Abstract
Eukaryotic selenocysteine (Sec) protein insertion machinery was thought to be restricted to animals, but the occurrence of both Sec-containing proteins and the Sec insertion system was recently found in Chlamydomonas reinhardtii, a member of the plant kingdom. Herein, we used RT-PCR to determine the sequence of C. reinhardtii Sec tRNA[Ser]Sec, the first non-animal eukaryotic Sec tRNA[Ser]Sec sequence. Like its animal counterpart, it is 90 nucleotides in length, is aminoacylated with serine by seryl-tRNA synthetase, and decodes specifically UGA. Evolutionary analyses of known Sec tRNAs identify the C. reinhardtii form as the most diverged eukaryotic Sec tRNA[Ser]Sec and reveal a common origin for this tRNA in bacteria, archaea, and eukaryotes.
Keywords: Phylogenetic tree, RT-PCR, selenium, selenocysteyl-tRNA, tRNA sequences
INTRODUCTION
Selenocysteine (Sec) is now recognized as the 21st amino acid in the genetic code. and its codeword is UGA (for review, see Hatfield and Gladyshev 2002). The tRNA for this amino acid, which is initially aminoacylated with serine and the serine moiety is then converted to Sec, is designated Sec tRNA[Ser]Sec. It is the only known tRNA that controls the expression of an entire class of proteins, the selenoproteins. Sec tRNA[Ser]Sec has therefore been described as the key molecule (Böck et al. 1991) or the central component (Hatfield et al. 1994) in selenoprotein biosynthesis. There are as few as one TGA encoding selenoprotein gene in the genome of some eukaryotes, such as Caenorhabditis elegans, to more than 20 in the genomes of other eukaryotes such as vertebrates, including those of humans and other mammals (Hatfield and Gladyshev 2002). Prokaryotes that contain the machinery for incorporating Sec into protein typically have a small number of selenoprotein genes; for example, there are three in Escherichia coli.
The use of Sec as the 21st amino acid in the genetic code is widespread in nature in the sense that it is found in the three life kingdoms, eubacteria, archaea, and eukaryotes. However, the number of organisms within the different life kingdoms that utilize the Sec insertion machinery varies considerably. For example, among the eukaryotic kingdom, yeast and higher plants do not appear to contain the machinery for inserting Sec into protein, whereas animals do utilize this means of incorporating Sec (for review, see Hatfield and Gladyshev 2002). In fact, there is evidence that this phenomenon occurs throughout the animal kingdom (Lee et al. 1990). Recently, Chlamydomonas reinhardtii, a green algae and a member of the plant kingdom, was surprisingly found to encode at least 10 selenoprotein genes in its genome, and the C. reinhardtii tRNA that specifically decodes UGA was detected (Novoselov et al. 2002).
Sec tRNA genes and/or the corresponding gene products have been sequenced from a variety of prokaryotic and eukaryotic organisms (for review, see Hatfield et al. 1999). However, their origin and evolutionary relationship are not clear, as they have very low sequence homology. Transfer RNA[Ser]Sec from E. coli is 95 nucleotides in length (Böck et al. 1991), whereas those from animals including lower and higher vertebrates, insects, and other lower animals are 90 nt in length (for review, see Hatfield et al. 1999). It should be noted that tRNA sequences are 3 nt longer than the corresponding genes, as the CCA terminus is added post-transcriptionally. Because the lower plant, C. reinhardtii, contains Sec tRNA (Novoselov et al. 2002), it is of interest to determine the sequence of this tRNA to assess whether it is more closely related to higher eukaryotes or to prokaryotes, and to determine its mechanism of aminoacylation. In the present study, we employed an RT-PCR technique to sequence C. reinhardtii Sec tRNA, and we found that it is aminoacylated with serine in the presence of C. reinhardtii or mammalian seryl-tRNA synthetases. Comparison of its sequence to those of other known eukaryotic and prokaryotic Sec tRNA[Ser]Sec genes revealed that it is the most divergent eukaryotic Sec tRNA sequenced to date, and that it is more closely related to animal Sec tRNAs.
RESULTS AND DISCUSSION
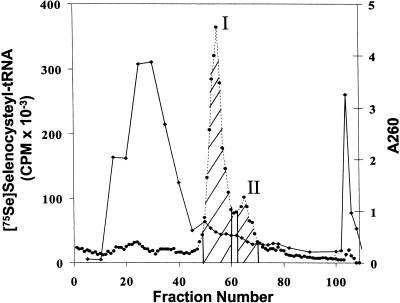
To isolate Sec tRNA for sequencing, C. reinhardtii cells were labeled with 75Se, the tRNA extracted and mixed with tRNA extracted from a larger amount of cells. The mixture was chromatographed on an RPC-5 column (Fig. 1 ▶). Sec-tRNA[Ser]Sec eluted from the column as two separate peaks, a major, initial eluting peak and a trailing smaller peak. Because Sec tRNA[Ser]Sec is more hydrophobic than most tRNAs, the tRNA[Ser]Sec population elutes from reverse-phase chromatographic columns later than the bulk of the tRNA (Lee et al. 1990). The peaks were pooled as shown in the figure, deacylated, and prepared for sequencing as described in Materials and Methods.
