Abstract
In an earlier study we evaluated innate immune responses to a first-generation adenoviral vector infused into the portal vein of rhesus monkeys who had never been exposed to adenovirus previously. In these animals, the systemic administration of E1/E3-deleted adenoviral vectors resulted in immediate activation of innate immunity and serious toxicity caused by targeting of vector to antigen-presenting cells and systemic inflammation. We analyze here how these responses are affected by vector-specific preexisting immunity that was induced by intramuscular immunization 6 months prior to evaluation. Our results show that preexposure to the vector substantially diminishes the transgene expression in most tissues but has little effect on gene transfer. Significantly, preimmunization does not eliminate systemic vector-induced toxicity. These conclusions are based on the presence of clinical features of coagulopathy and elevated levels of proinflammatory cytokine interleukin-6 in the serum of animals treated with vector after intramuscular immunization. Furthermore, preexisting immunity appears to induce a vector-specific inhibitory effect on erythroid progenitor development in the bone marrow that is not found when naive animals are challenged with vector.
First-generation recombinant adenovirus (Ad) vectors rendered replication-deficient by deletion of the viral early gene E1 have been widely utilized for in vivo gene expression, both in animal studies and in clinical trials (7, 14). Characteristic features of Ad vectors include broad cell and tissue tropism, high-efficiency vector transduction, and an ability to transduce nondividing cells. A major problem associated with the use of these vectors for in vivo applications is their strong immunogenicity leading to significant cellular and humoral immune responses (15, 16, 23, 25, 36). These responses not only limit the duration of desired transgene expression due to immune-mediated destruction of vector-transduced cells but also significantly reduce the efficiency of vector readministration due to neutralization of the vector by antibodies directed against capsid proteins of the vector.
The mechanisms and consequences of Ad-specific adaptive immune responses have been well characterized. Much less is known about innate inflammatory responses that occur immediately after vector administration. Early host responses to Ad are associated with release of inflammatory mediators (cytokines and chemokines) that recruit phagocytic cells (neutrophils and macrophages) to the sites of infection (1, 9, 21, 24, 34, 40). The resulting inflammation is frequently accompanied by tissue injury at the site of infection and acute systemic toxicity (1, 9, 21, 24, 27). The development of lethal systemic inflammatory response syndrome in a research subject with ornithine transcarbamylase deficiency (OTCD) who received systemic Ad vector is believed to be the result of vector-induced activation of the innate immune system (S. E. Raper, N. Chirmule, F. S. Lee, A. Bagg, G. P. Gao, R. Heidenreich, J. M. Wilson, and M. L. Batshaw, unpublished data).
In an attempt to understand the basis for innate immune responses after systemic Ad, we conducted a series of studies in mice and nonhuman primates (27, 37). We found that Ad vector was rapidly sequestered by antigen-presenting cells (APCs), such as macrophages and dendritic cells (DC), leading to the production of inflammatory cytokines. The clinical consequences of systemic dissemination of cytokines was best illustrated in nonhuman primates who, at high doses of vector, develop multiple organ damage, disseminated intravascular coagulation, and hemorrhage (23).
An issue that we did not address was how early host responses to the vector might be affected by preexisting immunity to vector. This is of particular importance with regard to Ad vector safety, since most of the human population is seropositive to human Ad, with a substantial fraction carrying Ad-specific neutralizing antibodies to serotypes 2 and 5, presumably as a result of the relatively high occurrence of the naturally acquired infections (4). Thus, the goal of current study was to assess the impact of preexisting immunity to Ad on the activation of innate immune responses to systemically administered Ad vector.
MATERIALS AND METHODS
Recombinant Ad vector.
Recombinant E1/E3-deleted human Ad (serotype 5) used in this study carried a cassette expressing the bacterial gene lacZ encoding β-galactosidase (β-Gal) gene under the control of cytomegalovirus early promoter (Ad-lacZ). The vector was prepared and evaluated for sterility, the presence of endotoxin, viral particles per PFU and per lacZ-forming unit ratios, the presence of replication-competent Ad (RCA), and genome structure as previously described (13, 27). For intraportal administration, each animal received a dose of vector from the same preparation, which was sterile, endotoxin-free, correct by restriction endonuclease analysis of viral DNA, contained <1 RCA in 107 particles of vector, was lacZ expression competent, and had a particle/PFU ratio of 25 and a particle/lacZ-forming unit ratio of 48.
Animals, immunization, surgical procedures, and specimen collection .
Rhesus macaques (Macaca mulatta) included in the study were negative for neutralizing antibodies to human serotype 5 Ad, as determined by in vitro analysis (see below). Six months prior to the evaluation two animals were injected intramuscularly (i.m.) with 2 × 1012 particles of Ad-lacZ vector per kg of body mass (preimmunization). At 24 h prior to the analysis, two preimmunized and two untreated (naive) monkeys were infused with 7.5 × 1012 Ad-lacZ particles per kg in 10 ml of phosphate-buffered saline (PBS) containing 10% glycerol into a mesenteric vein tributary of the portal vein. A negative control (control) monkey was administered with 10 ml of vector diluent. Anesthesia, laparotomy, catheterization, and necropsy procedures, as well as specimen collection, were performed as described previously (27).
Serum neutralizing antibody analysis.
Neutralizing capacity of sera from preimmunized monkeys was analyzed by assessing the ability of serum antibodies to inhibit transduction of HeLa cells with a reporter Ad vector expressing green fluorescent protein (GFP) as described previously (6). GFP expression was assessed by FluoroImaging (Molecular Dynamics), and neutralizing antibody titers were calculated by using ImageQuant software (Molecular Dynamics) as the serum dilution at which GFP fluorescence intensity was reduced by 50%.
Histology methods.
Hematoxylin and eosin (H&E) staining of paraffin-embedded liver and spleen sections and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining of cryosections were performed according to previously described protocols (27). For the preparation of high resolution epoxy semithin sections, cryosections of 50 μm were cut and fixed with 0.5% glutaraldehyde and 4% paraformadehyde for 10 min, followed by an X-Gal reaction at 37°C. At the end of the X-Gal reaction, as determined by microscopic monitoring, the sections were washed with cold PBS and refixed with 2.5% glutaraldehyde for 1 h. The sections were then washed and postfixed with 2% osmium tetroxide, dehydrated with ethanol, and directly infiltrated with 100% LX-112 epoxy medium (Ladd Research, Williston, Vt.) for 12 h, embedded in inverted BEEM capsules, and polymerized at 68°C for 72 h. Sections of 1.5 μm were cut with a Histo-Diamond diatome, mounted on coated microscopic slides, and counterstained with 0.8% toluidine blue. Images were digitally captured by using software from Phase3 (Sharon Hill, Pa.).
