Abstract
Using mutants (tgt, mnmA(asuE, trmU), mnmE(trmE), miaA, miaB, miaE, truA(hisT), truB) of either Escherichia coli or Salmonella enterica serovar Typhimurium and the trm5 mutant of Saccharomyces cerevisiae, we have analyzed the influence by the modified nucleosides Q34, mnm5s2U34, ms2io6A37, Ψ39, Ψ55, m1G37, and yW37 on −1 frameshifts errors at various heptameric sequences, at which at least one codon is decoded by tRNAs having these modified nucleosides. The frequency of −1 frameshifting was the same in congenic strains only differing in the allelic state of the various tRNA modification genes. In fact, in one case (deficiency of mnm5s2U34), we observed a reduced ability of the undermodified tRNA to make a −1 frameshift error. These results are in sharp contrast to earlier observations that tRNA modification prevents +1 frameshifting suggesting that the mechanisms by which −1 and +1 frameshift errors occur are different. Possible mechanisms explaining these results are discussed.
Keywords: Translation, tRNA modification, nucleoside, frameshifting
INTRODUCTION
Many retroviruses express Gag-Pol or Gag-Pro-Pol proteins by coupling their translation from overlapping reading frames with −1 ribosomal frameshifts (Farabaugh 2000). The frameshift event occurs on a distinctive heptameric sequence of the general form X-XXY-YYZ, where X is any nucleotide, Y is A or U, and Z can be any nucleotide (Dinman et al. 1991; Jacks et al. 1988). From studies on the Rous sarcoma virus gag-pol frameshifting region, a pretranslocation “simultaneous slippage” model was proposed to explain the mechanism of frameshifting at such sequences (Jacks et al. 1988). According to this model, the frameshifting occurs before translocation and simultaneously by the two tRNAs present in the P- and A-sites. Later, an alternative hypothesis was presented that suggested that the frameshifting event occurs after translocation when the codons in the heptameric sequence occupy the E- and P-sites (Horsfield et al. 1995). Another type of −1 frameshifting at sites not containing this kind of heptameric sequences occurs when the ribosome is stalled at a “hungry codon” induced by, for example, aminoacyl-tRNA limitation (Weiss and Gallant 1983) or at nonsense codons (Weiss et al. 1987, 1990). The peptidyl-tRNA was proposed first to slip in the 5′ direction allowing the amino acid-tRNA in the A-site to decode the codon now at the newly formed frame by cognate interaction (Weiss et al. 1990; Yelverton et al. 1994; Barak et al. 1996).
The most energy-consuming process in the cell is translation of the genetic message transmitted via mRNA. In this process, it is pivotal for the ribosome to maintain the reading frame. Because the message lacks punctuations that would identify the reading frame, the ribosome cannot correct a reading frame error. Therefore, the translation machinery has evolved to reduce such errors to at least a magnitude lower in frequency than missense errors (Kurland et al. 1996). Transfer RNA is the molecule that decodes the message, and therefore it may be an important contributor to the reading frame maintenance. Changes in the tRNA structure, such as those induced by lack of a modified nucleoside, may therefore affect the reading frame maintenance. Modified nucleosides are derivatives of the four major nucleosides U, C, A, and G. At present, 81 different modified nucleosides have been characterized (Rozenski et al. 1999). Although they are present at different positions of the tRNA, most of them are present in the anticodon region and especially at positions 34 (wobble position) and 37 (3′ of and adjacent to the anticodon). Recently, we have demonstrated that the presence of several different modifications in the tRNA prevents +1 frameshifting (Urbonavičius et al. 2001). In that work, we also described a model explaining how frameshifting is promoted by hypomodified tRNA (see Fig. 1 ▶). This model has features in common with three of the above described models (Weiss et al. 1990; Horsfield et al. 1995; Barak et al. 1996) and with a recently described model to explain how +1 frameshifts occur (Stahl et al. 2002). We suggested that the same mechanism might also apply for −1 frameshifting errors. In the present work, we tested this hypothesis for several tRNA modifications. Surprisingly, our results demonstrate that in contrast to the +1 frameshifting (Urbonavičius et al. 2001), most tRNA modifications tested have no or in one case a slight stimulatory effect on −1 frameshifting. We suggest that in sharp contrast to the pivotal role of tRNA modification in preventing +1 frameshift errors, tRNA modification has no major role in preventing −1 frameshifting and that intrinsic features of the ribosome prevent this kind of errors.
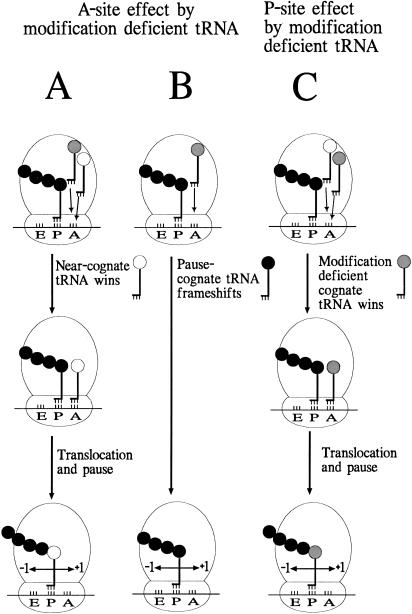
FIGURE 1.
A dual-error model for frameshifting (Urbonavičius et al. 2001). (A) Hypomodified cognate tRNA is defective in the amino acid-tRNA selection step, thereby allowing a wild-type near-cognate tRNA to be accepted instead at the A-site. After a normal 3-nt translocation, the near-cognate tRNA slips into either −1 or +1 frame depending on the sequence of the mRNA. (B) The hypomodified tRNA is very slow in entering the A-site, inducing a pause, thereby allowing the wild-type tRNA in the P-site to frameshift into either −1 or +1 frame. (C) As in A, but the hypomodified cognate tRNA is accepted in the A-site and when residing in the P-site, the hypomodification induces slippage into the −1 or +1 frame.
