Abstract
RNA interference is an evolutionarily conserved process in which expression of a specific gene is post-transcriptionally inhibited by a small interfering RNA (siRNA), which recognizes a complementary mRNA and induces its degradation. Currently, RNA interference is being used extensively to inhibit expression of specific genes for experimental and therapeutic purposes. For applications in mammalian cells, siRNAs are designed to be <~30 base pairs to avoid nonspecific effects that arise from inducing the cellular double-stranded RNA (dsRNA)-dependent protein kinase (PKR) response. Here we perform expression profiling in mammalian tissue-culture cells treated under standard conditions with conventional 21-bp siRNAs and find, unexpectedly, that >1000 genes involved in diverse cellular functions are nonspecifically stimulated or repressed. The effects on gene expression are dependent upon siRNA concentration and are stable throughout the course of siRNA treatment. Our results can be explained by previous studies showing that dsRNAs can affect multiple signaling and transcription pathways in addition to PKR. The potential for this widespread, nonspecific effect on mammalian gene expression must be carefully considered in the design of siRNA experiments and therapeutic applications.
Keywords: RNA interference, siRNA, interferon response, PKR response
INTRODUCTION
RNA interference (RNAi) is an evolutionarily conserved process of sequence-specific, post-transcriptional gene silencing that uses double-stranded RNA (dsRNA) as the signal to trigger the degradation of homologous mRNA (for review, see Fire 1999; Sharp 2001; Hannon 2002; Plasterk 2002; Zamore 2002). The mechanism by which dsRNA induces gene silencing involves a two-step process. First, long dsRNAs are recognized by the ribonuclease III–like enzyme Dicer, which cleaves the dsRNA into smaller RNAs of 21–23 nt. These small interfering RNAs (siRNAs) are then incorporated into a multicomponent nuclease complex known as the RNA-induced silencing complex (RISC), which recognizes and targets cognate mRNAs for destruction.
RNAi has proven to be an effective tool for studying gene function in numerous organisms, including Caenorhabditis elegans, Drosophila, and plants (Hannon 2002). In mammalian cells, however, the use of RNAi for targeted gene silencing has been limited due to nonspecific effects induced by long dsRNAs, which result in interferon (IFN) activation and induction of the cellular dsRNA-dependent protein kinase (PKR) response. Therefore, for applications in mammalian cells, siRNAs are designed to be <~30 bp to circumvent the PKR response and avoid nonspecific effects on gene silencing (see, for example, Caplen et al. 2001; Elbashir et al. 2001).
Currently, siRNAs are being used extensively in mammalian cells to inhibit expression of specific genes for experimental and therapeutic purposes. A critical assumption of this approach is that the siRNA will selectively inhibit the complementary gene. Here, by using expression profiling, we show that conventional siRNAs can in a concentration-dependent manner nonspecifically stimulate or repress expression of >1000 genes with protein products that are involved in diverse cellular functions.
RESULTS AND DISCUSSION
Expression profiling reveals nonspecific effects on mammalian gene expression by siRNAs
To identify targets of transcription factors, we had been performing expression profiling after siRNA treatment of mammalian tissue-culture cells. As a nonspecific control in these studies, we used a well-characterized siRNA directed against a luciferase reporter gene (Elbashir et al. 2001; Schwarz et al. 2002). This siRNA efficiently silences luciferase expression at a concentration of 1 nM (Schwarz et al. 2002). Unexpectedly, after transfection with 200 nm luciferase siRNA under standard conditions, we found that of the 33,000 genes represented on an Affymetrix U133 chip, expression of 1154 genes increased and expression of 689 genes decreased by ≥2.5-fold compared with untreated cells. Genes with expression that was altered by luciferase siRNA treatment encoded proteins involved in diverse cellular activities, including cell signaling, cytoskeletal organization, gene expression, metabolism, and cell adhesion (Table 1 ▶).
TABLE 1.
