Abstract
The RNA subunit of mitochondrial RNase P (mtP-RNA) is encoded by a mitochondrial gene (rnpB) in several ascomycete fungi and in the protists Reclinomonas americana and Nephroselmis olivacea. By searching for universally conserved structural elements, we have identified previously unknown rnpB genes in the mitochondrial DNAs (mtDNAs) of two fission yeasts, Schizosaccharomyces pombe and Schizosaccharomyces octosporus; in the budding yeast Pichia canadensis; and in the archiascomycete Taphrina deformans. The expression of mtP-RNAs of the predicted size was experimentally confirmed in the two fission yeasts, and their precise 5′ and 3′ ends were determined by sequencing of cDNAs generated from circularized mtP-RNAs. Comparative RNA secondary structure modeling shows that in contrast to mtP-RNAs of the two protists R. americana and N. olivacea, those of ascomycete fungi all have highly reduced secondary structures. In certain budding yeasts, such as Saccharomycopsis fibuligera, we find only the two most conserved pairings, P1 and P4. A P18 pairing is conserved in Saccharomyces cerevisiae and its close relatives, whereas nearly half of the minimum bacterial consensus structure is retained in the RNAs of fission yeasts, Aspergillus nidulans and Taphrina deformans. The evolutionary implications of the reduction of mtP-RNA structures in ascomycetes will be discussed.
Keywords: Evolution, phylogenetic modeling, RNA processing, organelle, rnpB, yeasts
INTRODUCTION
RNase P is a ribonucleoprotein (for exceptions, see below) that is universally present in eubacteria, archaebacteria, and eukaryotes, as well as in mitochondria and chloroplasts. It participates in the processing of tRNAs, 4.5 S RNAs, and other small RNAs, by endonucleolytic removal of 5′ leader sequences from RNA precursors (Peck-Miller and Altman 1991).
The RNA subunits of eubacterial RNase P (P-RNA) have been intensely studied and are currently best understood. Escherichia coli P-RNA is essential for the enzymatic activity in vivo and is the only subunit necessary for activity in vitro (Stark et al. 1978; Kole and Altman 1981; Guerrier-Takada et al. 1983). Comparisons of all known P-RNAs have revealed significant primary and secondary structure similarities, indicating that these molecules evolved from a common ancestral RNA. The in vitro activity of P-RNAs in the absence of a protein component has been demonstrated in numerous instances (Guerrier-Takada et al. 1983; Gardiner et al. 1985; Wagner et al. 2001). Nevertheless, the single eubacterial protein component of RNase P (P-protein) is essential for the enzymatic activity in vivo. It has been suggested that the protein participates in formation of an active-site architecture, by interacting with the 5′ leader of tRNAs (Crary et al. 1998), and that it increases the catalytic activity of RNase P by acting as an electrostatic shield between the negatively charged P-RNA and tRNAs (Guerrier-Takada et al. 1983; Gardiner et al. 1985; Christian et al. 2002). The P-protein also broadens the substrate specificity of RNase P, by enhancing its affinity for non-tRNA substrates. For example, 4.5 S RNA and C4 RNA precursors are processed more efficiently by a P-RNA/P-protein complex than by P-RNA alone (Peck-Miller and Altman 1991; Hartmann et al. 1995).
The P-RNA secondary structure model of Archaebacteria is strikingly similar to that of eubacteria, except for the lack of the P13, P14, and P18 helices (Brown and Haas 1995; Brown 1999). Archaeal P-RNAs were long thought to be inactive without their protein partner (see Brown and Haas 1995). However, the P-RNAs of Methanobacteria, Thermococci, and Halobacteria do have catalytic activity under extreme ionic conditions (Pannucci et al. 1999). In fact, deletion mutants show that the structural elements missing in archaeal P-RNAs are also dispensable for in vitro catalysis by the E. coli RNA molecule (Darr et al. 1992; Haas et al. 1994). Although archaebacterial P-RNA structures resemble those of eubacteria, the archaeal holoenzyme is much larger. It has at least four protein subunits that appear to be homologs of the eukaryotic P-proteins (Hall and Brown 2002).
Eukaryotic nuclear P-RNAs have been investigated in detail in ascomycete fungi and in animals. They have no catalytic activity in vitro and, as in archaebacteria, consist of an RNA subunit and several proteins, at least nine in yeast and 10 in human (Gopalan et al. 2002; Guerrier-Takada et al. 2002). A secondary structure model of the eukaryotic P-RNA conforms convincingly to the bacterial consensus structure with only minor deviations (Chen and Pace 1997; Frank et al. 2000). In plants, RNase P has been purified, and a P-RNA component has been suggested based on the sensitivity of the carrot RNase P activity to micrococcal nuclease treatment (Franklin et al. 1995). Although the nuclear RNase P from wheat is resistant to nuclease treatment, its density and its low isoelectric point also indicate the presence of a P-RNA subunit (Arends and Schön 1997). In fact, RNase P from rat liver (Jayanthi and Van Tuyle 1992) and the archaebacterium Sulfolobus solfataricus (Darr et al. 1990) resist micrococcal nuclease treatment but do contain a P-RNA subunit (Altman et al. 1993; Harris et al. 2001). This indicates that the RNA subunit is protected from digestion by P-proteins.
Mitochondria and chloroplasts contain distinct, organelle-specific RNase P activities. The analysis of organellar P-RNAs has been complicated by the patchy occurrence of the rnpB gene, both in chloroplast and mitochondrial DNAs (mtDNAs). The chloroplast-encoded P-RNA of Cyanophora paradoxa folds into a Cyanobacteria-like secondary structure (in agreement with the cyanobacterial origin of chloroplasts) and is essential for RNase P activity (Baum et al. 1996; Pascual and Vioque 1999). Chloroplast DNA (cpDNA)-encoded rnpB genes have been found only in the green alga Nephroselmis olivacea (Turmel et al. 1999), the red algae Porphyra purpurea (Reith and Munholland 1995), and Cyanidium caldarium. We identified the latter, previously unrecognized rnpB gene, at positions 99,959–100,305 of GenBank record AF022186, (Glöckner et al. 2000). All other known cpDNAs do not seem to encode a P-RNA. A recent prediction of a maize chloroplast rnpB gene (Collins et al. 2000) is based on rather weak secondary structure similarities to known homologs, does not consider otherwise highly conserved primary sequence motifs, and has not been confirmed experimentally. Inferences that do not take primary sequence conservation into account are compromised because A+T-rich chloroplast sequences can fit almost any given consensus structure and are, therefore, of little predictive value. Moreover, several biochemical studies indicate that chloroplast RNase P (cpRNase P) may not contain an organelle coded P-RNA. For instance, spinach cpRNase P activity resists micrococcal nuclease treatment and has physical properties consistent with the presence of a protein-only enzyme. These characteristics indicate that spinach cpRNase P is indeed a protein-only enzyme (Thomas et al. 2000).