RESULTS
The working model and the experimental systems used
Modification of tRNA may affect the accuracy of translation either by altering the rate tRNAs bind to the ribosome or by modulating the interaction of the tRNA•mRNA complex with the ribosome. Recruitment of each successive cognate, in-frame tRNA must occur in competition with all other tRNAs, including those that may decode out of frame; altering the rate of recruitment of cognate tRNA could change this competition to allow more frequent errant decoding. Alternatively, modification may modulate the interaction with the ribosome to ensure continued cognate, in-frame decoding by successive tRNAs. Figure 1 ▶ shows our model predicting how tRNA modification might influence reading frame maintenance. In all cases discussed below, the frameshift event occurs by peptidyl-tRNA slippage, although the identity of the tRNA that slips differs in each case. In the first case (Fig. 1A ▶), hypomodification of a cognate tRNA might reduce its affinity for the codon in the ribosomal A-site. Because of its decreased rate of binding to the A-site, a wild-type near-cognate tRNA might successfully outcompete it, and be accepted into the A-site. After a normal 3-nt translocation, this near-cognate peptidyl-tRNA might not be able to interact optimally with the ribosomal P-site. Such nonoptimal P-site interaction has been suggested to reduce the efficiency of in-frame decoding leading to +1 frameshift errors (Belcourt and Farabaugh 1990; Urbonavičius et al. 2001). These errors are thought to result from slippage of peptidyl-tRNA prior to A-site recruitment. In principle, slippage in either the +1 or −1 direction might be possible, resulting in +1 or −1 frameshift errors. (P-site effect of near-cognate tRNA). In the second case (Fig. 1B ▶), slow A-site recruitment of an undermodified cognate tRNA might induce frameshifting by causing a translational pause that allows a cognate wild-type peptidyl-tRNA in the P-site to slip either +1 or −1 (A-site effect by undermodified tRNA). Alternatively, in the third case, the defect of the undermodified cognate tRNA may not be in the A-site selection step, but rather undermodification may disrupt its interaction with the P-site after translocation (Fig. 1C ▶). Undermodification might make the anticodon–codon interaction less optimal, as with the interaction by a near-cognate tRNA, resulting in an increased frequency of frameshifting (P-site effect by undermodified tRNA). Of course, hypomodification may cause frameshifting by mediating both A-site and P-site effects. Thus, in contrast to the simultaneous slippage model (Jacks et al. 1988), this model does not require a simultaneous slippage of both A- and P-site-located tRNAs, and the frameshifting event occurs after translocation. Our model has features in common with the model presented by Horsfield et al. (1995) and by Gallant and coworkers (Weiss and Gallant 1986).
Recently, we have demonstrated that a common function of several modified nucleosides in tRNA is to prevent +1 frameshifting (Urbonavičius et al. 2001). In the present work, we have investigated the influence of various modified nucleosides on −1 frameshifting using several assay systems (Table 2 ▶, see below). To monitor −1 frameshifting in the bacteria Escherichia coli and Salmonella enterica, the HIV gag-pol programmed frameshifting site (pHIV-1; U-UUU-UUA), the mouse mammary tumor virus (MMTV) gag-pro programmed frameshifting site (A-AAA-AAC), and the nonprogrammed argI gene frameshifting site (G-UUU-UAU) were used. In these sequences, the first triplet denotes the codon in P-site, and the last triplet denotes the codon in A-site before the shift into the −1 frame has occurred according to the simultaneous slippage model (Jacks et al. 1988). In all cases, the lacZ gene is placed downstream from a short frameshifting window in such a way that the β-galactosidase activity is a direct measurement of the frequency by which the ribosome shifts to the −1 frame within this window (Table 1 ▶). Previously, both HIV and MMTV sequences were shown to stimulate frameshifting in E. coli (Weiss et al. 1989; Yelverton et al. 1994). Furthermore, in E. coli, a −1 frameshifting site in the argI gene was also demonstrated (Fu and Parker 1994). Therefore, these assay systems are suitable to test the influence of tRNA modifications on −1 frameshifting in bacteria. In the eukaryote Saccharomyces cerevisiae, the HIV gag-pol programmed frameshifting site (U-UUU-UUA) and the L-A virus frameshifting site (G-GGU-UUA) were tested. Both HIV and L-A sequences are prone to frameshift in S. cerevisiae (Stahl et al. 1995; Kurland et al. 1996). In this case, the activity of luciferase was the measurement of frequency by which the ribosome shifts frame.
TABLE 2.
Assay systems used to monitor various tRNA modifications in −1 frameshifting
| Site | Organism | Mutants used | Heptameric sequencea | tRNA in P-site | Modification tested in P-site tRNA | tRNA in A-site | Modification tested in A-site tRNA |
| HIV | S. typh. | miaA | U-UUU-UUA | tRNAPheGAA | ms2io6- of ms2io6A37 | tRNALeucmnm5UmAA | ms2io6- of ms2io6A37 |
| HIV | S. typh. | miaB | U-UUU-UUA | tRNAPheGAA | ms2- of ms2io6A37 | tRNALeucmnm5UmAA | ms2- of ms2io6A37 |
| HIV | S. typh. | miaE | U-UUU-UUA | tRNAPheGAA | o6- of ms2io6A37 | tRNALeucmnm5UmAA | o6- of ms2io6A37 |
| HIV | S. typh. | truA | U-UUU-UUA | tRNAPheGAA | Ψ39 | tRNALeucmnm5UmAA | Ψ39 |
| HIV | E. coli | truB | U-UUU-UUA | tRNAPheGAA | Ψ55 | tRNALeucmnm5UmAA | Ψ55 |
| argI | S. typh. | miaA, miaB | G-UUU-UAU | tRNAPheGmAA | ms2io6- of ms2io6A37 | tRNATyrQUA | ms2io6- of ms2io6A37 |
| argI | S. typh. | tgt | G-UUU-UAU | tRNAPheGmAA | None | tRNATyrQUA | Q34 |
| MMTV | E. coli | mnmA | A-AAA-AAC | tRNALysmnmn5s2UUU | s2- of mnm5s2U34 | tRNAAsnQUU | None |
| MMTV | E. coli | mnmE | A-AAA-AAC | tRNALysmnmn5s2UUU | mnm5- of mnm5s2U34 | tRNAAsnQUU | None |
| MMTV | S. typh. | tgt | A-AAA-AAC | tRNALysmnmn5s2UUU | None | tRNAAsnQUU | Q34 |
| MMTV | E. coli | truA | A-AAA-AAC | tRNALysmnmn5s2UUU | Ψ39 | tRNAAsnQUU | Ψ39 |
| MMTV | E. coli | truB | A-AAA-AAC | tRNALysmnmn5s2UUU | Ψ55 | tRNAAsnQUU | Ψ55 |
| HIV | S. cerev. | trm5 | U-UUU-UUA | tRNAPheGmAA | Y-base | tRNALeuU?AA | m1G37 |
| L-A | S. cerev. | trm5 | G-GGU-UUA | tRNAGlyU?CC | None | tRNALeuU?AA | m1G37 |
aThe first triplet denotes the codon in the P-site and the next triplet the codon in the A-site, if the frameshifting occurred as postulated by the “simultaneous slippage model.”