List of selected genes with expression that was either increased or decreased by luciferase siRNA treatment
| Name and biological function | Acc. no. | Gene product | Fold increase |
| Stimulated genes | |||
| Cytokine signaling | |||
| FGG | NM 000509 | fibrinogen, gamma polypeptide | 5.7 |
| HFL1 | X 56210 | H factor (complement)-like 1 | 5.4 |
| RAP2B | NM 002886 | member of RAS oncogene family | 4.9 |
| TRG@ | M 16768 | T cell receptor gamma locus | 4.2 |
| CCL23 | U 58913 | chemokine (C-C motif) ligand 23 | 4.0 |
| ISG20 | NM 002201 | interferon stimulated gene 20 kD | 3.5 |
| CXCL14 | NM 004887 | chemokine (C-X-C motif) ligand 14 | 3.3 |
| CCL3 | NM 002983 | chemokine (C-C motif) ligand 3 | 2.9 |
| IRF4 | U 52683 | interferon regulatory factor 4 | 2.9 |
| FGL1 | NM 004467 | fibrinogen-like 1 | 2.8 |
| IL5RA | M 96651 | interleukin 5 receptor, α | 2.6 |
| Cytoskeletal organization | |||
| LAMA4 | U 77706 | laminin, α 4 | 4.4 |
| BMP2 | NM 001200 | bone morphogenetic protein 2 | 3.0 |
| GOLGA6 | NM 018652 | golgin subfamily a, member 6 | 3.0 |
| COL15A1 | NM 001855 | collagen, type XV, α 1 | 2.7 |
| COL16A1 | NM 001856 | collagen, type XVI, α 1 | 2.7 |
| Apoptosis | |||
| REPRIMO | NM 019845 | p53 induced G2 arrest mediator | 3.8 |
| Extracellular matrix/cell adhesion | |||
| IGFBP5 | AW 007532 | insulin-like growth factor binding protein 5 | 4.8 |
| EDN2 | NM 001956 | endothelin 2 | 4.6 |
| CDH13 | NM 001257 | cadherin 13 | 4.2 |
| MRC1 | NM 002438 | mannose receptor, C type 1 | 3.9 |
| SEPP1 | NM 005410 | selenoprotein P, plasma, 1 | 3.8 |
| FGF13 | NM 004114 | fibroblast growth factor 13 | 3.6 |
| IGF1 | M 37484 | insulin-like growth factor 1 | 3.0 |
| Metabolism | |||
| PDK1 | NM 002610 | pyruvate dehydrogenase kinase, isoenzyme 1 | 4.1 |
| HPD | NM 002150 | 4-hydroxyphenylpyruvate dioxygenase | 3.6 |
| ARHU | NM 021205 | ras homolog gene family, member U | 3.4 |
| NDUFB7 | M 33374 | NADH dehydrogenase 1 beta subcomplex, 7 | 3.2 |
| DNMT3L | NM 013369 | DNA (cytosine-5)-methyltransferase 3-like | 3.1 |
| Other genes | |||
| CGI-60 | NM 016008 | CGI-60 protein | 4.1 |
| ZNF135 | NM 003436 | zinc finger protein 135 | 3.0 |
| FKBP8 | L 37033 | FK-506 binding protein 8 | 2.8 |
| MAGEA1 | NM 004988 | melanoma antigen, family A, 1 | 2.8 |
| Repressed genes | |||
| RNA synthesis | |||
| 18S rRNA | M 10098 | 18S ribosomal RNA | 3.1 |
| 28S rRNA | M 11167 | 28S ribosomal RNA | 2.8 |
| Metabolism | |||
| ACOX2 | NM 003500 | acyl-Coenzyme A oxidase 2 | 4.7 |
| ALPP | NM 001632 | alkaline phosphatase, placental | 4.2 |
| RODH | NM 003725 | 3-hydroxysteroid epimerase | 4.2 |
| MAOA | NM 000240 | monoamino oxidase A | 3.8 |
| GAD2 | M 81882 | glutamate decarboxylase 2 | 3.4 |
| DUSP6 | AB 013382 | dual specificity phosphatase 6 | 3.1 |
| Chromosome organization | |||
| HIST1H2AG | NM 021064 | histone 1, H2ag | 3.5 |
| HIST1H2AE | NM 021052 | histone 1, H2ae | 3.4 |
| H3FH | NM 003534 | histone H3 family, member H | 3.1 |