Determination of transgene expression in monkey tissues.
β-Gal expression in indicated tissues was quantified by using a β-Gal enzyme-linked immunosorbent assay (ELISA) kit from Boehringer Mannheim (Indianapolis, Ind.) according to the manufacturer's recommendations.
TaqMan real-time quantitative PCR and Southern blot hybridization analyses.
These analyses were used to quantitatively assess amounts of the vector (in copies per microgram of total DNA) present in different tissues 24 h after intraportal infusion. Total tissue or cellular DNAs were extracted by using Qiagen (Valencia, Calif.) Genomic Tips 20/G or 100/G as recommended by the manufacturer. TaqMan PCR analysis, targeting a 63-bp sequence from the Ad type 5 E2a region, was performed exactly as described earlier (27, 37). For Southern blot hybridization analysis, 10 μg of total DNA per sample was digested with BglII, separated on a 0.9% agarose gel, transferred to a Hybond-N+ nylon membrane (Amercontrol Pharmacia, Piscataway, N.J.), and hybridized to a 32P-labeled DNA probe corresponding to an 875-bp BglII-SmaI sequence from the Ad-lacZ E2a region. The probe was prepared by using Random Primed DNA Labeling Kit (Roche). The hybridized membane was exposed to Kodak BioMax MR film (Kodak, Rochester, N.Y.) in FBXC 810 autoradiography cassette (Fisher Scientific, Pittsburgh, Pa.). The vector copy number controls were 10 μg of DNA from the control monkey spleen spiked with 106, 107, or 108 copies of the vector DNA.
Isolation of splenic MNCs, flow cytometry, and FACS.
Single-cell suspensions from monkey spleens were prepared by teasing the tissue into small fragments in PBS containing 2% fetal bovine serum and 1 mM EDTA and pushing them through 50- to 70-μm-pore-size stainless steel mesh fabric (Sefar America, Inc., Kansas City, Mo.). Mononuclear cells (MNCs) were isolated by centrifugation through Ficoll-Paque Plus reagent (Amersham) as recommended by the manufacturer. For intracellular β-Gal staining, cells were permeabilized with Cytofix/Cytoperm (PharMingen, San Diego, Calif.) according to the manufacturer's recommendations. After a wash with Perm/Wash Buffer (PharMingen), the cells were incubated for 30 min on ice with biotin-conjugated β-Gal antibody (Sigma, St. Louis, Mo.), washed twice with Perm/Wash Buffer, and incubated with streptavidin-Cy-Chrome (PharMingen). To prepare the negative control samples, anti-β-Gal antibody was replaced with the buffer. The cells were fixed in 1% paraformaldehyde and kept at 4°C before flow cytometry analysis. Two specific cell populations were isolated from total splenic MNCs by fluorescence-activated cell sorting (FACS). First, the MNCs were stained with fluorescein isothiocyanate-conjugated anti-human HLA-DR antibody (PharMingen) and phycoerythrin-conjugated anti-human CD14 (Serotec, Inc., Raleigh, N.C.) and CD56 (PharMingen) antibodies to demarcate monkey monocyte/macrophage cells. Second, fluorescein isothiocyanate-conjugated anti-human HLA-DR and phycoerythrin-conjugated anti-human CD83 antibodies (both from PharMingen) were used to obtained mature DC-enriched cell population. Note that a substantial number of vector-transduced MNCs were apparently modified by vector transduction attaining a density great enough to migrate through the Ficoll-Paque layer, thereby segregating with granulocytes and erythrocytes. Therefore, absolute values presented for transgene expression and the presence of vector DNA are believed to be underestimates.
Serum cytokine analysis.
Concentrations of cytokines interleukin-6 (IL-6), IL-12 (total), IFN-γ, and tumor necrosis factor alpha (TNF-α) in the monkey sera 1 day prior to and 2, 6, and 24 h after vector administration were determined by using corresponding (human specific for IL-6 and monkey specific for IL-12, IFN-γ, and TNF-α) ELISA kits from BioSource International (Camarillo, Calif.) according to the manufacturer's recommendations.
Methylcellulose culture and histologic analysis of monkey bone marrow.
Collection, single-cell suspension preparation, and methylcellulose culture of monkey bone marrow-derived MNCs were performed as previously described (27). The cells were plated in quadruplicates at different concentrations in 0.5-ml aliquots of the culture medium and incubated at 37°C in 5% CO2 incubator. The numbers of burst-forming unit of erythroid (BFU-E), CFU of granulocyte-macrophage (CFU-GM), and CFU of granulocyte-erythrocyte-macrophage-megakaryocyte (CFU-GEMM) progenitor-derived colonies were determined at days 10 to 12 of culture under an inverted light microscope. To evaluate bone marrow histopathology, monkey femurs and/or sternums were decalcified, and the bone marrow aspirates were embedded in paraffin, sectioned, and subjected to H&E and periodic acid-Schiff stainings (performed by Colorado Histo-Prep, Fort Collins, Colo.).
RESULTS
All monkeys received 7.5 × 1012 vector particles per kg of body mass via intraportal infusion; this dose was selected based on previous studies as being just below that which causes lethal consequences (23, 27). Two monkeys were administered 2 × 1012 particles/kg of the same vector i.m. (preimmunized) 6 months prior to the analysis. Sensitization of these monkeys to the vector was confirmed by the presence in their sera of vector-specific neutralizing antibodies, with titers equal to 1/640 for preimmunized monkey 1 and 1/160 for preimmunized monkey 2. These values are in the same range as those typically found in humans seropositive by virtue of a naturally acquired Ad infection (4). Preimmunized monkeys were compared to two others which were not preimmunized prior to intraportal infusion of vector (naive animals). A fifth (control) monkey received intraportal infusion of vector diluent under the same experimental settings to control for effects of the surgical procedures. Blood samples were collected from all monkeys for analysis of hematologic parameters, serum chemistries, and the presence of cytokines in serum prior to and 2, 6, and 24 h after vector administration. All animals were necropsied 24 h after vector administration, and their tissues were analyzed for histopathology, transgene expression, and the presence of vector DNA.
Pathology.