TABLE 1.
Plasmids and strains used
| Plasmid | Sequence of the frameshifting window | Source/Reference |
| pHIV-1 | AUG-AAA-AGC-UUA-GCU-AAU-UUU-UUA-GG-GGA-GAU-CUG-GCC-UUC-lacZ | Weiss et al. 1989 |
| pMMTV1284 | AUG-AAA-AGC-UUA-GCU-GAA-AAU-UCA-AAA-AAC-UU-GUA-AAG-GGG-lacZ | Weiss et al. 1989 |
| pCFP3 | AAUUC-AUG-UCC-GGG-UUU-UAU-CAC-AAG-CAU-UUC-AUC-AAA-UA(AGC-lacZ | Fu and Parker 1994 |
| pAC1789 | lacZ-CAG-GCU=AAU-UUU-UUA-GG-GGA-GA---luc | Stahl et al. 1995 |
| pAC1790 | lacZ-CAG-GCU-AAU-UUU-UUA-AGG-GGA-GA---luc | Stahl et al. 1995 |
| pAC-LA | lacZ-UGG-CAG-CAG-GGU-UUA-GGA-GUG-GUA---luc | Bidou et al. 2000 |
| pAC-LAFF | lacZ-UGG-CAG-CAG-GGU-UUA-AGG-AGU-GGU-A---luc | This work |
| Strain | Relevant genotype or phenotype | Source/Reference |
| S. typhymurium | ||
| LT2 | wild type | J. Roth, Utah |
| TT5866 | truA(hisT)290::Tn5 | J. Roth, Utah |
| GT 5880 | tgt::Tn10dCm | Urbonavičius et al. 2001 |
| GT523 | miaA1 | Ericson and Bjök 1986 |
| GT2176 | miaB2508::Tn10dCm | Esberg and Björk 1995 |
| GT3034 | miaE2507::MudP | Persson and Björk 1993 |
| GT4946 | miaA1, miaB2508::Tn10dCm | This work |
| E. coli | ||
| TH98 | Δ(pro-lac), argE(am), (valR), ilv-135::Tn10, nalA, rif, mnmE+ | Urbonavičius et al. 2001 |
| TH99 | Δ(pro-lac), argE(am), (valR), ilv-135::Tn10, nalA, rif, mnmE | Urbonavičius et al. 2001 |
| TH193 | ara, Δ(pro-lac), nalA, argE (am), rif, thi, mnmA | Urbonavičius et al. 2001 |
| TH194 | ara, Δ(pro-lac), nalA, argE (am), rif, thi, mnmA+ | Urbonavičius et al. 2001 |
| GBEC384 | =CSH41 Δ(lac-pro), galE | CSH laboratories |
| GRB1488 | Δ(lac-pro), galE, truA(hisT)::kan | This work |
| GRB1490 | Δ(lac-pro), galE, truB2422::mini-Tn10Cm | Urbonavičius et al. 2002 |
| S. cerevisiae | ||
| GBY15 | Mat a, his3Δ1, leu Δ0, met15 Δ0, ura3 Δ0, trm5::kanMX4 | Research Genetics |
| GBY18 | Mat a, his3Δ1, leu Δ0, met15 Δ0, ura3 Δ0, TRM5 | Research Genetics |
According to our model (Fig. 1 ▶), lack of ms2io6A37 of, for example, the tRNALeucmnm5UmAA may affect the A-site selection step of tRNAs at the HIV frameshifting sites (Table 2 ▶). This would stimulate a frameshifting event at the P-site, mediated by tRNAPheGAA (A-site effect by the undermodified tRNA; Urbonavičius et al. 2001). Alternatively, the frameshifting can be stimulated by ms2io6A37 of, for example the tRNAPheGAA reading the UUU codon at the P-site, provided that the ribosome makes a pause at the A-site (P-site effect by the undermodified tRNA). Because the modified nucleoside ms2io6A37 is present in both of these tRNA, the effect monitored may be either caused by an A- or a P-site effect of the undermodified tRNA or a combined effect. Using the argI site (G-UUU-UAU) and the tgt mutant, which blocks formation of queuosine, we monitored specifically the influence of Q34 deficiency in tRNATyrQUA on reading the UAU codon in this sequence. Table 2 ▶ summarizes the assay systems used and indicates which modification was tested using the various mutants defective in tRNA modification. The assay systems, which are present on plasmids, were introduced into congenic strains of either E. coli, S. enterica, or S. cerevisiae, only differing in the allelic state of genes involved in the synthesis of various modified nucleosides. The frameshifting frequencies were compared with those in the wild type.
