Abstract
Signal recognition particle (SRP) guides secretory proteins to biological membranes in all organisms. Assembly of the large domain of mammalian SRP requires binding of SRP19 prior to the binding of protein SRP54 to SRP RNA. The crystal structure of the ternary complex reveals the parallel arrangement of RNA helices 6 and 8, a bridging of the helices via a hydrogen bonded A149–A201 pair and protein SRP19, and two A minor motifs between the asymmetric loop of helix 8 (A213 and A214) and helix 6. We investigated which residues in helix 8 are responsible for the SRP19-dependent binding of SRP54 by taking advantage of the finding that binding of human SRP54 to Methanococcus jannaschii SRP RNA is independent of SRP19. Chimeric human/M. jannaschii SRP RNA molecules were synthesized containing predominantly human SRP RNA but possessing M. jannaschii SRP RNA-derived substitutions. Activities of the chimeric RNAs were measured with respect to protein SRP19 and the methionine-rich RNA-binding domain of protein SRP54 (SRP54M). Changing A213 and A214 to a uridine has no effect on the SRP19-dependent binding of SRP54M. Instead, the two base pairs C189–G210 and C190–G209, positioned between the conserved binding site of SRP54 and the asymmetric loop, are critical for conveying SRP19 dependency. Furthermore, the nucleotide composition of five base pairs surrounding the asymmetric loop affects binding of SRP54M significantly. These results demonstrate that subtle, and not easily perceived, structural differences are of crucial importance in the assembly of mammalian SRP.
Keywords: RNP, protein–RNA interactions, site-directed mutagenesis
INTRODUCTION
Signal recognition particle (SRP) is an RNP that binds to ribosomes as they engage in the synthesis of secretory proteins. SRP interacts with the nascent signal sequence and subsequently with the SRP receptor in the membrane to deliver the protein to the proper cellular compartment (for review, see Keenan et al. 2001; Wild et al. 2002; Zwieb and Eichler 2002; Nagai et al. 2003). The prototypical mammalian SRP consists of an RNA molecule of ~300 nucleotides (SRP RNA) and the SRP9/14, SRP19, SRP54, and SRP68/72 proteins (Walter and Blobel 1983). The large SRP domain consists of proteins SRP19, SRP54, and SRP68/72 and a region of ~150 nt from the central region of the SRP RNA, which forms helices 6, 7, 8, and a portion of helix 5. The exceptionally conserved helix 8 and protein SRP54 are indispensable constituents of every SRP and central to signal peptide recognition in all organisms (Larsen and Zwieb 1991; Rosenblad et al. 2003).
Special attention has been given to the question as to how protein SRP19 and SRP RNA direct the binding of protein SRP54 (Walter and Blobel 1983). Our understanding of the protein–RNA interactions and structural changes that might occur during the assembly of the large SRP domain has been significantly enhanced by the recent availability of several high-resolution structures. Particularly relevant to the SRP assembly process are the NMR structure of Archaeoglobus fulgidus SRP19 (Pakhomova et al. 2002), the crystal structure of the ribonucleoprotein core of the Escherichia coli SRP (Batey et al. 2000), the structures of human SRP RNA (hSR) helix 6 with human SRP19 (Wild et al. 2001), M. jannaschii SRP19 with the large domain of hSR (Oubridge et al. 2002) or M. jannaschii SRP RNA (MjSR; Hainzl et al. 2002), and the human ternary complex of SRP RNA, SRP19, and SRP54 (Kuglstatter et al. 2002).
An important step in SRP assembly is the binding of the triple-stranded antiparallel β-sheet and loop 1 of protein SRP19 to the RNA tetraloop of helix 6. Another weaker interaction occurs between the loop 3 of SRP19 and the tip of SRP RNA helix 8. The adenosine at position 149, located at the third position of the tetraloop of hSR helix 6, is essential but only indirectly involved in the binding of SRP19, as it forms a conserved symmetric noncanonical base pair with the A201 of helix 8. This tertiary RNA–RNA interaction and the bridge formed by SRP19 arrange helices 6 and 8 in a parallel fashion and provide the structural framework for the subsequent binding of SRP54 (Oubridge et al. 2002). Time-resolved structure probing of SRP RNA confirms that SRP19 induces a conformational change in the large domain to arrange helices 6 and 8 in close proximity in preparation for the binding of SRP54 (Rose and Weeks 2001).