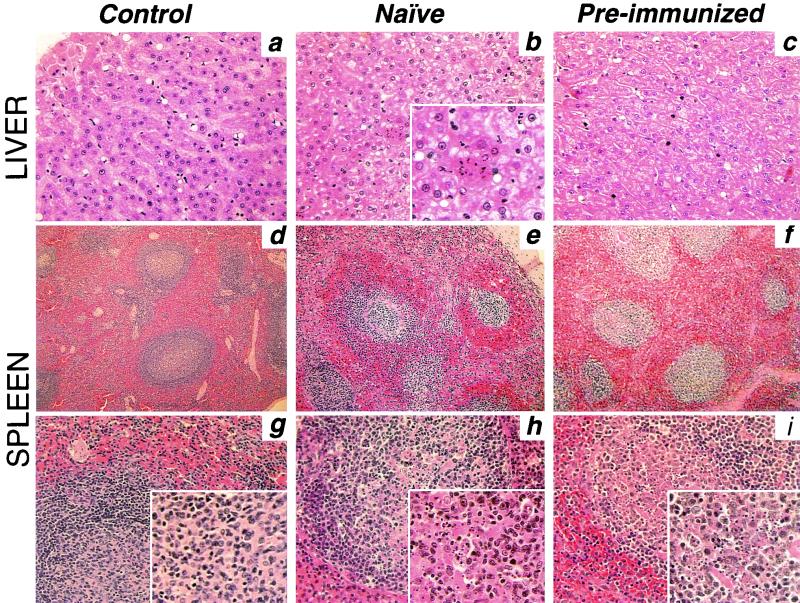
The clinical pathology data prior to and 24 h after vector administration are summarized in Table 1, whereas representative histopathology is shown in Fig. 1. The levels of transaminases (aspartate aminotransferase and alanine aminotransferase) and gamma glutamyl transpeptidase in serum were elevated after vector administration both in naive and preimmunized monkeys, a finding consistent with signs of liver inflammation, including neutrophil infiltrates and proapoptotic hepatocytes (Fig. 1b and c). The biochemical and histological evidence of liver damage was, however, reduced in preimmunized compared to naive animals. Leukopenia developed in naive but not in preimmunized animals. Slight declines in hemoglobin (Table 1) and hematocrit (data not shown) levels were observed in both naive and preimmunized groups. Consistent with vector-induced coagulopathy (27, 28), substantial reductions in platelet counts and an increase in fibrin split products were observed at comparable levels in naive and preimmunized animals; elevations in prothrombin time and partial thromboplastin time were more significant in naive animals.
TABLE 1.
Clinical pathology
| Parameter and time relative to vector administrationa | Results for individual animals
|
||||
|---|---|---|---|---|---|
| Control | Naive 1 | Naive 2 | Preimmunized 1 | Preimmunized 2 | |
| Aspartate aminotransferase concn (IU/liter) | |||||
| Pre | 18 | 29 | 24 | 18 | 38 |
| Post | 68 | 6,540 | 1,360 | 187 | 402 |
| Alanine aminotransferase concn (IU/liter) | |||||
| Pre | 16 | 51 | 28 | 37 | 46 |
| Post | 57 | 1,855 | 517 | 130 | 136 |
| Gamma glutamyl transpeptidase concn (IU/liter) | |||||
| Pre | 61 | 79 | 106 | 85 | 81 |
| Post | 55 | 578 | 125 | 137 | 99 |
| Hemoglobin concn (g/dl) | |||||
| Pre | 12.9 | 13.7 | 13.2 | 13.6 | 13.5 |
| Post | 12.6 | 11.9 | 6.9 | 11.6 | NDb |
| Platelet count (105/mm3) | |||||
| Pre | 4.2 | 3.7 | 5.6 | 4 | 2.9 |
| Post | 3 | 1.4 | 1.6 | 1.6 | ND |
| White blood cell count (103/mm3) | |||||
| Pre | 9.9 | 13.6 | 6.5 | 15.4 | 8.8 |
| Post | 13 | 9.2 | 4.2 | 15.4 | ND |
| Prothrombin time (s) | |||||
| Pre | 13 | 13 | 13.7 | 10.6 | 13.9 |
| Post | 12.9 | 16.6 | 18 | 13 | 14.6 |
| Partial thromboplastin time (s) | |||||
| Pre | 25.2 | 28.7 | 21.2 | 22.2 | 23.7 |
| Post | 26.2 | 42.5 | 36.4 | 25.3 | 30 |
| Fibrin split product concn (mg/ml) | |||||
| Pre | <2.5 | <2.5 | <2.5 | 20 | 10 |
| Post | 20 | 80 | 40 | 160 | 160 |
Pre, 1 day prior to vector administration; Post, 24 h after vector administration.\
ND, not determined due to the blood clot.
FIG. 1.
Analysis of histopathology in liver and spleen. Representative histologic sections of liver and spleen from control, naive, and preimmunized rhesus monkeys (as identified in the text) 24 h after intraportal infusion of Ad-lacZ vector are shown. Tissues were fixed in 10% buffered formalin, embedded in paraffin, sectioned, and stained with H&E. (a, b, and c) Liver sections from control, naive, and preimmunized monkeys, respectively. Magnifications: ×200, main panels; ×400, inset in panel b. (d and g, e and h, and f and i) Spleen sections from control, naive, and preimmunized monkeys, respectively. Magnifications: ×50, top row; ×100, bottom row; ×200, insets.
The histologic evidence of damage to the spleen was similar but slightly less intense in preimmunized compared to that observed in naive monkeys (compare Fig. 1e and h to Fig. 1f and i). In both groups, white pulp was less defined, with a somewhat homogeneous appearance and no distinct follicles and marginal zones, compared to the control animal (Fig. 1d and g). Also apparent in vector-administered animals were vascular congestion and red blood cells in the white pulp. The presence of numerous pyknotic nuclear fragments (see enlarged insets) was an indication of cellular destruction.
Preexisting immunity blocks transgene expression but not gene transfer.