Influence on −1 frameshifting by modification in position 34 (the wobble position)
Role of Q34
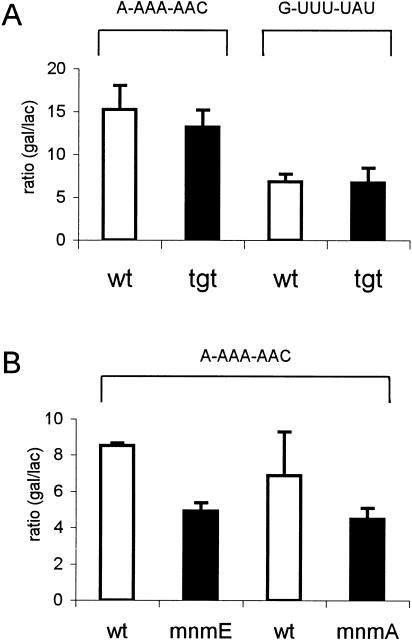
The hypermodified base 7-(((4.5-cis-dihydroxy-2-cyclopenten-1-yl)-amino) methyl)-7-deazaguanosine or queuosine (Q) is present in position 34 in tRNAs specific for Tyr, His, Asn, and Asp, encoding UAU/C, CAU/C, AAU/C, and GAU/C, respectively. In a tgt mutant, these tRNAs have an unmodified G34 instead of Q34. Of the −1 frameshifting sites available, the presence of G34 instead of Q34 may influence the A-site selection of tRNAAsnQUU at the A-AAA-AAC (pMMTV1284) or the A-site selection of tRNATyrQUA at the G-UUU-UAU (pCF3). To test this hypothesis, we introduced either plasmid pMMTV1284 or plasmid pCFP3 into the wild type and the tgt mutant, respectively, and measured the level of frameshifting. No difference in −1 frameshifting was observed between wild type and the tgt mutant, suggesting that the A-site selection of tRNAAsnQUU or tRNATyrQUA was not affected by Q34 (Fig. 2A ▶).
FIGURE 2.
Influence of the Q34 and mnm5s2U34 on frameshifting at A-AAA-AAC and G-UUU-UAU sites. (A) In the tgt mutant, the tRNALysmnm5s2UUU, reading AAA codon, is fully modified, whereas tRNAAsnQUU, reading AAC codon, contains G34; the tRNAPheGmAA, reading UUU codon, is fully modified, whereas tRNATyrQUA, reading UAU codon, contains G34. (B) In the mnmE mutant, AAA codon is read by tRNALysmnm5s2UUU, containing s2U34, whereas in the mnmA mutant, this tRNA contains mnm5U34; tRNAAsnQUU, reading AAC codon, is fully modified in both mutants. The frequency of frameshifting is expressed as the β-galactosidase activity normalized to the β-lactamase activity normalized encoded by the bla gene present on the same plasmid as that encoding lacZ gene.
Role of mnm5s2U34
The modified nucleoside 5-methylaminomethyl-2-thio-uridine (mnm5s2U) is present in position 34 in tRNAs specific for Gln, Lys, and Glu codons, which are part of the split codon boxes; that is, a codon box in which the four codons encode two amino acids. These tRNAs read codons CAA/G (Gln), AAA/G (Lys), and GAA/G (Glu). To test how the mnm5- or the s2- groups influence −1 frameshifting in the P-site by affecting the interaction of tRNALysmnm5s2UUU with the AAA codon, we introduced plasmid MMTV1284 (A-AAA-AAC) into wild type and mnmE (containing s2U34 instead of mnm5s2U34) or mnmA (containing mnm5U34) mutants, and measured the level of frameshifting. Lack of the mnm5-group, as in the mnmE mutant, reduced the rate of −1 frameshifting 1.7-fold, whereas lack of the s2-group, as in the mnmA mutant, did not affect the frequency of −1 frameshifting (Fig. 2B ▶).
Influence on −1 frameshifting by modification in position 37
Role of ms2io637
The modified nucleoside 2-(methylthio-N6-isopentenyl) adenosine ms2i6A37 is present in all E. coli tRNAs reading codons starting with U except tRNAGGASer I, V (Grosjean et al. 1985). In S. enterica, the hydroxylated derivative ms2io6A37 is present in the corresponding tRNAs. Three S. enterica genes involved in the synthesis of ms2io6A37 have been identified: miaA (Ericson and Björk 1986), miaB (Esberg and Björk 1995), and miaE (Persson and Björk 1993). The miaA mutant contains A37 instead of ms2io6A37 in its tRNA, whereas the miaB mutant tRNA lacks the ms2-group and therefore contains mainly i6A37 and a small amount of io6A37 (Esberg and Björk 1995). The miaE mutant lacks the hydroxyl-group and tRNA contains ms2i6A37 instead of ms2io6A37. We tested how the ms2io6- (as in miaA), ms2- (as in miaB), and o6- (as in miaE) groups influence the −1 frameshifting of tRNALeucmnm5UmAA, tRNATyrQUA, or tRNAPheGAA using the HIV (U-UUU-UUA) or the argI (G-UUU-UAU) frameshifting sites. We introduced the plasmids containing these sites into wild type and the miaA, miaB, and miaE mutants. We also introduced the plasmid containing the argI frameshifting site into wild type and the miaA-miaB double mutant, containing A37 instead of ms2io6A37. We measured the level of −1 frameshifting, and no difference between wild type and any of the mutants was observed (Fig. 3A ▶).
FIGURE 3.
Influence of ms2io6A37, yW37, and m1G37 on frameshifting at U-UUU-UUA, G-UUU-UAU, and G-GGU-UUA sites. (A) In the miaA mutant, both UUU and UUA codons at slippery sites are read by tRNAs containing A37. In the miaB mutant, those tRNAs contain io6A37, and the miaE mutant, they contain ms2i6A37. In the double miaA-miaB mutant, both UUU and UAU codons are read by tRNAs containing A37. The frequency of frameshifting is expressed as the β-galactosidase activity normalized to the β-lactamase activity normalized encoded by the bla gene present on the same plasmid as that encoding lacZ gene. (B) In the trm5 mutant, tRNAPheGmAA, reading the UUU codon, contains G37 instead of yW37; tRNALeuU?AA, reading the UUA codon, contains G37 instead of m1G37, and tRNAGlyU?CC, reading the GGU codon, contains m1G37. The frequency of frameshifting is expressed as the luciferase/β-galactosidase ratio of a test construct (pAC1789 or pAC-LA) normalized to the ratio obtained with the in-frame control (pAC1790 or pAC-LAFF).