The analysis of chimeric mutant SRP RNA molecules containing helices that are derived from both MjSR and hSR provided the first evidence that helix 8, or possibly a limited number of residues within helix 8, controls the SRP19-dependent binding of SRP54 (Yin et al. 2001). Indeed, significant structural differences in helix 8 are apparent when the structures of the SRP19–RNA complexes (Wild et al. 2001; Hainzl et al. 2002; Oubridge et al. 2002) are compared with the SRP54-containing ternary complex. Although essentially the same interactions are maintained between protein SRP19 and the SRP RNA, helix 8 collapses in the presence of SRP54, and the loop residues A213 and A214 extrude from helix 8 to form A minor motifs with helix 6 (Kuglstatter et al. 2002).
Here we have generated chimeric SRP RNA mutants to determine precisely which portion of helix 8 directs the assembly of the human SRP. Evidence is presented that the adjoining base pairs C189–G210 and C190–G209 play a crucial role in the SRP19-dependent binding of SRP54. These two base pairs are strategically placed between the major SRP54 binding site and the conformationally altered asymmetric loop of helix 8, suggesting that mammalian SRP assembly is not governed by the extensive structural changes suggested by the crystal structures, but by subtle changes in the geometry of the C190–G209 base pair.
RESULTS and DISCUSSION
Design of chimeric SRP RNAs
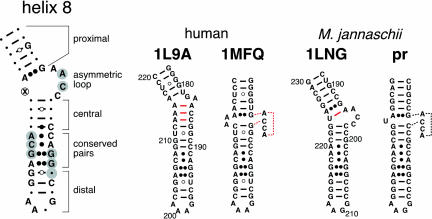
A comparison of hSR and MjSR (Fig. 1 ▶) shows that the proximal portion of helix 8 is composed of five canonical base pairs and an A–G pair. The adjacent asymmetric loop contains the sequence AACC on its 5′-side and an opposing dinucleotide (A213 and A214) in hSR or a single residue (U224) in MjSR. Four Watson-Crick and G–U pairs in the central region of helix 8 contain relatively variable residues. In contrast, the adjacent noncanonical A–C, G–G, and A–G pairs are extremely conserved. The distal portion of helix 8 is composed of three Watson-Crick base pairs and a tetraloop. The highly conserved residues form a flattened minor groove that is the predominant binding site for a helix–turn–helix motif of protein SRP54 (Batey et al. 2000; Kuglstatter et al. 2002). SRP54 is also in close proximity to the asymmetric loop.
FIGURE 1.
SRP RNA helix 8 secondary structures. On the left, residues that are conserved between human and MjSR are indicated as letters. The circled cross corresponds to two adenosine residues (A213 and A214) in human or a single uridine (U224) in MjSR. Watson-Crick pairings are indicated by lines, G–U interactions by circles, A–C pairings by dots, and purine–purine interactions by double dots. Grayed positions mark residues that are within 4 Å of protein SRP54 in the crystal structure of the ternary complex with SRP19 and SRP RNA (Kuglstatter et al. 2002). On the right, base pairs in the crystal structures 1L9A (Oubridge et al. 2002), 1MFQ (Kuglstatter et al. 2002), and 1LNG (Hainzl et al. 2002) in are indicated by red lines. The stacked conformation of the 5′-region of the asymmetric loop is indicated by dotted lines. The structure of M. jannaschii helix 8, labeled pr, is predicted to be similar to 1MFQ.
The construction of Ch1 to Ch9 has been described previously (Yin et al. 2001). The properties of mutant RNAs Ch1 to Ch19 are summarized in Table 1 ▶. Chimeric SRP RNA genes Ch10, Ch11, and Ch12 were synthesized by using PCR with a single mutagenic primer and Ch5 as the template DNA. A two-step strategy was used for the construction of Ch13 and Ch14 to obtain mutant RNAs with hSR-derived base pairs in the proximal region of helix 8. PCR overlap extension mutagenesis (Ho et al. 1989) was used to generate Ch15 to Ch19 (see Materials and Methods).
