Abstract
The 5′ untranslated regions (UTRs) of (+)-strand RNA viruses play a variety of roles in the reproductive cycles of these infectious agents. Tomato bushy stunt virus (TBSV) belongs to this class of RNA virus and is the prototype member of the genus Tombusvirus. Previous studies have demonstrated that a T-shaped domain (TSD) forms in the 5′ half of the TBSV 5′ UTR and that it plays a central role in viral RNA replication. Here we have extended our structure–function analysis to the 3′ half of the 5′ UTR. Investigation of this region in the context of a model viral replicon (i.e., a TBSV-derived defective interfering [DI] RNA) revealed that this segment contains numerous functionally relevant structural features. In vitro solution structure probing along with comparative and computer-aided RNA secondary structure analyses predicted the presence of a simple stem loop (SL5) followed by a more complex downstream domain (DSD). Both structures were found to be essential for efficient DI RNA accumulation when tested in a plant protoplast system. For SL5, maintenance of the base of its stem was the principal feature required for robust in vivo accumulation. In the DSD, both helical and unpaired regions containing conserved sequences were necessary for efficient DI RNA accumulation. Additionally, optimal DI RNA accumulation required a TSD–DSD interaction mediated by a pseudoknot. Modifications that reduced accumulation did not appreciably affect DI RNA stability in vivo, indicating that the DSD and SL5 act to facilitate viral RNA replication.
Keywords: RNA replication, RNA structure, Tombusvirus, (+)-strand RNA virus, plant virus, Tombusviridae, TBSV, DI RNA
INTRODUCTION
The genomes of (+)-strand RNA viruses are involved in many processes during viral infections. For many viruses, RNA elements involved in one or more process have been identified within the 5′ untranslated regions (UTR) of their genomes. For instance, several diverse plant and animal (+)-strand RNA viruses contain RNA structures in this region that facilitate viral protein translation (Gallie 2001; Guo et al. 2001; Vagner et al. 2001), genome replication (Andino et al. 1990; Miller et al. 1998; Chen et al. 2001; Mason et al. 2002; Luo et al. 2003) or subgenomic (sg) mRNA transcription (van Marle et al. 1999). In both filamentous and icosahedral viruses, RNA encapsidation signals that bind capsid protein and promote virus particle assembly have been identified in their 5′ UTRs (Sit et al. 1994; Bink et al. 2002; Sasaki and Taniguchi 2003). Given the importance and range of functions for viral 5′ UTRs, it is not surprising that modifications in this region can markedly influence viral pathogenicity (Sarnow 2003).
Many aspects of viral reproduction have been studied in members of the family Tombusviridae (Russo et al. 1994). Tomato bushy stunt virus (TBSV), the prototype member of this family, possesses a 4.8-kb (+)-strand RNA genome that encodes five proteins (Fig. 1A ▶; Hearne et al. 1990). These proteins are translated via a 5′-cap- and 3′-poly(A)-independent mechanism (Wu and White 1999). Productive genome replication requires viral protein p33 and its translational readthrough product p92 (the RNA-dependent RNA polymerase [RdRp]; Oster et al. 1998). In addition to these viral proteins (and unidentified host factors), this process uses RNA signals within the viral template to promote and regulate replication—a process that proceeds through a (−)-strand RNA intermediate (Buck 1996).
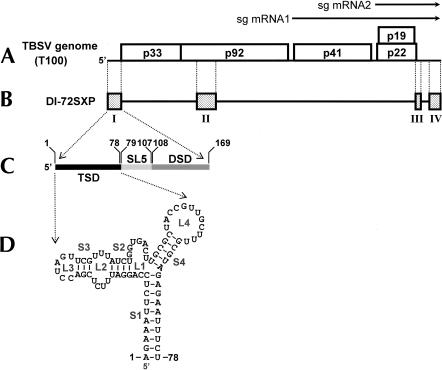
FIGURE 1.
(A) The TBSV RNA genome. The genome is represented by a thick black line with coding regions depicted as boxes with approximate molecular masses (in kDa) of the encoded proteins. Two subgenomic mRNAs produced during infection are shown as arrows above the genome. (B) A prototypical TBSV DI RNA (DI-72SXP). Shaded boxes represent TBSV genomic segments present in DI RNA, whereas black lines represent genomic segments that are absent. (C) Expanded linear representation of the TBSV 5′ UTR. The T-shaped domain (TSD; black) was defined previously (Wu et al. 2001). Elements defined in this study are stem–loop 5 (SL5; light gray) and the downstream domain (DSD; dark gray). Coordinates corresponding to the boundaries of each of the three elements are provided. (D) RNA secondary structure model for the TBSV TSD (Wu et al. 2001). RNA stem (S) and loop (L) structures are labeled and coordinates are given at the beginning and end of the sequence.
For TBSV, viral RNA replication has been studied extensively using defective interfering (DI) RNAs (White and Morris 1994a,b; Chang et al. 1995; Wu and White 1998; Ray and White 1999, 2003; Nagy and Pogany 2000; Panavas et al. 2002a,b; Panavas and Nagy 2003). DI RNAs are genome-derived deletion mutants that are noncoding but maintain important RNA replication elements that allow them to be amplified when p33 and p92 are provided in trans (White 1996). Thus, when coinoculated with the wild-type (wt) TBSV genome, DI RNAs are replicated very efficiently and provide convenient model templates to study cis-acting sequences involved specifically in replication.
A prototypical TBSV DI RNA is comprised of four regions (RI through RIV) derived from noncontiguous segments within the viral genome (Fig. 1B ▶; White 1996). RI corresponds to the TBSV 5′ UTR and includes the start codon for p33/p92. In vitro studies have shown that the core promoter for plus-strand synthesis is contained in the complement of this region and corresponds to the 3′-terminal 11 nt in the (−)-strand (Panavas et al. 2002a). Other in vivo studies have shown that the 5′-proximal portion of the 5′ UTR forms a T-shaped domain (TSD) in the (+)-strand and that this structure is crucial for DI RNA replication (Fig. 1C,D ▶; Wu et al. 2001). Interestingly, DI RNAs lacking the entire 5′ UTR are viable and accumulate to ~10% that of wild type (Wu and White 1998). However, when the 5′ UTR is present, mutations within this region act in a dominant-negative manner (Wu et al. 2001).
In this study, we used a TBSV DI RNA to define a structure–function model for the previously uncharacterized 3′ portion of the 5′ UTR. This region was found to contain two major structural elements, a simple stem–loop (SL), called SL5, and a more elaborate structure, termed the downstream domain (DSD). Formation of both SL5 and helical regions within the DSD were very important for DI RNA accumulation. Additionally, conserved unpaired sequences within the DSD contributed substantially to DI RNA amplification, and optimal accumulation required formation of a pseudoknot between the TSD and DSD. The results confirm an important role for structures in the 3′ half of the 5′ UTR in viral RNA replication.
RESULTS
RNA secondary structure of the 3′ half of the TBSV 5′ UTR
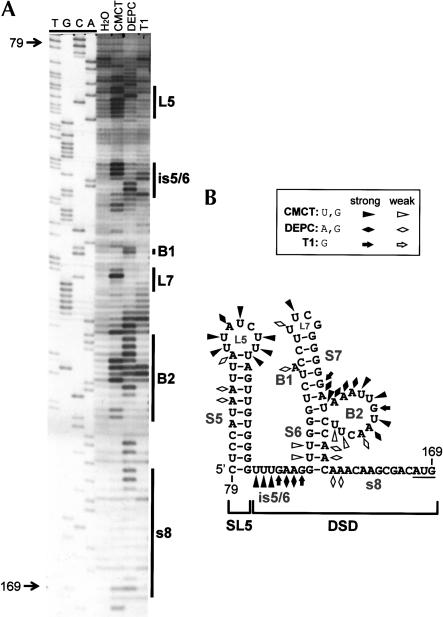
Previously, we investigated the structure of the 5′ half of the TBSV 5′ UTR (coordinates 1–78) and provided evidence that it folds into a functionally relevant TSD (Fig. 1D ▶; Wu et al. 2001). In the present study, we have extended our structural analysis to the 3′ half of the 5′ UTR (coordinates 79–169). Solution structure probing of this region, within the context of a prototypical DI RNA, predicts that it adopts two distinct helical regions, both of which are supported by MFOLD analysis (Fig. 2 ▶). At the extreme 5′ end of this segment, a relatively large hairpin, termed SL5, is predicted. This hairpin is separated from a more complex downstream helical region by a single-stranded (ss) intervening sequence (is), defined as is5/6. The second helical region consists of a lower helix (S6) separated by a 12-nt bulge (B2) from an upper helix (S7). S7 is interrupted by a single A-bulge and is capped by a UNCG-type super-stable tetraloop (L7). The 3′-proximal sequence, which ends with the start codon for p33/92, has been termed sequence 8 (s8) and, collectively, is5/6, S6, B1, B2, SL7, and s8 define the downstream domain (DSD; Fig. 2 ▶). Solution structure analysis of the sequence encompassing SL5 and the DSD revealed reactivities of single-strand (ss)-specific chemicals (CMCT and DEPC) and a ribonuclease (RNase T1) with predicted unpaired residues. These include strong modifications of L5, is5/6, L7, and B2 (Fig. 2 ▶). Additionally, weaker modifications were also observed for the bulged adenylate comprising B1 and also at the 5′ end of s8. The reactivities seen within S5, S6, and the lower half of S7 are likely the result of transient disruption of these helices at less stable locales. Interestingly, no significant modification of the majority of s8 was observed, indicating that this region may interact with other RNA sequences. In contrast, the extended unpaired segments in L5, is5/6, and B2, as well as the single A-bulge in B1, represent candidates for protein interactions.