| H3FL | NM 003537 | histone H3 family, member L | 2.9 |
| Extracellular matrix/cell adhesion | |||
| CDH5 | NM 001795 | cadherin 5, type 2 | 4.1 |
| CD5 | NM 014207 | CD5 antigen (p56-62) | 3.3 |
| PCDHGC3 | L 11372 | protocadherin gamma subfamily C, 3 | 3.1 |
| CD1D | NM 001766 | CD1D antigen, d polypeptide | 2.5 |
| CD38 | NM 001775 | CD38 antigen (p45) | 2.5 |
| PCDH11X | NM 014522 | protocadherin 11 X-linked | 2.5 |
| Cell signaling | |||
| TNFSF10 | NM 003810 | tumor necrosis factor superfamily, member 10 | 4.8 |
| IL11 | NM 000641 | interleukin 11 | 4.5 |
| SLC2A6 | NM 017585 | solute carrier family 2, member 6 | 4.5 |
| ZFP36L2 | NM 006887 | zinc finger protein 36, C3H type-like 2 | 4.4 |
| INHBB | NM 002193 | inhibin β B | 3.8 |
| HBP17 | NM 005130 | heparin-binding growth factor binding protein | 3.5 |
| RARRES1 | NM 002888 | retinoic acid receptor responder 1 | 3.0 |
| FGF18 | NM 003862 | fibroblast growth factor 18 | 2.8 |
| FGFR4 | AF 202063 | fibroblast growth factor receptor 4 | 2.8 |
| SLC21A12 | NM 016354 | solute carrier family 21, member 12 | 2.8 |
| SSI1 | U 88326 | suppressor of cytokine signaling 1 | 2.8 |
| IL7 | NM 000880 | interleukin 7 | 2.5 |
| Transcription | |||
| EHF | NM 012153 | ets homologous factor | 3.6 |
| ELF4 | NM 001421 | E74-like factor 4 | 3.3 |
| GLI2 | NM 030379 | GLI-Kruppel family member | 3.1 |
| SHOX2 | AF 022654 | short stature homeobox 2 | 2.9 |
| DLX2 | NM 004405 | distal-less homeobox 2 | 2.8 |
| PITX2 | NM 000325 | paired-like homeodomain transcription factor 2 | 2.8 |
| HOXC5 | NM 018953 | homeobox C5 | 2.5 |
| Other genes | |||
| CDC14A | NM 003672 | cell division cycle 14 homolog A | 6.4 |
| PSCA | NM 005672 | prostate stem cell antigen | 5.5 |
| SERPINB4 | AB 046400 | serine proteinase inhibitor, clade B, member 4 | 4.3 |
| MDA5 | NM 022168 | melanoma differentiation associated protein 5 | 4.1 |
| HSPA1A | NM 005345 | heat-shock 70-kD protein 1A | 3.8 |
| DPT | NM 001937 | dermatopontin | 3.1 |
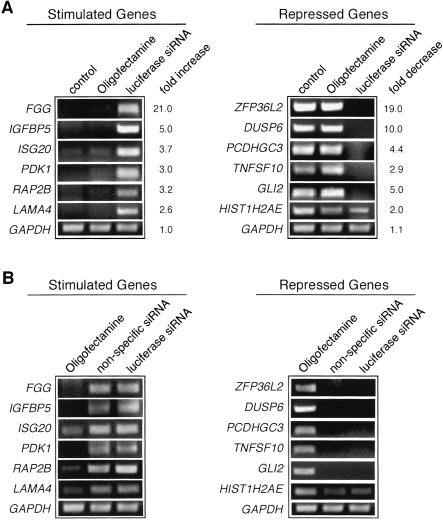
Twelve genes with expression that was either increased or decreased by luciferase siRNA treatment were randomly selected for further characterization. To confirm the results of the microarray experiment, we performed RT-PCR analysis. Figure 1A ▶ shows that expression of all six of the genes up-regulated in the microarray analysis were increased to varying extents by treatment with the luciferase siRNA. Likewise, expression of all six of the down-regulated genes in the microarray analysis were decreased to varying extents after treatment with the luciferase siRNA. Consistent with the microarray analysis, expression of GAPDH was unaffected. Significantly, treatment of cells with the transfection reagent (Oligofectamine) alone did not affect expression of any of the 12 genes analyzed, indicating the effect is attributable to the siRNA. We presume this is also the case for the other genes with expression that was affected in the microarray analysis.