FIGURE 1.
Fractionation of C. reinhardtii tRNA. 75Se-selenocysteyl-tRNA[Ser]Sec was obtained by labeling C. reinhardtii cells, extracting tRNA, mixing it with a larger amount of total C. reinhardtii tRNA, chromatographing the mixture over an RPC-5 column, pooling column fractions from the two peaks of 75Se-selenocysteyl-tRNA[Ser]Sec as shown in the figure, and preparing the pooled fractions for sequencing as given in Materials and Methods. (Closed circles) 75Se radioactivity; (closed diamonds) absorbance at 260 nm.
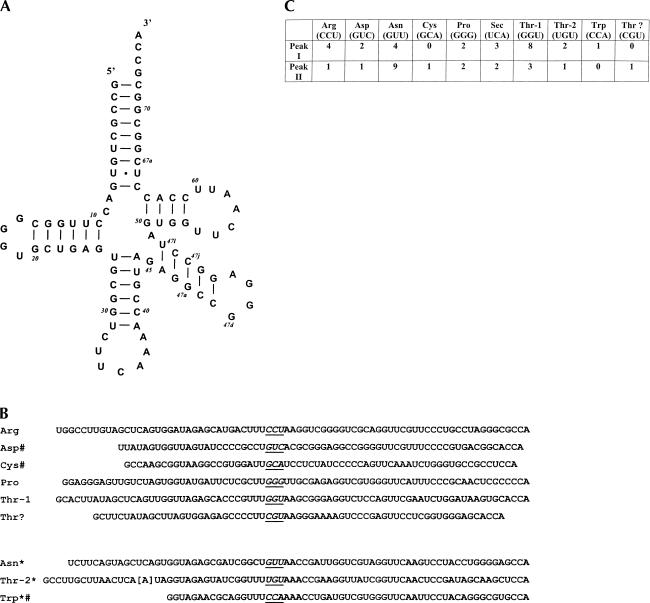
Constructs were generated by RT-PCR from C. reinhardtii tRNAs as described in Materials and Methods. Forty-seven bacterial colonies containing the cloned RT-PCR fragments obtained from each of the two pooled, RPC-5 column fractions (see Fig. 1 ▶) were selected for sequencing. The sequence of Sec tRNA is shown in Figure 2A ▶ in a cloverleaf model as a 9/4 representation (nine paired bases in the acceptor stem and four paired bases in the T stem; see Hubert et al. 1998). Sec tRNA in Peaks I and II from the RPC-5 column fractions (see Fig. 1 ▶) was identical and 90 nt in length, which is the same length of all eukaryotic Sec tRNAs sequenced to date (Hatfield and Gladyshev 2002).
FIGURE 2.
Sequences of C. reinhardtii Sec tRNA and other RNAs. (A) Cloverleaf model of Sec tRNA[Ser]Sec shown in a 9/4 representation (see text). The base at position 58 was determined to be T, but this position is shown as an A for reasons discussed in the text. The GenBank accession number for the nucleotide sequence of Sec tRNA[Ser]Sec is AY268554. (B) Sequences of other tRNAs. The upper six RNAs—Arg, Asp, Cys, Pro, Thr-1, and Thr?—are new sequences; the lower three—Asn, Thr-2, and Trp—which are designated with *, were reported previously (Yu et al. 1992; Maul et al. 2002). The sequence designated Thr? is an unknown RNA, but possibly a Thr tRNA (see text), and the sequences of the three previously reported tRNAs match those shown in the figure with the exception of the bracketed base at position 16 in Thr-2 tRNA (see text). Some of the sequences, indicated by #, are partial. Each tRNA sequence is aligned at the anticodon, which is in italics and underlined. The GenBank accession numbers for the nucleotide sequences of the new RNAs are AY268556 (Arg), AY268558 (Asp), AY268559 (Cys), AY268555 (Pro), AY268557 (Thr-1), and AY268560 (Thr?). (C) Number of times each tRNA clone was sequenced among 47 independently selected bacterial colonies from each fraction (see Fig. 1 ▶).
