH 7.9]-10 mM KCl-1.5 mM MgCl2-0.1 mM EDTA-0.1 mM EGTA-1 mM DTT-0.5 mM phenylmethylsulfonyl fluoride, and protease inhibitor tablet (Roche Molecular Biologicals) for 15 min on ice. Then 75 μl of 10% Nonidet P-40 was added, the sample was mixed vigorously, and intact nuclei were isolated by centrifugation. Nuclei were then shaken for 15 min at 4°C in buffer C (20 mM HEPES, pH 7.9; 400 mM KCl; 1 mM EDTA; 1 mM EGTA; 1 mM DTT; 1 mM phenylmethylsulfonyl fluoride; 10% glycerol; protease inhibitor tablet [Roche]) and then centrifuged at 15,000 rpm. The protein concentration was determined by Bradford assays (Bio-Rad). Double-stranded oligonucleotides with the sequence 5′-AGTTGAGGGGACTTTCCCAGGC-3′ were obtained (Promega), and the assay was performed according to the instructions provided in the Promega Gel Shift Assay Kit (Promega).
RESULTS
Ad E3 genes inhibit the signaling pathway responsible for NF-κB activation.
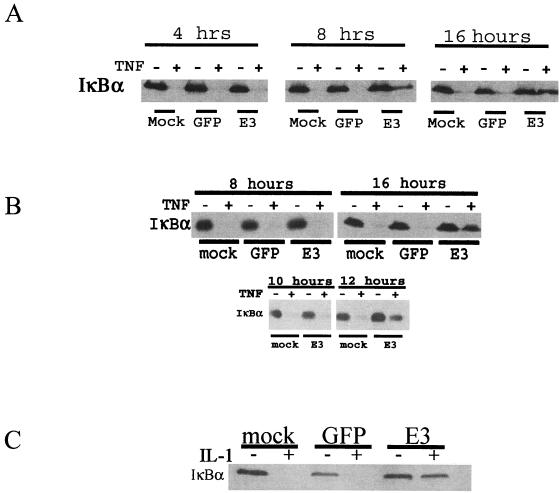
In order to determine whether Ad E3 could have an effect on the activation of NF-κB by TNF-α, 293 (Fig. 1A) and HeLa (Fig. 1B) cells were infected with either Ad CMV-GFP (E3−) or Ad CMV-E3 (E3+). The Ad mutants used for these studies are shown in Table 1, and uninfected cells served as additional negative controls. Cells were treated with TNF-α for 30 min prior to harvest at the indicated times postinfection. The lysates were electrophoresed on acrylamide gels, and Western blotting was performed with antibody against IκBα. TNF-α caused degradation of IκBα in uninfected cells and cells infected with Ad CMV-GFP, whereas degradation was inhibited only in cells infected with Ad CMV-E3. The prevention of IκBα degradation in 293 cells first occurred at ca. 8 h, whereas the same effect was seen in HeLa cells at ca. 12 h postinfection. Similar results were observed with wild-type (rec700) virus (data not shown). The kinetics of E3 inhibition of IκBα degradation paralleled the appearance and increase of the E3 proteins after Ad infection.
FIG. 1.
Ad E3 prevents the degradation of IκBα in cells treated with TNF-α or IL-1. A total of 106 293 (A) or HeLa (B) cells were infected in six-well dishes with Ad CMV-GFP (GFP) or Ad CMV-E3 (E3) at an MOI of 2,000 particles/cell or were mock infected and then harvested at 4, 8, 10, 12, or 16 h postinfection. At 30 min prior to harvest, cells as indicated (TNF+) were treated with TNF-α (20 ng/ml). (C) HUVECs were infected as described above for 16 h and treated with 10 ng of IL-1/ml for 15 min prior to harvest. The lysates were analyzed by SDS-PAGE and Western blotting with IκBα antibody. Gel loading controls were done by performing Western blotting for the β-tubulin protein (data not shown).
TABLE 1.