FIGURE 1.
Analysis of siRNA-induced alterations in gene expression by RT-PCR. (A) Representative set of up-regulated genes (left) or down-regulated genes (right) after treatment of HeLa cells with transfection reagent (Oligofectamine) alone or with luciferase siRNA. Untreated HeLa cells were used as a control. Fold differences in expression as measured by RT-PCR is indicated on the right. (B) siRNA-induced alteration in gene expression is independent of siRNA sequence. Comparison of luciferase siRNA and a random nonspecific siRNA on the expression of a selected set of stimulated genes (left) or repressed genes (right). Oligofectamine-treated cells were used as a control.
The luciferase siRNA used in the above experiments lacks significant sequence similarity to any human gene. It therefore seemed likely that the effects on gene expression we observed were independent of the specific siRNA sequence. To confirm this prediction, we analyzed expression of several genes after treatment with a randomized nonspecific control siRNA, which again lacks significant similarity to any human gene. The results of Figure 1B ▶ show that the luciferase siRNA and the nonspecific siRNA had comparable effects on all 12 genes analyzed.
siRNA treatment affects gene expression in a concentration-dependent manner
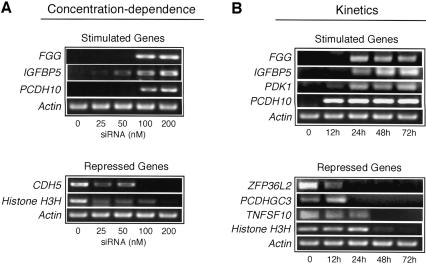
We sought to determine whether the nonspecific effects on gene expression were dependent upon siRNA concentration. We therefore analyzed the expression of a subset of genes at siRNA concentrations ranging from 0 to 200 nM. The results of Figure 2A ▶ show, as observed above, that at 200 nM luciferase siRNA, expression of FGG, IGFBP5, and PCDH10 were increased, whereas expression of CDH5 and Histone H3H were reduced. At 100 nm luciferase siRNA, expression of FGG, IGFBP5, PCDH10, and CDH5 were still significantly affected and expression of Histone H3H was affected modestly. When the concentration of luciferase siRNA was reduced to 50 nM, expression of IGBP5, CDH5, and Histone H3H remained modestly affected. Further reduction to 25 nM still affected CDH5 and Histone H3H expression, whereas IGBP5 expression was near normal. Thus, the nonspecific effects on gene expression are dependent upon siRNA concentration in a gene-specific manner.
FIGURE 2.
Concentration-dependence and kinetics of siRNA-induced alteration in gene expression. (A) Concentration-dependence. Comparison of varying concentrations of luciferase siRNA on the expression of a selected set of genes. (B) Kinetics. Time-course analysis by RT-PCR of selected genes with expression that increased or decreased after luciferase siRNA treatment.