FIGURE 2.
(A) Chemical and enzymatic probing of the 3′ half of the TBSV 5′ UTR. In vitro transcripts of full-length DI-72SXP DI RNA were treated with CMCT [1-cyclohexyl-3-(2-morpholinoethyl)carbodiimide], DEPC (diethylpyrocarbonate), or RNase T1, and the cleaved or modified RNAs were analyzed by primer extension (PE). The PE products were separated in an 8% acrylamide-urea gel along with a control H2O-treated sample (H2O) and a sequencing ladder. Nucleotide positions relative to the TBSV genome are indicated on the left, whereas the locations of various single-stranded RNA elements are delineated by vertical lines on the right. (B) RNA secondary structure model for the 3′ half of the 5′ UTR. Chemical and enzymatic modifications have been mapped onto the MFOLD-predicted structure and are labeled according to the figure legend. Coordinates are given at the beginning and end of the sequence, and stem–loop (SL) and bulged (B) structures are labeled.
Comparative sequence analysis of SL5 and the DSD
Sequence analysis of available Tombusvirus genomes was carried out to gain further insights into the structural model generated for TBSV in Figure 2B ▶. Initially, sequences corresponding to SL5 were aligned and evaluated (Fig. 3A ▶). The comparison revealed a significant degree of conservation for bases involved in the formation of the lower portion of S5, whereas those residues comprising the upper helical regions showed a high degree of mono- and covariation—supporting the existence of the S5 structure. The lengths of the stem regions varied considerably, with S5s ranging from 6 to 11 bp. Similarly, L5 lengths were variable and ranged from 3 to 12 nt, with an apparent bias for pyrimidines. The SL5 comparison was also extended to the genus Aureusvirus (family Tombusviridae), whose members are most closely related to tombusviruses (Miller et al. 1997; Rubino and Russo 1997). Again, conservation of SL5 and its general features was also observed (Fig. 3A ▶). Thus, SL5 represents a conserved but somewhat flexible structure that is maintained in both Tombusvirus and Aureusvirus genomes.
FIGURE 3.
Comparative sequence analysis of (A) SL5 and (B) DSD from members of the genera Tombusvirus and Aureusvirus. The tombusviruses are Tomato bushy stunt virus-statice isolate (TBSVs), Tomato bushy stunt virus-pepper isolate (TBSVp), Artichoke mottled crinkle virus (ACMV), Cucumber Bulgarian latent virus (CBLV), Carnation Italian ringspot virus (CIRV), Cymbidium ringspot virus (CymRSV), Cucumber necrosis virus (CNV), and Pear latent virus (PeLV). The aureusviruses are Cucumber leaf spot virus (CLSV) and Pothos latent virus (PoLV). Gaps (dashes) were introduced to maximize alignment. Subelements (i.e., stems, loops, and bulges) within the RNA structures are labeled above the sequences. Predicted single-stranded residues are depicted in black text (no highlighting) with loop regions underlined. Base-paired regions are in black text with light or dark gray highlighting. Residue differences in base-paired regions that maintain base-pairing via either mono- or covariation are indicated in white. Conserved single-stranded sequences in the DSD are depicted in white with black highlighting. In B the vertical line delineates the upper (on left) and lower (on right) portions of S7, and the open boxes enclose start codons for p33/92 (tombusviruses) and p25/84 (aureusviruses). The numbers on the right correspond to coordinates for the 3′-most residues shown.
Sequence comparisons of tombusviruses and aureusviruses also strongly supported the formation of the helical regions predicted in the DSD (Fig. 3B ▶). The existence of S6 and S7 was evident from multiple mono- and covariations that maintained base-pairing in the respective helices. Interestingly, the lengths of S6 and the lower portion of S7 were conserved at 5 and 4 bp, respectively. In contrast, the upper portion of S7 was variable. The L7 sequence was also variable with six UNCGs, one CUYG, and four other less common loop sequences. Further analysis of the DSD sequences revealed that the nucleotide identity of predicted ss regions is5/6, B1, and B2 were all highly conserved. This was not the case for s8, where, other than a short 5′-proximal A-tract, considerable variation was observed. The DSD is thus comprised of positionally conserved helical elements that could participate in the presentation of single stranded regions, including highly conserved residues. Overall, the comparative sequence analysis is in full agreement with our structural model for SL5 and the DSD presented in Figure 2B ▶.
The base of S5 is essential for efficient accumulation of DI RNAs
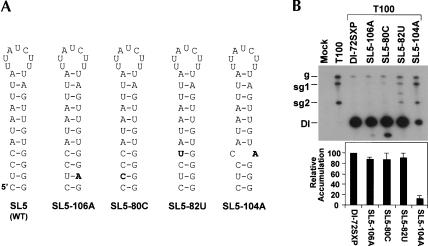
To determine if SL5 served any notable role in the accumulation of viral RNAs, this structure was deleted from a prototypical DI RNA, DI-72SXP. In vitro generated transcripts of mutant ΔSL5 were generated and coinoculated with transcripts of the TBSV genome (T100) into cucumber protoplasts. The effects of the deletion on DI RNA accumulation were then monitored by quantitative Northern blot analysis (Fig. 4B ▶). Examination of viral RNAs from infections showed that the wild-type DI-72SXP was amplified efficiently by the replication components provided in trans from the coinoculated TBSV helper virus (Fig. 4B ▶). In contrast, coinoculation with the ΔSL5 mutant resulted in accumulation to ~10% that of wild type, confirming an important role for SL5 (Fig. 4B ▶).
FIGURE 4.
Compensatory mutational analysis of SL5. (A) Depiction of SL5 in mutants. Nucleotide substitutions are in boldface. (B,C) Northern blot analysis of protoplast infections. Cucumber protoplasts were inoculated with H2O (mock), T100 (3 μg), or T100 and wild-type or mutant DI RNAs (1 μg) as indicated above the lanes. Total nucleic acids were isolated 24 h postinoculation, separated in 8M urea-4.5% polyacrylamide gels, transferred to nylon membranes, and probed with a 32P-end-labeled minus-sensed probe. Bands corresponding to TBSV genomic RNA (g), subgenomic mRNA1 (sg1), subgenomic mRNA2 (sg2), and DI RNA (DI) are indicated. The accumulation level of wild-type DI-72SXP was set at 100%, and mutant levels were normalized to this value. Relative DI RNA accumulation levels (with standard errors) are presented as histograms. The asterisk indicates the position of DI RNA head-to-tail dimers (Ray and White 1999, 2003).
The comparative sequence analysis in Figure 3A ▶ revealed that the lengths of S5 vary significantly between tombusviruses. For example, Cucumber necrosis virus (CNV) has an S5 only 6 bp long, compared with 11 bp for TBSV. However, despite this and other differences, the residues at the base of all SL5s contain the conserved base pairs CUC/GGG (Fig. 3A ▶). Thus, a critical feature of the SL5 structure could be these conserved nucleotides at its base. To test this hypothesis, compensatory mutational analysis—that included two of the conserved base pairs—was carried out on the base portion of S5 (Fig. 4A,C ▶). Substitutions that disrupted either half of this helical region (SL5-2a or SL5-2b) dramatically reduced DI RNA accumulation levels (~10% for both; Fig. 4A,C ▶). In contrast, combining these modifications in SL5-2c, so as to restore base-pairing potential, resulted in essentially full recovery of accumulation (Fig. 4A,C ▶). These results indicate that the identity of at least two of the three conserved base pairs is not important. However, the strong correlation between base-pairing potential and DI RNA accumulation clearly define a key role for stable formation of the lower portion of S5.