Ad mutants
| Virus | Expressiona of:
|
|||
|---|---|---|---|---|
| E1 | E3-14.7K | E3-10.4K/14.5K | E3-19K | |
| rec700 | + | + | + | + |
| CMV-GFP | − | − | − | − |
| CMV-E3 | − | + | + | ++ |
| dl7000 | + | ++ | − | − |
| dl7001 | + | − | − | − |
| dl762 | + | − | + | + |
| dl309 | + | − | − | + |
+, expression equal to that of wild-type virus; ++, expression greater than that of wild-type virus; −, no expression of the indicated protein.
To determine if this effect of Ad E3 was specific for TNF-α, HUVECs, which are primary cells that express the IL-1 receptor and the coxsackievirus and Ad receptor (CAR), were infected with Ad CMV-E3 or GFP for 16 h. Cells were then treated with IL-1 for 15 min prior to harvest (Fig. 1C). Stimulation of cells with IL-1 induced degradation of IκBα in both uninfected and Ad CMV-GFP-infected cultures. Similar to the results observed for cells stimulated with TNF-α, Ad CMV-E3 prevented the degradation of IκBα in cells stimulated with IL-1, indicating that the E3 inhibitory effect could be extended to other stimuli that activate NF-κB. Ad CMV-E3 also prevented degradation of IκBα in HUVECs stimulated with TNF-α (data not shown).
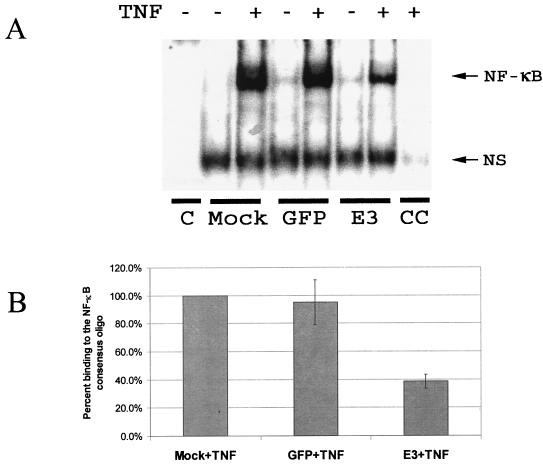
When IκBα is degraded, bound cytosolic NF-κB is released and is transported into the nucleus. In order to determine whether Ad E3 was able to block NF-κB shuttling into the nucleus, 293 cells were either left uninfected (control) or infected with Ad CMV-GFP or Ad CMV-E3. Cells were then treated with TNF-α for 30 min prior to harvest. Nuclear proteins were extracted, and EMSAs were carried out by using a DNA consensus-binding site for NF-κB. As expected, TNF-α caused an increase in the amount of NF-κB binding to the NF-κB consensus sequence in both the uninfected and the Ad CMV-GFP lanes. However, in the presence of Ad CMV-E3, this activity was significantly diminished (two- to threefold) (Fig. 2).
FIG. 2.
Ad E3 decreases NF-κB binding to its DNA consensus-binding site. 293 cells in 10-cm plates were left uninfected (Mock) or infected with either Ad-GFP or Ad CMV-E3 at an MOI of 2,000 particles/cell. (A) At 16 h, cells were treated with TNF-α (10 ng/ml) for 30 min prior to harvest, where indicated. Nuclear proteins were extracted, and EMSA was performed by using the NF-κB consensus double-strand binding site 5′AGTTGAGGGGACTTTCCCAGGC3′. Control (C) indicates probe in the absence of nuclear extract, while cold competitor (CC) denotes the addition of a nonlabeled NF-κB consensus binding site oligonucleotide (oligo) to the mock extract with TNF-α. NS, nonspecific band. The unbound probe at the bottom of the gel is not shown. (B) Binding of NF-κB to its consensus binding site oligonucleotide was quantitated at the position indicated (NF-κB), and the Mock+TNF lane was set equal to 100% binding. The other samples in which TNF-α was added were calculated based on the decrease of binding from the Mock+TNF lane.