The base at position 58 was observed to be a T in the sequencing procedure for C. reinhardtii Sec tRNA[Ser]Sec. 1-Methyladenosine (m1A) at this site is known to produce an artifact yielding A during the reverse transcription step (Warner et al. 1998). Because all eukaryotic Sec tRNAs and/or Sec tRNA genes sequenced to date contain A at this position, and all Sec tRNAs except that from Drosophila (Zhou et al. 1999) contain m1A, this position is most likely occupied by m1A. We have therefore indicated an A at position 58. All Sec tRNAs sequenced to date have pseudouridoine (ξ) at position 55 and isopentenyladenosine (i6A) at position 37. Animal Sec tRNAs that have been sequenced have methylcarboxylmethyl-5′-uridine (mcm5U) at position 34, and those of higher vertebrates have two Sec isoforms wherein the second isoform results from methylation of mcm5U to yield methylcarboxymethyl-5′-uridine-2′-O-methylriboside (mcm5Um). We propose that the hydrophobicity of C. reinhardtii Sec tRNA[Ser]Sec is due to i6A at position 37 (see below) and that this tRNA likely has ξ at position 55 and m1A at position 58. It will be of interest to determine the modified bases in the two isoforms of C. reinhardtii Sec tRNA[Ser]Sec that are evident from RPC-5 chromatography (see Fig. 1 ▶), because the two isoforms have identical primary structures.
The sequences of other C. reinhardtii tRNAs were also determined. Arg, Pro, and Thr-1 isoforms were newly identified tRNAs, as were the partial sequences of Asp and Cys tRNAs (Fig. 2B ▶). We tentatively identified another new sequence that has a CGU anticodon as Thr tRNA (designated Thr?). This sequence forms a cloverleaf model, but is short in length for a tRNA and has a small anticodon stem and loop. It may be a partial sequence of a new Thr tRNA, and its complete sequence would result in a standard tRNA cloverleaf model, but further work is required to resolve this possibility. Asn and Thr-2 tRNAs, and the partial sequence of Trp tRNA, which have been reported previously (Yu et al. 1992; Maul et al. 2002), were also detected. The sequence of Asn tRNA and the partial sequence of Trp tRNA matched those previously obtained from the corresponding C. reinhardtii gene sequences. In addition, the sequence of Thr-2 tRNA matched the corresponding C. reinhardtii gene sequence with the exception at position 16 where we found an A (see bracketed base in Fig. 2B ▶), and the gene sequence has a C. Presumably, this mismatch at position 16 is caused by a modified base in Thr-2 tRNA.
The number of times each tRNA was sequenced from the two RPC-5 column fractions is shown in Figure 3B ▶. The more frequently sequenced tRNAs, for example, Asn from Peak II and Thr-1 from Peak I, likely reflect an enrichment of these tRNAs in the respective fractions. Identical sequences were obtained each time that individual tRNAs, including those of the two Sec tRNA[Ser]Sec isoforms, were sequenced, further substantiating the reproducibility of this technique. However, it should be noted that the presence of partial tRNA sequences, which arise during the reverse transcription reaction, is likely due to modified bases, and complete sequences can be obtained by designing primers to complement partial sequences (Kapushoc et al. 2000). Furthermore, on some occasions, mismatches occur at the 5′-nucleotide, resulting in an insertion of an incorrect base (Warner et al. 1998). Because neither a partial sequence nor a mismatch at the 5′-nucleotide affected the sequence of Sec tRNA[Ser]Sec, we did not further pursue the complete resolution of the other RNA structures.
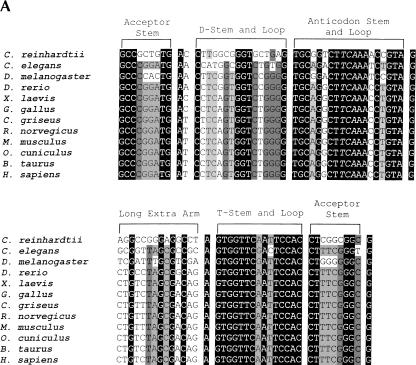
FIGURE 3.
Multiple alignment of known Sec tRNAs and/or Sec tRNA genes. (A) Sequences from eukaryotes. Animal Sec tRNAs[Ser]Sec were aligned with C. reinhardtii Sec tRNA[Ser]Sec based on secondary structure (see cloverleaf model in Fig. 2A ▶). The sources of animal Sec tRNA[Ser]Sec sequences are summarized elsewhere (for review, see Hatfield et al. 1999). (B) Sequences from C. reinhardtii, archaea, and bacteria. Prokaryotic Sec tRNAs were aligned with C. reinhardtii Sec tRNA[Ser]Sec based on secondary structure (see above). Sources of prokaryotic sequences are given in the legend to Figure 4 ▶. The Sec anticodon of each tRNA is shown in italics. Base positions shown with a black background indicate conserved sites, those in an intermediate background, a single evolutionary change, those in a light background, two evolutionary changes, and those not shaded, more than two evolutionary changes.