In the preceding analysis, the potential role of the conserved UG base pair near the bottom of S5 was not examined. As UG base pairs within helices can serve specific structural functions (Varani and McClain 2000), this pair was subjected to mutational analysis. Two mutants were generated, SL5-106A and SL5-80C, in which the UG pair was converted to either a UA or a CG pair, respectively (Fig. 5A ▶). In both cases, the substitutions are predicted to strengthen the helix in the modified region. Very minor reductions (~10%) in accumulation levels were observed for these mutants, but, interestingly, a faster-migrating product just below the positions of the DI RNAs was also observed (Fig. 5B ▶). This additional viral RNA likely represents a DI RNA-derived product that was generated by error during its replication. The accumulation of this secondary RNA product was minimal for the AU-containing mutant, but was more substantial for the CG-containing DI RNA (Fig. 5B ▶). The accumulation level of this additional product thus appears to correlate with the increased stability of the respective base pairs. However, the identity of the substitutions could also contribute to this effect. Regardless, the results implicate the conserved GU base pair as an important determinant for optimal accumulation and possibly for accurate DI RNA replication.
FIGURE 5.
Analysis of the conserved GU base pair in SL5 and strand-specific destabilization of SL5. (A) Depiction of SL5 in mutants. (B) Northern blot analysis and quantification of DI RNA accumulation levels.
SL5 functions in the (+)-strand
Inspection of the base pairs within S5 revealed the presence of three UG base pairs. This observation is indicative of (+)-strand function as the UG complements (i.e., AC mismatches) would be disruptive to formation of a structure complementary to SL5 in the (−)-strand. To test this concept directly, we introduced substitutions near the base of S5 that would preferentially destabilize this helical region in either the (−)-strand (mutant SL5-82U) or (+)-strand (mutant SL5-104A; Fig. 5A ▶). No significant loss in accumulation levels was observed for SL5-82U, whereas a dramatic drop in levels was seen for SL5-104A (Fig. 5B ▶). As DI RNA accumulation was relatively insensitive to a reduction in (−)-strand stability, but markedly susceptible to a decrease in (+)-strand stability, SL5 must function primarily in the (+)-strand.
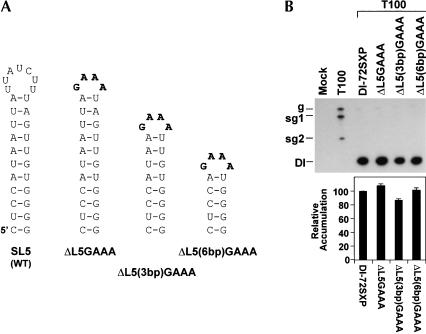
A miniaturized SL5 can mediate efficient DI RNA accumulation
A possible role for L5 in DI RNA accumulation was also examined. To this end, the 7-membered wild-type loop was replaced by a GAAA superstable tetraloop in mutant ΔL5GAAA (Fig. 6A ▶). This exchange did not appreciably alter accumulation levels, implying no major role for the wild-type loop sequence (Fig. 6B ▶). This altered loop was also tested in mutants, ΔL5(3bp)GAAA and ΔL5(6bp)GAAA, with 8-bp and 5-bp stems, respectively (Fig. 6A ▶). In both cases, near-wild-type levels of accumulation were observed (Fig. 6B ▶). This, combined with the other results on SL5 (Figs. 4 ▶ and 5 ▶), indicates that SL5 functions to facilitate DI RNA accumulation by acting in the (+)-strand as a primarily sequence-independent hairpin structure.
FIGURE 6.
Loop modifications and upper stem deletions in SL5. (A) Depiction of SL5 in mutants. (B) Northern blot analysis and quantification of DI RNA accumulation levels.
Helical regions within the DSD are essential for efficient accumulation of DI RNAs
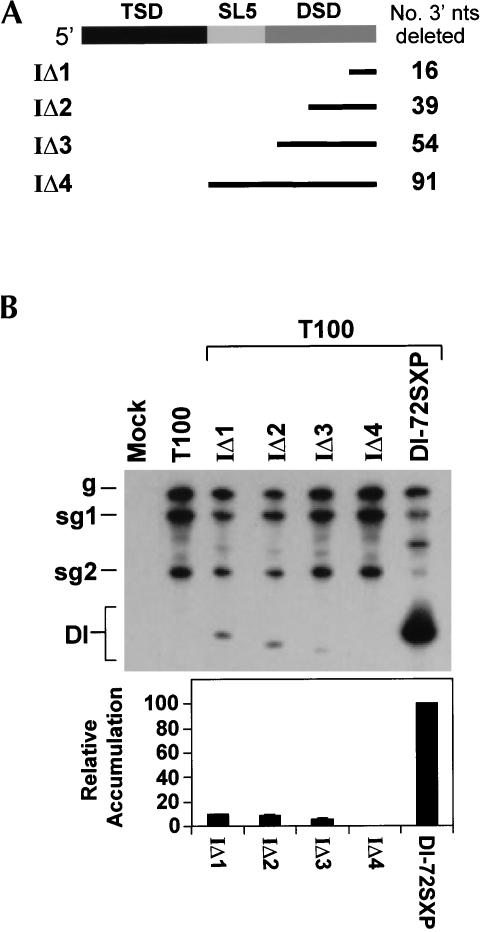
As an initial step to investigate the role of the DSD in DI RNA accumulation, we carried out 3′ deletion analysis of this region (Fig. 7A ▶). A deletion as small as 16 nt (IΔ1) led to dramatic reductions in DI RNA levels, with longer deletions being increasingly more inhibitory (Fig. 7B ▶). These results indicate an important role for the DSD in DI RNA accumulation and show that the TSD alone (IΔ4), or in combination with SL5 (IΔ3), is unable to mediate efficient DI RNA accumulation (Fig. 7 ▶).
FIGURE 7.
Deletion analysis of the 3′ portion of the 5′ UTR. (A) Mutant names are given on the left. The corresponding segments deleted are represented by the black lines, and the number of nucleotides deleted in each mutant is listed to the right. (B) Northern blot analysis and quantification of DI RNA accumulation levels.
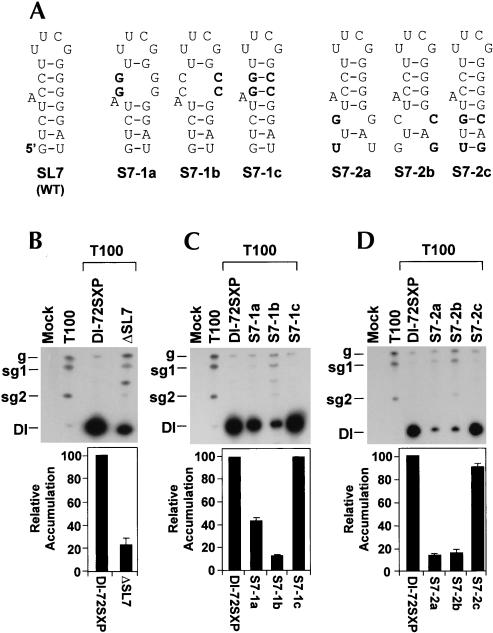
The importance of DSD subelement SL7 was first assessed by deletion analysis (Fig. 8 ▶). ΔSL7 accumulated to ~20% that of wild type, confirming its significance (Fig. 8B ▶). Next, the upper and lower parts of S7 were analyzed by compensatory mutational analysis (Fig. 8A ▶). In both mutant series (S7-1a, -1b, -1c and S7-2a, -2b, -2c), strong correlations between stem stabilities and DI RNA accumulation were observed (Fig. 8C,D ▶), demonstrating the necessity for both helical portions. S7 is predicted to function in the (+)-strand, as the relatively large proportion of GU pairs in it would preclude its formation in the (−)-strand. This idea is also supported by the presence of a superstable UUCG tetraloop (L7) in the (+)-strand (Fig. 2B ▶). Interestingly, L7 was found to play no major role because it could be replaced with GAAA without any loss of DI RNA accumulation (data not shown).