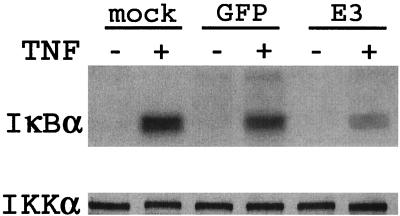
To further understand the mechanism responsible for the inhibition of IκBα degradation, we performed in vitro IκBα kinase assays with the endogenous IKK complex. HeLa cells were either left uninfected or were infected with Ad GFP or Ad CMV-E3 for 16 h and then treated with TNF-α for 10 min prior to harvest. IKKα was immunoprecipitated, and the associated kinase activity was assayed in cell extracts with glutathione S-transferase-IκBα as a substrate. IκBα was significantly phosphorylated in the presence of TNF-α in both uninfected cells and cells infected with Ad GFP. However, in cells that were infected with Ad CMV-E3 and treated with TNF-α, IκBα phosphorylation was decreased fivefold (Fig. 3). These experiments indicate that the E3 region is able to downregulate the kinase activity of the IKK complex and subsequently prevent IκBα from being degraded, thus blocking the translocation of NF-κB into the nucleus. These experiments demonstrate that an Ad expressing the E3 proteins inhibits key steps in the NF-κB activation pathway.
FIG. 3.
Ad E3 downregulates the activity of the IKK complex. To assay for IKK activity, HeLa cells were left uninfected (mock) or were infected with the indicated Ads for 16 h at an MOI of 2,000 particles/cell. Cells were then treated with TNF-α (20 ng/ml) for 10 min and lysed by using kinase lysis buffer. The endogenous IKK complex was immunoprecipitated by using anti-IKKα antibody and washed several times. The complex was resuspended in kinase reaction buffer and [γ-32P]ATP was added. Recombinant IκBα, amino acids 1 to 53, was used as a substrate for the kinase reaction. To control for equal amounts of IKK, Western blotting was performed for IKKα levels by using anti-IKKα, and the results are shown at the bottom of the figure.
Ad E3 inhibits NF-κB signaling by an E1-independent mechanism.
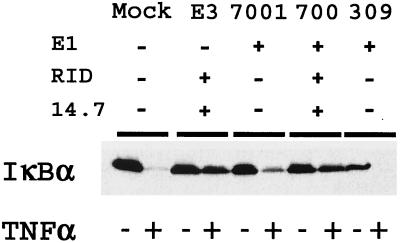
Both E1 and E3 regions of Ad have been observed to modify several cellular signaling cascades that may enable the virus to successfully evade host immune responses. Since 293 cells contain the E1A and E1B regions of Ad, we wanted to confirm that the E3 region alone was responsible for the prevention of NF-κB activation by TNF-α. HeLa cells were mock infected or infected with Ad CMV-E3 (E1− and E3+), dl7001 (E1+ and E3−), rec700 (E1+ and E3+), or dl309 (E1+ and E3-10.4K−/14.5K−/14.7K−) (Table 1). Treatment with TNF-α for 30 min prior to harvest caused extensive degradation of IκBα in the uninfected cells as well as in dl7001 and dl309 viruses. The wild-type virus, rec700, and Ad CMV-E3 prevented the degradation of IκBα (Fig. 4). This indicates that the region primarily responsible for the prevention of NF-κB activation by TNF-α is Ad E3 and, more specifically, either one or more of the genes found in the E3B region, 10.4K, 14.5K, or 14.7K. Although there was no IκBα protection after dl309 infection, there was a small band still present in cells that were infected with dl7001, indicating that the E1 region under certain conditions may also have a slight effect on the prevention of NF-κB activation.
FIG. 4.
The Ad E3 but not the Ad E1 region prevents the degradation of IκBα after TNF-α treatment. HeLa cells (106) were uninfected (Mock) or infected with Ad CMV-E3, dl7001, rec700 (wild type), or dl309 at an MOI of 2,000 particles/cell for 16 h. At 30 min prior to harvest, cells were treated with TNF-α (20 ng/ml). The lysates were analyzed by SDS-PAGE, and Western blotting was done with the IκBα antibody. Gel loading controls were done by performing Western blotting for the β-tubulin protein (data not shown).
Ad E3-10.4K/14.5K complex is responsible for downregulation of NF-κB.