TABLE 1.
Binding activities of human SRP54M (54M) toward human SRP RNA (hSR), SRP RNA of M. jannaschii (MjSR), and the chimeric mutants Ch1–Ch19.
| Name | Mutations in full-length human SRP RNA unless indicated otherwise | 54M % Act. (no p19) | 54M % Act. (with p19) | Dependency |
| hSR | none | 0 | 58 ± 16 | +++ |
| MjSR | — | 100 | 100 | − |
| Ch1 | large domain of MjSR | 100 | 100 | − |
| Ch2 | MjSR helices 6 and 8 | 100 | 100 | − |
| Ch3 | MjSR helix 6 | 0 | 100 | +++ |
| Ch4 | MjSR helix 8 | 100 | 100 | − |
| Ch5 | U224 of MjSR changed to AA in Ch4 | 100 | 100 | − |
| Ch6 | central and distal region of MjSR helix 8 | 0 | 31 ± 7 | ++ |
| Ch7 | proximal and distal region of MjSR helix 8 | 0 | 0 | N/A |
| Ch8 | proximal and central region of MjSR helix 8 | 63 ± 13 | 94 ± 7 | + |
| Ch9 | 213-AA-214 replaced with U in Ch3 | 0 | 98 ± 3 | +++ |
| Ch10 | proximal region of MjSR helix 8 | 0 | 10 ± 4 | N/A |
| Ch11 | G200, 220-AC-0221 of MjSR in Ch7 | 0 | 8 ± 2 | N/A |
| Ch12 | 198-CC-199, 222-GG-223 of MjSR in Ch7 | 0 | 97 ± 4 | +++ |
| Ch13 | 188-CU-189, 228-CAG-230 of MjSR in Ch6 | 0 | 8 ± 4 | N/A |
| Ch14 | 191-CC-192, 226-GG-227 of MjSR in Ch6 | 94 ± 10 | 96 ± 5 | − |
| Ch15 | G200, C221 of MjSR in Ch12 | 80 ± 12 | 85 ± 11 | − |
| Ch16 | A220 of MjSR in Ch12 | 95 ± 4 | 100 | − |
| Ch17 | 213-AA-214 changed to U | 0 | 75 ± 10 | +++ |
| Ch18 | G200, 220-AC-221 of MjSR | 0 | 44 ± 8 | ++ |
| Ch19 | 199-CG-200, 220-ACG-222 of MjSR | 26 ± 9 | 78 ± 9 | + |
The degree to which binding of 54M depends on protein SRP19 (p19) is summarized in the column to the right; a minus indicates SRP19-independent binding. No assessment (N/A) is made for mutant RNAs that are incapable of binding SRP54M. Measurements are taken from two or more independent experiments.
Protein binding activities
SRP RNAs were synthesized in vitro by run-off transcription with T7 RNA polymerase. The ability of the various RNAs to bind to purified recombinant human SRP19 and SRP54M was measured by the affinity of the RNP complexes to DEAE Sepharose. RNA-bound protein was removed at elevated ionic strength, and the polypeptides in the flowthrough (F) and eluate (E) fractions were visualized by SDS-PAGE (see Materials and Methods).
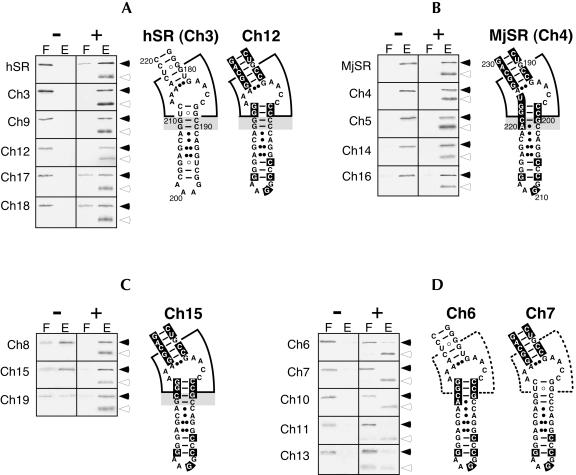
Approximately 60 percent of the added SRP54M polypeptides form complexes with hSR in the presence of protein SRP19. The less efficient binding of human SRP54 to its own SRP RNA may be due to the dissociation of SRP54 as required for SRP function (Huang et al. 2002). In contrast, SRP54M binds to MjSR completely and independently of SRP19 (Fig. 2 ▶; Table 1 ▶). The indistinguishable protein binding activities of MjSR and the Ch4 mutant RNA confirms that the SRP19 dependency resides within helix 8 of human SRP RNA (Yin et al. 2001).