FIGURE 8.
Compensatory mutational analysis of S7. (A) Depiction of S7 in mutants. (B,C,D) Northern blot analysis and quantification of DI RNA accumulation levels.
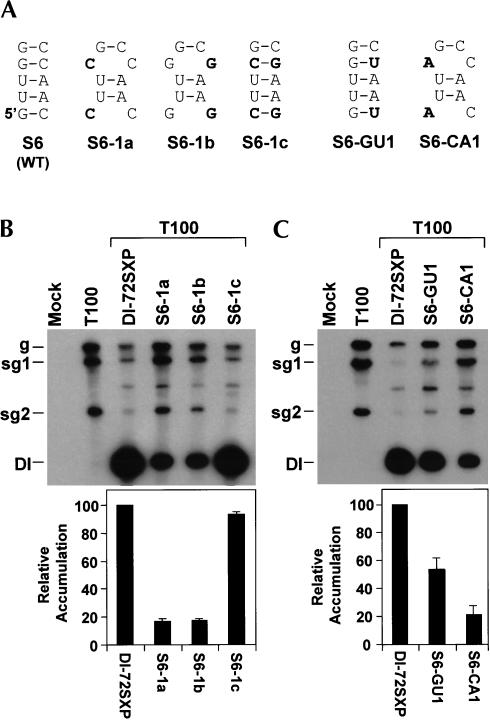
Compensatory analysis of S6 with mutant series S6-1a, -1b, and -1c verified a prominent role for this helix (Fig. 9A,B ▶). Unlike S7, no GU base pairs were present in S6 to aid in the deduction of strand-specific activity. Consequently, S6 was tested as described previously for S5 by preferentially destabilizing it in either the (−)-strand (mutant S6-GU1) or (+)-strand (mutant S6-CA1; Fig. 9A ▶). The enhanced sensitivity observed for (+)-strand destabilization indicates a primarily (+)-strand function (Fig. 9C ▶). Thus, both S6 and S7 are functionally relevant and are active in the (+)-sense.
FIGURE 9.
Compensatory and strand-specific mutational analysis of S6. (A) Depiction of S6 in mutants. (B,C) Northern blot analysis and quantification of DI RNA accumulation levels.
Other regions of the TSD contribute to DI RNA accumulation
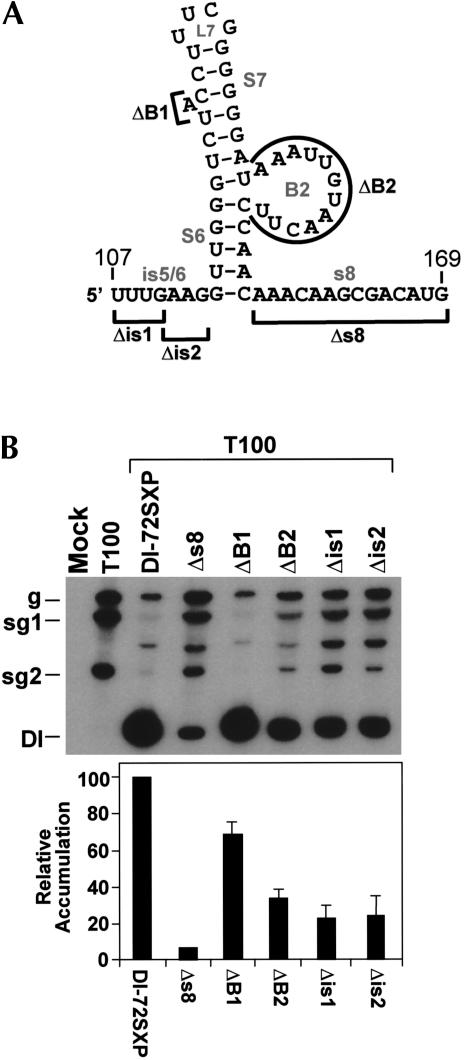
The preceding mutational analyses confirmed the importance of helical structures within the DSD. In our model for the DSD (Fig. 10A ▶), these helical structures are integrated with other adjacent sequences, several of which are highly conserved (i.e., is5/6, B1, and B2). Mutants containing deletions that targeted these conserved sequences were generated and tested (Fig. 10A,B ▶). Removal of either half of is5/6 in Δis1 or Δis2 led to reduced levels of DI RNA (Fig. 10B ▶). Deletion of the larger B2 had a similar inhibitory effect, whereas elimination of the bulge-A of B1 was less detrimental (Fig. 10B ▶). Analysis of mutant Δs8, containing a precise deletion of s8, was also performed (Fig. 10A ▶). In this latter case, a very severe defect in DI RNA accumulation was witnessed (Fig. 10B ▶).
FIGURE 10.
Deletion of conserved and nonconserved single-stranded RNA regions in the DSD. (A) Regions deleted in the DSD (indicated by bold lines). (B) Northern blot analysis and quantification of DI RNA accumulation levels.
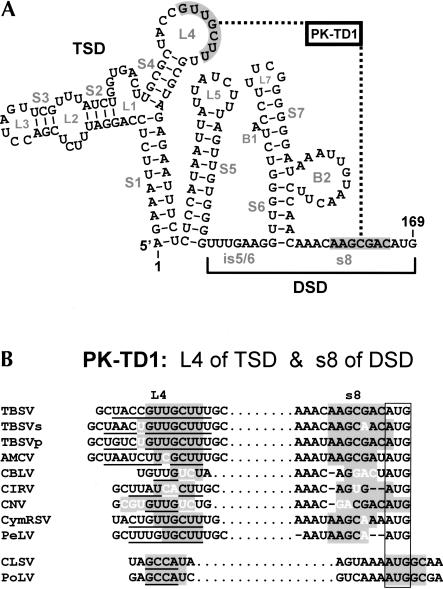
A TSD–DSD interaction is required for optimal DI RNA accumulation
Visual inspection of the secondary structure of the 5′ UTR revealed the possibility of a base-pairing interaction between L4 of the TSD and s8 of the DSD (Fig. 11A ▶). Importantly, comparative sequence analysis of corresponding regions in other tombusviruses and aureusviruses revealed mono- and covariations that maintained base pairing of the participating segments (Fig. 11B ▶). These data prompted us to test directly the importance of this potential interdomain interaction, termed pseudoknot TD1 (PK-TD1). Compensatory mutations were designed to disrupt (PK-TD1a and PK-TD1b) and then restore (PDK-TD1c) pairing of the PK between L4 and s8 (Fig. 12A ▶). The results of this analysis showed a clear dependence on formation of this interdomain interaction for efficient DI RNA accumulation (Fig. 12B ▶). Furthermore, the requirement for this interaction is also supported by the reduced activity observed when s8 was deleted (Fig. 10 ▶) and the resistance of the 3′ portion of s8 to ss modification (Fig. 2B ▶). Thus, with respect to the DSD, both intra- and interdomain interactions are required for optimal activity.
FIGURE 11.
(A) Depiction of a potential pseudoknot, PK-TD1, between the TSD and DSD. RNA sequences that form PK-TD1 are shaded gray and joined by a dotted line. (B) Comparative sequence analysis showing conservation of PK-TD1 as described in the legend to Figure 3 ▶.
FIGURE 12.
Compensatory mutational analysis of PK-TD1. (A) Depiction of PK-TD1 interaction in mutants. (B) Northern blot analysis and quantification of DI RNA accumulation levels.
SL5 and DSD defects affect accumulation of both (+)- and (−)-strands but not DI RNA stability
Although the DSD appears to function in the (+)-strand, its activity could facilitate either (+)-strand or (−)-strand synthesis, or both. To investigate whether one or both of these processes was affected in our mutants, the compensatory mutant series targeting S5, S6, S7, and PK-TD1 was subjected to (−)-strand analysis (Fig. 13 ▶). DI RNA (−)-strand accumulation at 24 h postinfection was quantified and plotted with corresponding (+)-strand accumulation. The comparisons revealed no major differences in relative accumulation levels, indicating that both (+)- and (−)-strand synthesis is affected by the modifications to the same relative degree (Fig. 13 ▶).
FIGURE 13.
Northern blot analysis of minus-strand DI RNA accumulation for mutants with compensatory mutations in (A) SL5, (B) SL7, (C) S6, and (D) PK-TD1. Northern blot analysis and quantification of DI RNA (−)-strand accumulation levels. For comparison, corresponding (+)-strand accumulation levels (black bars) have been included along with (−)-strand accumulation levels (white bars).
