Abstract
In mammals, the mRNAs encoding many proteins involved in inflammation bear destabilizing AU-rich elements (AREs) in the 3′-untranslated region. The exosome, a complex of 3′ → 5′ exonucleases, is rate limiting in the destruction of such mRNAs in a mammalian in vitro system, but a role in vivo has not been demonstrated. The phenomenon of ARE-mediated degradation also occurs in the protist parasite Trypanosoma brucei. Messenger RNAs with 3′-untranslated region U-rich elements, which strongly resemble AREs, are extremely unstable in the trypanosome form that parasitizes mammals. The first step in degradation of these mRNAs in vivo is rapid destruction of the 3′-untranslated region; subsequently the mRNA is destroyed by exonucleases acting in both 5′ → 3′ and 3′ → 5′ directions. We here investigated the roles of three subunits of the trypanosome exosome complex, RRP45, RRP4, and CSL4, in this process, depleting the individual subunits in vivo by inducible RNA interference. RRP45 depletion, which probably disrupts exosome integrity, caused a delay in the onset of degradation of the very unstable RNAs, but did not affect degradation of more stable species. Depletion of RRP4 or CSL4 does not affect the stability of the residual exosome and did not change mRNA degradation kinetics. We conclude that the exosome is required for the initiation of rapid degradation of unstable mRNAs in trypanosomes.
Keywords: Exosome, Trypanosoma, mRNA, degradation, turnover
INTRODUCTION
Control of mRNA stability is essential for growth and differentiation of eukaryotic cells, and the disruption of mRNA degradation mechanisms leads to defects in development and homeostasis (Carballo et al. 2000; Keene 2001; Wang et al. 2001). Although there are exceptions, eukaryotic mRNA half-lives are usually determined by sequences in the 3′-untranslated regions (3′-UTRs) and degradation is generally initiated by shortening of the poly(A) tail (Beelman and Parker 1995; Mitchell and Tollervey 2000 Mitchell and Tollervey 2001; van Hoof and Parker 2002). In yeast, the next step is removal of the 5′-cap structure, which is followed by progressive degradation mainly from the 5′-end (Beelman and Parker 1995). Thus mutations in yeast that affected either the decapping complex or the major 5′ → 3′ exonuclease, Xrn1p, delayed mRNA degradation, and the inclusion of degradation-resistant G 18secondary structures in test transcripts resulted in preferential accumulation of degradation products lacking the 5′ portion of the mRNA (Beelman and Parker 1995). Degradation from the 3′-end of deadenylated yeast mRNAs is effected by a complex of 3′ → 5′ exonucleases called the exosome. Because the exosome is also involved in the nuclear processing of many stable mRNAs (Allmang et al. 1999; Zanchin and Goldfarb 1999; van Hoof et al. 2000), depletion of exosome activity in conditional mutants inhibits yeast cell growth. Despite this, exosome depletion in yeast does not have any measurable effects on the turnover of mRNAs unless additional mutations in mRNA transport or processing are present (for examples, see Jacobs et al. 1998; Hilleren et al. 2001; Torchet et al. 2002).
In mammals, many experiments have concentrated on degradation of mRNAs that contain a destabilizing AU-rich element (ARE) in the 3′-UTR (Chen and Shyu 1995). These include mRNAs involved in lymphocyte and granulocyte differentiation and in control of the cell cycle. Regulation of the degradation is effected by ARE-binding proteins such as HuR and related ELAV family members, which stabilize the mRNAs, and TTP (HnRNP-D) and AUF1, which can promote degradation (Kiledjian et al. 1997; Lai et al. 1999; Loflin et al. 1999; Mahtani et al. 2001). Again, degradation of both unstable and stable mRNAs is initiated by deadenylation. Results obtained using mammalian in vitro extracts suggest, however, that the subsequent degradation proceeds predominantly in the 3′ → 5′ direction, and in vivo data are also consistent with this (Ford et al. 1999; Chen et al. 2001; Wang and Kiledjian 2001; Mukherjee et al. 2002). The in vitro results implicate the exosome as a major contributor to degradation (Chen et al. 2001; Mukherjee et al. 2002). The exosome interacts in vitro with the decapping machinery (Wang and Kiledjian 2001), with regulatory binding proteins, and possibly also with the AREs (Chen et al. 2001; Mukherjee et al. 2002). No conditional knockouts of the degradative machinery have been described in mammalian cells, so the roles of the exosome and of 5′ → 3′ exonucleases in vivo have not been directly tested.
Trypanosoma brucei, the sleeping sickness trypanosome, parasitizes the blood and tissue fluids of mammals, and is transmitted in sub-Saharan Africa by Tsetse flies. Considerable adaptations in morphology, surface composition and metabolism—and therefore in gene expression—are required for T. brucei to survive the disparate environments in the mammal and the fly. Remarkably, there is no evidence at all for regulation of RNA synthesis by RNA polymerase II in trypanosomes (reviewed in Clayton 2002). Instead, the protein-coding genes are arranged in long arrays that are continuously transcribed. The mRNAs encoding single proteins are generated by posttranscriptional processing: trans splicing of a capped, 39-nt “spliced leader” RNA to the 5′-end and cleavage and polyadenylation at the 3′-end. Thus, the final levels of protein products have to be determined almost exclusively at the posttranscriptional level. The amounts of mRNAs are determined by splicing efficiency and by the rate of mRNA degradation, and the levels of proteins are further influenced by the rates of translation and by protein stability (Clayton 2002). This makes trypanosomes an interesting model for the study of posttranscriptional regulation.
The degradation of unstable mRNAs in bloodstream trypanosomes is, according to all results obtained so far, remarkably similar to the process in mammalian cells. The mRNAs encoding the EP1 major surface protein, the cytosolic phosphoglycerate kinase (PGKB), and pyruvate phosphate dikinase (PPDK) are all stable in procyclic (Tsetse fly midgut) forms and unstable in bloodstream forms. Each of these trypanosome mRNAs contains, in the 3′-UTR, a U-rich element (URE) that resembles mammalian AREs in both sequence and predicted secondary structure (Drozdz and Clayton 1999; Quijada et al. 2002). (The trypanosome UREs lack the regularly-spaced A residues found in many AREs.) Mutation or deletion of the UREs impaired stage-specific degradation of reporter transcripts containing the PGKB, EP1, or PPDK 3′-untranslated regions (Hotz et al. 1997; Schürch et al. 1997; Quijada et al. 2002). A detailed analysis of EP1reporter mRNA turnover in bloodstream forms showed that although degradation appeared to proceed from both ends, the 3′-untranslated region was degraded before the 5′-end (Irmer and Clayton 2001). In addition, overexpression of human HuR in bloodstream trypanosomes increased the abundance of the three URE-containing reporter transcripts, whereas control mRNAs lacking the UREs were unaffected (Quijada et al. 2002). In concordance with this, extracts from the Leptomonas seymouri, a kinetoplastid that is related to T. brucei, were found to behave very similarly to mammalian extracts with regard to the degradation of RNA substrates: Degradation was predominantly from the 3′-end, and was stimulated by a mammalian ARE sequence (Milone et al. 2002).