The sequence of C. reinhardtii Sec tRNA[Ser]Sec was compared to those of Sec tRNA genes sequenced from other eukaryotes, archaea, and bacteria (Fig. 3 ▶). C. reinhardtii Sec tRNA[Ser]Sec has several regions that are homologous to those of other eukaryotes (Fig. 3A ▶), but little homology to those of bacteria or archaea (Fig. 3B ▶). The most highly conserved region of all Sec tRNAs appears to be the anticodon loop. In eukaryotes, the acceptor and anticodon stems and the long extra arm contain several conserved bases. Because several bases, particularly in the acceptor stem and long extra arm, have to do with Sec tRNA[Ser]Sec discrimination by seryl-tRNA synthetase (Wu and Gross 1993; Ohama et al. 1994), many of these bases most certainly play a role in this process. Interestingly, in the 9/4 model, the U:U base pairing in this stem is a highly conserved feature within eukaryotic Sec tRNAs. The intragenic promoter region that occurs at bases 6–18 (see numbering system in the cloverleaf model) is conserved at its 5′ (TGA) and 3′ ends (GG) and manifests some homology with the consensus A box promoter sequence common to all eukaryotic tRNAs (Murphy and Baralle 1983; Traboni et al. 1984).
The T stem and loop are highly conserved among the Sec tRNA[Ser]Sec genes (Fig. 3A ▶). In fact, the T loop is a highly conserved region in many tRNAs, and the bases occupying positions 51 through 63 serve as the internal B box promoter in eukaryotic tRNA (Murphy and Baralle 1983; Traboni et al. 1984) and tRNA[Ser]Sec gene transcription (for review, see Hatfield et al. 1999). The role of the internal A and B box promoter regions in Sec tRNA gene transcription has been investigated by two groups (Myslinski et al. 1993; Park et al. 1995), and this transcription has been reviewed in detail elsewhere (Hatfield et al. 1999). Most certainly, many of the conserved bases in Sec tRNAs play roles in secondary and tertiary structure (Hubert et al. 1998; Kim et al. 2000) and in the interaction with other factors involved in aminoacylation (Wu and Gross 1993; Ohama et al. 1994), Sec biosynthesis (Amberg et al. 1996), and Sec incorporation into protein (Fagegaltier et al. 2000; Tujebajeva et al. 2000).
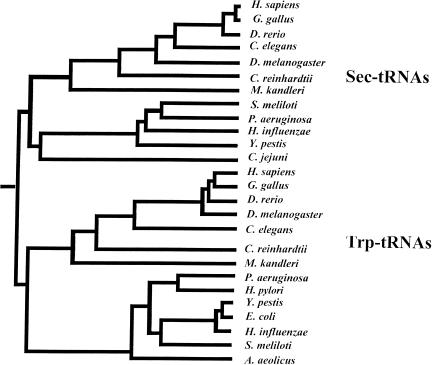
The phylogeny of Sec tRNAs and/or Sec tRNA genes sequenced to date was assessed and compared to that of Trp tRNA, which appears to be related to Sec tRNA in sequence. A phylogenetic tree summarizing these findings is shown in Figure 4 ▶. The tree permits us to trace the origin and evolution of Sec tRNA[Ser]Sec genes. It appears that eukaryotic and bacterial Sec tRNA sequences form a branch on the tree, indicating that they are more closely related to each other than to the more distantly related Trp tRNAs, which form a separate branch in the tree. These data reveal a common origin of known Sec tRNAs and suggest that Sec tRNA evolved prior to separation of the major domains of life, that is, bacteria, archaea, and eukaryotes. C. reinhardtii Sec tRNA[Ser]Sec resided in the eukaryotic part of the Sec tRNA[Ser]Sec branch, but highly diverged from those of animals, suggesting that this tRNA evolved prior to the origin of animals.
FIGURE 4.
Phylogenetic tree of known Sec and Trp tRNAs. Sequences used to generate the tree were extracted from nonredundant and microbial databases at NCBI or were obtained as described in Fig. 3 ▶ and its legend. Nucleotide sequence alignments were generated using the PileUP program. Distances between sequences were calculated from these alignments by the DISTANCE program with the Kimura distance-measuring methods. Finally, unrooted phylogenic trees were visualized by the GrowTree program. The trees were used to trace the evolutionary origin of Sec tRNAs.