Role of m1G37 and yW37
The Trm5p protein is involved in the formation of modified nucleosides m1G and wybutosine (yW; nucleoside of the Y-base) at position 37 in a subset of tRNAs in yeast S. cerevisiae. The tricyclic nucleoside yW is present only in tRNAPheGmAA, and the first step in its synthesis is the formation of m1G37 (Droogmans and Grosjean 1987). We have earlier shown that the trm5 mutant is devoid of yW and has an unmodified G37 in its tRNAPheGAA (Björk et al. 2001). The m1G in position 37 is present in several tRNA species, including tRNALeuU?AA. The trm5 mutant of S. cerevisiae contains G37 in those tRNAs.
To test how m1G37 influences reading frame maintenance by affecting A-site selection of tRNALeuU?AA, we introduced the L-A site (G-GGU-UUA) into wild type and the trm5 mutant, and measured the level of frameshifting. We found no difference in frameshifting (Fig. 3B ▶). Thus, lack of m1G37 in tRNALeuU?AA had no influence on −1 frameshifting compared to the fully modified tRNA. The combined deficiency of yW in tRNAPheGmAA and of m1G37 in tRNALeuU?AA had no impact on −1 frameshifting when monitored at the HIV-site (U-UUU-UUA) (Fig. 3B ▶).
Influence on −1 frameshifting by other modifications outside the anticodon
Role of Ψ39
The modified nucleoside pseudouridine (Ψ) is present in the anticodon stem and loop of many bacterial tRNAs, including tRNALeucmnm5UmAA, tRNAAsnQUU, tRNAPheGAA, and tRNALysmnm5s2UUU. The truA (hisT) gene product catalyzes the formation of Ψ in position 39 in those tRNA species. To test how Ψ39 influences reading frame maintenance by affecting A-site selection of tRNALeucmnm5UmAA and/or P-site codon–anticodon interaction of the tRNAPheGAA, the HIV frameshifting site (U-UUU-UUA) was introduced into wild-type strain and the truA mutant, and the level of frameshifting was measured. We found no difference in −1 frameshifting between the wild type and the truA mutant (Fig. 4A ▶). To test how Ψ39 influences reading frame maintenance by affecting A-site selection of tRNAAsnQUU and/or P-site codon–anticodon interaction of tRNALysmnm5s2UUU, the MMTV frameshifting site (A-AAA-AAC) was introduced into wild type and the truA mutant, and the level of frameshifting was measured. We observed no difference in −1 frameshifting between these strains, as in the case of pHIV-1 (Fig. 4A ▶). Thus, Ψ39 did not influence −1 frameshifting in any case.
FIGURE 4.
Influence of Ψ39 and Ψ55 on frameshifting at U-UUU-UUA and A-AAA-AAC sites. (A) In the truA mutant, all tRNAs, reading codons at slippery sites, contain U39 instead of Ψ39. (B) In the truB mutant, all tRNAs, reading codons at slippery sites, contain U55 instead of Ψ55. The frequency of frameshifting is expressed as the β-galactosidase activity normalized to the β-lactamase activity normalized encoded by the bla gene present on the same plasmid as that encoding the lacZ gene.
Role of Ψ55
The modified nucleoside Ψ55 is part of the TΨC loop and thus is present in all tRNA species of E. coli and S. typhimurium. To test whether Ψ55 influences frameshifting, either the HIV frameshifting site (U-UUU-UUA) or the MMTV frameshifting site (A-AAA-AAC) was introduced into wild type and the truB mutant, and the level of frameshifting was measured. We found no influence of Ψ55 on the frameshifting at both these frameshifting sites (Fig. 4B ▶).
DISCUSSION
We have earlier shown that a common function for several tRNA modifications is to improve the reading frame maintenance by preventing +1 frameshift (Björk et al. 1989; Hagervall et al. 1993; Li et al. 1997; Urbonavičius et al. 2001). Contrary to this observation, here we show that several different modified nucleosides do not influence or only slightly increase the frequency of −1 frameshifting (summarized in Table 3 ▶). Taken together, these observations suggest that the mechanism by which tRNA modification exerts its effect on the reading frame maintenance is different for shifts of the reading frame in the 5′ (−1) and the 3′ (+1) direction.
TABLE 3.
Comparison of the effect of tRNA modification on −1 and +1 frameshifting
| Increased −1 fs | Increased +1 fs | ||||
| Modification tested | Codon tested | P-site | A-site | P-site | A-site |
| Q34 | UAU | ND | No | No | Yesa |
| Q34 | AAC | ND | No | ND | ND |
| s2- of mnm5s2U34 | AAA | No | ND | Yesa | Yesa |
| mnm5- of mnm5s2U34 | AAA | Nob | ND | Yesa | Yesa |
| ms2io6- of ms2io6A37 | UUU | No | ND | Yesa | Noc |
| ms2- of ms2io6A37 | UUU | No | ND | Yesa | Noc |
| o6- of ms2io6A37 | UUU | No | ND | Yesa | ND |
| ms2io6- of ms2io6A37 | UUA | ND | No | ND | ND |
| ms2- of ms2io6A37 | UUA | ND | No | ND | ND |
| o6- of ms2io6A37 | UUA | ND | No | ND | ND |
| ms2io6- of ms2io6A37 | UAU | ND | No | Yesa | Yesc |
| Y-base | UUU | No | ND | ND | ND |
| m1G37 | UUA | ND | No | ND | ND |
| Ψ39 | UUU | No | ND | Nod | ND |
| Ψ39 | UUA | ND | No | ND | ND |
| Ψ39 | AAA | No | ND | ND | ND |
| Ψ39 | AAC | ND | No | ND | ND |
| Ψ55 | UUU | No | ND | Noe | ND |
| Ψ55 | UUA | ND | No | ND | ND |
| Ψ55 | AAA | No | ND | ND | Noe |
| Ψ55 | AAC | ND | No | ND | ND |
(ND) Not determined.
aAdapted from Urbonavičius et al. (2001).
bReduced level of frameshifting was observed.
cAdapted from Li et al. (1997).
dAdapted from Qian (1997).
eAdapted from Urbonavičius et al. (2002).