The trypanosome exosome consists of at least 11 subunits (Estévez et al. 2001, 2003). It resembles both the yeast and human exosomes in overall structure (Estévez et al. 2003) although RRP44, the trypanosome homolog of the Rrp44p subunit of the yeast exosome, does not copurify with the trypanosome exosome (Estévez et al. 2001). We previously showed that RNA-interference-mediated depletion of eight individual exosome components inhibited procyclic trypanosome growth and affected 5.8S rRNA processing (Estévez et al. 2001). We have now extended this anlaysis to study the effects of exosome depletion on mRNA degradation in bloodstream trypanosomes.
MATERIALS AND METHODS
Trypanosomes, plasmids, and transfections
Bloodstream forms of T. brucei Lister 427 were cultured and transfected as described (Biebinger et al. 1997; van Deursen et al. 2001). All plasmids used are listed in Table 1 ▶, and more details including reconstructed sequences are available from the authors. Transfection with pHD 1313 and pHD 514 (Table 1 ▶) resulted in constitutive expression of the Tn10 tetracycline repressor and T7 RNA polymerase. The resulting cells were transfected with tetracycline-inducible RNA interference plasmids designed to give stem–loop RNAs (pHD 1153, 1154, 1199; Table 1 ▶; Estévez et al. 2001). Cloned cell lines showing maximal RNA interference by Northern blot or Western blot were chosen for further study. They were subsequently transfected with plasmids containing the open reading frame of chloramphenicol acetyltransferase (CAT) gene fused to various downstream 3′-UTRs using puromycin as a selectable marker (Table 1 ▶; Fig. 2 ▶, below). To make these plasmids, the hygromycin resistance cassette (HYG) marker in pHD 774 was replaced with the puromycin resistance cassette (PAC) marker from pHD 1034, and the single tet operator in pHD 774 was deleted by BglII digestion and religation. In plasmids pHD 1344, 1413, 1345, 1414, and 1416, the 3′-UTR downstream from the chloramphenicol acetyltransferase (CAT) gene was replaced by the ACT 3′-UTR, the ep1ΔURE 3′-UTR, versions containing G30C30 sequences, or the PPDK 3′-UTR (see Table 1 ▶). In plasmids 1415 and 1417, the entire intergenic region was exchanged for wild-type or mutant versions of the PGKB-PGKC intergenic region.
TABLE 1.
Plasmids used in this study
| Transgene | Selectable marker | |||||||||
| Plasmid (pHD #) | 5′-UTR | ORF | 3′ UTR | 5′ UTR | ORF | 3′ UTR | Promoter | Integration locus | Parent plasmid (pHD #) | Referencesa |
| 1313 | EP1 | 2× TetR | ACT, VSG | ACT | BLE | ACT | TUB | 449 | 1, 2 | |
| 514 | EP1 | T7 RNA POL | ACT | ACT | NEO | ACT | TUB | 514 | 3 | |
| 1153b | EP1 | RRP4 RNAi | VSG | ACT | HYG | ACT | EPIT1 | RRNA | 1146 | 4, 5 |
| 1154b | EP1 | RRP45 RNAi | VSG | ACT | HYG | ACT | EPITi | RRNA | 1146 | 4, 5 |
| 1199 | EP1 | CSL4 RNAi | ACT | ACT | HYG | ACT | EPITi | RRNA | 1146 | 4 |
| 1413c | EP1 | CAT | EP1 | ACT | PAC | ΔALD | T7 | RRNA | 774 | 5, 6 |
| 1344c | EP1 | CAT | ACT | ACT | PAC | ΔALD | T7 | RRNA | 789, 1034 | 5, 6 |
| 1414c | EP1 | CAT | ep1ΔURE | ACT | PAC | ΔALD | T7 | RRNA | 775 | 5, 6 |
| 1415c | EP1 | CAT | PGKB | PGKC | PAC | ΔALD | T7 | RRNA | 869 | 5, 7 |
| 1417c | EP1 | CAT | pgkbΔURE | PGKC | PAC | ΔALD | T7 | RRNA | 1044 | 5, 7 |
| 1416c | EP1 | CAT | PPDK | ACT | PAC | ΔALD | T7 | RRNA | 1317 | 5, 7 |
| 1343c | EP1 | CAT | G30C30EP1 | ACT | PAC | ΔALD | T7 | RRNA | 1413, 921 | 5, 6 |
a(1) Biebinger et al. 1997; (2) L. Storm, D. Horn, and C. Clayton, in prep.; (3) C. Hartmann and C. Clayton, unpubl.; (4) Estévez et al. 2001; (5) this study; (6) Irmer and Clayton 2001; (7) Quijada et al. 2002.
bThese plasmids are similar to those described in Estévez et al. (2001) except that the 3′ UTRs following the RNAi stem-loop sequences are from a VSG locus.
cThese plasmids are similar to those described in Irmer and Clayton (2001) or Quijada et al. (2002) except that the selectable marker is PAC and the Tet operator sequence is absent.
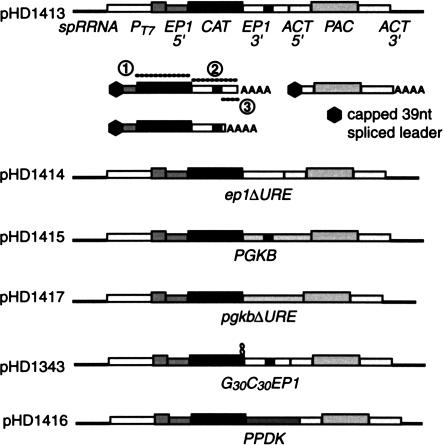
FIGURE 2.