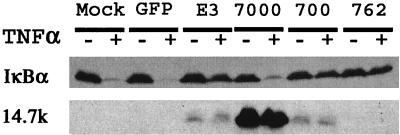
The E3 region of Ad encodes multiple proteins that appear to play a role as modulators of the host immune response. Since the NF-κB regulatory function mapped to the E3B region, we wanted to determine which gene(s) were responsible for the observed effects. HeLa cells were either left uninfected or infected with Ad CMV-GFP, Ad CMV-E3, Ad dl7000 (E3− except for 14.7K), or dl762 (E3+ and 14.7−) for 16 h and then treated for 30 min with TNF-α prior to harvest (Fig. 5). As expected, TNF-α treatment caused degradation of IκBα in mock- and Ad GFP-infected cells. Degradation was also observed in cells infected with dl7000, which has deletions of all genes in E3 except for E3-14.7K. Infection of cells with Ad CMV-E3, rec700, and dl762 blocked degradation of IκBα. Our results show that Ad E3 suppression of IκBα degradation required the E3-10.4K/14.5K complex and not E3-14.7K. Similar results were also observed for HUVECs stimulated with IL-1 (data not shown).
FIG. 5.
Ad E3 RID is responsible for the prevention of IκBα degradation upon treatment with TNF-α. HeLa cells (106) were uninfected (mock) or infected with Ad CMV-GFP, Ad CMV-E3, Ad dl7000, rec700, or dl762 at an MOI of 2,000 particles/cell for 16 h (see Table 1). At 30 min prior to harvest, cells were treated with TNF-α (20 ng/ml). Lysates were analyzed by SDS-PAGE and by Western blotting, which was performed with the IκBα antibody or the 14.7K antibody. Gel loading controls were done by performing Western blotting for the β-tubulin protein (data not shown).
DISCUSSION
TNF-α appears to play a key role in the clearance of Ads from the host organism (12). Upon Ad intravenous infection with first-generation vectors, the majority of virus is eliminated within the first 24 to 48 h of infection, most likely through innate immunity pathways mediated via TNF-α release by Kupffer and dendritic cells (25). The cellular and the humoral arm of the immune response, along with inhibition of viral replication, is stimulated by the release of TNF-α. By removing the TNF-α response through the use of TNF-α-null mice or viruses that express soluble TNFR1, prolonged transgene expression has been demonstrated. Therefore, in order to utilize Ad successfully as a delivery system for a transgene, the TNF-α-induced immune response must be circumvented.
When cells are exposed to TNF-α or IL-1, the transcription factor NF-κB, which is a key mediator of the inflammatory response, is activated in a rapid manner with peak activation occurring within several minutes. By infecting cells with Ad E3 containing viruses prior to cytokine treatment, we observed that there are viral genes that prevent the activation of NF-κB. This effect was demonstrated in experiments that have shown us that in the presence of TNF-α the IκBα molecule is not degraded, NF-κB does not enter the nucleus and bind to its consensus binding sequence, and the IKK complex is not activated. Ad E3 also inhibited NF-κB activation by the proinflammatory molecule IL-1. This indicates that the mechanism for preventing the activation of NF-κB by Ad E3 may occur at a point common to both the IL-1 and the TNF-α pathways. Furthermore, the effect was observed in 293 and HeLa cells, as well as in primary HUVECs, indicating that this is neither a cell-specific effect nor an effect that requires an immortalized cell line.
The inhibition of NF-κB activity occurred only after the Ad E3 genes were sufficiently expressed, i.e., at approximately 8 h postinfection in 293 cells and 12 h postinfection in HeLa cells. Several studies have demonstrated that replication-competent or -incompetent first-generation Ad vectors, as well as third-generation vectors (deleted in E1, E2, E3, and various late proteins), upregulate NF-κB almost immediately postinfection (5, 25, 29). This immediate upregulation of NF-κB is probably due to the interaction of either the CAR receptor or the integrins with a viral structural protein on the input virus, because inhibitors of viral transcription such as UV light and psoralen do not prevent this very early induction of NF-κB in sensitive cell lines. The E3 proteins cannot inhibit this initial activation of NF-κB since they are not structural proteins brought into the cell with the virus, nor are they expressed in sufficient amounts until 8 to 12 h postinfection. However, since the E3 promoter contains an NF-κB binding site and this site is important for E3 transcription (21), we propose that this NF-κB activation might lead to increased expression of E3 proteins in vivo. Furthermore, the loop is completed when the E3 proteins inhibit NF-κB, turning off the promoter and maintaining a balance of NF-κB signaling versus other signals such as TNF-α-induced cytolysis.