Kinetics of altered gene expression
We next analyzed the time-course of altered expression of several genes after siRNA treatment. The results of Figure 2B ▶ show that for FGG, IGFBP5, PDK1, and PCDH10, a significant increase in expression was first detected 12 to 24 h after siRNA treatment and remained constant throughout the remainder of the 72-h time-course. For ZFP36L2 and PCDHGC3, a decrease in expression was first observed 24 h after siRNA treatment and was maintained, whereas a decrease in expression of TNFSF10 and Histone H3H first occurred 48 h after siRNA treatment. Thus, the nonspecific effects on gene expression induced by siRNAs are not transient and, once initiated, are sustained throughout the course of siRNA treatment.
siRNAs, long dsRNAs, and IFN affect overlapping pathways
The nonspecific effects we observed by using siRNAs raise the possibility that dsRNAs can affect gene expression through pathways other than PKR. Of particular significance, polyI.polyC, a long dsRNA homopolymer, can still activate expression of specific genes in knockout mice lacking both copies of the PKR gene (Yang et al. 1995). This result is consistent with reports that dsRNA can activate several protein kinases such as p38, JNK2, and IKK (Kumar et al. 1994; Chu et al. 1999; Williams 1999) in addition to PKR. Induction of these signaling pathways can alter gene expression by regulating the activity of transcription factors such as NF-κB, IRF-3, and ATF-1 (Williams 1999). Because siRNAs do not trigger the PKR response, the most likely explanation for the nonspecific effects on gene expression that we have observed is that siRNAs can affect gene expression through one of these other pathways. As an initial investigation of the pathway(s) affected by siRNAs, we compared the results of our expression profiling analysis with those obtained after treatment with type 1 IFN (Der et al. 1998) or polyI.polyC in cells lacking the type I IFN locus (Table 2 ▶; Geiss et al. 2001). Interestingly, most of the genes were affected by two but not all three of the inducing agents. As expected, a number of genes were affected by treatment with IFN and long dsRNAs but not by siRNAs. This is presumably because IFN and long dsRNAs induce expression of these genes through the PKR pathway, which is not activated by siRNAs. Consistent with this conclusion, RT-PCR analysis revealed that several genes with expression that is induced by dsRNA, IFNβ, and 2′-5′ oligoadenylate synthetase (OAS), or by IFN, myxovirus-resistance-1 (MXA), and IFN-stimulated gene 54 (ISG-54), were unaffected by siRNA treatment (data not shown). Thus, the pathways affected by IFN, long dsRNAs, and siRNAs appear to be overlapping but nonidentical.
TABLE 2.
Comparison of selected genes affected by siRNA, poly(I):poly(C), and interferon type 1
| Gene description | siRNA | long dsRNA | IFN–α/β |
| Guanylate binding protein 1, interferon inducible (GBP1) | • | • | • |
| Cell division cycle 14 homolog A (CDC14A) | • | • | |
| Chemokine (C-C motif) ligand 2 (CCL2) | • | • | |
| Fibroblast growth factor 2 (FGF2) | • | • | |
| Proteoglycan 1, secretory granule (PRG1) | • | • | |
| Caspase 8 (CASP8) | • | • | |
| CCAAT/enhancer binding protein delta (C/EBPD) | • | • | |
| Chemokine (C-X-C motif) ligand 11 (CXCL11)a | • | • | |
| Collagen, type XVI, alpha 1 (COL16A1) | • | • | |
| Glucose monophosphate reductase (GMPR) | • | • | |
| Interferon stimulated gene 20kDa (ISG20)a | • | • | |
| Zinc finger protein 36, C3H type-like 2 (ZFP36L2) | • | • | |
| Adenosine deaminase, RNA-specific (ADAR) | • | • | |
| Complement component 1, s subcomponent (C1S) | • | • | |
| Interferon-induced protein with tetratricopeptide repeats 1 (IFIT1) | • | • | |
| Interferon regulatory factor 1 (IRF1) | • | • | |
| Interferon regulatory factor 2 (IRF2) | • | • | |
| Mothers against decapentaplegic homolog 4 (MADH4) | • | • | |
| N-myc (and STAT) interactor (NMI) | • | • | |
| Plasminogen activator, urokinase receptor (PLAUR) | • | • | |
| Transporter 1, ATP-binding cassette, subfamily B (TAP1) | • | • |
Genes found to be up- or down-regulated twofold or more by microarray analysis after treatment with a nonspecific siRNA (this study), long dsRNA [poly(I):poly(C)] (Geiss et al. 2001), or IFNα or IFNβ (Der et al. 1998).