Plasmid constructs used to study the effects of exosome subunit depletion on mRNA degradation. The elements indicated are carried in a pGEM backbone. Constructs were linearized within the rRNA spacer region before transfection. The mRNAs produced from the CAT-EP1 plasmid are shown beneath the construct. The CAT mRNAs from the constructs with alternative 3′-UTRs are similar; for ep1ΔURE, only one major polyadenylation site was found whereas for the PGKB mRNAs, several were used (Quijada et al. 2002). Probes used for Figures 3 ▶ and 4 ▶ are shown as dotted lines with circled numbers: (1) CAT probe; (2) EP1 3′-UTR probe; (3) probe specific for longer EP1 3′-UTR.
RNA extraction, RNase-H digestion, RT-PCR, and Northern blots
To induce RNA interference, cells at initial density of 1.0 × 104 were cultured for 48 h with or without 100 ng/mL of tetracycline. For RNA turnover studies, cells at a density of less than 1.2 × 106 were divided into appropriate volumes (usually 50 mL). One volume each, from control and tetracycline-induced, was used for RNA extractions. This portion was referred as time 0. To each of the remaining volumes, actinomycin was added and RNA was extracted at appropriate time points. Total RNA was isolated using peqGOLDTrifast (peqLab) according to the manufacturer’s instructions. Ten to 20 μg RNA were either directly separated on formaldehyde-agarose gels or 5% urea-polyacrylamide gels. RNase H digestion (to detect poly(A) tails) was done as described (Irmer and Clayton 2001) except that RNA and oligos were first denatured at 65°C for 10 min. RNA was transferred onto Nytran membranes (Schleicher and Schuell) by downward cappilary blotting or semidry blotting from agarose or polyacrylamide gels, repectively. Blots were hybridised with 32P-labeled DNA (random-primer labeling) or RNA (in vitro transcription) probes and bands were quantified using a PhosphorImager (Molecular Dynamics).
For mapping of polyadenylation sites, 5 μg of total RNA were used as a template for first-strand cDNA synthesis using Superscript Reverse Transcriptase (Invitrogen) according to the manufacturer’s instruction and CZ1584 (5′-TTGAATTCGCATTGAGC ACCTGC(T)18NN-3′) as a primer. Subsequently, PCR was done as described (Hug et al. 1994) using CZ1584 as antisense primer.
The processing of 5.8S rRNA was studied as described previously (Estévez et al. 2001).
Protein analysis
For Western blots, 0.5–2.0 × 106 cells were lysed, separated on SDS-PAGE, and transferred onto a nitrocellulose membrane (Optitran BA-S85, Schleicher & Schuell). Blots were incubated with rabbit polyclonal anti-TbRRP4, anti-TbRRP44, anti-TbRRP45, or anti-TbCSM (Estévez et al. 2001; Guerra-Giraldez et al. 2002). Blots were subsequently incubated with donkey horseradish peroxidase linked antirabbit IgG (Amersham Biosciences). Proteins were detected using the ECL system (Amersham Biosciences) according to the manufacturer’s instructions.
Metabolic labeling of proteins was done as described (Quijada et al. 2002).
RESULTS
Depletion of exosome components in bloodstream trypanosomes
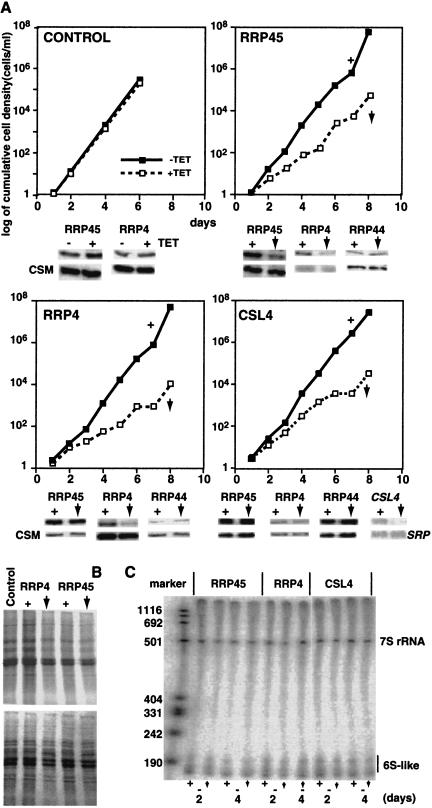
The UREs that have been characterized so far cause developmentally regulated mRNA instability in bloodstream forms. To determine the role of the exosome in URE-mediated mRNA degradation, we therefore first had to generate conditional exosome mutants in that life-cycle stage. We started with bloodstream trypanosomes that expressed the tet repressor (for inducible production of dsRNAs) and bacteriophage T7 polymerase (for transcription of reporter genes, see below). These were transfected with plasmids designed for expression of various stem–loop RNAs under the control of a tetracycline-inducible promoter. The sequences of the double-stranded stems corresponded to parts or mRNAs encoding different exosome components (Estévez et al. 2001). Addition of tetracycline to the medium resulted in induction of double-stranded RNAs that initiated destruction of the cognate mRNAs by RNA interference. The effects of depletion of RRP45, RRP4, and CSL4 on bloodstream trypanosome growth are shown in Figure 1A ▶. Western blot analyses showed that protein levels were reduced to about 10% of normal levels 24 h after tetracycline addition (Fig. 1A ▶), and remained at that level for several days (not shown). These reductions are similar to those previously reported for RNAi of exosome components in procyclic forms (Estévez et al. 2001).
FIGURE 1.
(A) Effect of exosome subunit depletion on trypanosome growth. Cumulative growth curves are shown for depletion of the subunits shown. Below each curve is shown the effect of the depletion of RRP45, RRP4, or CSL4 on the abundances of RRP45, RRP44, and RRP4. The plus sign (+) indicates that normal levels of exosome are present. The vertical, downward-pointing arrow indicates that the level of the exosome subunit indicated above the growth curve is reduced. All panels are Western blots except that for CSL4, which is a Northern blot. CSM is an unrelated protein and SRP is the signal recognition particle RNA, both used as controls. RRP44 is an exonuclease that is not associated with the exosome. (B) The effects of RRP45 depletion on metabolic incorporation of 35S-labeled methionine. The upper panel shows autoradiography and the lower panel the corresponding Coommassie-stained gel. (C) The effects of RRP45, RRP4, or CSL4 depletion on 5.8 S rRNA maturation, analyzed as in Estévez et al. (2001).