Although we initially mapped the NF-κB effect to the E3 region of Ad in both 293 and HeLa cells, E1A proteins were previously shown to sensitize cells to TNF-α-induced cell death by inhibiting the IKK complex and preventing NF-κB activation (35). Since the 293 cell line is immortalized by transformation with E1A and E1B, we wanted to confirm that the E3 and not the E1 region, when expressed by the virus, was responsible for our observed results. By the use of viral mutants, we were able to show that Ad E3 was the region primarily responsible for the prevention of NF-κB activation, although E1 had a slight effect as seen in experiments performed with Ad dl7001. Furthermore, studies that were done subsequently with primary HUVECs (E1−) with Ad CMV-E3 confirmed our hypothesis that the Ad E3 was the region primarily responsible for the inhibition of NF-κB activation by TNF-α. We believe that the previously reported effects on IKK of isolated E1A are most likely counteracted in the presence of other viral genes such as E1B in a manner similar to the ability of E1B to prevent E1A-induced apoptosis through p53 (32).
Prior work from our lab demonstrated that the Ad E3-14.7K protein, which inhibits TNF-α-induced cytolysis, interacts with a protein we named FIP-3 (14.7K interacting protein 3). FIP-3 is a human protein identical to IKKγ and similar to the mouse protein NEMO. We showed that FIP-3/NEMO/IKKγ also interacts with RIP, IKKα, and IKKβ, which are important molecules in the NF-κB signal transduction pathway (23). Other groups also demonstrated that FIP-3/NEMO/IKKγ was an essential molecule on this pathway and was part of a large kinase complex responsible for the phosphorylation of IκBα (26, 34, 45). We therefore expected that Ad E3-14.7K would be responsible for our observed inhibitory effect on NF-κB activation. However, by using several deletions of the Ad E3 region we mapped the inhibitory effect on the NF-κB signaling pathway stimulated by both TNF-α and IL-1 to the E3-10.4K/14.5K complex.
Three different studies have demonstrated a decrease in the levels of Fas by the E3-10.4K/14.5K complex via internalization and degradation of the receptor in the lysosomes (13, 36, 40). Cell surface levels of the TRAIL receptor, another TNF-α receptor family member, have also been reported to be reduced by an E3-10.4K/14.5K/6.7K complex (3). However, there appears to be disagreement on whether TNFR1 is also internalized by E3-10.4K/14.5K (36, 42). Since our results could have been explained by the complete downregulation of the receptor, we measured the amount of TNFR1 on the surface of HeLa cells by fluorescence-activated cell sorting analysis but only observed a small decrease (5 to 10%) of TNFR1 levels at 16 h postinfection accompanied by a significant decrease (50 to 60%) in the amount of Fas by E3-10.4K/14.5K (data not shown). We believe that the decrease in TNFR1 does not explain the inhibition of NF-κB by Ad E3-10.4K/14.5K and that the mechanism of NF-κB downregulation is downstream of the receptor.
TNF-α has been shown to play a central role in the immune response and clearance of Ad vectors currently being used in gene therapy protocols. Our study indicates that the Ad E3-10.4K/14.5K complex is important for the escape of the virus from TNF-α-induced responses, primarily the signaling pathway leading to activation of the immunomodulatory factor NF-κB. Inhibiting NF-κB may also make Ad vectors safer by decreasing the production of inflammatory chemokines that can lead to circulatory and organ failure. By further understanding and utilizing the Ad E3 region, we should be able to realize the goal of developing less-immunogenic Ad vectors capable of expressing transgenes for prolonged periods of time.
Acknowledgments
This research was supported by NIH grants RO1 CA72963 and RO1 AI42295 (M.S.H.), NIAID training grant 2 T32 AI07506 (J.M.F.), and Cancer Center of the Albert Einstein College of Medicine grant P30-CA13330.