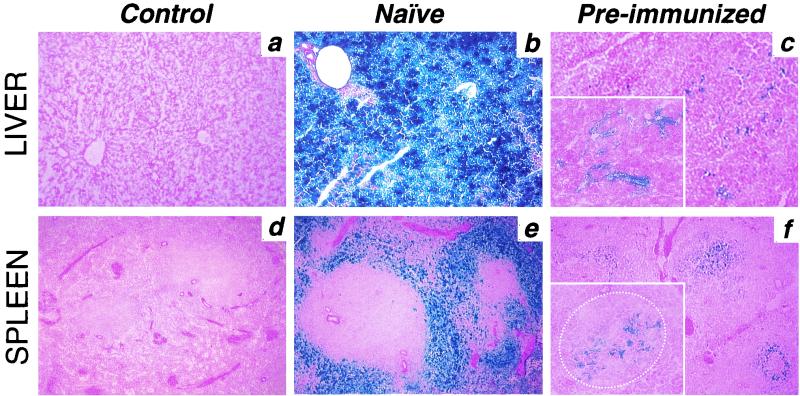
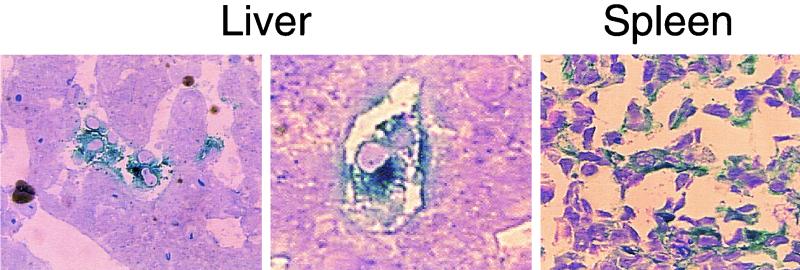
As expected, the levels of transgene (β-Gal) expression in the liver and spleen of preimmunized monkeys was significantly reduced compared to those in naive animals (Fig. 2, Table 2), presumably reflecting antibody-mediated vector neutralization, as has been described elsewhere (16, 17, 20, 36). This was also true for other tissues where low-level transgene expression was noted, including lungs and kidneys (Table 2). More than 95% of all hepatocytes were transduced in naive animals (Fig. 2b), a finding in contrast to the preimmunized animals, in which transgene expression was restricted to cells in the sinusoids (Fig. 2c), most of which had macrophage-like morphology (Fig. 3, left and middle panels) resembling Kupffer cells. Analysis of the spleens from naive animals revealed a preferential transduction of cells in the marginal zone and some in the red pulp, with follicular cells being essentially negative for transgene expression (Fig. 2e). The transduction pattern was very different in the spleens of preimmunized monkeys, where β-Gal-expressing cells with abundant cytoplasm were primarily localized within the follicular areas (Fig. 2f and 3 [right panel]).
FIG. 2.
Vector-mediated transgene expression in liver and spleen. Representative sections of β-Gal expression in liver and spleen from control, naive, and preimmunized monkeys 24 h after intraportal infusion of Ad-lacZ vector are shown. Tissues were preserved at −80°C, sectioned, fixed in 0.5% glutaraldehyde, and stained overnight in X-Gal solution as described in Materials and Methods. (a, b, and c) Liver sections from control, naive, and preimmunized monkeys, respectively. Magnifications: ×100, main panels; ×300, inset in panel c. (d, e, and f) Spleen sections from control, naive, and preimmunized monkeys, respectively. Magnifications: ×100, main panels; ×300, inset in panel f. The dotted line in the panel f inset encircles the follicular space of the white pulp inside the marginal zone.
TABLE 2.
β-Gal expression in various monkey tissues
| Animal | β-Gal expression (ng/1 mg of total protein) ± SD in:
|
|||
|---|---|---|---|---|
| Liver | Spleen | Lung | Kidney | |
| Control | <0.03 | <0.03 | NT | NT |
| Naive | 3,558 ± 435 | 731 ± 39 | 4.1 ± 0.2 | 2.1 ± 0.3 |
| Preimmunized | ||||
| 1 | <0.06 | NTa | NT | NT |
| 2 | 0.91 ± 0.02 | 0.18 ± 0.02 | <0.06 | <0.06 |
NT, not tested.
FIG. 3.
Transgene expression in liver and spleen of preimmunized monkeys. High-resolution epoxy 1.5-μm sections from the liver and spleen of preimmunized monkeys stained with X-Gal and counterstained with 0.8% toluidine blue (as described in Materials and Methods) are shown. (Left and middle) Liver sections. Magnifications: left panel, ×744; middle panel, ×1,395. (Right) Spleen germinal center. Magnification, ×744.
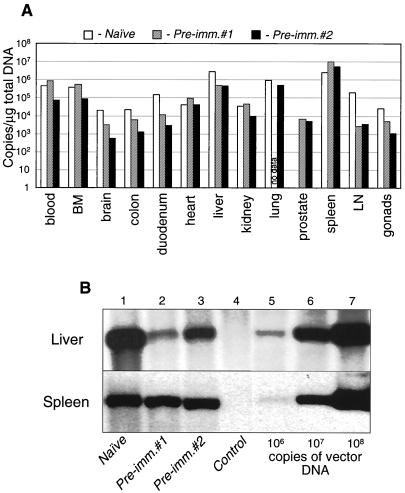
The distribution of vector DNA in tissues harvested 24 h after vector infusion was evaluated by real-time quantitative PCR analysis and then confirmed by Southern blot hybridization analysis (Fig. 4A and B, respectively) for the liver and spleen. These analyses are based on detection of the vector DNA in transfected tissues and thus served as indications of gene transfer. The vector was widely distributed, with the liver and spleen harboring the highest quantities (Fig. 4A). Surprisingly, despite the significant decrease in transgene expression in most analyzed tissues from preimmunized compared to that of naive animals (Table 2 and Fig. 2 for the liver and spleen results), not all of these tissues showed the corresponding reduction in vector gene transfer (Fig. 4A). Thus, in some tissues of preimmunized animals, including spleen, lung, kidney, and bone marrow, the amount of vector was not reduced in concert with the decrease in transgene expression. There was some discrepancy between the two methods of determination of vector DNA presence regarding the liver DNA value for preimmunized monkey 1, although this did not change the final conclusion that the reduction of transgene expression was out of proportion to the change in vector DNA.
FIG. 4.
Biodistribution of Ad-lacZ vector after intraportal infusion. Total DNA was extracted from the indicated tissues of one naive and two preimmunized monkeys and analyzed by TaqMan real-time quantitative PCR (A) or by Southern blot hybridization (B) for the presence of Ad-lacZ DNA. The bars in panel A represent average values of triplicate measurements, and the results are presented as the numbers of vector DNA copies per microgram of total tissue DNA.