Procyclic forms with 90% reduction of RRP45 or RRP4 show severe growth defects, which culminate in cell death (Estévez et al. 2001); CSL4 reduction results in very slow growth. The depleted bloodstream forms, in contrast, showed only a reduced growth rate (Fig. 1A ▶). As previously seen (Helfert et al. 2001; Guerra-Giraldez et al. 2002), tetracycline did not affect the growth of the parent cells, which lack the RNAi plasmids but are otherwise identical to the RRP45, RRP4, and CSL4 cell lines (Fig. 1A ▶). Figure 1B ▶ shows that the induction of double-strand RNAs, at least in the cases of RRP4 and RRP45, did not significantly affect overall protein synthesis despite the reduced growth rate. This lack of obligate coupling of protein synthesis with growth rate has also been previously observed (Quijada et al. 2002).
The depleted procyclic forms had clear defects in processing of 5.8S rRNA (Estévez et al. 2001). In contrast, we found no defects in 5.8S rRNA processing in the depleted bloodstream forms (Fig. 1C ▶). Quantitative analysis of several mature and precursor mRNAs by RNAase protection also revealed no changes after RRP45 depletion (data not shown).
Structural predictions, and the results of two-hybrid analyses with the human exosome, suggest that RRP45 forms part of an exosome “core” containing six RNAase PH subunits (Aloy et al. 2002; Raijmakers et al. 2002). Our results from procyclic forms are consistent with this. For example, depletion of RRP45 resulted in codepletion of RRP4, whereas depletion of either RRP4 or CSL4 had no effect on the abundance of RRP45 (Estévez et al. 2003). To test that this was also so in our bloodstream-form lines, we probed Western blots from the different depleted cell lines with appropriate antibodies. The results (Fig. 1 ▶) were consistent with those obtained in procyclics.
Cell lines for determining the effects of depletion on turnover of very unstable reporter mRNAs
The three URE-containing mRNAs that we have previously characterized in trypanosomes have very low abundance. To study their turnover kinetics, it is therefore necessary to overproduce reporter mRNAs carrying their destabilizing 3′-UTRs. Our reporter constructs (Fig. 2 ▶; Table 1 ▶) contained the following components (in addition to the plasmid backbone):
A segment of the nontranscribed spacer from the ribosomal RNA locus (spRRNA) containing a unique restriction site. Linearization within this region results in the insertion of the transfected plasmid DNA in the nontranscribed spacer by homologous recombination.
A T7 promoter. This ensures much higher production of the reporter mRNA, by T7 polymerase, than is possible using endogenous trypanosome polymerases (Irmer and Clayton 2001). The T7 polymerase is constitutively expressed from an integrated transgene in the recipient trypanosomes. We have previously shown that the overproduction of reporter mRNAs does not affect their posttrancriptional regulation (Irmer and Clayton 2001).
A trans splicing signal from the EP1 locus, which has no regulatory properties but which ensures accurate 5′ trans-splicing of the CAT RNA.
A CAT gene.
Different 3′-UTRs. The 3′-UTR from a constitutively expressed actin (ACT) gene served as a control; the remaining 3′-UTRs were from the EP1, PPDK, or PGKB genes (Irmer and Clayton 2001; Quijada et al. 2002). Mutant versions of the 3′-untranslated regions lacking the UREs—ep1ΔURE and pgkbΔURE—were also included (Fig. 2 ▶).
A second trans-splicing signal (from the ACT locus) and 5′-UTR. This drives trans-splicing of the downstream RNA and ensures polyadenylation of the CAT transcript.
A puromycin resistance (PAC) marker with an ACT 3′-UTR.
Each of these plasmids was transfected into the RRP45 RNAi cell line and permanent transformants were selected. Similar lines were made using the CAT-ACT, CAT-PGKB, CAT-EP1, CAT-EP1ΔURE, and CAT-pgkbΔURE plasmids in the CSL4 and RRP4 RNAi cell lines. As an additional control, the plasmid with the PGKB 3′-UTR was transfected into cells expressing the tet repressor and T7 polymerase, but without an RNAi plasmid.
Careful examination of our Northern blots for the CAT-EP1 cell lines (for example, see Fig. 3 ▶), revealed that—in contrast to all the other lines—there were two CAT mRNA bands. The lower band was of variable intensity and was not always clearly resolved. The RNA in this lower band was polyadenylated, as it was present in poly(A)+ RNA (data not shown) and decreased in length when treated with oligo d(T) and RNAase H (Irmer and Clayton 2001; data not shown). To determine the identity of the two mRNAs, we performed RT-PCR using oligo d(T) and CAT primers. This yielded products of two lengths, whose structures were confirmed by restriction digestion and by sequencing (not shown). One of the products represented RNA that was polyadenylated in the usual position for EP1 transcripts, and the other represented transcripts that were polyadenylated just downstream from the URE (see Fig. 2 ▶). Similar polyadenylation positions were previously seen in transcripts from deletion constructs (Hug et al. 1994); they are probably directed by a polypyrimidine tract that is upstream of the normal polyadenylation site (Hug et al. 1994).
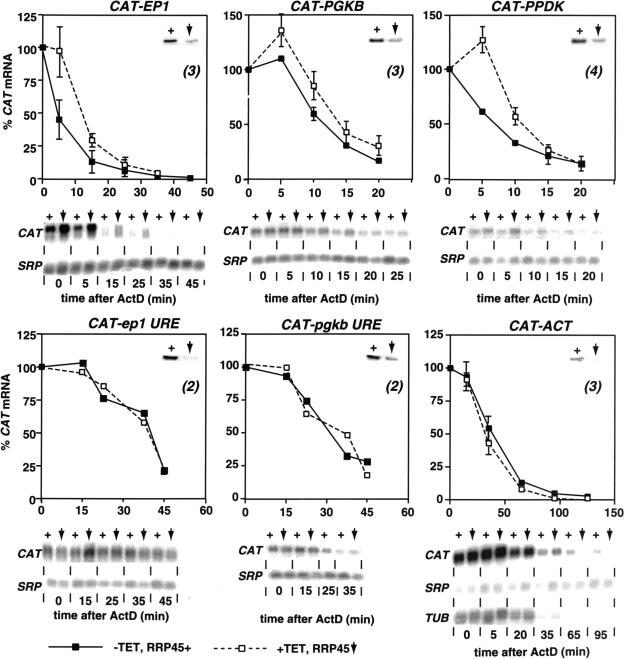
FIGURE 3.