aGenes affected by siRNA treatment that have previously been reported to be regulated by IFN (ISG20,Gongora et al. 2000; CXCL11,Cole et al. 1998) although they were not reported in the microarray analysis carried out by Der et al. (1998).
In this report, we have found that in addition to the well-known ability of siRNAs to silence specific genes, there is also a widespread nonspecific effect on mammalian gene expression. Interestingly, expression of some genes was increased, whereas others were reduced. These nonspecific effects must be considered in the design of controls for siRNA-mediated loss-of-function experiments.
Previous studies examining siRNA specificity have generally monitored expression of only a few genes and probably would not have detected the nonspecific effects described here. However, several recent reports have used expression profiling to reveal nonspecific effects on human gene expression by siRNAs (Jackson et al. 2003; Semizarov et al. 2003) or short hairpin RNAs (shRNAs; Bridge et al. 2003). Below we discuss the relationship and implications of our results and those in these related studies.
What is the mechanism(s) by which siRNAs induce nonspecific effects on gene expression? Jackson et al. (2003) attributed the nonspecific effects they observed to off-target gene regulation, in which the expression of nontarget genes are suppressed due to cross-hybridization of transcripts containing regions of partial homology with the siRNA sequence. The effects we observed, however, cannot be explained by off-target regulation because the siRNAs used in our experiments lacked significant sequence similarity to any human gene. Moreover, for all of the genes tested, expression was affected by two siRNAs (luciferase and nonspecific) with sequences that are completely unrelated.
Bridge et al. (2003) reported that shRNAs can affect the expression of many genes, including several IFN targets, and therefore suggested that shRNAs induce an IFN response. Because shRNAs are processed to siRNAs in the cell, these observations raised the possibility that siRNAs might also induce an IFN response. However, a comparison of genes affected by siRNAs and IFN (Table 2 ▶) revealed that siRNAs affect pathways that are overlapping but not identical to those regulated by IFN. Thus, siRNAs do not trigger a true IFN response. More importantly, Bridge et al. (2003) reported that the nonspecific effects occurred with an shRNA expressed from an RNA polymerase III promoter but not with a synthetic siRNA. In contrast, the nonspecific effects we observed occurred with synthetic siRNAs.
A characteristic feature of the nonspecific effects on gene expression we observed was dependence on siRNA concentration. Semizarov et al. (2003) reported nonspecific effects that occurred at an siRNA concentration of 100 nM but not at 20 nM. Thus, we suspect that the nonspecific effects reported by Semizarov et al. (2003) are related to our results. However, for several reasons it is not yet clear that the basis for altered gene expression in our study is identical to that of Semizarov et al. (2003). Specifically, unlike our study, Semizarov et al. used only microarray analysis to study expression, did not test whether different unrelated siRNAs comparably altered the expression of specific genes, and did not analyze the kinetics of altered expression. Finally, Semizarov et al. (2003) did not report genes with expression that was nonspecifically reduced by siRNAs in a concentration-dependent fashion.
Gene silencing experiments in mammalian cells have used siRNAs at varying concentrations, typically ranging from 20 nM (Semizarov et al. 2003) to 200 nM (Wu et al. 2003). Many published studies report using siRNAs at 100 nM, a concentration that is also suggested by manufacturers of siRNAs and siRNA-related products. Significantly, 100 nM siRNA is a concentration at which we found nonspecific effects occur. Unfortunately, however, lowering the concentration of a particular siRNA <100 nM can reduce silencing efficiency. These considerations underscore the importance of determining the rules for siRNA design so that optimal silencing efficiency can be achieved at minimal siRNA concentrations to avoid nonspecific effects.