The Q34 deficiency in tRNAAsnQUU acting at the slippery sequence A-AAA-AAC did not influence the −1 frameshifting (Fig. 2A ▶), as was observed for +1 frameshifting (Urbonavičius et al. 2001). Similarly, the absence of Q34 in tRNAAsn did not influence the level of −1 frameshifting at U-UUA-AAC/U sites in the cos-cells and in vitro using the rabbit reticulocyte lysate (RRL) system (Marczinke et al. 2000). Using the same in vitro system, the level of −1 frameshifting at the A-AAA-AAC site only increased 1.5-fold when the Q-deficient tRNAAsnGUU from yeast was added to the lysate (Carlson et al. 2001). When the effect by Q34 was analyzed in vivo using a Q34 deficient mutant of E. coli, frameshifting by tRNALeuU?AA decreased 2-fold at the U-UUA-AAC site (i.e., when tRNALeuU?AA interacted with the AAC codon) and increased 2-fold at the U-UUA-AAU site (i.e., when tRNALeuU?AA interacted with the AAU codon; Brierley et al. 1997). The difference between those and our (Fig. 2A ▶) results may be explained by presence of different tRNAs (tRNALeucmnm5UmAA versus tRNALysmnm5s2UUU or tRNAPheGAA) in the P-site. Apparently, Q34 in tRNAAsnQUU interacting with the AAC codon slightly stimulates −1 frameshift errors, whereas when interacting with the AAU codon, it slightly prevents −1 frameshifts. Therefore, presence of Q34 in the tRNA stimulates −1 frameshifting when it interacts with C, whereas it prevents the same error if interacting with U as the third nucleoside of the codon. Similarly, when monitoring +1 frameshifting, Q34 prevented such errors when interacting with a U-ending codon (Urbonavičius et al. 2001). Q34 deficiency of tRNAHis does not influence −1 frameshifting on the sequence U UUC AUA in vivo in E. coli (Masucci et al. 2002), consistent with our observation and that of Brierly et al. (1997) that Q34 does not influence −1 frameshifting. Because in most cases no effect on −1 frameshift that could be attributed to Q was observed and if an effect was observed, it was small, these results show that Q34 has no major role in preventing −1 frameshifting.
Lack of either the mnm5- or the s2-group of mnm5s2U34 increases the frequency of +1 frameshifting for the tRNALysmnm5s2UUU independently if the codons AAA/G are in the A- or in the P-site (Urbonavičius et al. 2001). In contrast, frequency of −1 frameshifting decreased at the A-AAA-AAC site when tRNALysmnm5s2UUU was lacking the mnm5-group, whereas lack of the s2-group did not make any difference (Fig. 2B ▶). Similarly, in E. coli, the s2- or the mnm5-group each has very little or no influence on −1 frameshifting at a U-UUA-AAG site, and the s2-group has no influence on −1 frameshifting at the U-UUA-AAA site (Brierley et al. 1997). However, lack of the mnm5-group increases the −1 frameshifting at the slippery U-UUA-AAA sites twofold (Brierley et al. 1997). Thus, contrary to the large impact of mnm5s2U34 to prevent +1 shifts, its role in −1 frameshifting is minor, and, in fact, in some cases the presence of it increases the −1 frameshift error.
Transfer RNA from all organisms frequently contains modified nucleosides at position 37 (3′ and adjacent to the anticodon). Two such modified nucleosides are ms2io6A37 and m1G37, which both prevent +1 frameshift errors (Björk et al. 1989; Hagervall et al. 1993; Li et al. 1997; Urbonavičius et al. 2001). However, ms2io6A37 did not influence −1 frameshifting (Fig. 3A ▶) at any sequence tested. We found also no effect of m1G37 in yeast tRNALeuU?AA on −1 frameshifts (Fig. 3B ▶). Thus, whereas m1G37 and ms2io6A37 are very important in preventing +1 frameshifts, they apparently have no role in preventing −1 frameshifts.
The highly complex tricyclic modified nucleoside wybutosine (yW) is present at position 37 only in eukaryotic tRNAPheGmAA. Using the rabbit reticulocyte lysate (RRL) in vitro translation system, a threefold increase in −1 frameshifting at the A-AAU-UUU site was attributed to the exchange of yW37 to m1G37 in tRNAPheGmAA, reading UUU in the A-site (Carlson et al. 1999, 2001). However, the level of −1 frameshifting at U-UUU-UUU/C sites, which have codons read by tRNAPheGmAA both in P- and A-sites, was the same using yW or m1G37 containing tRNAPheGmAA. Similarly, we observed in vivo no influence by yW37 on −1 frameshifting at the U-UUU-UUA site (Fig. 3B ▶), which has UUU in the P-site, similarly to the U-UUU-UUU/C sequence. Thus, lack of yW37 of tRNAPheGmAA decoding UUU in the P-site did not influence −1 frameshifting either in vivo or in vitro. Because lack of yW37 of tRNAPheGmAA increased −1 frameshifting at A-AAU-UUU but not at U-UUU-UUU/C (Carlson et al. 1999, 2001), the yW37-mediated effect on tRNAPheGmAA decoding UUU in the A-site may be sensitive to which tRNA is occupying the P-site (peptidyl-tRNALys versus peptidyl-tRNAPhe).
Taken together, whereas modifications such as ms2io6A37 and m1G37 at position 37 have a profound influence on preventing +1 frameshifting, these modifications and yW37 have no or only a minor effect on −1 frameshift errors.