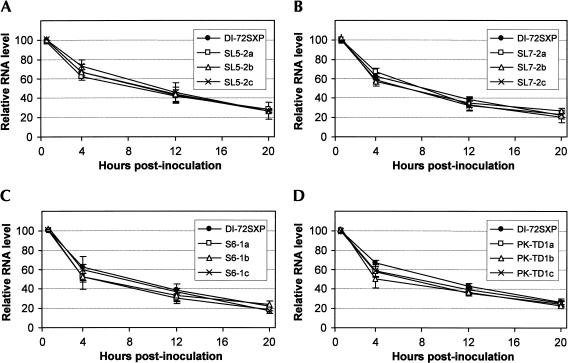
The in vivo stabilities of these mutant series were also assessed. Different DI RNAs were inoculated into protoplasts (without T100), and their decay was monitored over a 20-h period (Fig. 14 ▶). No substantial differences in the degradation profiles were observed. This result indicates that the decreases in DI RNA accumulation observed in the assays containing T100 are related to defects in replication.
FIGURE 14.
In vivo stability analysis of DI RNAs for mutants with compensatory mutations in (A) SL5, (B) SL7, (C) S6, and (D) PK-TD1. Protoplasts that were inoculated with DI RNAs only and total nucleic acids were extracted at 1, 4, 12, and 20 h postinoculation. DI RNA levels were determined by Northern blot analysis and quantified by radioanalytical scanning of membranes. The graphs represent relative DI RNA levels as a function of time. The values represent means from two separate experiments.
DISCUSSION
In this study we have characterized the 3′ portion of the TBSV 5′ UTR within the context of a prototypical DI RNA. The results establish the existence of two major structural features, SL5 and the DSD, as key elements for efficient DI RNA accumulation in vivo. For SL5, formation and stability of the base of its stem are critical features for function. In contrast, both helical and unpaired sequences within the DSD contribute to its activity and, like SL5, these structures operate primarily in the (+)-strand. Additionally, the DSD must interact via base pairing with the previously defined TSD to facilitate optimal DI RNA accumulation. The possible functions and mechanisms of action of the newly defined SL5 and DSD are considered in relation to the TSD and various viral processes.
Possible roles for SL5
We have shown, through sequence, structural, and functional analysis, that SL5 represents a critical structure for efficient DI RNA accumulation. Deletion of this hairpin led to a dramatic decrease in accumulation (Fig. 4B ▶), indicating that SL5 is not merely an element that functions solely to unite the TSD and the DSD. The large loop sequence in L5 was not important (Fig. 6 ▶); instead, the key feature required for optimal activity was pairing of the base of its stem (Fig. 4 ▶). As this structure was shown to function in the (+)-strand (Fig. 5 ▶), and considering its position directly adjacent to S1 of the TSD, one likely role for the base of SL5 is to coaxially stack on the base of S1 (Fig. 11A ▶). This interaction could in turn serve several different functions. First, previous studies have shown that S1 formation and stability in the (+)-strand is critical for DI RNA accumulation (Wu et al. 2001). This coaxial stacking could therefore act to further stabilize S1. Second, the stacking interaction would lead to the incorporation of the 5′ terminus into a quasihelix. Such an arrangement could be of particular importance as Tombusvirus genomes are not capped (Russo et al. 1994), and therefore their 5′ termini may be more susceptible to exoribonuclease attack. Accordingly, burying the terminus in a helix may be one way of limiting RNase access to these residues. The results of DI RNA stability assay performed on SL5 mutants (Fig. 14A ▶) do not preclude this function as this method does not have sufficient resolution to detect the loss of one or a few 5′-terminal residues. A third possible function of SL5 could be to properly orient the TSD and/or DSD elements. A service such as this could conceivably facilitate interactions such as PK-TD1. These examples represent a sampling of some of the more obvious roles that SL5 could perform, and they are being investigated experimentally.
Another feature of SL5 found to be of less importance was the conserved UG pair near its base. This wobble pair is present in a conserved block of 3 bp (Fig. 3A ▶); however, compensatory mutational analysis demonstrated that the identity of the two flanking CG pairs was not important (Fig. 4 ▶). In contrast, converting the UG pair to either a UA or CG pair resulted in a slight reduction in accumulation and, for the CG pair, the appearance of a secondary DI RNA product (Fig. 5 ▶). The U-to-C modification in SL5-80C would change both the identity of the 5′ partner in the pair and strengthen the base of the helix (Fig. 5 ▶). Strengthening the helix could alter the dynamics of its formation and/or ability to stack coaxially with S1. Alternatively, a requirement for U at position 80 would indicate an important alternate role for this residue. Further analysis will be required to determine which of these features is responsible for the production of the secondary product.
Although the loop sequence of SL5 was shown clearly to be flexible in terms of efficient DI RNA accumulation, within the genomic context the wild-type sequence could be beneficial to other processes (e.g., translation). The size and sequence in the L5s in different tombusviruses vary, but they are for the most part very pyrimidine-rich (Fig. 3A ▶). This characteristic could be related to a possible function such as binding to a polypyrimidine-tract binding protein (Valcarcel and Gebauer 1997). Indeed, such proteins have been implicated in facilitating the translation of (+)-strand RNA viruses such a picornavirus (Belsham and Sonenberg 1996). For TBSV, if S5 does indeed stack coaxially on S1, then L5 would be presented away from the upper portion of the TSD (Fig. 11A ▶). Depending on the tombusvirus and its sequence, the length of S5 may be adjusted accordingly to extend and present L5 within the different structural contexts.
Possible roles for the DSD
In vitro and in vivo analysis of the DSD has allowed us to propose a secondary structural model for this domain. The functionally relevant structure contains several quasicontinuous helices and several conserved nonhelical elements. The strong requirement for the maintenance of both types of structures indicates that they work as an integrated unit in which the conserved residues are presented in ss form by adjacent helical regions. Relative positioning may also be important, as strict conservation of 5 bp in S6 and 4 bp in S7 is observed (Fig. 3B ▶). However, the presentation of these conserved sequences may not be the only role for the helical regions, as they themselves could form generic portions of a recognition motif(s).
Two major conserved sequences, is5/6 (5′-UUUGAAG) and B2 (5′-AAAUUGUAACUU), are present in primarily ss regions of the DSD (Fig. 2B ▶). Both of these sequences are important, as deletion of either significantly compromises DI RNA accumulation (Fig. 10 ▶). It is possible that a secondary effect of their deletion could be alteration of the spatial configuration of other adjacent elements. However, in the case of B2, this would likely be minimal as flanking S6 and S7 would remain in the same relative positions, although flexibility at this previously “hinged” region would be lost. In the case of is5/6, 5′ and 3′ halves of this segment were deleted to minimize size-related effects. Although we cannot preclude the possibility that the phenotypes observed are related to effects other than loss of a particular sequence, the observation that these sequences are highly conserved and are present in primarily ss regions supports a direct and important role for them.
If their function was only to interact with other RNA elements, one would expect some degree of variation that would be compensated by substitutions in the partner sequences (i.e., base pair covariation). Instead, these sequences are invariant, indicating that, if they do have RNA partners, nucleotide identity either within the interaction or independent of the interaction is also important. Alternatively, a more likely role for these conserved sequences is in recruitment of protein factors. Indeed, internally positioned linear RNA sequences have been shown to be important for satellite RNA replication in Turnip crinkle virus (TCV), a related Carmovirus (family Tombusviridae; Guan et al. 1997, 2000a,b). In the case of TBSV, the B2 sequence could also act as a linear ligand; however, base-pairing within this sequence is also possible (data not shown) and thus intraelement interactions could potentially contribute to its activity. Also, it is interesting to note that the B2 sequence shares some limited identity (underlined) to the core (+)-strand promoter [(−)- 5′-UGGAGAAUUUCU-OH] defined in vitro previously (Panavas et al. 2002a). If this connection is relevant, the sequence could be involved in recruitment of the RdRp complex.
The third highly conserved element in the DSD is the bulged-A (B1) in S7. This type of motif is known to be important for capsid protein binding in various RNA viruses (Witherell et al. 1991; Fujimura and Esteban 2000). For the TBSV DI RNA, deletion of B1 resulted in a 30% decrease in accumulation (Fig. 10 ▶), indicating that it does contribute to fitness in vivo. This benefit could be related to facilitating replication, or possibly encapsidation, via protein binding. In general, Tombusvirus DI RNAs are not packaged very efficiently into stable particles (Russo et al. 1994), but it is possible that binding of capsid protein could confer some protection and a selective advantage in vivo.