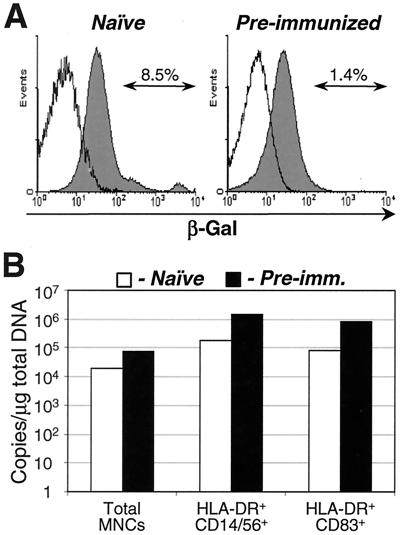
To further analyze vector distribution, MNCs were isolated from the spleen of each animal, fractionated into different cell types by FACS, and analyzed for the presence of transgene product and vector DNA (Fig. 5). Note that since a substantial amount of vector-transduced cells was eliminated from this analysis during cell isolation (see Materials and Methods for details), the absolute values shown for transgene expression and the presence of vector DNA are believed to be underestimates. Total splenic MNCs from naive and preimmunized monkeys were stained with an antibody to β-Gal to assess the amount of transgene product. Consistent with the histochemical analysis (Fig. 2), more cells from the naive group expressed the transgene than those from the preimmunized group (Fig. 5A). To assess the amount of vector DNA transferred to different cell types in the spleen, total MNCs were separated by FACS into HLA-DR+ CD14+ CD56+ monocyte/macrophage-enriched (3, 12, 18) and HLA-DR+ CD83+ mature DC-enriched (18, 29, 38, 39) populations. Total DNA was extracted from these populations and subjected to real-time PCR analysis. Despite a substantially reduced level of the transgene expression in cells from preimmunized monkeys, the amount of vector DNA was comparable between the two groups (Fig. 5B). Furthermore, vector was selectively taken up by macrophages and DCs.
FIG. 5.
Vector-mediated transgene expression and presence of vector DNA in splenic MNCs. (A) Flow cytometry analysis of transgene (β-Gal) expression in splenic MNCs from monkeys receiving intraportally administered Ad-lacZ vector. The cells were permeabilized and stained with biotin-conjugated β-Gal antibody, followed by incubation with streptavidin-Cy-Chrome (solid histograms). In preparation of control samples (open histograms) biotin-β-Gal antibody was replaced with the buffer. (B) TaqMan Ad-lacZ DNA-specific PCR analysis of splenic MNC fractions. Splenic MNCs were fractionated by FACS to isolate HLA-DR+ CD14+ CD56+ monocyte/macrophage- and HLA-DR+ CD83+ mature DC-enriched populations (see Materials and Methods for details). Total cellular DNA was extracted from the MNC fractions and subjected to the vector DNA-specific TaqMan PCR analysis (results are presented as the numbers of vector DNA copies per microgram of total cellular DNA). These data were generated from one out of two monkeys from each (naive or preimmunized) group.
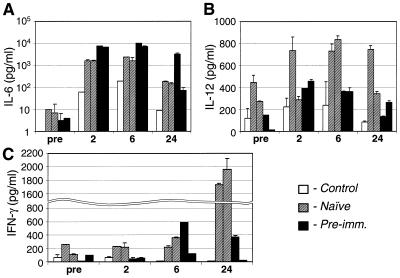
IL-6 levels in serum are increased in preimmunized monkeys.
Systemic levels of cytokines immediately after vector infusion were used as a measure of the activation of innate immunity. Previously we reported an acute induction of proinflammatory cytokines IL-6, IL-12, and TNF-α by 1 day after systemic administration of Ad vector in vector-naive mice (37) and of IL-6 in vector-naive rhesus monkeys (27). Quite unexpectedly, vector-induced elevations of IL-6 in serum were significantly higher in preimmunized animals than in naive animals (Fig. 6A). In one preimmunized monkey, the level of IL-6 in serum remained very high through 24 h after vector infusion. The levels of IL-12 in serum were moderately increased after vector infusion, with somewhat higher values in naive monkeys, values which returned to baseline values by 24 h after vector administration, although there was considerable variation (Fig. 6B). Systemic IFN-γ was not significantly induced in preimmunized monkeys within the evaluation period, which differed from naive animals that showed a substantial increase at the 24-h point (Fig. 6C). Levels of TNF-α were low for both experimental groups and not significantly different from the control injected animal (data not shown).
FIG. 6.
Concentrations of cytokines IL-6, IL-12, and IFN-γ in sera of control, two naive, and two preimmunized monkeys at the indicated time points after the intraportal infusion of Ad-lacZ vector, as determined by using human-specific IL-6 and monkey-specific IL-12 and IFN-γ ELISAs. Each serum sample was evaluated in triplicate. The error bars represent one standard deviation from the mean values.
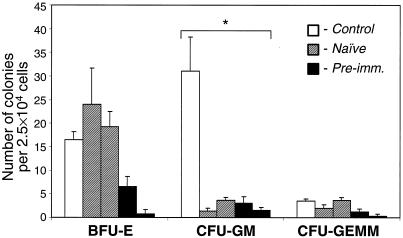
The number of bone marrow erythroid progenitors is decreased in preimmunized animals.
In a previous nonhuman primate study, we demonstrated growth inhibition of the myeloid progenitor cells of the bone marrow harvested after intraportal Ad vector administration in naive animals (27). In the present study we analyzed the growth of bone marrow progenitor cells (Fig. 7) and evaluated the histology of the bone marrow 24 h after Ad vector administration in the presence of preexisting anti-vector immunity. As in the preceding study with naive animals (27), the number of CFU-GM was significantly reduced in both naive and preimmunized animals compared to the control animal. Moreover, the number of BFU-E was substantially decreased in preimmunized monkeys but not in naive monkeys. Histologic analysis showed no significant abnormalities, except for a mild to moderate global reduction in bone marrow cellularity in all vector-treated (naive and preimmunized) animals (data not shown). In one preimmunized animal, however, there was a greater suppression of erythropoiesis than of granulopoiesis (data not shown).
FIG. 7.