It is important to determine whether Sec tRNA is aminoacylated with serine when this tRNA and the Sec insertion machinery are found in a novel organism such as C. reinhardtii, because the possibility always exists that Sec may be biosynthesized independently of a nucleic acid carrier and attached directly to its tRNA by a selenocysteyl-tRNASec synthetase. Here, total C. reinhardtii tRNA was aminoacylated with 3H-serine in the presence of aminoacyl-tRNA synthetases from either C. reinhardtii or mammals and chromatographed separately on an RPC-5 column to determine whether C. reinhardtii Sec tRNA is initially aminoacylated with serine. The majority of the 3H-serine is attached to seryl-tRNA, and the elution profiles of seryl-tRNA aminoacylated in the presence of either C. reinhardtii or mammalian synthetases were virtually the same (data not shown). To detect the position of 3H-seryl-tRNA[Ser]Sec elution, a small portion of each fraction was dot-blotted onto a filter, and the filter was hybridized with 32P-oligonucleotide probe. Hybridization of the filter gave a major 32P-signal and a trailing shoulder that comigrated with minor, late-eluting peaks of 3H-seryl-tRNA (data not shown).
To further confirm the aminoacylation of C. reinhardtii Sec tRNA[Ser]Sec by mammalian synthetases and to determine the amount of this tRNA relative to that of the serine tRNA population, 3H-serine was attached to total tRNA from this organism with rabbit reticulocyte synthetases, and the resulting 3H-seryl-tRNA was chromatographed on a reverse-phase chromatographic column twice, first in the absence of Mg++ and then in the presence of Mg++ (data not shown). We have used this column procedure to cleanly separate 3H-seryl-tRNA[Ser]Sec from 3H-seryl-tRNA (see Kumaraswamy et al. 2003 and references therein). Hybridization of filters dot-blotted with a small portion of each column fraction demonstrated the point of elution of 3H-seryl-tRNA[Ser]Sec. A minor eluting peak of 3H-seryl-tRNA comigrated with the peak of hybridization on the second chromatographic run. This minor peak represented 2.2% of the total 3H-seryl-tRNA fractionated by chromatography. These results demonstrate that C. reinhardtii contains tRNA[Ser]Sec, which is also aminoacylated by mammalian seryl-tRNA synthetase, and that tRNA[Ser]Sec represents only a small proportion of the total seryl-tRNA population.
The studies reported herein used RT-PCR to determine the sequence of the first non-animal, eukaryotic Sec tRNA, which is from a member of the plant kingdom, C. reinhardtii. This tRNA is 90 nt in length and, like all other Sec tRNAs analyzed to date, it is aminoacylated initially with serine and specifically decodes UGA (Novoselov et al. 2002). Thus, Sec in C. reinhardtii is biosynthesized on its tRNA. Comparison of the primary structures of all Sec tRNAs and/or their genes sequenced to date shows that C. reinhardtii Sec tRNA is highly diverged from animal Sec tRNAs. However, phylogenetic analyses firmly placed this tRNA within the eukaryotic Sec tRNA family and established a common origin of Sec tRNA genes in all major domains of life.
MATERIALS AND METHODS
C. reinhardtii, strain cwd/ARG, was obtained as given (Novoselov et al. 2002). 75Selenious acid (190 Ci/mmole) was purchased from the Research Reactor Facility, University of Missouri, Columbia, MO; γ-32P-ATP, 3H-serine (30 Ci/mmole) and Ex taq DNA polymerase PCR beads were from Amersham Biosciences; E. coli polymerase A was from USB; yeast polymerase A, reverse transcriptase (Superscript II), terminal nucleotidyl transferase, T4 polynucleotidyl kinase, DH5α-competent cells, EcoRI and the pCR-2.1-TOPO cloning kit were from Gibco/Invitrogen Corp.; the PCR purification kit, QIAquick spin columns, and miniprep kits were from QIAGEN Inc.; T4 RNA ligase was from Promega Corp. All other reagents and chemicals were of the highest grade available.
Isolation of C. reinhardtii tRNA
C. reinhardtii cells were grown in TAP medium supplemented with 5 mg/L arginine and 5 × 10−7 M sodium selenite at 25°C (Novoselov et al. 2002) and stored at −80°C until ready for use. Total tRNA was isolated from frozen cells (Hatfield et al. 1992; Novoselov et al. 2002) and either stored at −20°C (920 A260 units from ~20 g of cells) or mixed (~250 A260 units from ~5 g of cells) with a similar tRNA extract obtained from 2.5 g of freshly grown cells that were labeled with 5.0 mCi of 75Se (neutralized with KOH; Novoselov et al. 2002). The pooled fractions were chromatographed on an RPC-5 column (Kelmers and Heatherly 1971); the elution of Sec tRNA was monitored by counting column fractions in an Automatic Gamma Counter (Model 1470; Wallac), and the individual 75Se-containing fractions were pooled (see Fig. 1 ▶) and collected as described (Hatfield et al. 1992; Novoselov et al. 2002). The collected fractions were deacylated (Hatfield et al. 1992) and then electrophoresed on 17.5% polyacrylamide TBE-urea gels (20 × 20 cm × 1.5 mm in 2.0-cm-wide wells with 0.5 A260/well). The band that migrated at the same position on developed gels as a purified sample of bovine liver tRNA[Ser]Sec (Diamond et al. 1981) was eluted from gels and prepared for sequencing (see below).