Relevance of the TSD–DSD interaction
Previous analysis of the TSD allowed for development of a structural model for this domain (Wu et al. 2001). S1 was shown to be essential for DI RNA accumulation through mutational analysis, and the other SL structures in the TSD were deduced from sequence and solution structure analysis. We have since confirmed the existence and importance of SL4 in the (+)-strand for TSD activity (D. Ray and K.A. White, unpubl.), and this finding is fully consistent with the identified interdomain PK-TD1 interaction involving L4 and s8 (Fig. 11A ▶). Thus, although the TSD and DSD represent discrete entities in terms of secondary structure, these domains need to interact in order to operate proficiently. The formation of this larger RNA complex could optimally position and present individual ligands within the complex or could unite intradomain elements to form a multidomain binding site. Additionally, as formation of this interdomain interaction strongly influences accumulation of both (+)- and (−)-strand DI RNA accumulation (Fig. 13 ▶), it could represent an effective means of regulating replication.
The 5′ UTR and viral RNA replication
The complement of the 5′ UTR contains an element essential for TBSV RNA replication, namely, the (+)-strand promoter (Panavas et al. 2002a). However, previous studies (Wu et al. 2001) and the present results support a role for the entire (+)-strand of the 5′ UTR in viral RNA replication. We have shown that DI RNA stability was not affected by various modifications introduced into SL5 and the DSD (Fig. 14 ▶). This result indicates that the observed defects are related to reduced replication activity rather than increased degradation. Both (+)- and (−)-strand synthesis were affected proportionally (Fig. 13 ▶), indicating that the defect is equally detrimental to synthesis of both strands. This could be related to a defect that affects both (+)- and (−)-strand synthesis. However, previous studies have shown that the synthesis of both strands is tightly coupled (Ray and White 1999, 2003), thus a defect lowering synthesis of only one of the strands could indirectly lower the production of the other strand. The precise role(s) played by the structures in the 5′ UTR remain to be determined. Based on other systems, possible functions include facilitating (−)-strand synthesis (Barton et al. 2001; Herold and Andino 2001), (+)-strand synthesis (Miller et al. 1998), or targeting RNA templates to replication centers (Chen et al. 2001). Efforts are under way to investigate the step(s) at which the 5′ UTR participates.
Conclusion
This study completes the general structural analysis of the entire 5′ UTR of TBSV within the context of a DI RNA. The analyses revealed two additional functional structural elements, SL5 and the DSD, and defined important features within each. Additionally, the investigation uncovered an interdomain interaction between the TSD and DSD that was critical for optimal activity. Taken together with previous studies on the TSD (Wu et al. 2001), the work establishes a central role for the 5′ UTR in mediating viral RNA replication.
MATERIALS AND METHODS
Viral constructs
Construction of the full-length TBSV genome, pT100 (Hearne et al. 1990), and DI RNA pDI-72SXP (Wu et al. 2001) have been described previously. Mutations were introduced into PCR products by oligonucleotide-mediated PCR mutagenesis using DI-72SXP templates (Table 1 ▶). Mutant S6-CA1 was constructed by ligating a PCR product (generated using primer pairs PF7/PR154) into pDI-72SXP after digesting PCR products and vector with AccI and XbaI. Mutants S6-1a, S6-1b, S7-2b, Δis1, and Δis2 were constructed by subcloning PCR products (using PF7 and phosphorylated PR150, PR149, PR147, PR144, and PR145, respectively) into pDI-72SXP after digestion with SacI/SmaI and subsequently religating AccI/XbaI-digested (S6-1b and S7-2b) or SacI/AccI-digested (S6-1a, Δis1, and Δis2) fragments into similarly digested pDI-72SXP vectors. PCR products corresponding to mutants ΔSL7, IΔ1, IΔ2, IΔ3, IΔ4, S7-1a, S7-1b, S7-1c, S7-2a, S7-2c, S6-GU1, PK-TD1b, Δs8, ΔB1, and ΔB2 (using primer pairs PF7 and PR117, PR75, PR76, PR77, PR78, PR115, PR116, PR117, PR146, PR148, PR153, PR152, PR75A, PR118, and PR119, respectively) were digested with SacI and XbaI and subsequently ligated into a pDI-72SXP vector digested with identical restriction enzymes. SL5 mutants ΔSL5, SL5-2a, SL5-2b, SL5-2c, SL5-106A, SL5-80C, SL5-82U, SL5-104A, ΔL5GAAA, ΔL5(3bp)GAAA, and ΔL5(6bp)GAAA were cloned into the SacI and XbaI sites in pDI-72SXP using three-part ligations. Here, wild-type PCR fragments (using PF7 and phosphorylated PR66) were digested with SacI, whereas mutant PCR products were generated (using PF5 and phosphorylated PR67, PR92, PR93, PR120, PR121, PR122, PR123, PR124, PR125, PR126, respectively) and digested with XbaI. Similarly, for PK-TD1a, a wild-type PCR fragment (using PF7 and phosphorylated PR83) and mutant fragment (using PF5 and phosphorylated PR151) were digested with SacI and XbaI, respectively, and ligated into a similarly digested pDI-72SXP vector. Compensatory mutants S6-1c and PK-TD1c were constructed by isolating mutant fragments from vectors digested with SacI/AccI (S6-1a and PK-TD1a) and AccI/XbaI (S6-1b and PK-TD1b) and ligating them into a pDI-72SXP vector digested with SacI and XbaI.
Table 1.
Oligonucleotides used in the study
| Oligo | Positiona | REb site | Sequencec | Sensed |
| P9 | 4757–4776 | SphI/Smaf | GGCGGCCCGCATCCCGGGCTGCATTTCTGCAATGTTCC | − |
| P50 | 4754–4776 | - | GGAACATTGCAGAAATGCAGCCC | + |
| P51 | 1420–1443 | BamHI | GCGCCGGATCCAGCGGTGCGAAACTCCGTACTTAC | + |
| PF4 | 1285–1304 | XbaI | GCGCGTCTAGAAGAAACGGGAAGCTCGCTCG | + |
| PF6 | 4398–4417 | PstI | GCGCGCTGCAGAGCGAGTAAGACAGACTCTT | + |
| PF7 | 1–20 | SacI | GGCGGAGCTCTAATACGACTCACTATAGGAAATTCTCCAGGATTTCTC | + |
| PR21 | 4645–4668 | NsiI | CCGCGCATGCATATTCCTGTTTACGAAAGTTAGG | + |
| PR35 | 4437–4467 | - | GGAGATGAGTGTAAATCTGGCATAGCATACAGGTTACTGCA | + |
| PR37 | 4686–4708 | - | GACCCAGACACGGTTGATCTCAC | + |
| PR38 | 4727–4739 | - | GATCGCTGGAAGCACTACCGGAC | + |
| PR63 | 1426–1445 | - | GCGAAACTCCGTACTTACAC | + |
| PR66 | 64–78 | - | AGAAATTCTCTACGCAAAGCAACGG | − |
| PR67 | 108–126 | - | TTTGAAGGTTGGGTCTACC | + |
| PR75 | 135–153 | XbaI | GCGGCTCTAGATGGAAGTTACAATTTATCC | − |