REFERENCES
- 1.Baeuerle, P. A., and T. Henkel. 1994. Function and activation of NF-κB in the immune system. Annu. Rev. Immunol. 12:141-179. [DOI] [PubMed] [Google Scholar]
- 2.Baichwal, V. R., and P. A. Baeuerle. 1997. Activate NF-κB or die? Curr. Biol. 7:R94-R96. [DOI] [PubMed] [Google Scholar]
- 3.Benedict, C. A., P. S. Norris, T. I. Prigozy, J. L. Bodmer, J. A. Mahr, C. T. Garnett, F. Martinon, J. Tschopp, L. R. Gooding, and C. F. Ware. 2001. Three adenovirus E3 proteins cooperate to evade apoptosis by tumor necrosis factor-related apoptosis-inducing ligand receptor-1 and -2. J. Biol. Chem. 276:3270-3278. [DOI] [PubMed] [Google Scholar]
- 4.Bix, M., N. Liao, M. Zijlstra, J. Loring, R. Jaenisch, and D. Raulet. 1991. Rejection of class I MHC deficient haemopoietic cells by irradiated MHC-matched mice. Nature 349:329-331. [DOI] [PubMed] [Google Scholar]
- 5.Borgland, S. L., G. P. Bowen, N. C. Wong, T. A. Libermann, and D. A. Muruve. 2000. Adenovirus vector-induced expression of the C-X-C chemokine IP-10 is mediated through capsid-dependent activation of NF-κB. J. Virol. 74:3941-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, Z. J., L. Parent, and T. Maniatis. 1996. Site-specific phosphorylation of IκBα by a novel ubiquitination-dependent protein kinase activity. Cell 84:853-862. [DOI] [PubMed] [Google Scholar]
- 7.Dai, Y., E. M. Schwarz, D. Gu, W. W. Zhang, N. Sarvetnick, and I. M. Verma. 1995. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc. Natl. Acad. Sci. USA 92:1401-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delhase, M., M. Hayakawa, Y. Chen, and M. Karin. 1999. Positive and negative regulation of IκB kinase activity through IKKβ subunit phosphorylation. Science 284:309-313. [DOI] [PubMed] [Google Scholar]
- 9.Devin, A., A. Cook, Y. Lin, Y. Rodriguez, M. Kelliher, and Z. Liu. 2000. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity 12:419-429. [DOI] [PubMed] [Google Scholar]
- 10.Efrat, S., G. Fejer, M. Brownlee, and M. S. Horwitz. 1995. Prolonged survival of pancreatic islet allografts mediated by adenovirus immunoregulatory transgenes. Proc. Natl. Acad. Sci. USA 92:6947-6951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Efrat, S., D. V. Serreze, A. Svetlanov, C. M. Post, E. A. Johnson, K. Herold, and M. S. Horwitz. 2001. Adenovirus early region 3 (E3) immunomodulatory genes decrease the incidence of autoimmune diabetes in nonobese diabetic (NOD) mice. Diabetes 50:980-984. [DOI] [PubMed] [Google Scholar]
- 12.Elkon, K. B., C.-C. Liu, J. G. Gall, J. Trevejo, M. W. Marino, K. A. Abrahamsen, X. Song, J. L. Zhou, L. J. Old, R. G. Crystal, and E. Falck-Pedersen. 1997. Tumor necrosis factor α plays a central role in immune-mediated clearance of adenoviral vectors. Proc. Natl. Acad. Sci. USA 94:9814-9819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elsing, A., and H. G. Burgert. 1998. The adenovirus E3/10.4K-14.5K proteins down-modulate the apoptosis receptor Fas/Apo-1 by inducing its internalization. Proc. Natl. Acad. Sci. USA 95:10072-10077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 15.Gooding, L. R. 1994. Regulation of TNF-mediated cell death and inflammation by human adenoviruses. Infect. Agents Dis. 3:106-115. [PubMed] [Google Scholar]
- 16.Graham, F. L., and L. Prevec. 1991. Manipulation of adenovirus vectors, p. 109-128. In E. J. Murray (ed.), Methods in molecular biology. The Humana Press, Clifton, N.J. [DOI] [PubMed]
- 17.Hsu, H., B. H. Shu, M. G. Pan, and D. V. Goeddel. 1996. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell 84:299-308. [DOI] [PubMed] [Google Scholar]