Sequencing of C. reinhardtii tRNA
Partially purified tRNAs obtained after RPC-5 column chromatography and one-dimensional polyacrylamide gel electrophoresis (see above) were polyadenylated according to Sampson and Saks (1996). The resulting polyadenylated tRNAs were denatured at 65°C for 10 min with the primer 5′-TTGAATTCGCATTGAGCAC CTGCTTTTTTTTTTTTTTTTTTGG-3′ and a mixture of dNTPs, cooled on ice, briefly centrifuged, and then transcribed with reverse transcriptase according to the manufacturer’s protocol (Gibco). Unreacted tRNA was digested with RNAse A and RNAse H by incubating at 37°C for 20 min (Kapushoc et al. 2000), and the cDNA was purified on QIAquick spin columns that separated fragments more than 100 bp in length from the other components in the reaction mix.
To achieve the complete sequence of tRNA, its 3′-end was extended by ligation using an anchor-oligonucleotide primer (Edwards et al. 1991; Morse and Bass 1997). The anchor oligonucleotide, 3′-CCGTTAATTGGGAGTGATTTCT-5′, was initially phosphorylated on its 5′-end using T4 polynucleotidyl kinase and ATP, and then blocked on its 3′-end by incubating with ddATP and terminal nucleotidyl transferase according to the manufacturer’s protocol (Gibco). The phosphorylated, blocked anchor-oligonucleotide, 3′-ddACCGTTAATTGGGAGTGATTTCTp-5′, was ligated to the 3′-terminus of cDNA using T4 RNA ligase (Kapushoc et al. 2002), and the resulting cDNA-anchor-oligonucleotide product was purified on a QIAquick spin column to remove protein and unreacted primers. The purified cDNA-anchor-oligonucleotide PCR was amplified with the forward primer 3′-CGTCCACGA GTTACGCTTAAGTT-5′ and reverse primer 5′-GGCAATTAACC CTCACTAAAG-3′ using Ex taq DNA polymerase PCR beads under the following conditions: 4 min at 94°C; 5 cycles of 94°C for 30 sec, 40°C for 1 min, 55°C for 1 min; 30 cycles of 94°C for 30 sec, 50°C for 1 min, 70°C for 1 min; 10 min at 72°C. The primary PCR products of 100–200 bp sizes were purified on 1% agarose gels; secondary PCR was performed under the same thermal conditions, and the resulting products were purified from agarose gels as described (Kapushoc et al. 2002).
Taq polymerase-amplified, secondary PCR products were cloned into the pCR-2.1-TOPO vector using the TOPO TA cloning kit (Invitrogen), and the construct was used to transfect competent E. coli DH5α cells by standard techniques. Forty-seven bacterial clones were isolated that had been generated from each of the two original tRNA fractions from the RPC-column (see Fig. 1 ▶), and the plasmids were isolated from each clone using a Miniprep kit according to the manufacturer’s instructions (QIAGEN). The presence of the insert in the vector was confirmed by digestion with EcoR1, followed by electrophoresis on 1% agarose gels. The plasmid DNA constructs encoding the cloned tRNAs were then sequenced from both ends using M13 forward and reverse primers.
Northern blot analysis
Transfer RNA was dot-blotted onto a nylon membrane, and then the membrane was crossed-linked with UV and hybridized with 5′-end, 32P-labeled TTTCCACCTCGGCGGCGCCA-3′. This oligonucleotide was 5′-end labeled with γ-32P-ATP and T4 polynucleotidyl kinase using the vendor’s protocol.
Preparation of aminoacyl-tRNA synthetases, and aminoacylation and chromatography of tRNA
Aminoacyl-tRNA synthetases were prepared from rabbit reticulocytes or from C. reinhardtii cells as described (Hatfield et al. 1979). C. reinhardtii cells were sonicated at a maximum setting for four 20-sec intervals using a 550 Sonic Dismembrator (Fisher Scientific). The cell debris was removed by centrifugation at 15,000 rpm for 15 min, and the ribosomes were removed by ultracentrifugation at 45,000 rpm for 2 h before applying the extract to a DE-52 column (Hatfield et al. 1979). tRNA was aminoacylated with 3H-serine under limiting tRNA conditions as described (Hatfield et al. 1979), and then chromatographed on an RPC-5 column (Kelmers and Heatherly 1971) in the presence or absence of Mg++ as described (Kumaraswamy et al. 2003 and references therein). Elution of 3H-seryl-tRNA[Ser]Sec was detected by dot-blotting 10 μl of each fraction on a nitrocellulose membrane and hybridizing the UV-cross-linked filter with 32P-labeled oligonucleotide probe as described above.