According to our model (Fig. 1 ▶; Urbonavičius et al. 2001; Stahl et al. 2002), +1 frameshifts occur after translocation and by slippage of the P-site tRNA when the A-site is empty. According to the simultaneous slippage model (Jacks et al. 1988), −1 frameshifts occur after the A-site is filled, by simultaneous slippage of the tRNAs present in both A- and P-sites, that is prior to translocation. In general, our results (Table 3 ▶) and those of others (Hagervall et al. 1993; Brierley et al. 1997; Carlson et al. 1999; Marczinke et al. 2000) suggest that tRNA modifications have no or only a minor effect on −1 frameshifts. In fact, in some cases, the presence of the modified nucleoside actually stimulates −1 frameshifting (Fig. 2B ▶) in sharp contrast to earlier observations that the presence of several modifications in tRNA prevents +1 frameshifts (Urbonavičius et al. 2001). These results suggest that −1 and +1 frameshifting occur by distinct mechanisms. It is known that +1 frameshifting is quite sensitive to the rate of recognition of the codon in the empty A-site, for example, because of slow selection of mutant tRNAs (Li et al. 1997; Qian and Björk 1997; Urbonavičius et al. 2001) or low concentration of cognate tRNA (Belcourt and Farabaugh 1990; Farabaugh et al. 1993). Such slow A-site decoding increases the probability of a forward shift of the reading frame. In contrast, a −1 slippage by the peptidyl-tRNA would depend on a movement of the apparently strongly bound deacylated tRNA in the E-site (for a review, see Ramakrishnan 2002), suggesting that such a peptidyl-tRNA slippage is unlikely. According to the simultaneous slippage model, the E-site is empty at the time of slippage. Still, both tRNAs interact with various parts of the ribosome, making the presence of modified nucleoside of minor importance to inhibit a simultaneous slippage in the 5′ direction. Perhaps interactions between the two tRNAs, the mRNA, and the ribosome during −1 simultaneous slippage frameshifting are epistatic to small structural changes in the tRNA, such as those induced by hypomodification. Such considerations could explain our inability to show an effect of hypomodification on −1 frameshifting and why tRNA modification is more important to prevent +1 than −1 frameshift errors. Our results are therefore consistent with the predictions of the simultaneous slippage model (Jacks et al. 1988).
MATERIALS AND METHODS
Plasmids, strains, and growth conditions
Plasmids and strains, which were constructed by standard genetic methods (Davis et al. 1980), are listed in Table 1 ▶. S. enterica serovar Typhimurium strains harboring various plasmids were grown at 37°C in the rich medium NB + AV + ADE, Difco nutrient broth (0.8%; Difco Laboratories) supplemented with the aromatic amino acids (A), aromatic vitamins (V), and adenine (ADE; Davis et al. 1980). Strains of E. coli were grown at 37°C in LB medium (Bertani 1951). Strains of S. cerevisiae were grown at 30°C in SD medium supplemented with appropriate amino acids or uracil (Adams et al. 1997).
Determination of the β-galactosidase, β-lactamase, and luciferase activity
In bacteria, β-galactosidase and β-lactamase activity and the frequency of frameshifting were measured as described earlier (Hagervall et al. 1993; Li et al. 1997). In yeast, β-galactosidase and luciferase activities and the frequency of frameshifting were measured as described previously (Stahl et al. 1995). Statistical variation was calculated using the t test with two tails. A difference in the frequency of frameshifting was considered significant when p < 0.05.
Acknowledgments
This work was supported by grants to G.R.B. from the Swedish Cancer Foundation (project 680) and Science Research Council (project B-BU 2930) and to P.J.F. from the U.S. National Institute of General Medical Sciences (GM-29480). We thank D. Ågren and A. Raman for assistance in some experiments, and T. Hagervall for critical reading of the manuscript.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.rnajournal.org/cgi/doi/10.1261/rna.5210803.
REFERENCES
- Adams, A., Gottschling, D.E., Kaiser, C.A., and Stearns, T. 1997. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York.
- Barak, Z., Lindsley, D., and Gallant, J. 1996. On the mechanism of leftward frameshifting at several hungry codons. J. Mol. Biol. 256: 676–684. [DOI] [PubMed] [Google Scholar]
- Belcourt, M.F. and Farabaugh, P.J. 1990. Ribosomal frameshifting in the yeast retrotransposon Ty: tRNAs induce slippage on a 7 nucleotide minimal site. Cell 62: 339–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani, G. 1951. Studies on lysogenesis. J. Bacteriol. 62: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidou, L., Stahl, G., Hatin, I., Namy, O., Rousset, J.P., and Farabaugh, P.J. 2000. Nonsense-mediated decay mutants do not affect programmed −1 frameshifting. RNA 6: 952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk, G.R., Wikström, P.M., and Byström, A.S. 1989. Prevention of translational frameshifting by the modified nucleoside 1-methylguanosine. Science 244: 986–989. [DOI] [PubMed] [Google Scholar]
- Björk, G.R., Jacobsson, K., Nilsson, K., Johansson, M.J., Byström, A.S., and Persson, O.P. 2001. A primordial tRNA modification required for the evolution of life? EMBO J. 20: 231–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brierley, I., Meredith, M.R., Bloys, A.J., and Hagervall, T.G. 1997. Expression of a coronavirus ribosomal frameshift signal in Escherichia coli: Influence of tRNA anticodon modification on frameshifting. J. Mol. Biol. 270: 360–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson, B.A., Kwon, S.Y., Chamorro, M., Oroszlan, S., Hatfield, D.L., and Lee, B.J. 1999. Transfer RNA modification status influences retroviral ribosomal frameshifting. Virology 255: 2–8. [DOI] [PubMed] [Google Scholar]
- Carlson, B.A., Mushinski, J.F., Henderson, D.W., Kwon, S.Y., Crain, P.F., Lee, B.J., and Hatfield, D.L. 2001. 1-Methylguanosine in place of Y base at position 37 in phenylalanine tRNA is responsible for its shiftiness in retroviral ribosomal frameshifting. Virology 279: 130–135. [DOI] [PubMed] [Google Scholar]
- Davis, W., Botstein, D., and Roth, J.R. 1980. A manual for genetic engineering: Advanced bacterial genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York.