Effect of RRP45 depletion on mRNA abundance and stability. Trypanosomes expressing different reporter RNAs (indicated above the graphs) were treated with actinomycin D, and RNA was isolated at the various times thereafter. RNAs were detected by Northern blotting and hybridization with [32P]-labeled probes, and quantitated by phosphorImager using the SRP RNA as an internal control. For each cell line, we show a control Western blot showing typical RRP45 depletion for the cell line. Beneath the graphs are typical Northern blots. Point 0 is before actinomycin D addition; the first time point corresponds to cells that were centrifuged immediately after actinomycin D addition. (Processing takes 5–10 min.) As for Figure 1 ▶, the plus sign (+) indicates that normal levels of RRP45 are present and the vertical, downward-pointing arrow indicates that the level of RRP45 is reduced. Results from three or more experiments are displayed as mean and standard deviation.
To study mRNA decay, we took each cell line and induced RNA interference by addition of tetracycline. Cells without tetracycline served as controls. Twenty-four hours later, we inhibited transcription by addition of Actinomycin D, and followed transcript fates by Northern blotting (Irmer and Clayton 2001). The SRP RNA, which is stable over the time course of the experiments (Quijada et al. 2002), served as an internal control. Addition of tetracycline to the cell line expressing the tet repressor, T7 polymerase, and the CAT-PGK mRNA, without any RNAi, had no effect on CAT mRNA degradation (not shown).
Depletion of CSL4 or RRP4 had no effect on the degradation of the various CAT mRNAs (not shown). Thus, either these subunits are not required for rapid degradation in vivo, or the RNAi-mediated depletion was not sufficiently effective to make the levels of these subunits rate limiting.
Effect of RRP45 depletion on mRNA turnover kinetics
Depletion of RRP45 causes more extensive exosome disruption than depletion of RRP4 or CSL4. Correspondingly, there were clear effects on mRNA degradation. Results for cell lines expressing all the different reporter mRNAs are shown in Figure 3 ▶. (For the PGKB, pgkbΔURE, and ep1ΔURE plasmids, results for one additional cell line each gave similar results.) In the cells that had not been treated with tetracycline (solid lines, filled squares), the degradation kinetics for the CAT mRNAs were similar to those previously reported: mRNAs with URE-bearing 3′-UTRs (EP1, PGKB, and PPDK) decayed with half-lives of around 5 min, whereas mRNAs lacking UREs (ep1ΔURE, pgkbΔURE, and ACT) showed an initial increase in abundance before decaying with half-lives of around 15 min. (Hotz et al. 1997; Irmer and Clayton 2001; Quijada et al. 2002). The mechanism of the apparent initial rise, which is very reproducible, is not understood. Somewhat to our surprise, the depletion of RRP45 for 48 h had no reproducible effect on the steady-state abundance of CAT mRNAs, independent of the 3′-UTR.
The decay kinetics of the mRNAs lacking UREs (including the endogenous tubulin mRNA, TUB) were unaffected by RRP45 depletion (Fig. 3 ▶, lower panels). In contrast, the depletion of RRP45 caused a significant delay in the degradation of CAT mRNAs with 3′ UREs. These now showed a brief rise in abundance, like their more stable counterparts, before degradation was initiated (Fig. 3 ▶, upper panels). These results suggest that RRP45 (or the core exosome) had become rate limiting for the initiation of mRNA degradation.
Quantitation of the CAT-EP1 transcripts was usually done by pooling the signals of both species: First, the two bands were insufficiently resolved to allow separate quantitation, and second, we expect the deadenylated full-length transcript to comigrate with the shorter polyadenylated species. In the absence of tetracycline, the overall half-life of the pooled bands was between 5 and 10 min, as previously seen (Hotz et al. 1997; Irmer and Clayton 2001). When we reevaluated the bands separately, the upper band had a half-life of less than 5 min, whereas the lower band had a half-life of nearly 10 min, perhaps because the close proximity of the poly(A) tail affected the conformation or accessibility of the URE. This species was nevertheless less stable than the CAT-ep1ΔURE transcript.
Effect of RRP45 depletion on degradation intermediates
We previously found that (G)30(C)30 structures were transiently able to inhibit the action of trypanosome 3′ → 5′ and 5′ → 3′ exonucleases in vivo (Irmer and Clayton 2001). In particular, transcripts that contained (G)30(C)30 structures on each side of the CAT gene produced a transient intermediate containing only the CAT coding region with the surrounding protective structures. Other results also suggested that degradation was occuring from both ends (Irmer and Clayton 2001).
To find out if RRP45 depletion affected the degradation pattern, we followed the degradation of an RNA with a (G)30(C)30 structure between the CAT gene and the EP1 3′-UTR. Figure 4A ▶ shows a Northern blot in which all the major RNA products were detected. Their identities (diagrammed on the left) were determined using specific CAT and EP1 3′-UTR probes. The slowest-migrating band, a, represents the full-length polyadenylated transcript, which was degraded with a half-life of about 7 min. Band b, immediately below it and corresponding to the transcript polyadenylated just after the URE, was more stable. Below this was band c, which did not hybridize with an EP1 3′-UTR probe and was—as shown previously (Irmer and Clayton 2001)—only seen when the (G)30(C)30 structure was present in the reporter RNA. This species migrated in the position expected for the product of 3′ → 5′ degradation, terminating at the (G)30(C)30 structure. Two (G)30(C)30-EP1 3′-UTR fragments were present: These hybridized with the 3′-UTR probe but not the CAT probe and were the products of 5′ → 3′ degradation of the full-length (d) and truncated (e) mRNAs. Using polyacrylamide gels, we could also detect the stub containing only the (G)30(C)30 sequence (not shown).
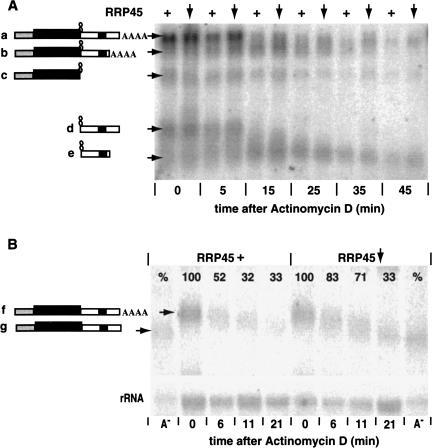
FIGURE 4.