| PR75A | 135–155 | XbaI | GCGGCTCTAGAGTTGGAAGTTACAATTTATCC | − |
| PR76 | 111–129 | XbaI | GCGCCTCTAGAAAAGGTAGACCCAACCTTC | − |
| PR77 | 94–114 | XbaI | CGCGGTCTAGACTTCAAACCCCACAACTAAAG | − |
| PR78 | 64–78 | XbaI | CGCCGTCTAGAAGAAATTCTCTACGCAAAGC | − |
| PR92 | 79–107 | - | GTGGATAATTATTATCTTTAGTTGTGGGG | + |
| PR93 | 79–133 | - | CTCCATAATTATTATCTTTAGTTGTCCGCTTTGAAGGTTGGGTCTACCTTTCGG | + |
| PR115 | 102–168 | XbaI | CCGCGTCTAGACATGTCGCTTGTTTGTTGGAAGTTACAATTTATCCGGCCGAAAGGTAGACCCAACCTTCAAACCCCAC | − |
| PR116 | 102–168 | XbaI | CCGCGTCTAGACATGTCGCTTGTTTGTTGGAAGTTACAATTTATCCCCCCGAAAGGAGACCCAACCTTCAAACCCCAC | − |
| PR117 | 71–168 | XbaI | CCGCGTCTAGACATGTCGCTTGTTTGTTGGAAGTTACAATTTCCAACCTTCAAACCCCACAACTAAAGATAATAATTATGG | − |
| PR118 | 102–168 | XbaI | CCGCGTCTAGACATGTCGCTTGTTTGTTGGAAGTTACAATTTATCCCCCCGAAAGGAGACCCAACCTTCAAACCCCAC | − |
| PR119 | 102–168 | XbaI | CCGCGTCTAGACATGTCGCTTGTTTGTTGGATCCCCCCGAAAGGTAGACCCAACC | − |
| PR120 | 79–133 | - | CTCCATAATTATTATCTTTAGTTGTGGAGTTTGAAGGTTGGGTCTACCTTTCGG | + |
| PR121 | 79–107 | - | CCCCATAATTATTATCTTTAGTTGTGGGG | + |
| PR122 | 79–107 | - | CTCTATAATTATTATCTTTAGTTGTGGGG | + |
| PR123 | 79–133 | - | CTCCATAATTATTATCTTTAGTTGTAGGGTTTGAAGGTTGGGTCTACCTTTCGG | + |
| PR124 | 79–120 | - | CTCCATAATTAGAAATAGTTGTGGGGTTTGAAGGTTGGG | + |
| PR125 | 79–120 | - | CTCCATAAGAAATTGTGGGGTTTGAAGGTTGGG | + |
| PR126 | 79–120 | - | CTCCAGAAATGGGGTTTGAAGGTTGGGTCTACC | + |
| PR 144 | 79–125 | AccI | CCGCCGGTAGACCCAACCTTCCCCACAACTAAAGATAATAATTATGGAG | − |
| PR145 | 79–125 | AccI | CCGCCGGTAGACCCAACCAAACCCCACAACTAAAGATAATAATTATGGAG | − |
| PR146 | 102–168 | XbaI | GGCCGTCTAGACATGTCGCTTGTTTGTTGGAAGTTACAATTTATCCCCCCGAAAGGTACTCCCAACCTTCAAACCCCAC | − |
| PR147 | 120–162 | AccI | CCGCGCGTCTACCTTTCGGGGGCTTAAATTGTAACTTCCAACAAACAAGCG | + |
| PR148 | 79–168 | XbaI | GCGCGTCTAGACATGTCGCTTGTTTGTTGGAAGTTACAATTTAAGCCCCCGAAAGGTACTCCCAACCTTCAAACCCCAC | − |
| PR149 | 120–168 | AccI | CCGCGCGTCTACCTTTCGGGGGGATAAATTGTAACTTCGAAGAAACAAGCGACATGTC | + |
| PR150 | 130–168 | AceI | CGCGGCGTAGACCGAAGCTTCAAACCCCAC | − |
| PR151 | 48–81 | - | TGCGCTACCGTGGGATTGCGTAGAGAATTTCTCTCC | + |
| PR152 | 130–168 | XbaI | CCGCGTCTAGACATGTGGAATGTTTGTTGGAAGTTACAATTTATCCCCCCG | − |
| PR153 | 102–168 | XbaI | GGCCGTCTAGACATGTCGCTTGTTTATTGAAAGTTACAATTTATCCCCCCGAAAGGTAGAC | − |
| PR154 | 82–125 | AccI | CCGCACGCAGCCGCGTAGACTCAATCTTCAAACCCCACAACTAAAGATAATAATTATGG | − |
aCoordinates correspond to those of the TBSV genome (Hearne et al. 1990).
bRestriction enzyme site.
cViral sequences corresponding to the coordinates shown are underlined. Restriction enzyme sites are italicized and individual mutations are in boldface.
dRefers to the sense of the oligonucleotide in reference to the plus-sense viral RNA.
Computer-aided analysis of viral RNA
The nucleotide sequences for TBSV (NC_001554), TBSVs (AJ249740), TBSVp (U80935), AMCV (X62493), CBLV (AY163842), CymRSV (X15511), CNV (M25270), CIRV (X85215), PeLV (AY100482), and PoLV (X87115) were obtained from the National Center for Biotechnology Information’s GenBank genetic sequence database. The nucleotide sequence for CLSV has been published (Miller et al. 1997). RNA secondary structures were predicted at 37°C using MFOLD version 3.1. (Mathews et al. 1999; Zuker et al. 1999).
In vitro transcription
Viral transcripts were synthesized in vitro by transcription of SmaI-linearized DNAs using an Ampliscribe T7 RNA polymerase transcription kit (Epicentre Technologies) as described previously (Oster et al. 1998). Transcript concentrations were determined spectrophotometrically, and RNA integrity was verified using agarose gel electrophoresis.
RNA secondary structure probing
For the in vitro analysis of RNA secondary structure, DI-72SXP RNA transcripts (3 μg) were added to yeast RNA (3 μg) and modification buffer (25 mM Tris-HCl at pH 7.5, 200 mM NaCl, 5 mM MgCl2, and 1 mM EDTA), and were equilibrated (95°C for 2 min, 60°C for 10 min, and 37°C for 10 min), treated with various RNA structure probing chemicals (diethyl pyrocarbonate [DEPC] and 1-cyclohexyl-3-(2-morpholinoethyl) carbodiimide [CMCT]), or RNase T1, and analyzed by primer extension as described previously (Wu et al. 2001).
Isolation and inoculation of protoplasts
Protoplasts were prepared from 6- to 8-day-old cucumber cotyledons (var. Straight 8) as described previously (White and Morris 1994b). Isolated protoplasts were inoculated, using polyethylene glycol-CaCl2, as described (White and Morris 1994b) with viral RNA transcripts (1 μg for DI RNA and 2–5 μg for genomic transcripts), and were incubated in a growth chamber under fluorescent lighting at 22°C for 22–24 h.
Analysis of viral RNA accumulation in vivo
Total nucleic acids were harvested from protoplasts as described previously (White and Morris 1994b). One-fifth of the total nucleic acid was separated in denaturing 4.5% polyacrylamide gels containing 8 M Urea. Gels were stained with ethidium bromide to ensure even loading of samples. Northern blot analysis of viral plus-strand accumulation was conducted by transferring total nucleic acid to nylon, followed by hybridization with a 32P-end-labeled oligonucleotide probe (P9) complementary to the 3′-terminal 23 nt of the TBSV genome. The intensity of the hybridization signal was quantified by radioanalytical scanning using an InstantImager (Packard Instrument Co.) and are represented graphically with standard error derived from three independent experiments unless otherwise noted. Analysis of viral minus-strands was performed as described previously (Ray and White 2003). Northern blot analysis was conducted using 32P-end-labeled oligonucleotide probes P50, PR21, PR37, PR38, PF4, PF6, PF7, P51, PR35, and PR63.
DI RNA stability assay
Analysis of DI RNA in vivo stability was performed as described previously (Ray and White 1999). Briefly, protoplasts were inoculated with 5 μg of DI RNA transcripts only (i.e., without T100) and subsequently treated with RNase A (final concentration 10 μg/mL) to remove transcripts outside of the protoplasts. Total nucleic acids were extracted from protoplasts at 1, 4, 12, and 20 h postinoculation, and DI RNA levels were analyzed by Northern blotting using 32P-end-labeled P9 probe.
Acknowledgments
We thank members of our laboratory of reviewing the manuscript. This work was supported by NSERC and PREA.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.rnajournal.org/cgi/doi/10.1261/rna.5630203.