Acknowledgments
This work was supported in part by U.S. NIH GM061603 and U.S. DOE DE-FG07-02ID14380 to V.N.G.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.rnajournal.org/cgi/doi/10.1261/rna.5510503.
REFERENCES
- Amberg, R., Mizutani, T., Wu, X.Q., and Gross, H.J. 1996. Selenocysteine synthesis in mammalia: an identity switch from tRNA(Ser) to tRNA(Sec). J. Mol. Biol. 263: 8–19. [DOI] [PubMed] [Google Scholar]
- Böck, A., Forchhammer, K., Heider, J., and Baron, C. 1991. Selenoprotein synthesis: an expansion of the genetic code. Trends Biochem. Sci. 16: 463–467. [DOI] [PubMed] [Google Scholar]
- Diamond, A., Dudock, B., and Hatfield, D. 1981. Structure and properties of a bovine liver UGA suppressor serine tRNA with a tryptophan anticodon. Cell 25: 497–506. [DOI] [PubMed] [Google Scholar]
- Edwards, J.B., Delort, J., and Mallet, J. 1991. Oligodeoxyribonucleotide ligation to single-stranded cDNAs: a new tool for cloning 5′ ends of mRNAs and for constructing cDNA libraries by in vitro amplification. Nucleic Acids Res. 19: 5227–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagegaltier, D., Hubert, N., Yamada, K., Mizutani, T., Carbon, P., and Krol, A. 2000. Characterization of mSelB, a novel mammalian elongation factor for selenoprotein translation. EMBO J. 19: 4796–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield, D.L. and Gladyshev, V.N. 2002. How selenium has altered our understanding of the genetic code. Mol. Cell. Biol. 22: 3565–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield, D., Matthews, C., and Rice, M. 1979. Aminoacyl-transfer RNA populations in mammalian cells: Chromatographic profiles and patterns of codon recognition. Biochim. Biophys. Acta 564: 414–423. [DOI] [PubMed] [Google Scholar]
- Hatfield, D., Choi, I.S., Mischke, S., and Owens, L.D. 1992. Selenocysteyl-tRNAs recognize UGA in Beta vulgaris, a higher plant, and in Gliocladium virens, a filamentous fungi. Biochem. Biophys. Res. Comm. 184: 254–259. [DOI] [PubMed] [Google Scholar]
- Hatfield, D., Choi, I.S., Ohama, T., Jung, J.E., and Diamond, A. 1994. Selenocysteine tRNA[Ser]Sec isoacceptors as central components in selenoproteins biosynthesis in eukaryotes. In: Selenium in Biology and Human Health (ed. R.F. Burk), pp. 25–44. Springer-Verlag, New York.
- Hatfield, D., Gladyshev, V., Park, J.M., Park, S.I., Chittum, H.S., Huh, J.R., Carlson, B.A., Kim, K., Moustafa, M.E., and Lee, B.J. 1999. Biosynthesis of selenocysteine and its incorporation into protein as the 21st amino acid. In Comprehensive Natural Products Chemistry (ed. Kelly, J.F.), pp. 353–380. Elsevier, Oxford, UK.