Mitochondrial RNase P activities have been studied in various yeasts (see below), the ascomycete fungus Aspergillus nidulans (Lee et al. 1996a), human (Doersen et al. 1985; Rossmanith and Karwan 1998; Puranam and Attardi 2001; Rossmanith and Potuschak 2001), Trypanosoma brucei (Salavati et al. 2001), potato (Marchfelder and Brennicke 1994), wheat (Hanic-Joyce and Gray 1990), and carrot (Franklin et al. 1995). The most detailed information on the biochemical and genetic properties of mitochondrial RNase P (mtP-RNA) is available for Saccharomyces cerevisiae. Its RNA subunit was first identified as a mitochondrially encoded molecule by analyzing yeast mitochondrial mutants deficient in mitochondrial tRNA processing and protein synthesis (Miller and Martin 1983; Underbrink-Lyon et al. 1983). The unusually large protein subunit has been shown to be nucleus-encoded (Morales et al. 1992; Dang and Martin 1993).
Additional rnpB genes have been identified by sequence similarity in mtDNAs of numerous budding yeasts (T. glabrata, Clark-Walker et al. 1985; Saccharomycopsis fibuligera, Wise and Martin 1991a; Kluyveromyces lactis, Wilson et al. 1989; Saccharomyces exiguus, Wise and Martin 1991b; Saccharomyces douglasii; Ragnini et al. 1991; Saccharomyces chevalieri, Saccharomyces ellipsoideous, and Saccharomyces diastaticus, Sbisa et al. 1996; and Saccharomyces castellii, Petersen et al. 2002), the protist Reclinomonas americana (Lang et al. 1997), and the prasinophyte green alga N. olivacea (Turmel et al. 1999), but not in the mtDNAs of plants, animals, a great number of protists, or nonascomycete fungi (Lang et al. 1999). From an evolutionary standpoint, it is puzzling that the occurrence of mitochondrially encoded rnpB genes is so patchy. Have these genes been lost from the mtDNAs of plants, animals, most fungi, and protists, or do we fail to identify them because they are so extremely derived? In fact, highly derived, extremely A+U-rich mtP-RNAs are characteristic in yeast. Consequently, predictions of yeast mtP-RNA secondary structures are difficult, despite the availability of comparative data. In addition, the drastic size variations of these RNA molecules obscure the identification of RNA secondary structure elements (Wise and Martin 1991a). For example, the respective lengths of predicted mtP-RNAs are 423 nt for S. cerevisiae (Stribinskis et al. 1996), 227 nt for Torulopsis glabrata (Shu et al. 1991), and as short as 140 nt for Saccharomycopsis fibuligera (Wise and Martin 1991a).
The mitochondrially encoded mtP-RNA of the protist Reclinomonas americana was the first identified to contain all structural elements defined in the eubacterial P-RNA consensus structure (Lang et al. 1997). Its features served as a hallmark for reanalyzing the mtP-RNA secondary structure of A. nidulans. This analysis reveals a secondary structure that is substantially more similar to both the Reclinomonas mitochondrial and to the eubacterial consensus (Martin and Lang 1997) than to the previously published model (Lee et al. 1996b). In this article, we present the results of our ongoing efforts to identify ascomycete rnpB genes, and to infer their RNA secondary structures by comparative phylogenetic modeling. We provide evidence that the mitochondrial rnpB genes have been lost several times independently in fungi.
RESULTS
Search for ascomycete mitochondrial rnpB genes
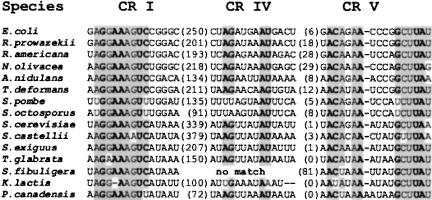
To identify previously unrecognized, possibly highly derived rnpB genes in fungal mtDNAs, we used two equally productive in silico procedures. The more straightforward of the two was based on sequence pattern recognition (PERL regular expression matching), including the most conserved rnpB sequence motifs CRI and CRV (GGAAAGTC...50–500 nt...ACANAANNNNGCTTAT; Fig. 1 ▶; Chen and Pace 1997). Potential deviations from the consensus sequence were explored by gradually changing the primary sequence motifs, and subsequently, all matches were validated by folding of the highly conserved P4 helix (Fig. 2A,B ▶). Only those candidate rnpB sequences that fall into a noncoding region of a given mtDNA were further investigated. The second approach used the dedicated pattern matching tool, RNAMOT (Laferriére et al. 1994), which searches for both sequence similarity and RNA secondary structure folding. Because this program does not allow errors, possible deviations from the consensus sequence were also explored by systematic changes of the sequence descriptor. All publicly available fungal mtDNAs were analyzed accordingly.
FIGURE 1.
Alignment of the universally conserved regions (CRs) I, IV, and V. CRs of the universally conserved nucleotides are in bold, following the analysis by Chen and Pace 1997. Shaded nucleotides are conserved at >75%, in E. coli, R. prowazekii, and mitochondrial P-RNAs. The numbers between brackets indicate the number of nucleotides that separate the conserved regions.
FIGURE 2.
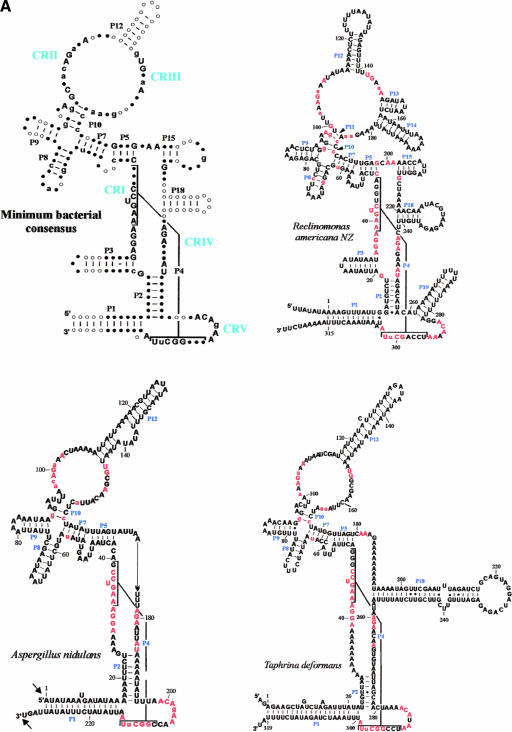
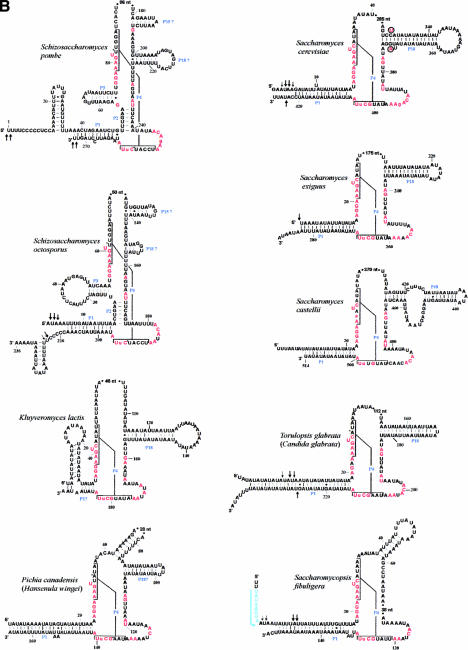
Minimum bacterial P-RNA consensus and mtP-RNA secondary structure models of R. americana and ascomycete fungi. (A) Positions in red are invariant in the minimum bacterial consensus; uppercase letters in the mtP-RNAs indicate 100%, lowercase letters at least 90%, conservation of the minimum bacterial consensus sequence. The conserved motifs CRI through CRV are indicated in blue. The arrows indicate experimentally determined RNA extremities; arrow thickness is proportional to the percentage of molecules ending at this position. (B) mtP-RNA secondary structures from nine additional ascomycetes. In the S. cerevisiae structure, nucleotides that co-vary with the Saccharomyces douglasii sequence are in red circled letters. A putative promoter in S. fibuligera is highlighted in blue. Asterisks next to nucleotides in the P4 of S. pombe, S. octosporus, C. glabrata, and P. canadensis indicate mispairs in these helices. Note that the Candida structure P1 helix can be further extended by 9 to 12 bp from its mature ends. It is possible that the noncanonical A–G pair adjacent to the RNA processing sites in this mtP-RNA serves as a signal for RNA maturation.