- Dinman, J.D., Icho, T., and Wickner, R.B. 1991. A −1 ribosomal frameshift in a double-stranded RNA virus of yeast forms a gag-pol fusion protein. Proc. Natl. Acad. Sci. 88: 174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans, L. and Grosjean, H. 1987. Enzymatic conversion of guanosine 3′ adjacent to the anticodon of yeast tRNAPhe to N1-methylguanosine and the wye nucleoside: Dependence on the anticodon sequence. EMBO J. 6: 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson, J.U. and Björk, G.R. 1986. Pleiotropic effects induced by modification deficiency next to the anticodon of tRNA from Salmonella typhimurium LT2. J. Bacteriol. 166: 1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esberg, B. and Björk, G.R. 1995. The methylthio group (ms2) of N6-(4-hydroxyisopentenyl)-2-methylthioadenosine (ms2io6A) present next to the anticodon contributes to the decoding efficiency of the tRNA. J. Bacteriol. 177: 1967–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh, P.J. 2000. Translational frameshifting: Implications for the mechanism of translational frame maintenance. Prog. Nucleic Acid Res. Mol. Biol. 64: 131–170. [DOI] [PubMed] [Google Scholar]
- Farabaugh, P.J., Zhao, H., and Vimaladithan, A. 1993. A novel programed frameshift expresses the POL3 gene of retrotransposon Ty3 of yeast: Frameshifting without tRNA slippage. Cell 74: 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, C. and Parker, J. 1994. A ribosomal frameshifting error during translation of the argI mRNA of Escherichia coli. Mol. Gen. Genet. 243: 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean, H., Nicoghosian, K., Haumont, E., Soll, D., and Cedergren, R. 1985. Nucleotide sequences of two serine tRNAs with a GGA anticodon: The structure–function relationships in the serine family of E. coli tRNAs. Nucleic Acids Res. 13: 5697–5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagervall, T.G., Tuohy, T.M., Atkins, J.F., and Björk, G.R. 1993. Deficiency of 1-methylguanosine in tRNA from Salmonella typhimurium induces frameshifting by quadruplet translocation. J. Mol. Biol. 232: 756–765. [DOI] [PubMed] [Google Scholar]
- Horsfield, J.A., Wilson, D.N., Mannering, S.A., Adamski, F.M., and Tate, W.P. 1995. Prokaryotic ribosomes recode the HIV-1 gag-pol-1 frameshift sequence by an E/P site post-translocation simultaneous slippage mechanism. Nucleic Acids Res. 23: 1487–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks, T., Madhani, H.D., Masiarz, F.R., and Varmus, H.E. 1988. Signals for ribosomal frameshifting in the Rous sarcoma virus gag-pol region. Cell 55: 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurland, C.G., Hughes, D., and Ehrenberg, M. 1996. Limitations of translational accuracy. In Escherichia coli and Salmonella: Cellular and molecular biology (eds. F.C. Neidhardt et al.), pp. 979–1004. ASM Press, Washington, DC.
- Li, J., Esberg, B., Curran, J.F., and Björk, G.R. 1997. Three modified nucleosides present in the anticodon stem and loop influence the in vivo aa-tRNA selection in a tRNA-dependent manner. J. Mol. Biol. 271: 209–221. [DOI] [PubMed] [Google Scholar]
- Marczinke, B., Hagervall, T., and Brierley, I. 2000. The Q-base of asparaginyl-tRNA is dispensable for efficient −1 ribosomal frameshifting in eukaryotes. J. Mol. Biol. 295: 179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masucci, J.P., Gallant, J., Lindsley, D., and Atkinson, J. 2002. Influence of the relA gene on ribosomal frameshifting. Mol. Genet. Genomics 268: 81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson, B.C. and Björk, G.R. 1993. Isolation of the gene (miaE) encoding the hydroxylase involved in the synthesis of 2-methylthio-cis-ribozeatin in tRNA of Salmonella typhimurium and characterization of mutants. J. Bacteriol. 175: 7776–7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, Q. 1997. Transfer RNA modification and translational frameshifting. Solfjädern Offset AB, Umeå, Sweden.
- Qian, Q. and Björk, G.R. 1997. Structural alterations far from the anticodon of the tRNAProGGG of Salmonella typhimurium induce +1 frameshifting at the peptidyl-site. J. Mol. Biol. 273: 978–992. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan, V. 2002. Ribosome structure and the mechanism of translation. Cell 108: 557–572. [DOI] [PubMed] [Google Scholar]
- Rozenski, J., Crain, P.F., and McCloskey, J.A. 1999. The RNA Modification Database: 1999 update. Nucleic Acids Res. 27: 196–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl, G., Bidou, L., Rousset, J.P., and Cassan, M. 1995. Versatile vectors to study recoding: Conservation of rules between yeast and mammalian cells. Nucleic Acids Res. 23: 1557–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl, G., McCarty, G.P., and Farabaugh, P.J. 2002. Ribosome structure: Revisiting the connection between translational accuracy and unconventional decoding. Trends Biochem. Sci. 27: 178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, R., and Gallant, J. 1983. Mechanism of ribosome frameshifting during translation of the genetic code. Nature 302: 389–393. [DOI] [PubMed] [Google Scholar]
- Weiss, R.B., and Gallant, J.A. 1986. Frameshift suppression in aminoacyl-tRNA limited cells. Genetics 112: 727–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, R., Dunn, D.M., Atkins, J.F., and Gesteland, R.F. 1987. Slippery runs, shifty stops, backward steps, and forward hops: −2, −1, +1, +2, +5, and +6 ribosomal frameshfifting. Cold Spring Harbor Symp. Quant. Biol. 52: 687–693. [DOI] [PubMed] [Google Scholar]
- Weiss, R.B., Dunn, D.M., Shuh, M., Atkins, J.F., and Gesteland, R.F. 1989. E. coli ribosomes re-phase on retroviral frameshift signals at rates ranging from 2 to 50 percent. New Biol. 1: 159–169. [PubMed] [Google Scholar]
- Weiss, R.B., Dunn, D.M., Atkins, J.F., and Gesteland, R.F. 1990. Ribosomal frameshifting from −2 to +5 nucleotides. Progr. Nucl. Acid Res. Mol. Biol. 39: 159–183. [DOI] [PubMed] [Google Scholar]
- Yelverton, E., Lindsley, D., Yamauchi, P., and Gallant, J.A. 1994. The function of a ribosomal frameshifting signal from human immunodeficiency virus-1 in Escherichia coli. Mol. Microbiol. 11: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]