(A) The effects of RRP45 depletion on degradation of an RNA containing a strong secondary structure, G30C30, at the 3′-boundary of the CAT cassette. The experiment was performed as for Figure 3 ▶, and RNAs were identified using a probe hybridizing with the CAT gene (probe 1 in Fig. 2 ▶) and the EP1 3′-UTR (probe 2 in Fig. 2 ▶). The identities of the bands were determined using individual CAT and EP1 3′-UTR probes (not shown). As before, the plus sign (+) indicates that RRP45 was present at approximately normal levels (no tetracycline addition, no RNAi induction). The downward arrow indicates that the level of RRP45 was reduced (tetracycline present). (B) The effects of RRP45 depletion on degradation of the full-length CAT-EP1 RNA (analagous mRNA “a” in panel A, but without G30C30). The experiment was performed as for Figure 3 ▶, except that samples were run on a 2% agarose gel. Samples that were predigested with RNase H in the presence of oligo dT serve as markers for fully deadenylated transcripts (A−). The bands were detected with an RNA probe to the portion of the 3′-UTR that is absent in the shorter CAT-EP1 transcript (probe 3 in Fig. 2 ▶). The RNA probe cross-hybridized with ribosomal RNA, so we used this signal (lower panel) as a standard for phosphoImager quantitation, which is shown as percentage values on the lanes.
At the 5-min and 15-min time points, species a was reproducibly more abundant in the exosome-depleted cells, confirming that the exosome was required for the initiation of degradation. Band b showed a similar increase, particularly at the 15-min time point. It therefore seemed likely that deadenylation was affected. But neither the abundance of the 3′-degradation product c, nor the degradation kinetics of the 5′-degradation products d and e were affected by RRP45 depletion. Initially we found this surprising. There are, however, two possible explanations. First, the exosome-depleted cells still contained roughly 10% of the normal exosome activity. It may well be that this was sufficient for normal degradation kinetics once deadenylation had occurred. Second, the exosome-depleted trypanosomes retained normal levels of other 3′–5′ exonucleases, including RRP44 (Fig. 1 ▶). These exonucleases might be able to take over degradation once deadenylation is complete. Both explanations suggest that the exosome might affect the rate of removal of the poly(A) tail by a deadenylase or an endonuclease.
Our previous results provided no evidence for a discrete deadenylation step preceding degradation of unstable mRNAs containing UREs (Irmer and Clayton 2001). This was in contrast to the pathway seen for more stable mRNAs. To check the influence of the exosome on deadenylation, we specifically examined the effect of RRP45 depletion on degradation of the full-length CAT-EP1 mRNA. RNA degradation was studied as before (Fig. 3 ▶), but the blot was probed with a labeled RNA complementary to the far 3′-end and specific for the longer CAT-EP1 transcript (Fig. 4B ▶). With wild-type levels of RRP45, some deadenylation was clearly detected within 6 min of actinomycin D addition. Almost complete removal of the poly(A) tail was seen only after 30 min. There was no evidence for a deadenylated intemediate. In RRP45 depleted cells, deadenylation kinetics were similar although the complete degradation was—as always—delayed. It is possible that the gradually deadenylated RNAs detected in this experiment were actually RNAs that had so far escaped the normal mechanism of rapid URE-mediated decay. Results for the CAT-PGKB mRNA also provided no indication of any effects of exosome depletion on deadenylation kinetics (data not shown).
In an attempt to determine the role of RRP44, we introduced an additional plasmid-mediating RRP44 RNAi into the RRP45 RNAi cell line. Addition of tetracycline to this line was shown by Western blot to result in depletion of RRP45 and RRP44, and cell growth stopped within 4 days, indicating that the depletion of the exosome and RRP44 is more deleterious than exosome depletion alone (not shown). Unfortunately we could not study these cells because the RNAi phenotype was unstable. Either continuous cultivation or freezing resulted in the selection of cells that no longer gave RRP45 depletion upon tetracycline addition.
DISCUSSION
The direction of mRNA degradation in mammals and kinetoplastids
In mammalian in vitro systems, mRNA degradation proceeds predominantly in the 3′ → 5′ direction (Chen et al. 2001; Wang and Kiledjian 2001; Mukherjee et al. 2002); experiments involving electroporation of synthetic substrates into mammalian cells give similar results (Wang and Kiledjian 2001). Although careful examination of the data reveals that 5′ → 3′ exonucleases were active, 5′-digested intermediates have not been seen. It is not clear whether these results truly reflect the situation of endogenously synthesized mRNAs in intact cells: The synthetic substrates used in both types of experiment are stripped of the proteins present on messenger RNPs and the in vitro systems may lack contributing components or structures (Sheth and Parker 2003). Experiments to determine the direction of degradation of endogenously synthesized mRNAs in mammalian systems also showed mostly 3′ → 5′ activity, but attempts to find intermediates have been hampered by the fact that the insertion of G16 tracts within mRNAs appears to be unable to inhibit the exonucleases.
Experiments using an extract from Leptomonas seymouri, a parasite related to trypanosomes, yielded very similar results to those from mammalian extracts (Milone et al. 2002). In particular, decapping activities were detected and the predominant direction of mRNA degradation, as determined by the inclusion of a G18 tract within the substrate, was 3′ → 5′. Degradation was stimulated by the inclusion of a mammalian destabilizing AU-rich element. It is interesting that—as in mammalian cells, and in contrast with the in vitro result—degradation of endogenously synthesized mRNAs containing internal G16 or G30 tracts in intact trypanosomes (in vivo) results in no detectable intermediates (Irmer and Clayton 2001), indicating that degradation must be more processive in vivo than in vitro. For intermediates to be detectable in trypanosomes, it was necessary to use G30 C30 sequences. Once this was done, it was apparent that degradation proceeded in both directions (Irmer and Clayton 2001; this paper). G30C30 tracts have, to our knowledge, not been tried in mammalian cells.
The role of the exosome in mRNA degradation
Several sets of experiments have shown that the exosome is critical in mRNA degradation in mammalian in vitro systems. Chen et al. (2001) found that removal of the exosome from a Jurkat cell extract, using a variety of different antibodies, strongly inhibited the degradation, but not the deadenylation, of ARE-containing substrates; effects on non-ARE substrates were not apparent. They found that a purified exosome preparation alone preferentially degraded ARE-containing substrates, but had little effect on substrates lacking an ARE, and that the ARE-binding proteins KSRP and TTP, which were associated with the exosome preparation, were required for rapid degradation. Very similar results were described for HeLa extracts (Mukherjee et al. 2002), although in this system it appeared that the exosome might be recognizing the ARE directly. Exosome depletion did not entirely prevent ARE-dependent degradation in either system; the remaining activity could have been due either to residual exosome that had escaped depletion or to other exonucleases. Attempts to reduce exosome activity in whole mammalian cells have not yet been reported.