Significant CRI and CRV matches turned up for the three known rnpB genes in S. cerevisiae (Miller and Martin 1983), S. castellii (Petersen et al. 2002), and A. nidulans (Lee et al. 1996a), and four new candidates emerged in Pichia canadensis (Sekito et al. 1995), Schizosaccharomyces pombe (Lang et al. 1983), Schizosaccharomyces octosporus (Bullerwell et al. 2003b), and Taphrina deformans, a fungus classified within Archiascomycota (or Taphrinomycetales, according to the National Center for Biotechnology Information [NCBI] nomenclature). Sequence alignments are shown in Figure 1 ▶. However, we failed to detect mitochondrial rnpB genes in six other ascomycetes and in all basidiomycete and chytridiomycete fungi for which complete, or near-complete, mtDNA sequences are available (Table 1 ▶), even at search pattern stringency close to background levels.
TABLE 1.
Occurrence and features of rnpB in complete fungal mtDNA sequences
| Organismal group | mtDNA-encoded rnpBa | P2 | P3 | P15 | P18 | P5,7,8,9,10,12 | Accession # |
| Ascomycota (Euascomycota) | |||||||
| Aspergillus nidulans | ▪ | ▪ | □ | □ | □ | ▪ | b |
| Hypocrea jecorina | □ | NC_003388 | |||||
| Neurospora crassa | □ | c | |||||
| Podospora anserina | □ | NC_001320 | |||||
| Verticillium lecanii | □ | AF487277 | |||||
| Ascomycota (Hemiascomycota) | |||||||
| Candida albicans | □ | NC_002653 | |||||
| Candida glabrata | ▪ | □ | □ | □ | ▪ | □ | AJ511533 |
| Pichia canadensis | ▪ | □ | □ | □ | ▪ | □ | NC_001762 |
| Saccharomyces castellii | ▪ | □ | □ | □ | ▪ | □ | AF437291 |
| Saccharomyces cerevisiae | ▪ | □ | □ | □ | ▪ | □ | NC_001224 |
| Yarrowia lipolytica | □ | NC_002659 | |||||
| Ascomycota (others) | |||||||
| Schizosaccharomyces japonicus | □ | NC_004332 | |||||
| Schizosaccharomyces octosporus | ▪ | ▪ | ▪ | ▪ | ▪ | □ | AF275271 |
| Schizosaccharomyces pombe | ▪ | ▪ | ▪ | ▪ | ▪ | □ | NC_001326 |
| Taphrina deformans | ▪ | ▪ | □ | □ | ▪ | ▪ | AY262107d |
| Basidiomycotae | □ | ||||||
| Chytridiomycotae | □ | ||||||
Filled and empty squares stand for presence or absence of the gene or helices, respectively.
aAll mtP-RNAs contain P1 and P4.
bThe A. nidulans sequence is almost complete: ODAS1, CAA33481, AAA99207, AAA31737, CAA25707, AAA31736, CAA23994, X15442, P15956, CAA23995, CAA33116, X00790, X15441, X06960, J01387, and X01507.
cCompleted at Whitehead Institute, currently available at http://pages.slu.edu/faculty/kennellj/genbank.html.
dThe GenBank record documents the rnpB sequence, the complete sequence is unpublished.
eMitochondrial rnpB genes have not been identified in basidiomycete (Cantharellus cibarius, unpubl.; Cryptococcus neoformans, NC00_4336; Microbotryum violaceum, unpubl.; Schizophyllum commune, NC_003049) or chytridiomycete fungi (Allomyces macrogynus, NC_001715; Harpochytrium94, AY182005; Harpochytrium105, AY182006; Hyaloraphidium curvatum, NC_003048).
Modeling of fungal mtP-RNA secondary structures
Fungal mitochondrial rnpB genes, including those newly identified, vary considerably in size and primary sequence. In addition, most are exceedingly rich in A and T, which makes RNA modeling an intricate task. It requires substantial comparative sequence information to determine a biologically meaningful solution among the many alternative structure predictions. To deal with the dilemma that in the absence of primary sequence conservation, A+T-rich sequences can be fitted to any given consensus structure, we have based our predictions on three criteria, namely, similarity to (1) the bacterial minimum consensus structure, (2) the universally conserved motifs in bacterial P-RNAs, and (3) the secondary structure models of R. americana and A. nidulans mtP-RNA.
From studies of eubacterial P-RNAs, it is known that the P1 and P4 helices are the functionally most important regions of this RNA molecule (i.e., most sensitive to mutations; see the bacterial consensus structure in Fig. 2A ▶; Schlegel et al. 1994). This is reflected by the presence of the highly conserved mitochondrial CRI and CRV motifs in all mitochondrial rnpB sequences (Fig. 1 ▶), which together fold into a P4 helix. In addition, here we demonstrate for the first time that P1 is also conserved in all known mtP-RNAs (Fig. 2A,B ▶), with the possible exception of K. lactis, as discussed below. However, several other helical regions that characterize the bacterial consensus structure are lost in mtP-RNAs, as outlined below.
Budding yeast mtP-RNAs
To define a consensus RNA secondary structure model for budding yeast mtP-RNAs, we performed a comparative analysis of all available sequences, except those from S. chevalieri, S. douglasii, S. ellipsoideous, and S. diastaticus, because they are almost identical to the S. cerevisiae sequence.