- 18.Hsu, H., J. Xiong, and D. V. Goeddel. 1995. The TNF receptor 1-associated protein TRADD signals cell death and NF-κB activation. Cell 81:495-504. [DOI] [PubMed] [Google Scholar]
- 19.Ilan, Y., G. Droguett, N. RoyChowdhury, Y. Li, K. Sengupta, N. R. Thummala, A. Davidson, J. RoyChowdhury, and M. S. Horwitz. 1997. Insertion of the adenoviral E3 region into a recombinant viral vector prevents antiviral humoral and cellular immune responses and permits long term gene expression. Proc. Natl. Acad. Sci. USA 94:2587-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones, N., and T. Shenk. 1978. Isolation of deletion and substitution mutants of adenovirus type 5. Cell 13:181-188. [DOI] [PubMed] [Google Scholar]
- 21.Korner, H., U. Fritzsche, and H. G. Burgert. 1992. Tumor necrosis factor α stimulates expression of adenovirus early region 3 proteins: implications for viral persistence. Proc. Natl. Acad. Sci. USA 89:11857-11861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, Y., and M. S. Horwitz. 1997. Use of green fluorescent protein in studies of apoptosis of transfected cells. Biotechnology 23:1026-1029. [DOI] [PubMed] [Google Scholar]
- 23.Li, Y., J. Kang, J. Friedman, L. Tarassishin, J. Ye, A. Kovalenko, D. Wallach, and M. S. Horwitz. 1999. Identification of a cell protein (FIP-3) as a modulator of NF-κB activity and as a target of an adenovirus inhibitor of tumor necrosis factor alpha-induced apoptosis. Proc. Natl. Acad. Sci. USA 96:1042-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lieber, A., C.-Y. He, L. Meuse, C. Himeda, C. Wilson, and M. A. Kay. 1998. Inhibition of NF-κB activation in combination with Bcl-2 expression allows for persistence of first-generation adenovirus vectors in the mouse liver. J. Virol. 72:9267-9277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieber, A., C.-Y. He, L. Meuse, D. Schowalter, I. Kirillova, B. Winther, and M. A. Kay. 1997. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 71:8798-8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mercurio, F., B. W. Murray, A. Shevchenko, B. L. Bennett, D. B. Young, J. W. Li, G. Pascual, A. Motiwala, H. Zhu, M. Mann, and A. M. Manning. 1999. IκB kinase (IKK)-associated protein 1, a common component of the heterogeneous IKK complex. Mol. Cell. Biol. 19:1526-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mercurio, F., H. Zhu, B. W. Murray, A. Shevchenko, B. L. Bennett, J. Li, D. B. Young, M. Barbosa, M. Mann, A. Manning, and A. Rao. 1997. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science 278:860-866. [DOI] [PubMed] [Google Scholar]
- 28.Miyamoto, S., and I. M. Verma. 1995. Rel/NF-κB/IκB story. Adv. Cancer Res. 66:255-292. [PubMed] [Google Scholar]
- 29.Morelli, A. E., A. T. Larregina, R. W. Ganster, A. F. Zahorchak, J. M. Plowey, T. Takayama, A. J. Logar, P. D. Robbins, L. D. Falo, and A. W. Thomson. 2000. Recombinant adenovirus induces maturation of dendritic cells via an NF-κB-dependent pathway. J. Virol. 74:9617-9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prevec, L., M. Schneider, K. L. Rosenthal, L. W. Belbeck, J. B. Derbyshire, and F. L. Graham. 1989. Use of human adenovirus-based vectors for antigen expression in animals. J. Gen. Virol. 70(Pt. 2):429-434. [DOI] [PubMed] [Google Scholar]