Methylcellulose culture of bone marrow cells. Bone marrow cells from control, two naive, and two preimmunized monkeys were cultured in quadruplicates. The numbers of developed colonies were determined at days 10 to 12 of incubation. The error bars represent one standard deviation from the mean values. BFU-E, burst-forming unit of erythroid; CFU-GM, CFU of granulocyte-macrophage; CFU-GEMM, CFU of granulocyte-erythrocyte-macrophage-megakaryocyte. ✽, number of colonies per 1.25 × 104 bone marrow cells.
DISCUSSION
Ad vectors have been considered in many applications of in vivo gene therapy due in part to the efficiency with which they transduce nondividing cells. Administration of Ad vectors is associated with dose-dependent toxicity (5, 10, 23, 32), the nature of which is dependent on the route of vector administration (2, 8, 19, 31, 33, 35). We evaluated here the impact of preexisting immunity to the vector on the efficiency and safety of the Ad vector, targeted to the liver of rhesus monkeys via intraportal infusion.
An important consequence of in vivo administration of Ad vectors is targeting APCs and vibrant activation of T cells to vector-expressed antigens (4, 9, 15, 16, 25, 26, 27, 36, 37). The consequence of APC targeting is cytotoxic-T-lymphocyte-mediated elimination of vector-transduced cells which contributes to the extinction of transgene expression and a mononuclear cell-dominated inflammatory response at the site of gene transfer. Examples of this include pneumonia after intratracheal vector instillation (2, 8, 33), hepatitis after intraportal vector infusion (19, 31), and myositis after i.m. injection of vector (35). Toxicity associated with antigen-specific immune responses is directly linked to the site and extent of vector-mediated gene expression. It was predicted, therefore, that the presence of preexisting immunity to the vector in the form of neutralizing antibodies would diminish both gene expression and toxicity. In fact, we observed that hepatocyte transduction, as measured by β-Gal expression, was substantially diminished in preimmunized animals. Accordingly, there was less liver damage in preimmunized animals, as judged by serum transaminase levels and histopathology, suggesting that the toxic effect of vector transduction was inhibited by preexisting immunity.
More recently, attention has focused on another mechanism of toxicity that has direct consequences following vector-targeting of APCs. We and others have demonstrated the distribution of Ad vector to macrophages and DCs with immediate release of inflammatory cytokines after in vivo administration (9, 21, 24, 27, 37, 40; Raper et al., unpublished). A surprising finding in the current study was that preexisting immunity did not substantially alter the activation of innate immunity, which previously was ascribed to the targeting of APCs. The levels of IL-6 in serum after vector administration were actually higher in preimmunized relative to those in naive animals. Since the analysis was performed very early after vector administration, this escalation of IL-6 secretion was likely associated with activation of the innate immune system. We could not, however, rule out the possibility that the increased IL-6 secretion in preimmunized animals reflected release by preactivated T cells. The proposed association between an elevated IL-6 level in serum and clinical manifestations of systemic inflammation that emerged from our previous studies of liver-directed gene transfer in human and nonhuman primates (27; Raper et al., unpublished) was demonstrated in the current study with both naive and preimmunized animals developing thrombocytopenia, coagulopathy, and hepatic damage. We do not suggest that IL-6 is causally related to the toxicity but rather that it is a marker of activation of innate immunity.
Our previous experiments have shown that activation of an innate immune response by Ad is the consequence of targeting and activation of APCs, which is independent of the expression of vector encoded genes in these cells (27, 37). Although the neutralizing antibodies clearly inhibited vector-mediated gene expression in cells of the liver and spleen, they did not diminish the total vector uptake in the spleen, a finding consistent with the biochemical and clinical evidence of innate immune responses at comparable levels in naive and preimmunized animals. This suggests that the pathway of vector transduction is distinct from the pathway of vector-APC interaction that activates innate immunity and indicates that this activation is not a (direct) consequence of vector-mediated gene expression, as evidenced in our earlier study with UV-inactivated Ad (27). The presence of vector-specific antibodies may provide alternative vector uptake pathways, such as Fc receptor-mediated internalization of antibody-coated vector by the cells of the immune system. Further studies will focus on the role of capsid-directed antibodies in facilitating the Ad-APC interaction.
One unexplained finding of the recent OTCD clinical trial was the development of apparent red cell aplasia in a subject who experienced lethal systematic inflammatory response syndrome after administration of a high-dose intraportal vector (Raper et al., unpublished). The assessment of bone marrow histopathology was performed 4 days after vector infusion, and it remains unclear whether this finding was directly related to the vector administration or simply reflected a preexisting abnormality in the subject. In the present study we discovered that the development of erythroid progenitors, as reflected by the in vitro propagation of bone marrow cells, was impaired in preimmunized but not naive monkeys. This is interesting since the OTCD patient who was noted to have erythroid aplasia did have preexisting neutralizing antibodies to Ad (Raper et al., unpublished). Even though the assessment of bone marrow progenitor growth was not performed in the OTCD trial, our finding in monkeys may reflect similar pathological mechanisms to those observed in humans. The apparent discordance between the BFU-E data (growth inhibition) and histologic findings (no specific effect on erythropoiesis, except for one animal) may be due to the timing of the studies (bone marrow from the monkeys was analyzed 24 h after vector administration, whereas the patient's tissue was harvested 4 days after vector administration). The pathway(s) leading to the BFU-E growth inhibition, as well as its long-term consequence, is currently unknown. The mechanism is probably not secondary to the transduction of bone marrow cells, since these cells were transduced more efficiently in naive than in preimmunized monkeys (data not shown). It is possible that repression of the progenitor growth was influenced and/or mediated by certain cytokine(s) differentially induced by the vector in the presence of neutralizing antibodies. Earlier, one study indicated that IL-6 might have some influences on bone marrow cell proliferation (22). More recently, it was discovered that inhibition of erythroid progenitor cell proliferation in human bone marrow culture could be inhibited by macrophage inflammatory protein 1α (30), a chemokine induced during Ad infection (24, 40).
In summary, preimmunization of rhesus monkeys with recombinant Ad vector significantly blocked vector-mediated gene expression, but not vector gene transfer, upon vector readministration. Mechanisms of the vector uptake and/or its intracellular pathways may be influenced or altered by the presence of (neutralizing) immune mediators. The exact nature of these alterations remains to be determined. Based on clinical pathology and histology, we concluded that preexisting immunity did not eliminate Ad vector-induced systemic toxicity. The levels of proinflammatory cytokine IL-6 within 24 h after vector administration in the sera of preimmunized monkeys were elevated compared to those of naive monkeys. Finally, erythroid progenitor development in bone marrow was significantly impaired in preimmunized but not in naive animals. These results provide new insight into safety issues regarding the use of Ad as vectors in humans.