In addition to the CRI and CRV motifs, we found a potential, previously unrecognized CRIV motif (AGNNNNAU; Figs. 1 ▶, 2A ▶). Identification of this motif was based on the assumption that a P2 helix would be absent in all yeast mtP-RNAs, such that it would be positioned directly adjacent to the CRV motif. By using these criteria, CRIV motifs were found in all but the S. fibuligera and K. lactis rnpB sequences (Figs. 1 ▶, 2B ▶). In addition, in K. lactis the P1 helix is formed by only few base pairs (labeled “P1?” in Fig. 2B ▶), the CRI motif has a deletion of a universally conserved A, and CRV has a U in the third position that is otherwise a C (Fig. 1 ▶). We confirmed the unconventional deletion of an A in the K. lactis CRI motif, by resequencing the clone containing the rnpB gene. Although a transcript of the expected size is found in K. lactis mitochondria (Wilson et al. 1989), the high incidence of noncanonical features raises the question whether or not rnpB codes for a functional mtP-RNA.
Further comparative analysis revealed another conserved base pairing, P18 (Fig. 2A ▶), in the five phylogenetically closely related organisms S. cerevisiae, S. castellii, T. glabrata, S. exiguus, and K. lactis (Fig. 2B ▶). These helical regions start at the same position as P18 of R. americana and as P18 in the bacterial minimum consensus model, 2 nt upstream of the CRIV motif (Fig. 1B ▶). Additional support for this P18 helix comes from a compensatory exchange of a base pair (G–C to A–U) between the S. cerevisiae and S. douglasii mtP-RNAs (see the circled nucleotides of the S. cerevisiae structure in Fig. 2B ▶). In P. canadensis, a P18 pairing is possible (labeled “P18?” in Fig. 2B ▶); however, it is not located at the same position relative to the CRIV motif. Finally, we did not detect a CRIV motif or a P18 pairing in the S. fibuligera mtP-RNA.
Fission yeasts
The S. pombe rnpB gene was only identified recently, although the complete sequence of mtDNA has been available for more than a decade. Differences in both the CRI and CRV motifs, relative to the consensus (Fig. 1 ▶), made it difficult to recognize this gene. After identifying potential CRI, CRIV, and CRV motifs in an intergenic region of the S. pombe mtDNA, we confirmed the presence of an rnpB homolog in S. octosporus at the same location in the mtDNA, flanked by the same tRNA genes, trnA and trnG.
The comparative structural modeling of the two Schizosaccharomyces sequences reveals not only the presence of P1 and P4, as in budding yeasts, but also P2 and P3 pairings that distinguish them from most other fungal RNA structures (Fig. 2B ▶). Moreover, we identified potential P15 and P18 pairings. However, only the S. pombe P18 is located at the same distance from CRIV as in other species, and the potential S. octosporus P18 helix would be only 3 bp long (Fig. 2B ▶). The central sequence of rnpB contains three motifs that are identical in the fission yeasts (4, 6, and 9 nt long; Fig. 2B ▶), but without similarity to the bacterial consensus.
T. deformans has been grouped together with Schizosaccharomyces species in phylogenetic analyses (termed archiascomyetes, Nishida and Sugiyama 1993; for an alternative view, see Bullerwell et al. 2003b; Leigh et al. 2003), but the mtP-RNA secondary structure of T. deformans exhibits striking similarity only to the euascomyete A. nidulans (Fig. 2B ▶). The only principal structural difference is the extra P18 pairing in T. deformans.
Mapping of mtP-RNAs
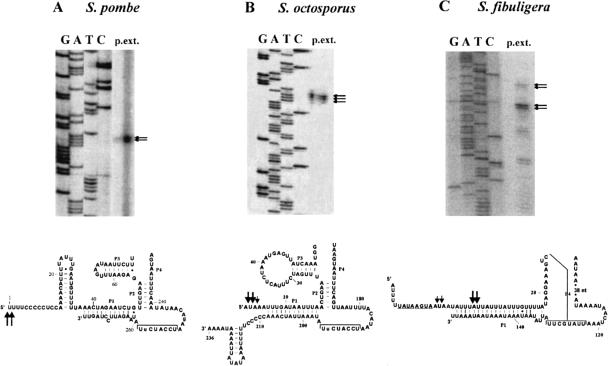
As indicated in the bacterial consensus structure (Fig. 2A ▶), the 5′ and 3′ extremities of most mapped P-RNA molecules are directly adjacent, or at least close to, the P1 helix of P-RNAs. We show here by primer extension that the putative S. fibuligera mtP-RNA is processed at its 5′ end to an RNA molecule with a perfect terminal P1 helix (Figs. 2B ▶, 3 ▶). In S. pombe and S. octosporus, the 5′ ends were initially mapped by primer extension experiments (Fig. 3 ▶) and confirmed by RT-PCR of the circularized RNAs and sequencing of the PCR product. The sequencing results confirmed the primer extension results shown in Figure 2B ▶ and revealed that there is only a marginal heterogeneity of mtP-RNA extremities.
FIGURE 3.
RNA mapping of S. pombe, S. octosporus, and S. fibuligera mtP-RNAs. (A) S. pombe primer extension experiment. The bottom part of the figure shows the partial, corresponding region of the mtP-RNA structure. The main bands are consistent with sequencing of RT-PCR product of in vitro circularized P-RNA. The thickness of the arrows is proportional to the proportion of RNA extremities of the RT-PCR experiment. (B) S. octosporus primer extension experiment. The main bands are consistent with sequencing of RT-PCR product of in vitro circularized P-RNA. The thickness of the arrows is proportional to the proportion of RNA extremities of the RT-PCR experiment. (C) Primer extension for S. fibuligera mtP-RNA. The weak upper signal matches the transcription initiation site at the potential promoter sequence (underlined). The strongest signal corresponds to the mature end of the mtP-RNA.
Results further show that the 3′ end of S. pombe and the 5′ end of S. octosporus mtP-RNAs correspond well to the predicted RNA structure model (Fig. 2B ▶). The 5′ end of S. pombe and the 3′ end of S. octosporus are somewhat longer than expected, at sequence motifs rich in cytidines and uridines. Similar motifs are present at most 3′ ends of mitochondrial protein-coding genes in S. pombe, S. octosporus (Bullerwell et al. 2003b), and several zygomycete and basidiomycete mitochondrial genomes (B.F. Lang, unpubl.). These sequence motifs may be RNA processing signals (Lang et al. 1983; Trinkl et al. 1989), and protect the RNA extremities against further exonuclease digestion.