- Hubert, N., Sturchler, C., Westhof, E., Carbon, P., and Krol, A. 1998. The 9/4 secondary structure of eukaryotic selenocysteine tRNA: More pieces of evidence. RNA 4: 1029–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapushoc, S.T., Alfonzo, J.D., Rubio, M.A., and Simpson, L. 2000. End processing precedes mitochondrial importation and editing of tRNAs in Leishmania tarentolae. J. Biol. Chem. 275: 37907–37914. [DOI] [PubMed] [Google Scholar]
- Kapushoc, S.T., Alfonzo, J.D., and Simpson, L. 2002. Differential localization of nuclear-encoded tRNAs between the cytosol and mitochondrion in Leishmania tarentolae. RNA 8: 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelmers, A.D. and Heatherly, D.E. 1971. Columns for rapid chromatographic separation of small amounts of tracer-labeled transfer ribonucleic acids. Anal. Biochem. 44: 486–495. [DOI] [PubMed] [Google Scholar]
- Kim, L.K., Matsufuji, T., Matsufuji, S., Carlson, B.A., Kim, S.S., Hatfield, D.L., and Lee, B.J. 2000. Methylation of the ribosyl moiety at position 34 of selenocysteine tRNA[Ser]Sec is governed by both primary and tertiary structure. RNA 6: 1306–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaraswamy, E., Carlson, B.A., Morgan, F., Miyoshi, K., Robinson, G.W., Su, D., Wang, S., Southon, E., Tessarollo, L., Lee, B.J., et al. 2003. Selective removal of the selenocysteine tRNA[Ser]Sec gene (Trsp) in mouse mammary epithelium. Mol. Cell. Biol. 23: 1477–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, B.J., Rajagopalan, M., Kim, Y.S., You, K.H., Jacobson, K.B., and Hatfield, D. 1990. Selenocysteine tRNA[Ser]Sec gene is ubiquitous within the animal kingdom. Mol. Cell. Biol. 10: 1940–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul, J.E., Lilly, J.W., Cui, L., dePamphilis, C.W., Miller, W., Harris, E.H., and Stern, D.B. 2002. The Chlamydomonas reinhardtii plastid chromosome: Islands of genes in a sea of repeats. Plant Cell 14: 2659–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse, D.P. and Bass, B.L. 1997. Detection of inosine in messenger RNA by inosine-specific cleavage. Biochemistry 36: 8429–8434. [DOI] [PubMed] [Google Scholar]
- Murphy, M.H. and Baralle, F.E. 1983. Directed semisynthetic point mutational analysis of an RNA polymerase III promoter. Nucleic Acids Res. 22: 7695–7700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myslinski, E., Schuster, C., Huet, J., Sentenac, A., Krol, A., and Carbon, P. 1993. Point mutations 5′ to the tRNA selenocysteine TATA box alter RNA polymerase III transcription by affecting the binding of TBP. Nucleic Acids Res. 21: 5852–5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoselov, S.V., Rao, M., Onoshko, N.V., Zhi, H., Kryukov, G.V., Xiang, Y., Weeks, D.P., Hatfield, D.L., and Gladyshev, V.N. 2002. Selenoproteins and selenocysteine insertion system in the model plant cell system, Chlamydomonas reinhardtii. EMBO J. 21: 3681–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohama, T., Yang, D., and Hatfield, D. 1994. Selenocysteine tRNA and serine tRNA are aminoacylated by the same synthetase, but may manifest different identities with respect to the long extra arm. Arch. Biochem. Biophys. 315: 293–301. [DOI] [PubMed] [Google Scholar]
- Park, J.M., Choi, I.S., Kang, S.G., Lee, J.Y., Hatfield, D.L., and Lee, B.J. 1995. Upstream promoter elements are sufficient for selenocysteine tRNA[Ser]Sec gene transcription and determine the transcription start site. Gene 162: 13–19. [DOI] [PubMed] [Google Scholar]
- Sampson, J.R. and Saks, M.E. 1996. Selection of aminoacylated tRNAs from RNA libraries having randomized acceptor stem sequences: Using old dogs to perform new tricks. Methods Enzymol. 267: 384–410. [DOI] [PubMed] [Google Scholar]
- Traboni, C., Ciliberto, G., and Cortese, R. 1984. Mutations in Box B of the promoter of a eucaryotic tRNAPro gene affect rate of transcription, processing, and stability of the transcripts. Cell 36: 179–187. [DOI] [PubMed] [Google Scholar]
- Tujebajeva, R.M., Copeland, P.R., Xu, X.M., Carlson, B.A., Harney, J.W., Driscoll, D.M., Hatfield, D.L., and Berry, M.J. 2000. Decoding apparatus for eukaryotic selenocysteine insertion. EMBO Reports 1: 158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner, G.J., Rusconi, C.P., White, I.E., and Faust, J.R. 1998. Identification and sequencing of two isopentenyladenosine-modified transfer RNAs from Chinese hamster ovary cells. Nucleic Acids Res. 26: 5533–5535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, X.Q. and Gross, H.J. 1993. The long extra arms of human tRNA((Ser)Sec) and tRNA(Ser) function as major identify elements for serylation in an orientation-dependent, but not sequence-specific manner. Nucleic Acids Res. 21: 5589–5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, W., Zhang, D., and Spreitzer, R.J. 1992. Sequences of the Chlamydomonas reinhardtii chloroplast genes encoding tRNA ser and ribosomal protein L20. Plant Physiol. 100: 1079–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, X., Park, S., Moustafa, M.E., Carlson, B.A., Crain, P.F., Diamond, A., Hatfield, D.L., and Lee, B.J. 1999. Selenium metabolism in Drosophila: Characterization of the selenocysteine tRNA population. J. Biol. Chem. 274: 18729–18734. [DOI] [PubMed] [Google Scholar]