MATERIALS AND METHODS
siRNA preparation
The siRNAs were chemically synthesized by the UMass CFAR (Center for AIDS Research) Molecular Biology Core using 2′-TOM-RNA phosphoramidites. The luciferase siRNA oligoribonucleotides (5′-CGUACGCGGAAUACUUCGATT-3′ and 5′-UC GAAGUAUUCCGCGUACGTT-3′) correspond to the coding region 153–173 relative to the first nucleotide of the start codon of the Photinus pyralis luciferase gene (Elbashir et al. 2001). The nonspecific siRNA oligoribonucleotides (5′-AAUUUUUUUCCC CAAAGGGGG-3′ and 5′-AACCCCCUUUGGGGAAAAAAA-3′) were randomly synthesized and did not correspond to any known gene in the human genome database. To anneal the siRNAs, 20 μM single-stranded RNAs were incubated in annealing buffer (100 mM potassium acetate, 2 mM magnesium acetate, 30 mM Hepes-KOH at pH 7.4) for 1 min at 90°C followed by incubation for 2 h at 37°C. DsRNAs were stored at −20°C until transfection.
Cell culture and transfection
HeLa S3 cells were propagated at 37°C in DMEM (Life Technologies) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were regularly passed to maintain exponential growth. Transfections were carried out by using Oligofectamine (Life Technologies) and 200 nM duplex siRNA per well, as per the manufacturer’s instructions. Cells were transfected one to three times at ~24-h intervals.
Microarray analysis
Total RNA was prepared from test (siRNA treated) and control (untreated) cells by using the RNeasy kit (Qiagen). Targets for hybridization to the microarrays were prepared as described (Eisen and Brown 1999). Hybridization and scanning of human U133 GeneChip Arrays (Affymetrix) were performed as recommended by the manufacturer. The complete list of genes induced or repressed by luciferase siRNA treatment is available upon request.
RNA isolation and RT-PCR analysis
RT-PCR analysis was performed according to standard protocols (Ausubel et al. 2001), except that total RNA was prepared by using the RNeasy extraction kit (Qiagen). RT-PCR products were separated by agarose gel electrophoresis and visualized by ethidium bromide staining. The density of the ethidium bromide-stained bands was quantitated by using NIH Image analysis software. Fold differences were calculated by comparing the intensity of the signal in the luciferase siRNA-treated sample relative to that obtained by using transfection reagent alone. Primer sequences used for RT-PCR analysis are available upon request.
Acknowledgments
We are grateful to P. Zamore for discussions during the course of this work. This work was supported by a grant to M.R.G. from the National Institutes of Health. M.R.G. is an investigator of the Howard Hughes Medical Institute. The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.rnajournal.org/cgi/doi/10.1261/rna5160904.
REFERENCES
- Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., and Struhl, K. 2001. Current protocols in molecular biology. Wiley, New York.