DISCUSSION
Highly reduced mtP-RNA structures in fungi
We report here the identification of four previously undetected mitochondrial rnpB genes in ascomycetes. Comparative RNA secondary structure modeling of previously and newly discovered sequences allowed us to establish a minimum consensus secondary structure for fungal mtP-RNAs (for a complete collection of mtP-RNA structures, see http://megasun.bch.umontreal.ca/People/lang/rnpB/). The fungal mitochondrial consensus includes only two out of the five conserved motifs (CRI and CRV) and two out of 11 conserved helices (P1 and P4; Fig. 2A ▶) found in the minimum bacterial consensus structure. The presence of additional conserved structural elements appears to tightly correlate with the phylogenetic relatedness. For example, P2, P3, P15, and P18 are found in the two Schizosaccharomyces species, but only P18 is found in most (but not all) budding yeasts (Table 1 ▶). Accordingly, the occurrence of P2, P5, P7–10, and P12 in both T. deformans and A. nidulans possibly indicates that T. deformans is an ancestral fungus that branches at the base of the ascomycetes. Although it has been proposed to be related to Schizosaccharomyces species based on rRNA phylogenies (Nishida and Sugiyama 1993), it does not appear to be related based on either mtP-RNA structure or from mitochondrial protein sequences (Leigh et al. 2003).
The reduction of the structural complexity of mtP-RNAs is more extensive in fungal mitochondria than in nuclear (Frank et al. 2000) and archaeal P-RNAs (Harris et al. 2001). It is likely compensated by increase in size and/or number of P-proteins (e.g., the large size of the mitochondrial P-protein in S. cerevisiae; Morales et al. 1992; Dang and Martin 1993) and the possibility of multiple mitochondrial P-proteins in A. nidulans (Lee et al. 1996b). An investigation of fungal mitochondrial P-proteins and of native mtP-RNA enzymes could be useful in shedding light on the principles related to the replacement of RNA by protein structure. Despite the substantial loss of structural complexity, the fungal mtP-RNAs are likely to be the catalytic component of RNase P. Deletion analysis shows that bacterial P1 and P4 elements are intimately involved in catalytic function (Guerrier-Takada and Altman 1992; Schlegl et al. 1994). This hypothesis is also supported by genetic and biochemical studies in S. cerevisiae and A. nidulans, showing that the RNA subunit is essential for mtP-RNA activity (Miller and Martin 1983; Underbrink-Lyon et al. 1983; Lee et al. 1996a).
Processing of fungal mtP-RNAs
The processing of precursor molecules of structural RNAs usually depends on highly specific biochemical activities that recognize RNA structure. This prompted the question whether this also applies to mtP-RNAs. The RNA mapping data presented here indicate two types of mtP-RNA processing. First, in S. pombe, S. octosporus, and several budding yeasts (including S. cerevisiae and C. glabrata; Shu and Martin 1991), rnpB genes are flanked by tRNA genes. The removal of tRNA sequences from the RNA precursor directly liberates mature mtP-RNA molecules in some instances (e.g., as shown here in S. pombe, Fig. 2B ▶; and as predicted in P. canadensis, S. exiguus, and K. lactis from the positioning of the postulated processing sites). It follows the tRNA “punctuation model” first described as the key principle of human mitochondrial RNA processing (Ojala et al. 1980), as well as in S. pombe and a few other fungi (Paquin et al. 1997). Note that RNA processing by tRNA punctuation is not a general principle in budding yeast mitochondria, although applied in maturation of the mtP-RNA. In fact, there is a high overall incidence of mitochondrial rnpB genes that are flanked on both sides, or at least one side (and then most frequently at the 3′ end), by tRNA genes. This implies that in numerous instances, RNase P itself has a defining role in 3′ processing of its RNA subunit. Second, in some cases, 3′ and 5′ end processing occurs subsequent to tRNA removal. For example, processing of 3′ ends of transcripts containing cytidine-uridine–rich motifs occurs in S. octosporus (Fig. 2B ▶). The primary transcript in S. cerevisiae includes tRNAMetf, mt-PRNA (also called Rpm1r), and tRNAPro. The tRNAs are first separated from the mtP-RNA precursor, and secondary maturation occurs at both P-RNA ends (Stribinskis et al. 1996), with the possible involvement of the mitochondrial P-protein (Stribinskis et al. 2001). The rnpB gene of S. fibuligera is flanked only downstream by tRNAPro, but upstream by a protein coding gene (cox2). The sequence immediately upstream of rnpB contains a conserved octanucleotide promoter motif (TATAAGTA; Fig. 2 ▶) that serves as a promoter in S. cerevisiae. Our primer extension analysis in S. fibuligera indicates that rnpB is indeed transcribed from this putative promoter (Fig. 3 ▶), so that starting transcription close to the 5′ end is yet another potential mechanism leading to the definition of mtP-RNA extremities. The same mechanism potentially applies to S. cerevisiae, as an alternative pathway to maturation by tRNA punctuation (Biswas 1996). Finally, in A. nidulans and T. deformans none of the previously discussed mechanisms seem to apply.
Mitochondrial rnpB genes in other fungi, plants, or protists?
We failed to detect mitochondrial rnpB genes in 18 out of 26 complete fungal mtDNAs. These negative matches included all studied basidiomycete and chytridiomycete fungi, four euascomycete fungi, two budding yeasts, and one fission yeast (Table 1 ▶). In these species, a mitochondrial rnpB either migrated to the nucleus, or had its function replaced by a nuclear encoded gene, or, less likely, diverged to a degree that it is no longer recognizable. There is evidence to indicate that rnpB is indeed absent from most of the above-listed mtDNAs. In the chytridiomycete fungi Harpochytrium94 and Harpochytrium105 (Bullerwell et al. 2003a), intragenic regions are either too short or filled with repeats so there is no space to fit an rnpB gene. The chytridiomycte fungus, A. macrogynus, has the most ancestral (underived) mtDNA sequence among all known fungi, with respect to both gene content and gene sequence similarity. Therefore, although one might expect that A. macrogynus would have an easily identifiable, eubacterial-like mitochondrial rnpB, we were unable to locate even a highly derived version of this gene. S. pombe and S. octosporus mtDNAs contain rnpB sequences that are highly similar and easily identified by sequence comparisons. Yet, there is no significant match with a third fission yeast mtDNA, that of S. japonicus. P. anserina, H. jecorina, and Verticillium lecanii are closely related to A. nidulans. A. nidulans is the only one of the four that has a eubacteria-like rnpB sequence. Otherwise, their mitochondrial gene content is identical, which indicates that none of these four species has undergone accelerated evolution. Evidently, sequences from more deeply diverging members of the euascomycetes need to be investigated to support the idea that loss of mitochondrial rnpB is typical for this fungal group.
This widespread loss raises the question of how the RNase P function is provided in the absence of a mitochondrial encoded rnpB gene. One possibility is that a protein-only enzyme, coded by nuclear gene(s) and imported into the organelle, is responsible for 5′ processing of precursor tRNAs, a situation postulated for spinach chloroplast (Thomas et al. 2000). An alternate possibility is that an RNA is required for catalysis but that it is encoded in the nucleus and imported into mitochondria to function. Biochemical characterization of the RNase P activity isolated from mitochondria lacking an endogenous rnpB will be necessary to differentiate between these possibilities. Such studies have been undertaken for human mtP-RNA, but there is an unresolved debate whether or not the absence of mitochondrially encoded rnpB is compensated by the nuclear P-RNA, or by a protein-only enzyme (Rossmanith and Karwan 1998; Puranam and Attardi 2001; Rossmanith and Potuschak 2001).