REFERENCES
- Andino, R., Rieckhof, G.E., and Baltimore, D.A. 1990. A functional ribonucleoprotein complex forms around the 5′ end of poliovirus RNA. Cell 63: 369–380. [DOI] [PubMed] [Google Scholar]
- Barton, D.J., O’Donnell, B.J., and Flanegan, J.B. 2001. 5′ cloverleaf in poliovirus RNA is a cis-acting replication element required for negative-strand synthesis. EMBO J. 20: 1439–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsham, G.J. and Sonenberg, N. 1996. RNA–protein interactions in regulation of picornavirus RNA translation. Microbiol. Rev. 60:499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bink, H.H., Hellendoorn, K., van der Meulen, J., and Pleij, C.W. 2002. Protonation of non-Watson–Crick base pairs and encapsidation of turnip yellow mosaic virus RNA. Proc. Natl. Acad. Sci. 99: 13465–13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck, K.W. 1996. Comparison of the replication of positive-stranded RNA viruses of plants and animals. Adv. Virus Res. 47: 159–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, Y.C., Borja, M., Scholthof, H.B., Jackson, A.O., and Morris, T.J. 1995. Host effects and sequences essential for accumulation of defective interfering RNAs of cucumber necrosis and tomato bushy stunt tombusviruses. Virology 210: 41–53. [DOI] [PubMed] [Google Scholar]
- Chen, J., Noueiry, A., and Ahlquist P. 2001. Brome mosaic virus Protein 1a recruits viral RNA2 to RNA replication through a 5′ proximal RNA2 signal. J. Virol. 75: 3207–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimura, T. and Esteban, R. 2000. Recognition of RNA encapsidation signal by the yeast L-A double-stranded RNA virus. J. Biol. Chem. 275: 37118–37126. [DOI] [PubMed] [Google Scholar]
- Gallie, D.R. 2001. Cap-independent translation conferred by the 5′ leader of tobacco etch virus is eukaryotic initiation factor 4G dependent. J. Virol. 75: 12141–12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, H., Song, C., and Simon, A.E. 1997. RNA promoters located on (−)-strands of a subviral RNA associated with turnip crinkle virus. RNA 3: 1401–1412. [PMC free article] [PubMed] [Google Scholar]
- Guan, H., Carpenter, C.D., and Simon, A.E. 2000a. Analysis of cis-acting sequences involved in plus-strand synthesis of a turnip crinkle virus-associated satellite RNA identifies a new carmovirus replication element. Virology 268: 345–354. [DOI] [PubMed] [Google Scholar]
- ———. 2000b. Requirement of a 5′-proximal linear sequence on minus strands for plus-strand synthesis of a satellite RNA associated with turnip crinkle virus. Virology 268: 355–363. [DOI] [PubMed] [Google Scholar]
- Guo, L., Allen, E.M., and Miller, W.A. 2001. Base-pairing between untranslated regions facilitates translation of uncapped, nonpolyadenylated viral RNA. Mol. Cell 7: 1103–1109. [DOI] [PubMed] [Google Scholar]
- Hearne, P.Q., Knorr, D.A., Hillman, B.I., and Morris, T.J. 1990. The complete genome structure and synthesis of infectious RNA from clones of tomato bushy stunt virus. Virology 177: 141–151. [DOI] [PubMed] [Google Scholar]
- Herold, J. and Andino, R. 2001. Poliovirus RNA replication requires genome circularization through a protein–protein bridge. Mol. Cell 7: 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, G., Xin, S., and Cai, Z. 2003. Role of the 5′-proximal stem–loop structure of the 5′ untranslated region in replication and translation of hepatitis C virus RNA. J. Virol. 77: 3312–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason, P.W., Bezborodova, S.V., and Henry, T.M. 2002. Identification and characterization of a cis-acting replication element (cre) adjacent to the internal ribosome entry site of foot-and-mouth disease virus. J. Virol. 76: 9686–9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews, D.H., Sabina, J., Zuker, M., and Turner, D.H. 1999. Expanded sequence dependence of thermodynamic parameters provides robust prediction of RNA secondary structure. J. Mol. Biol. 288: 911–940. [DOI] [PubMed] [Google Scholar]
- Miller, J.S., Damude, H., Robbins, M.A., Reade, R.D., and Rochon, D.M. 1997. Genome structure of cucumber leaf spot virus: Sequence analysis suggests it belongs to a distinct species within the Tombusviridae. Virus Res. 52: 51–60. [DOI] [PubMed] [Google Scholar]
- Miller, E.D., Plante, C.A., Kim, K.H., Brown, J.W., and Hemenway, C. 1998. Stem–loop structure in the 5′ region of potato virus X genome required for plus-strand RNA accumulation. J. Mol. Biol. 284: 591–608. [DOI] [PubMed] [Google Scholar]
- Nagy, P.D. and Pogany, J. 2000. Partial purification and characterization of Cucumber necrosis virus and Tomato bushy stunt virus RNA-dependent RNA polymerases: Similarities and differences in template usage between Tombusvirus and Carmovirus RNA-dependent RNA polymerases. Virology 276: 279–288. [DOI] [PubMed] [Google Scholar]
- Oster, S.K., Wu, B., and White, K.A. 1998. Uncoupled expression of p33 and p92 permits amplification of tomato bushy stunt virus RNAs. J. Virol. 72: 5845–5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray, D. and White, K.A. 1999. Enhancer-like properties of an RNA element that modulates Tombusvirus RNA accumulation. Virology 256: 162–171. [DOI] [PubMed] [Google Scholar]
- ———. 2003. An internally located RNA hairpin enhances replication of Tomato bushy stunt virus RNAs. J. Virol. 77: 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino, L. and Russo, M. 1997. Molecular analysis of the pothos latent virus genome. J. Gen. Virol. 78: 1219–1226. [DOI] [PubMed] [Google Scholar]
- Russo, M., Burgyan, J., and Martelli, G.P. 1994. Molecular biology of Tombusviridae. Adv. Virus Res. 44: 381–428. [DOI] [PubMed] [Google Scholar]
- Sarnow, P. 2003. Viral internal ribosome entry site elements: Novel ribosome–RNA complexes and roles in viral pathogenesis. J. Virol. 77: 2801–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, J. and Taniguchi, K. 2003. The 5′-end sequence of the genome of Aichi virus, a picornavirus, contains an element critical for viral RNA encapsidation. J. Virol. 77: 3542–3548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit, T.L., Leclerc, D., and AbouHaidar, M.G. 1994. The minimal 5′ sequence for in vitro initiation of papaya mosaic potexvirus assembly. Virology 199: 238–242. [DOI] [PubMed] [Google Scholar]
- Valcarcel, J. and Gebauer, F. 1997. Post-transcriptional regulation: The dawn of PTB. Curr. Biol. 7: R705–R708. [DOI] [PubMed] [Google Scholar]
- Vagner, S., Galy, B., and Pyronnet, S. 2001. Irresistible IRES. Attracting the translation machinery to internal ribosome entry sites. EMBO Rep. 2: 893–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marle, G., Dobbe, J.C., Gultyaev, A.P., Luytjes, W., Spaan, W.J., and Snijder, E. J. 1999. Arterivirus discontinuous mRNA transcription is guided by base pairing between sense and antisense transcription-regulating sequences. Proc. Natl. Acad. Sci. 96: 12056–12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani, G. and McClain, W.H. 2000. The GU wobble base pair. A fundamental building block of RNA structure crucial to RNA function in diverse biological systems. EMBO Rep. 1: 18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, K.A. 1996. Formation and evolution of tombusvirus defective interfering RNAs. Sem. Virol. 7: 409–416. [Google Scholar]
- White, K.A. and Morris, T.J. 1994a. Enhanced competitiveness of Tomato bushy stunt virus defective interfering RNAs by segment duplication or nucleotide insertion. J. Virol. 68: 6092–6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 1994b. Nonhomologous RNA recombination in tombusviruses: Generation and evolution of defective interfering RNAs by stepwise deletions. J. Virol. 68: 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherell, G.W., Gott, J.M., and Uhlenbeck, O.C. 1991. Specific interaction between RNA phage coat proteins and RNA. Prog. Nucleic Acid Res. Mol. Biol. 40: 185–220. [DOI] [PubMed] [Google Scholar]
- Wu, B. and White, K.A. 1998. Formation and amplification of a novel tombusvirus defective RNA which lacks the 5′ nontranslated region of the viral genome. J. Virol. 72: 9897–9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 1999. A primary determinant of cap-independent translation is located in the 3′-proximal region of the Tomato bushy stunt virus genome. J. Virol. 73: 8982–8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, B., Vanti, W.B., and White, K.A. 2001. An RNA domain within the 5′ untranslated region of the Tomato bushy stunt virus genome modulates viral RNA replication. J. Mol. Biol. 305: 741–756. [DOI] [PubMed] [Google Scholar]
- Zuker, M., Mathews, D.H., and Turner, D.H. 1999. Algorithms and thermodynamics for RNA secondary structure prediction: A practical guide. In RNA biochemistry and bio/technology (eds. J. Barciszewski and B.F.C. Clark), pp. 11–43. Kluwer Academic Publishers, Dordrecht/Norwell, MA.