In vivo depletion of the exosome components RRP45, RRP4, and CSL4 decelerated growth of bloodstream-form trypanosomes, but there were no detectable effects on rRNA processing. These results contrast with the more severe defects we had previously seen with procyclic trypanosomes. In bloodstream forms, the residual exosome activity is apparently sufficient for basic exosome housekeeping functions such as 5.8S rRNA processing. It may be that the parasites adapt their growth to allow RNA processing to keep pace; it may also be that in bloodstream forms, more extensive depletion of the exosome is very rapidly lethal, so that only those cells with tolerable exosome levels survive. Similar adaptation and “escape” phenomena have been seen before in conditionally mutant trypanosomes. We have also seen that many essential components can relatively easily be depleted by about 80%–90%, but beyond that, a threshold is reached where either the cells die or further depletion is technically impossible (see, e.g., Helfert et al. 2001; Guerra-Giraldez et al. 2002). It would clearly be useful to study mRNA degradation in the procyclic RNAi mutants. There is no evidence that UREs promote degradation in this stage, so we are currently trying to identify both suitable substrate mRNAs that are stable in bloodstream forms and unstable in procyclics, and the relevant destabilizing 3′-UTR elements.
Although the overall abundance of URE-containing mRNAs was not affected by RRP45 depletion, after actinomycin D addition we observed a very clear delay in the initiation of their degradation. The degradation of mRNAs lacking UREs was, in contrast, not affected, as in the mammalian in vitro system (Chen et al. 2001). One possible interpretation is that—as in mammalian cells—the URE recruits the exosome to the mRNA, either directly or through intermediate RNA-binding proteins. Once the exosome is loaded onto the mRNA, it forms a stable, efficient, and processive degradation complex. (The exosome-containing complex might also recruit an endonuclease, although we have no evidence for this.) In exosome-depleted cells, only the loading of the exosome is rate limiting; URE-RNAs that lack exosome are, in the meantime, subject to more gradual deadenylation by the constitutive pathway. The role of dedicated deadenylases—if any—in the URE-mediated pathway remains to be elucidated.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft, CL112/7 and Graduiertenkolleg 300. We thank Thomas Rausch (Institut für Pflanzenwissenschaften, Universität Heidelberg), Roy Parker (University of Arizona, Tucson), and David Tollervey (Institute of Cell & Molecular Biology, University of Edinburgh) for useful comments on the degradation kinetics.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
EP1, EP1 procyclin
PGKB, cytosolic phosphoglycerate kinase
PPDK, pyruvate phosphate dikinase
URE, U-rich element
ARE, AU-rich element: UTR, untranslated region
CAT, chloramphenicol acetyltransferase
PAC, puromycin resistance cassette
HYG, hygromycin resistance cassette
Article and publication are at http://www.rnajournal.org/cgi/doi/10.1261/rna.5940703.
REFERENCES
- Allmang, C., Kufel, J., Chanfreau, G., Mitchell, P., Petfalski, E., and Tollervey, D. 1999. Functions of the exosome in rRNA, sno RNA and snRNA synthesis. EMBO J. 18: 5399–5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloy, P., Ciccarelli, F.D., Leutwein, C., Gavin, A.-C., Superti-Furga, G., Bork, P., Böttcher, B., and Russell, R.B. 2002. A complex prediction: Three-dimensional model of the yeast exosome. EMBO Rep. 3: 628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beelman, C.A. and Parker, R. 1995. Degradation of mRNA in eukaryotes. Cell 81: 179–184. [DOI] [PubMed] [Google Scholar]
- Biebinger, S., Wirtz, L.E., and Clayton, C.E. 1997. Vectors for inducible over-expression of potentially toxic gene products in bloodstream and procyclic Trypanosoma brucei. Mol. Biochem. Parasitol. 85: 99–112. [DOI] [PubMed] [Google Scholar]
- Carballo, E., Lai, W.S., and Blackshear, P.J. 2000. Evidence that tristetraprolin is a physiological regulator of granulocyte-macrophage colony-stimulating factor messenger RNA deadenylation and stability. Blood 95: 1891–1899. [PubMed] [Google Scholar]
- Chen, C.Y. and Shyu, A.B. 1995. AU-rich elements: Characterization and importance in mRNA degradation. Trends Biochem. Sci. 20: 465–470. [DOI] [PubMed] [Google Scholar]
- Chen, C.Y., Gherzi, R., Ong, S.E., Chan, E.L., Raijmakers, R., Pruijn, G.J., Stoecklin, G., Moroni, C., Mann, M., and Karin, M. 2001. AU binding proteins recruit the exosome to degrade ARE-containing mRNAs. Cell 107: 451–464. [DOI] [PubMed] [Google Scholar]
- Clayton, C.E. 2002. Developmental regulation without transcriptional control? From fly to man and back again. EMBO J. 21: 1881–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozdz, M. and Clayton, C.E. 1999. Structure of a regulatory 3′-untranslated region from Trypanosoma brucei. RNA 5: 1632–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estévez, A., Kempf, T., and Clayton, C.E. 2001. The exosome of Trypanosoma brucei. EMBO J. 20: 3831–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estévez, A.M., Lehner, B., Sanderson, C.M., Ruppert, T., and Clayton, C. 2003. The roles of inter-subunit interactions in exosome stability. J. Biol. Chem. 278: 34943–34951. [DOI] [PubMed] [Google Scholar]
- Ford, L.P., Watson, J., Keene, J.D., and Wilusz, J. 1999. ELAV proteins stabilize deadenylated intermediates in a novel in vitro mRNA deadenylation/degradation system. Genes & Dev. 13: 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Giraldez, C., Quijada, L., and Clayton, C.E. 2002. Compartmentation of enzymes in a microbody, the glycosome, is essential in Trypanosoma brucei. J. Cell Sci. 115: 2651–2658. [DOI] [PubMed] [Google Scholar]