MATERIALS AND METHODS
DNA and RNA extraction, PCR, and sequencing
S. pombe strain ade7-50h− (kindly provided by U. Leupold, University of Bern, Switzerland) S. octosporus (ATCC 2479), and T. deformans (NRRL T-857) were grown in 1% yeast extract plus 3% glycerol liquid medium. For mitochondrial DNA and RNA extractions, cells were broken mechanically, and a mitochondrial fraction was isolated by differential centrifugation (Lang et al. 1977). This fraction was lysed in the presence of 1% SDS and 100 μg/mL proteinase K for 1 h at 37°C, and after phenol-chloroform extraction, the nucleic acids were precipitated with ethanol. For RNA extractions of Schizosaccharomyces strains, the cell walls were digested with lytic enzymes from Trichoderma harzianum (Sigma L-2265), and mitochondria were purified by differential centrifugation. The mitochondrial fraction was solubilized in the presence of 1% SDS and 100 μg/mL proteinase K for 1 h at 37°C. After a phenol-chloroform extraction, the high-molecular-weight RNA fraction was precipitated with 2M LiCl, redissolved in RNase-free water, and ethanol-precipitated.
Primer extension
Primer extensions were performed with primers 5′-CCCTCT TGGGTTTCTTTTTTA-3′ (S. pombe) and 5′-GATGGATTATG TAAAATTAACTG-3′ (S. octosporus). The primers were labeled at the 5′ end with γ-P32 ATP and T4 polynucleotide kinase (Boehringer). The mitochondrial RNA was incubated for 30 min at 37°C in the presence of the respective primers, dNTPs, AMV buffer (Boehringer), and AMV reverse transcriptase (Boehringer 1495062). The resulting product was ethanol-precipitated and loaded on a high resolution sequencing gel (see above). The DNA sequencing ladder that served as a size marker was produced with the same primer from a DNA template containing the rnpB region from the organism being examined.
RNA ligation and RT-PCR
RNA ligation of mtP-RNAs, followed by RT-PCR amplification, was performed to determine precise 5′ and 3′ end(s). The protocol was essentially the same used to circularize tRNAs (Yokobori and Pääbo 1995), except that ~10 μg of RNA was used as starting material. The primers used for RT-PCR were 5′-CCCTCTTG GGTTTCTTTTTTA-3′ and 5′-TTTTAGTAATTTCAAATATAA CAG-3′ for S. pombe, and 5′-TCCAAACTTTCCATTTGATAAC3′ and 5′-CAGTTAATTTTACATAATCCATC-3′ for S. octosporus.
Acknowledgments
We thank G. Burger and C. Bullerwell for comments on the manuscript, Wang Zhang for participation in DNA sequencing, Marlene Steffen for technical assistance, and H. Fukuhara (Orsay, France) for sharing information on K. lactis rnpB and providing a clone carrying its sequence. This project was supported by operating grants from the Canadian Institute for Health Research (CIHR) to B.F.L. and the National Institutes of Health (NCM). Salary and interaction support (to B.F.L.) from the Canadian Institute for Advanced Research is gratefully acknowledged. The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
REFERENCES
- Altman, S., Wesolowski, D., and Puranam, P.S. 1993. Nucleotide sequences of the RNA subunit of RNase P from several mammals. Genomics 18: 418–422. [DOI] [PubMed] [Google Scholar]
- Arends, S. and Schön, A. 1997. Partial purification and characterization of nuclear ribonuclease P from wheat. Eur. J. Biochem. 244: 635–645. [DOI] [PubMed] [Google Scholar]
- Baum, M., Cordier, A., and Schön, A. 1996. RNase P from a photosynthetic organelle contains an RNA homologous to the cyanobacterial counterpart. J. Mol. Biol. 257: 43–52. [DOI] [PubMed] [Google Scholar]
- Biswas, T.K. 1996. Expression of the mitochondrial RNase P RNA subunit-encoding gene from a variant promoter sequence in Saccharomyces cerevisiae. Gene 170: 23–30. [DOI] [PubMed] [Google Scholar]
- Brown, J.W. 1999. The Ribonuclease P Database. Nucleic Acids Res. 27: 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, J.W. and Haas, E.S. 1995. Ribonuclease P structure and function in Archaea. Mol. Biol. Rep. 22: 131–134. [DOI] [PubMed] [Google Scholar]
- Bullerwell, C.E., Forget, L., and Lang, B.F. 2003a. Evolution of monoblepharidalean fungi based on complete mitochondrial genome sequences. Nucleic Acids Res. 31: 1614–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullerwell, C.E., Leigh, J., Forget, L., and Lang, B.F. 2003b. A comparison of three fission yeast mitochondrial genomes. Nucleic Acids Res. 31: 759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J.L. and Pace, N.R. 1997. Identification of the universally conserved core of ribonuclease P RNA. RNA 3: 557–560. [PMC free article] [PubMed] [Google Scholar]
- Christian, E.L., Zahler, N.H., Kaye, N.M., and Harris, M.E. 2002. Analysis of substrate recognition by the ribonucleoprotein endonuclease RNase P. Methods 28: 307–322. [DOI] [PubMed] [Google Scholar]
- Clark-Walker, G.D., McArthur, C.R., and Sriprakash, K.S., 1985. Location of transcriptional control signals and transfer RNA sequences in Torulopsis glabrata mitochondrial DNA. EMBO J. 4: 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, L.J., Moulton, V., and Penny, D. 2000. Use of RNA secondary structure for studying the evolution of RNase P and RNase MRP. J. Mol. Evol. 51: 194–204. [DOI] [PubMed] [Google Scholar]
- Crary, S.M., Niranjanakumari, S., and Fierke, C.A. 1998. The protein component of Bacillus subtilis ribonuclease P increases catalytic efficiency by enhancing interactions with the 5′ leader sequence of pre-tRNAAsp. Biochemistry 37: 9409–9416. [DOI] [PubMed] [Google Scholar]