- Bridge, A.J., Pebernard, S., Ducraux, A., Nicoulaz, A.L., and Iggo, R. 2003. Induction of an interferon response by RNAi vectors in mammalian cells. Nat. Genet. 34: 263–264. [DOI] [PubMed] [Google Scholar]
- Caplen, N.J., Parrish, S., Imani, F., Fire, A., and Morgan, R.A. 2001. Specific inhibition of gene expression by small double-stranded RNAs in invertebrate and vertebrate systems. Proc. Natl. Acad. Sci. 98: 9742–9747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, W.M., Ostertag, D., Li, Z.W., Chang, L., Chen, Y., Hu, Y., Williams, B., Perrault, J., and Karin, M. 1999. JNK2 and IKKβ are required for activating the innate response to viral infection. Immunity 11: 721–731. [DOI] [PubMed] [Google Scholar]
- Cole, K.E., Strick, C.A., Paradis, T.J., Ogborne, K.T., Loetscher, M., Gladue, R.P., Lin, W., Boyd, J.G., Moser, B., Wood, D.E., et al. 1998. Interferon-inducible T cell α chemoattractant (I-TAC): A novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J. Exp. Med. 187: 2009–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der, S.D., Zhou, A., Williams, B.R., and Silverman, R.H. 1998. Identification of genes differentially regulated by interferon α, β, or γ using oligonucleotide arrays. Proc. Natl. Acad. Sci. 95: 15623– 15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen, M.B. and Brown, P.O. 1999. DNA arrays for analysis of gene expression. Methods Enzymol. 303: 179–205. [DOI] [PubMed] [Google Scholar]
- Elbashir, S.M., Harborth, J., Lendeckel, W., Yalcin, A., Weber, K., and Tuschl, T. 2001. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411: 494–498. [DOI] [PubMed] [Google Scholar]
- Fire, A. 1999. RNA-triggered gene silencing. Trends Genet. 15: 358–363. [DOI] [PubMed] [Google Scholar]
- Geiss, G., Jin, G., Guo, J., Bumgarner, R., Katze, M.G., and Sen, G.C. 2001. A comprehensive view of regulation of gene expression by double-stranded RNA-mediated cell signaling. J. Biol. Chem. 276: 30178–30182. [DOI] [PubMed] [Google Scholar]
- Gongora, C., Degols, G., Espert, L., Hua, T.D., and Mechti, N. 2000. A unique ISRE, in the TATA-less human Isg20 promoter, confers IRF-1-mediated responsiveness to both interferon type I and type II. Nucleic Acids. Res. 28: 2333–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon, G.J. 2002. RNA interference. Nature 418: 244–251. [DOI] [PubMed] [Google Scholar]
- Jackson, A.L., Bartz, S.R., Schelter, J., Kobayashi, S.V., Burchard, J., Mao, M., Li, B., Cavet, G., and Linsley, P.S. 2003. Expression profiling reveals off-target gene regulation by RNAi. Nat. Biotechnol. 21: 635–637. [DOI] [PubMed] [Google Scholar]
- Kumar, A., Haque, J., Lacoste, J., Hiscott, J., and Williams, B.R. 1994. Double-stranded RNA-dependent protein kinase activates transcription factor NF-κB by phosphorylating I κB. Proc. Natl. Acad. Sci. 91: 6288–6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasterk, R.H. 2002. RNA silencing: the genome’s immune system. Science 296: 1263–1265. [DOI] [PubMed] [Google Scholar]
- Schwarz, D.S., Hutvágner, G., Haley, B., and Zamore, P.D. 2002. Evidence that siRNAs function as guides, not primers, in the Drosophila and human RNAi pathways. Mol. Cell 10: 537–548. [DOI] [PubMed] [Google Scholar]
- Semizarov, D., Frost, L., Sarthy, A., Kroeger, P., Halbert, D.N., and Fesik, S.W. 2003. Specificity of short interfering RNA determined through gene expression signatures. Proc. Natl. Acad. Sci. 100: 6347–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, B.R. 1999. PKR: A sentinel kinase for cellular stress. Oncogene 18: 6112–6120. [DOI] [PubMed] [Google Scholar]
- Wu, H., Hait, W.N., and Yang, J.M. 2003. Small interfering RNA-induced suppression of MDR1 (P-glycoprotein) restores sensitivity to multidrug-resistant cancer cells. Cancer Res. 63: 1515–1519. [PubMed] [Google Scholar]
- Yang, Y.L., Reis, L.F., Pavlovic, J., Aguzzi, A., Schafer, R., Kumar, A., Williams, B.R., Aguet, M., and Weissmann, C. 1995. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 14: 6095–6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamore, P.D. 2002. Ancient pathways programmed by small RNAs. Science 296: 1265–1269. [DOI] [PubMed] [Google Scholar]