Furthermore, our studies highlight the problem of using a vector derived from a virus which naturally infects the target population of individuals (i.e., preexisting immunity). It may be prudent to consider vectors based on nonhuman viruses not recognized by serologic responses to human pathogens (11).
Acknowledgments
We are grateful to the Vector, Cell Morphology, and QC cores of the Institute for Human Gene Therapy and specifically to Julio Sanmiguel, Barbara Chambers, and Roberto Calcedo, as well as to Jeffrey S. Faust (Wistar Flow Cytometry Facility, Wistar Institute, Philadelphia, Pa.), for technical assistance and to Bill Reenstra for critical review of the manuscript.
This work was supported by grants from the National Institutes of Health (NHLBI P01 HL59407-03 and NIDDK P30 DK47757-09), the Juvenile Diabetes Research Foundation, and GlaxoSmithKline.
J.M.W. holds equity in Targeted Genetics.
REFERENCES
- 1.Adesanya, M. R., R. S. Redman, B. J. Baum, and B. C. O'Connell. 1996. Immediate inflammatory responses to adenovirus-mediated gene transfer in rat salivary glands. Hum. Gene Ther. 7:1085-1093. [DOI] [PubMed] [Google Scholar]
- 2.Brody, S. L., M. Metzger, C. Danel, M. A. Rosenfeld, and R. G. Crystal. 1994. Acute responses of non-human primates to airway delivery of an adenovirus vector containing the human cystic fibrosis transmembrane conductance regulator cDNA. Hum. Gene Ther. 5:821-836. [DOI] [PubMed] [Google Scholar]
- 3.Carter, D. L., T. M. Shieh, R. L. Blosser, K. R. Chadwick, J. B. Margolick, J. E. Hildreth, J. E. Clements, and M. C. Zink. 1999. CD56 identifies monocytes and not natural killer cells in rhesus macaques. Cytometry 37:41-50. [PubMed] [Google Scholar]
- 4.Chirmule, N., K. Propert, S. Magosin, Y. Qian, R. Qian, and J. Wilson. 1999. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 6:1574-1583. [DOI] [PubMed] [Google Scholar]
- 5.Christ, M., B. Louis, F. Stoeckel, A. Dieterle, L. Grave, D. Dreyer, J. Kintz, D. Ali Hadji, M. Lusky, and M. Mehtali. 2000. Modulation of the inflammatory properties and hepatotoxicity of recombinant adenovirus vectors by the viral E4 gene products. Hum. Gene Ther. 11:415-427. [DOI] [PubMed] [Google Scholar]
- 6.Croyle, M. A., N. Chirmule, Y. Zhang, and J. M. Wilson. 2001. “Stealth” adenoviruses blunt cell-mediated and humoral immune responses against the virus and allow for significant gene expression upon readministration in the lung. J. Virol. 75:4792-4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crystal, R. G. 1995. Transfer of genes to humans: early lessons and obstacles to success. Science 270:404-410. [DOI] [PubMed] [Google Scholar]
- 8.Crystal, R. G., N. G. McElvaney, M. A. Rosenfeld, C. S. Chu, A. Mastrangeli, J. G. Hay, S. L. Brody, H. A. Jaffe, N. T. Eissa, and C. Danel. 1994. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat. Genet. 8:42-51. [DOI] [PubMed] [Google Scholar]
- 9.Driesse, M. J., M. C. Esandi, J. M. Kros, C. J. Avezaat, C. Vecht, C. Zurcher, I. van der Velde, D. Valerio, A. Bout, and P. A. Sillevis Smitt. 2000. Intra-CSF administered recombinant adenovirus causes an immune response-mediated toxicity. Gene Ther. 7:1401-1409. [DOI] [PubMed] [Google Scholar]
- 10.Durham, H. D., H. Lochmuller, A. Jani, G. Acsadi, B. Massie, and G. Karpati. 1996. Toxicity of replication-defective adenoviral recombinants in dissociated cultures of nervous tissue. Exp. Neurol. 140:14-20. [DOI] [PubMed] [Google Scholar]
- 11.Farina, S. F., G. P. Gao, Z. Q. Xiang, J. J. Rux, R. M. Burnett, M. R. Alvira, J. Marsh, C. J. Hildegund, and J. Wilson. 2001. Replication-defective vector based on a chimpanzee adenovirus. J. Virol. 75:11603-11613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freudenthal, P. S., and R. M. Steinman. 1990. The distinct surface of human blood dendritic cells, as observed after an improved isolation method. Proc. Natl. Acad. Sci. USA 87:7698-7702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao, G. P., Y. Yang, and J. M. Wilson. 1996. Biology of adenovirus vectors with E1 and E4 deletions for liver-directed gene therapy. J. Virol. 70:8934-8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hackett, N. R., and R. G. Crystal. 2000. Adenovirus vectors for gene therapy, p. 17-40. In N. S. Templeton and D. D. Lasic (ed.), Gene therapy: therapeutic mechanisms and strategies. Marcel Dekker, Inc., New York, N.Y.
- 15.Jooss, K., H. C. Ertl, and J. M. Wilson. 1998. Cytotoxic T-lymphocyte target proteins and their major histocompatibility complex class I restriction in response to adenovirus vectors delivered to mouse liver. J. Virol. 72:2945-2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juillard, V., P. Villefroy, D. Godfrin, A. Pavirani, A. Venet, and J. G. Guillet. 1995. Long-term humoral and cellular immunity induced by a single immunization with replication-defective adenovirus recombinant vector. Eur. J. Immunol. 25:3467-3473. [DOI] [PubMed] [Google Scholar]
- 17.Kass-Eisler, A., L. Leinwand, J. Gall, B. Bloom, and E. Falck-Pedersen. 1996. Circumventing the immune response to adenovirus-mediated gene therapy. Gene Ther. 3:154-162. [PubMed] [Google Scholar]
- 18.Kipps, T. J. 1995. The cluster of differentiation (CD) antigens, p. 113-140. In E. Beutler, M. A. Lichtman, B. S. Coller, and T. J. Kipps (ed.), Williams hematology. McGraw-Hill Book Co., Inc., New York, N.Y.