- Dang, Y.L. and Martin, N.C. 1993. Yeast mitochondrial RNase P sequence of the RPM2 gene and demonstration that its product is a protein subunit of the enzyme. J. Biol. Chem. 269: 19791–19796. [PubMed] [Google Scholar]
- Darr, S.C., Pace, B., and Pace, N.R. 1990. Characterization of ribonuclease P from the archaebacterium Sulfolobus solfataricus. J. Biol. Chem. 265: 12927–12932. [PubMed] [Google Scholar]
- Darr, S.C., Zito, K., Smith, D., and Pace, N.R. 1992. Contributions of phylogenetically variable structural elements to the function of the ribozyme ribonuclease P. Biochemistry 31: 328–333. [DOI] [PubMed] [Google Scholar]
- Doersen, C.J., Guerrier-Takada, C., Altman, S., and Attardi, G. 1985. Characterization of an RNase P activity from Hela cell mitochondria: Comparison with the cytosol RNase P activity. J. Biol. Chem. 260: 5942–5949. [PubMed] [Google Scholar]
- Frank, D.N., Adamidi, C., Ehringer, M.A., Pitulle, C., and Pace, N.R. 2000. Phylogenetic-comparative analysis of the eukaryal ribonuclease P RNA. RNA 6: 1895–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin, S.E., Zwick, M.G., and Johnson, J.D. 1995. Characterization and partial purification of two pre-tRNA 5′-processing activities from Daucus carrota (carrot) suspension cells. Plant J. 7: 553–563. [DOI] [PubMed] [Google Scholar]
- Gardiner, K.J., Marsh, T.L., and Pace, N.R. 1985. Ion dependence of the Bacillus subtilis RNase P reaction. J. Biol. Chem. 260: 5415–5419. [PubMed] [Google Scholar]
- Glöckner, G., Rosenthal, A., and Valentin, K. 2000. The structure and gene repertoire of an ancient red algal plastid genome. J. Mol. Evol. 51: 382–390. [DOI] [PubMed] [Google Scholar]
- Gopalan, V., Vioque, A., and Altman, S. 2002. RNase P: Variations and uses. J. Biol. Chem. 277: 6759–6762. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada, C., and Altman, S. 1992. Reconstitution of enzymatic activity from fragments of M1 RNA. Proc. Natl. Acad. Sci. 89: 1266–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrier-Takada, C., Gardiner, K., Marsh, T., Pace, N., and Altman, S. 1983. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 35: 849–857. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada, C., Eder, P.S., Gopalan, V., and Altman, S. 2002. Purification and characterization of Rpp25, an RNA-binding protein subunit of human ribonuclease P. RNA 8: 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas, E.S., Brown, J.W., Pitulle, C., and Pace, N.R. 1994. Further perspective on the catalytic core and secondary structure of ribonuclease P RNA. Proc. Natl. Acad. Sci. 91: 2527–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall, T.A. and Brown, J.W. 2002. Archaeal RNase P has multiple protein subunits homologous to eukaryotic nuclear RNase P proteins. RNA 8: 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanic-Joyce, P.J. and Gray, M.W. 1990. Processing of transfer RNA precursors in a wheat mitochondrial extract. J. Biol. Chem. 265: 13782–13791. [PubMed] [Google Scholar]
- Harris, J.K., Haas, E.S., Williams, D., Frank, D.N., and Brown, J.W. 2001. New insight into RNase P RNA structure from comparative analysis of the archaeal RNA. RNA 7: 220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann, R.K., Heinrich, J., Schlegl, J., and Schuster, H. 1995. Precursor of C4 antisense RNA of bacteriophages P1 and P7 is a substrate for RNase P of Escherichia coli. Proc. Natl. Acad. Sci. 92: 5822–5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayanthi, G.P. and Van Tuyle, G.C. 1992. Characterization of ribonuclease P isolated from rat liver cytosol. Arch. Biochem. Biophys. 296: 264–270. [DOI] [PubMed] [Google Scholar]
- Kole, R. and Altman, S. 1981. Properties of purified ribonuclease P from Escherichia coli. Biochemistry 20: 1902–1906. [DOI] [PubMed] [Google Scholar]
- Laferriere, A., Gautheret, D., and Cedergren, R. 1994. An RNA pattern matching program with enhanced performance and portability. Comput. Appl. Biosci. 10: 211–212. [DOI] [PubMed] [Google Scholar]
- Lang, B.F., Burger, G., Doxiadis, I., Thomas, D.Y., Bandlow, W., and Kaudewitz, F. 1977. A simple method for the large-scale preparation of mitochondria from microorganisms. Anal. Biochem. 77: 110–121. [DOI] [PubMed] [Google Scholar]
- Lang, B.F., Ahne, F., Distler, S., Trinkl, H., Kaudewitz, F., and Wolf, K. 1983. Sequence of the mitochondrial DNA, arrangement of genes and processing of their transcripts in Schizosaccharomyces pombe. In Molecular biology of the fission yeast (eds. A. Nasim, et al.), pp. 3118–3119, Academic Press, San Diego, CA.
- Lang, B.F., Burger, G., O’Kelly, C.J., Cedergren, R., Golding, G.B., Lemieux, C., Sankoff, D., Turmel, M., and Gray, M.W. 1997. An ancestral mitochondrial DNA resembling a eubacterial genome in miniature. Nature 387: 493–497. [DOI] [PubMed] [Google Scholar]
- Lang, B.F., Gray, M.W., and Burger, G. 1999. Mitochondrial genome evolution and the origin of eukaryotes. Annu. Rev. Genet. 33: 351–397. [DOI] [PubMed] [Google Scholar]
- Lee, Y.C., Lee, B.J., Hwang, D.S., and Kang, H.S. 1996a. Purification and characterization of mitochondrial ribonuclease P from Aspergillus nidulans. Eur. J. Biochem. 235: 289–296. [DOI] [PubMed] [Google Scholar]
- Lee, Y.C., Lee, B.J., and Kang, H.S. 1996b. The RNA component of mitochondrial ribonuclease P from Aspergillus nidulans. Eur. J. Biochem. 235: 297–303. [DOI] [PubMed] [Google Scholar]
- Leigh, J., Seif, E., Rodriguez, N., Jacob, Y., and Lang, B.F. 2003. Fungal evolution meets fungal genomics. In Handbook of fungal biotechnology, 2nd ed. (ed. Arora D.). Marcel Dekker Inc., New York (in press).