- 31.Ranheim, T. S., J. Shisler, T. M. Horton, L. J. Wold, L. R. Gooding, and W. S. M. Wold. 1993. Characterization of mutants within the gene for the adenovirus E3 14.7-kilodalton protein which prevents cytolysis by tumor necrosis factor. J. Virol. 67:2159-2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao, L., M. Debbas, P. Sabbatini, D. Hockenbery, S. Korsmeyer, and E. White. 1992. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc. Natl. Acad. Sci. USA 89:7742-7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Regnier, C. H., H. Y. Song, X. Gao, D. V. Goeddel, Z. Cao, and M. Rothe. 1997. Identification and characterization of an IκB kinase. Cell 90:373-383. [DOI] [PubMed] [Google Scholar]
- 34.Rothwarf, D. M., E. Zandi, G. Natoli, and M. Karin. 1998. IKK-γ is an essential regulatory subunit of the IκB kinase complex. Nature 395:297-300. [DOI] [PubMed] [Google Scholar]
- 35.Shao, R., M. C. Hu, B. P. Zhou, S. Y. Lin, P. J. Chiao, R. H. von Lindern, B. Spohn, and M. C. Hung. 1999. E1A sensitizes cells to tumor necrosis factor-induced apoptosis through inhibition of IκB kinases and nuclear factor κB activities. J. Biol. Chem. 274:21495-21498. [DOI] [PubMed] [Google Scholar]
- 36.Shisler, J., C. Yang, B. Walter, C. F. Ware, and L. R. Gooding. 1997. The adenovirus E3-10.4K/14.5K complex mediates loss of cell surface Fas (CD95) and resistance to Fas-induced apoptosis. J. Virol. 71:8299-8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siebenlist, U., G. Franzoso, and K. Brown. 1994. Structure, regulation and function of NF-κB. Annu. Rev. Cell Biol. 10:405-455. [DOI] [PubMed] [Google Scholar]
- 38.Sonenshein, G. E. 1997. Rel/NF-κB transcription factors and the control of apoptosis. Semin. Cancer Biol. 8:113-119. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi, M., Y. Ilan, N. Roy-Chowdury, J. Guida, M. S. Horwitz, and J. Roy-Chowdhury. 1996. Long term correction of bilirubin-UDP-glucuronosyltransferase deficiency in Gunn rats by administration of a recombinant adenovirus during the neonatal period. J. Biol. Chem. 271:1-7. [DOI] [PubMed] [Google Scholar]
- 40.Tollefson, A. E., T. W. Hermiston, D. L. Lichtenstein, C. F. Colle, R. A. Tripp, T. Dimitrov, K. Toth, C. E. Wells, P. C. Doherty, and W. S. Wold. 1998. Forced degradation of Fas inhibits apoptosis in adenovirus-infected cells. Nature 392:726-730. [DOI] [PubMed] [Google Scholar]
- 41.von Herrath, M., S. Efrat, M. B. A. Oldstone, and M. S. Horwitz. 1997. Expression of adenoviral E3 transgenes in β cells prevents autoimmune diabetes. Proc. Natl. Acad. Sci. USA 94:9808-9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wold, W. S., K. Doronin, K. Toth, M. Kuppuswamy, D. L. Lichtenstein, and A. E. Tollefson. 1999. Immune responses to adenoviruses: viral evasion mechanisms and their implications for the clinic. Curr. Opin. Immunol. 11:380-386. [DOI] [PubMed] [Google Scholar]
- 43.Wold, W. S. M., S. L. Deutscher, N. Takemori, B. M. Bhat, and S. C. Magie. 1986. Evidence that AGUAUAUGA and CCAAGAUGA initiate translation in the same mRNA in region E3 of adenovirus. Virology 148:168-180. [DOI] [PubMed] [Google Scholar]
- 44.Woronicz, J. D., X. Gao, Z. Cao, M. Rothe, and D. V. Goeddel. 1997. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science 278:866-869. [DOI] [PubMed] [Google Scholar]
- 45.Yamaoka, S., G. Courtois, C. Bessia, S. T. Whiteside, R. Weil, F. Agou, H. E. Kirk, R. J. Kay, and A. Israel. 1998. Complementation cloning of Nemo, a component of the IκB kinase complex essential for NF-κB activation. Cell 93:1231-1240. [DOI] [PubMed] [Google Scholar]
- 46.Yang, Y., Q. Li, H. C. Ertl, and J. M. Wilson. 1995. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J. Virol. 69:2004-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zandi, E., D. M. Rothwarf, M. Delhase, M. Hayakawa, and M. Karin. 1997. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell 91:243-252. [DOI] [PubMed] [Google Scholar]