- 19.Li, Q., M. A. Kay, M. Finegold, L. D. Stratford-Perricaudet, and S. L. Woo. 1993. Assessment of recombinant adenoviral vectors for hepatic gene therapy. Hum. Gene Ther. 4:403-409. [DOI] [PubMed] [Google Scholar]
- 20.Mack, C. A., W. R. Song, H. Carpenter, T. J. Wickham, I. Kovesdi, B. G. Harvey, C. J. Magovern, O. W. Isom, T. Rosengart, E. Falck-Pedersen, N. R. Hackett, R. G. Crystal, and A. Mastrangeli. 1997. Circumvention of anti-adenovirus neutralizing immunity by administration of an adenoviral vector of an alternate serotype. Hum. Gene Ther. 8:99-109. [DOI] [PubMed] [Google Scholar]
- 21.Muruve, D. A., M. J. Barnes, I. E. Stillman, and T. A. Libermann. 1999. Adenoviral gene therapy leads to rapid induction of multiple chemokines and acute neutrophil-dependent hepatic injury in vivo. Hum. Gene Ther. 10:965-976. [DOI] [PubMed] [Google Scholar]
- 22.Nemunaitis, J., D. F. Andrews, D. Y. Mochizuki, M. B. Lilly, and J. W. Singer. 1989. Human marrow stromal cells: response to interleukin-6 (IL-6) and control of IL-6 expression. Blood 74:1929-1935. [PubMed] [Google Scholar]
- 23.Nunes, F. A., E. E. Furth, J. M. Wilson, and S. E. Raper. 1999. Gene transfer into the liver of nonhuman primates with E1-deleted recombinant adenoviral vectors: safety of readministration. Hum. Gene Ther. 10:2515-2526. [DOI] [PubMed] [Google Scholar]
- 24.Otake, K., D. L. Ennist, K. Harrod, and B. C. Trapnell. 1998. Nonspecific inflammation inhibits adenovirus-mediated pulmonary gene transfer and expression independent of specific acquired immune responses. Hum. Gene Ther. 9:2207-2222. [DOI] [PubMed] [Google Scholar]
- 25.Peeters, M. J., G. A. Patijn, A. Lieber, L. Meuse, and M. A. Kay. 1996. Adenovirus-mediated hepatic gene transfer in mice: comparison of intravascular and biliary administration. Hum. Gene Ther. 7:1693-1699. [DOI] [PubMed] [Google Scholar]
- 26.Sarukhan, A., S. Camugli, B. Gjata, H. von Boehmer, O. Danos, and K. Jooss. 2001. Successful interference with cellular immune responses to immunogenic proteins encoded by recombinant viral vectors. J. Virol. 75:269-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnell, M. A., Y. Zhang, J. Tazelaar, G. P. Gao, Q. C. Yu, R. Qian, S. J. Chen, A. N. Varnavski, C. LeClair, S. E. Raper, and J. M. Wilson. 2001. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol. Ther. 3:708-722. [DOI] [PubMed] [Google Scholar]
- 28.Seligsohn, U. 1995. Disseminated intravascular coagulation, p. 1497-1516. In E. Beutler, M. A. Lichtman, B. S. Coller, and T. J. Kipps (ed.), Williams hematology. McGraw-Hill Book Co., Inc., New York, N.Y.
- 29.Steinman, R. M., M. Pack, and K. Inaba. 1997. Dendritic cells in the T-cell areas of lymphoid organs. Immunol. Rev. 156:25-37. [DOI] [PubMed] [Google Scholar]
- 30.Su, S., N. Mukaida, J. Wang, Y. Zhang, A. Takami, S. Nakao, and K. Matsushima. 1997. Inhibition of immature erythroid progenitor cell proliferation by macrophage inflammatory protein-1α by interacting mainly with a C-C chemokine receptor, CCR1. Blood 90:605-611. [PubMed] [Google Scholar]
- 31.Sullivan, D. E., S. Dash, H. Du, N. Hiramatsu, F. Aydin, J. Kolls, J. Blanchard, G. Baskin, and M. A. Gerber. 1997. Liver-directed gene transfer in non-human primates. Hum. Gene Ther. 8:1195-1206. [DOI] [PubMed] [Google Scholar]
- 32.Thomas, C. E., D. Birkett, I. Anozie, M. G. Castro, and, P. R. Lowenstein. 2001. Acute direct adenoviral vector cytotoxicity and chronic, but not acute, inflammatory responses correlate with decreased vector-mediated transgene expression in the brain. Mol. Ther. 3:36-46. [DOI] [PubMed] [Google Scholar]
- 33.Wilmott, R. W., R. S. Amin, C. R. Perez, S. E. Wert, G. Keller, G. P. Boivin, R. Hirsch, J. De Inocencio, P. Lu, S. F. Reising, S. Yei, J. A. Whitsett, and B. C. Trapnell. 1996. Safety of adenovirus-mediated transfer of the human cystic fibrosis transmembrane conductance regulator cDNA to the lungs of nonhuman primates. Hum. Gene Ther. 7:301-318. [DOI] [PubMed] [Google Scholar]
- 34.Worgall, S., G. Wolff, E. Falck-Pedersen, and R. G. Crystal. 1997. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum. Gene Ther. 8:37-44. [DOI] [PubMed] [Google Scholar]
- 35.Yang, Y., S. E. Haecker, Q. Su, and J. M. Wilson. 1996. Immunology of gene therapy with adenoviral vectors in mouse skeletal muscle. Hum. Mol. Genet. 5:1703-1712. [DOI] [PubMed] [Google Scholar]
- 36.Yang, Y., Q. Li, H. C. Ertl, and J. M. Wilson. 1995. Cellular and humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J. Virol. 69:2004-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang, Y., N. Chirmule, G. P. Gao, R. Qian, M. Croyle, B. Joshi, J. Tazelaar, and, J. M. Wilson. 2001. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol. Ther. 3:697-707. [DOI] [PubMed] [Google Scholar]
- 38.Zhou, L. J., and T. F. Tedder. 1996. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc. Natl. Acad. Sci. USA 93:2588-2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou, L. J., and T. F. Tedder. 1995. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J. Immunol. 154:3821-3835. [PubMed] [Google Scholar]
- 40.Zsengeller, Z., K. Otake, S. A. Hossain, P. Y. Berclaz, and B. C. Trapnell. 2000. Internalization of adenovirus by alveolar macrophages initiates early proinflammatory signaling during acute respiratory tract infection. J. Virol. 74:9655-9667. [DOI] [PMC free article] [PubMed] [Google Scholar]