- Marchfelder, A. and Brennicke, A. 1994. Characterization and partial purification of tRNA processing activities from potato mitochondria. Plant Physiol. 105: 1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, C.A. and Lang, B.F. 1997. Mitochondrial RNase P: The RNA family grows. Nucleic Acids Symp. Ser. 36: 42–44. [PubMed] [Google Scholar]
- Miller, D.L. and Martin, N.C. 1983. Characterization of the yeast mitochondrial locus necessary for tRNA biosynthesis: DNA sequence analysis and identification of a new transcript. Cell 3: 911–917. [DOI] [PubMed] [Google Scholar]
- Morales, M.J., Dang, Y.L., Lou, Y.C., Sulo, P., and Martin, N.C. 1992. A 105 k-Da protein is required for yeast mitochondrial RNase P activity. Proc. Natl. Acad. Sci. 89: 9875–9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida, H. and Sugiyama, J. 1993. Phylogenetic relationships among Taphrina, Saitoella, and other higher fungi. Mol. Biol. Evol. 10: 431–436. [DOI] [PubMed] [Google Scholar]
- Ojala, D., Merkel, C., Gelfand, R., and Attardi, G. 1980. The tRNA genes punctuate the reading of genetic information in human mitochondrial DNA. Cell 22: 393–403. [DOI] [PubMed] [Google Scholar]
- Pannucci, J.A., Haas, E.S., Hall, T.A., Harris, J.K., and Brown, J.W. 1999. RNase P RNAs from some archaea are catalytically active. Proc. Natl. Acad. Sci. 96: 7803–7808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquin, B., Laforest, M.J., Forget, L., Roewer, I., Wang, Z., Longcore, J., and Lang, B.F. 1997. The fungal mitochondrial genome project: Evolution of fungal mitochondrial genomes and their gene expression. Curr. Genet. 31: 380–395. [DOI] [PubMed] [Google Scholar]
- Pascual, A. and Vioque, A. 1999. Functional reconstitution of RNase P activity from a plastid RNA subunit and a cyanobacterial protein subunit. FEBS Lett. 442: 7–10.9923593 [Google Scholar]
- Peck-Miller, K.A. and Altman, S. 1991. Kinetics of the processing of the precursor to 4.5S RNA, a naturally occurring substrate for RNase P from Escherichia coli. J. Mol. Biol. 221: 1–5. [DOI] [PubMed] [Google Scholar]
- Petersen, R.F., Langkjaer, R.B., Hvidtfeldt, J., Gartner, J., Palmen, W., Ussery, D.W., and Piskur, J. 2002. Inheritance and organisation of the mitochondrial genome differ between two Saccharomyces yeasts. J. Mol. Biol. 318: 627–636. [DOI] [PubMed] [Google Scholar]
- Puranam, R.S. and Attardi, G. 2001. The RNase P associated with HeLa cell mitochondria contains an essential RNA component identical in sequence to that of the nuclear RNase P. Mol. Cell. Biol. 21: 548–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragnini, A., Grisanti, P., Rinaldi, T., Frontali, L., and Palleschi, C. 1991. Mitochondrial genome of Saccharomyces douglasii: Genes coding for components of the protein synthetic apparatus. Curr. Genet. 19: 169–174. [DOI] [PubMed] [Google Scholar]
- Reith, M. and Munholland, J. 1995. Complete nucleotide sequence of the Porphyra purpurea chloroplast genome. Plant Mol. Biol. Reptr. 13: 333–335. [Google Scholar]
- Rossmanith, W. and Karwan, R.M. 1998. Characterization of human mitochondrial RNase P: Novel aspects in tRNA processing. Biochem. Biophys. Res. Commun. 247: 234–241. [DOI] [PubMed] [Google Scholar]
- Rossmanith, W. and Potushak, T. 2001. Difference between mitochondrial RNase P and nuclear RNase P. Mol. Cell. Biol. 21: 8236–8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salavati, R., Panigrahi, A.K., and Stuart, K.D. 2001. Mitochondrial ribonuclease P activity of Trypanosoma brucei. Mol. Biochem. Parasitol. 115: 109–117. [DOI] [PubMed] [Google Scholar]
- Sbisa, E., Pesole, G., Tullo, A., and Saccone, C. 1996. The evolution of the RNase P- and RNase MRP- associated RNAs: Phylogenetic analysis and nucleotide substitution rate. J. Mol. Evol. 43: 46–57. [DOI] [PubMed] [Google Scholar]
- Schlegl, J., Hardt, W.D., Erdmann, V.A., and Hartmann, R.K. 1994. Contribution of structural elements to Thermus thermophilus ribonuclease P RNA function. EMBO J. 13: 4863–4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekito, T., Okamoto, K., Kitano, H., and Yoshida, K. 1995. The complete mitochondrial DNA sequence of Hansenula wingei reveals new characteristic of yeast mitochondria. Curr. Genet. 28: 39–53. [DOI] [PubMed] [Google Scholar]
- Shu, H.H. and Martin, N.C. 1991. RNase P RNA in Candida glabrata mitochondria is transcribed with substrate tRNAs. Nucleic Acids Res. 19: 6221–6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu, H.H., Wise, C.A., Clark-Walker, G.D., and Martin, N.C. 1991. A gene required for RNase P activity in Candida (Torulopsis) glabrata mitochondria codes for a 227-nucleotide RNA with homology to bacterial RNase P RNA. Mol. Cell. Biol. 11: 1662–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark, B.C., Kole, R., Bowman, E.J., and Altman, S. 1978. Ribonuclease P: An enzyme with an essential RNA component. Proc. Natl. Acad. Sci. 75: 3717–3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stribinskis, V., Gao, G.J., Sulo, P., Dang, Y.L., and Martin, N.C. 1996. Yeast mitochondrial RNase P RNA synthesis is altered in an RNase P protein subunit mutant insights into the biogenesis of a mitochondrial RNA-processing enzyme. Mol. Cell. Biol. 16: 3429–3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stribinskis, V., Gao, G.J., Ellis, S.R., and Martin, N.C. 2001. Rpm2, the protein subunit of mitochondrial RNase P in Saccharomyces cerevisiae, also has a role in the translation of mitochondrially encoded subunits of cytochrome c oxidase. Genetics 158: 573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, B.C., Li, X., and Gegenheimer, P. 2000. Chloroplast ribonuclease P does not utilize the ribozyme-type pre-tRNA cleavage mechanism. RNA 6: 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinkl, H., Lang, B.F., and Wolf, K. 1989. Nucleotide sequence of the gene encoding the small ribosomal RNA in the mitochondrial genome of the fission yeast Schizosaccharomyces pombe. Nucleic Acids Res. 17: 6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turmel, M., Lemieux, C., Burger, G., Lang, B.F., Otis, C., Plante, I., and Gray, M.W. 1999. The complete mitochondrial DNA sequences of Nephroselmis olivacea and Pedinomonas minor. Two radically different evolutionary patterns within green algae. Plant Cell 11: 1717–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underbrink-Lyon, K., Miller, D.L., Ross, N.A., Fukuhara, H., and Martin, N.C. 1983. Characterization of a yeast mitochondrial locus necessary for tRNA biosynthesis: Deletion mapping and restriction mapping studies. Mol. Gen. Genet. 191: 512–518. [DOI] [PubMed] [Google Scholar]
- Wagner, M., Fingerhut, C., Gross, H.J., and Schon, A. 2001. The first phytoplasma RNase P RNA provides new insights into the sequence requirements of this ribozyme. Nucleic Acids Res. 29: 2661–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, C., Ragnini, A., and Fukuhara, H. 1989. Analysis of the regions coding for transfer RNAs in Kluyveromyces lactis mitochondrial DNA. Nucleic Acids Res. 17: 4485–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise, C.A. and Martin, N.C. 1991a. Dramatic size variation of yeast mitochondrial RNAs suggests that RNase P RNAs can be quite small. J. Biol. Chem. 266: 19154–19157. [PubMed] [Google Scholar]
- ———. 1991b. Sequence analysis of Saccharomyces exiguus mitochondrial DNA reveals an RNase P RNA flanked by two tRNA genes. Nucleic Acids Res. 19: 4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokobori, S. and Pääbo, S. 1995. Transfer RNA editing in land snail mitochondria. Proc. Natl. Acad. Sci. 92: 10432–10435. [DOI] [PMC free article] [PubMed] [Google Scholar]