- Helfert, S., Estévez, A., Bakker, B., Michels, P., and Clayton, C.E. 2001. The roles of triosephosphate isomerase and aerobic metabolism in Trypanosoma brucei. Biochem. J. 357: 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilleren, P., McCarthy, T., Rosbash, M., Parker, R., and Jensen, T.H. 2001. Quality control of mRNA 3′-end processing is linked to the nuclear exosome. Nature 413: 538–542. [DOI] [PubMed] [Google Scholar]
- Hotz, H.-R., Hartmann, C., Huober, K., Hug, M., and Clayton, C.E. 1997. Mechanisms of developmental regulation in Trypanosoma brucei: A polypyrimidine tract in the 3′-untranslated region of a trypanosome surface protein mRNA affects RNA abundance and translation. Nucleic Acids Res. 25: 3017–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug, M., Hotz, H.R., Hartmann, C., and Clayton, C.E. 1994. Hierarchies of RNA processing signals in a trypanosome surface antigen mRNA precursor. Mol. Cell. Biol. 14: 7428–7435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irmer, H. and Clayton, C.E. 2001. Degradation of the EP1 mRNA in Trypanosoma brucei is initiated by destruction of the 3′-untranslated region. Nucleic Acids Res. 29: 4707–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs, J.S., Anderson, A.R., and Parker, R.P. 1998. The 3′ to 5′ degradation of yeast mRNAs is a general mechanism for mRNA turnover that requires the SKI2 DEVH box protein and 3′ to 5′ exonucleases of the exosome complex. EMBO J. 17: 1497–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene, J.D. 2001. Ribonucleoprotein infrastructure regulating the flow of genetic information between the genome and the proteome. Proc. Natl. Acad. Sci. 98: 7018–7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiledjian, M., DeMaria, C.T., Brewer, G., and Novick, K. 1997. Identification of AUF1 (heterogeneous nuclear ribonucleoprotein D) as a component of the alpha-globin mRNA stability complex. Mol. Cell Biol. 17: 4870–4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, W.S., Carballo, E., Strum, J.R., Kennington, E.A., Phillips, R.S., and Blackshear, P.J. 1999. Evidence that tristetraprolin binds to AU-rich elements and promotes the deadenylation and destabilization of tumor necrosis factor alpha mRNA. Mol. Cell Biol. 19: 4311–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loflin, P.A., Chen, C.-Y.A., and Shyu, A.-B. 1999. Unraveling a cytoplasmic role for hnRNP D in the in vivo mRNA destabilization directed by the AU-rich element. Genes & Dev. 13: 1884–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahtani, K.R., Brook, M., Dean, J.L.E., Sully, G., Saklatvala, J., and Clark, A.R. 2001. Mitogen-activated protein kinase p38 controls the expression and posttranslational modification of tristetraprolin, a regulator of tumor necrosis factor alpha mRNA stability. Mol. Cell. Biol. 21: 6461–6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milone, J., Wilusz, J., and Bellofatto, V. 2002. Identification of mRNA decapping activities and an ARE-regulated 3′ to 5′ exonuclease activity in trypanosome extracts. Nucleic Acids Res. 30: 4040–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, P. and Tollervey, D. 2000. mRNA stability in eucaryotes. Curr. Opin. Genet. Dev. 10: 193–198. [DOI] [PubMed] [Google Scholar]
- ———. 2001. mRNA turnover. Curr. Opin. Cell Biol. 13: 320–325. [DOI] [PubMed] [Google Scholar]
- Mukherjee, D., Gao, M., O’Connor, J.P., Raijmakers, R., Pruijn, G., Lutz, C.S., and Wilusz, J. 2002. The mammalian exosome mediates the efficient degradation of mRNAs that contain AU-rich elements. EMBO J. 21: 165–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quijada, L., Hartmann, C., Guerra-Giraldez, C., Drozdz, M., Irmer, H., and Clayton, C.E. 2002. Expression of the human RNA-binding protein HuR in Trypanosoma brucei induces differentiation-related changes in the abundance of developmentally regulated mRNAs. Nucleic Acids Res. 30: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raijmakers, R., Ebgerts, W.V., van Venrooij, W.J., and Pruijn, G.J.M. 2002. Protein-Protein Interactions between Human Exosome Components Support the Assembly of RNase PH-type Subunits into a Six-membered PNPase-like Ring. J. Mol. Biol. 323: 653–663. [DOI] [PubMed] [Google Scholar]
- Schürch, N., Furger, A., Kurath, U., and Roditi, I. 1997. Contribution of the procyclin 3′ untranslated region and coding region to the regulation of expression in bloodstream forms of Trypanosoma brucei. Mol. Biochem. Parasit. 89: 109–121. [DOI] [PubMed] [Google Scholar]
- Sheth, U. and Parker, R. 2003. Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science 300: 805–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchet, C., Bousquet-Antonelli, C., Milligan, L., Thompson, E., Kufel, J., and Tollervey, D. 2002. Processing of 3′-extended read-through transcripts by the exosome can generate functional mRNAs. Mol. Cell 9: 1285–1296. [DOI] [PubMed] [Google Scholar]
- van Deursen, F.J., Shahi, S.K., Turner, C.M, Hartmann, C., Guerra-Giraldez, C., Matthews, K.R., and Clayton, C.E. 2001. Characterisation of the growth and differentiation in vivo and in vitro of bloodstream-form Trypanosoma brucei strain TREU 927. Mol. Biochem. Parasit. 112: 163–172. [DOI] [PubMed] [Google Scholar]
- van Hoof, A. and Parker, R. 2002. Messenger RNA degradation: Beginning at the end. Curr. Biol. 12: R285–R287. [DOI] [PubMed] [Google Scholar]
- van Hoof, A., Lennertz, P., and Parker, R. 2000. Yeast exosome mutants accumulate 3′-extended polyadenylated forms of U4 small nuclear RNA and small nucleolar RNAs. Mol. Cell. Biol. 20: 441–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z. and Kiledjian, M. 2001. Functional link between the mammalian exosome and mRNA decapping. Cell 107: 751–762. [DOI] [PubMed] [Google Scholar]
- Wang, W., Yang, X., Cristofalo, V.J., Holbrook, N.J., and Gorospe, M. 2001. Loss of HuR is linked to reduced expression of proliferate genes during replicative senescense. Mol. Cell. Biol. 21: 5889–5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanchin, N.I. and Goldfarb, D.S. 1999. The exosome subunit Rrp43p is required for the efficient maturation of 5.8S, 18S and 25S rRNA. Nucleic Acids Res. 27: 1283–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]