FIGURE 2.
SDS PAGE of human SRP54M (solid arrowhead) in the flowthrough (F) and high-salt eluate (E) of DEAE-columns in the absence (−) or presence (+) of SRP19 (open arrowhead). The RNAs used in the binding reactions are labeled on the left of each panel. (A) Mutant RNAs with assembly properties similar to those observed with human SRP RNA (hSR). (B) Mutant RNAs that behave like M. jannaschii SRP RNA (MjSR). (C) Functional mutant RNAs that exhibit a residual dependency on SRP19. (D) Mutant RNAs in which binding of SRP54M is significantly reduced or abolished. Secondary structure examples are given for hSR, MjSR, and mutant RNAs Ch3, Ch4, Ch6, Ch7, Ch12, and Ch15. The asymmetric loop and its surroundings are marked by a wedge, which is drawn with dashed line in D. (A–C) The 2 bp (C189–G210 and C190–G209 of hSR, or G200–C221 and C201–A220 of MjSR) crucial for SRP19 dependency are highlighted in gray.
A noticeable difference between hSR and MjSR exists in the shorter strand of the asymmetric loop of helix 8. This site is composed of two adenosines (A213/A214) in hSR, or a single uridine residue (U224) in MjSR. In the structure of the binary complex with SRP19 (PDP accession 1L9A; Oubridge et al. 2002), A213/A214 base pairs intrahelically with C186/C185 (Fig. 1 ▶). However, in the ternary complex with SRP54M, A213/A214 forms A minor motifs with helix 6, presumably to stabilize the collapsed state of helix 8 (PDP accession 1MFQ; Kuglstatter et al. 2002). Replacing the single uridine of the asymmetric loop of Ch4 with two adenosine residues (A213/A214) in Ch5 has no effect on the SRP19-independent binding of SRP54M, rendering the Ch4 and Ch5 mutant RNAs indistinguishable from MjSR (Table 1 ▶). This result would be expected if helix 8 of the mutants exists in the collapsed state in the absence of SRP19. Alternatively, SRP54 might be able to induce the collapse of helix 8 without SRP19. In either case, the A minor motif interactions between helices 8 and 6 have no critical role for the helical collapse and the ability of SRP54 to bind. Possibly, A213/A214 provides additional stability for the collapsed conformation of the ternary complex after the binding of SRP54.
Conversely, in the Ch9 mutant RNA, the two adenosines of the asymmetric loop of Ch3 are changed to a uridine. In Ch17, the same change from AA to U has been introduced into the hSR wild-type background. Binding of SRP54M to mutant RNAs Ch9 and Ch17 remains dependent on the addition of SRP19, demonstrating that the uridine does not provoke the collapsed state. Combined with the fact that hSR and MjSR contain the same AACC loop sequence, this finding supports the conclusion that the asymmetric loop has no role in triggering the transition from the base paired to the collapsed conformation. The Ch17 RNA binds slightly more (75±10% versus 58±16%) SRP54 than hSR (Table 1 ▶), suggesting that the removal of a residue might have a minor favorable influence on the helical collapse.
Helix 8 of the binary complex composed of human SRP RNA and SRP19 (1L9A; Oubridge et al. 2002) contains 3 bp (shown connected by red lines in Fig. 1 ▶), which are disrupted in the ternary complex with SRP54 (1MFQ.pdb; Kuglstatter et al. 2002). The proximal and central regions of helix 8 stack on each other and thus provide a site that is suitable for the binding of SRP54. The ability of the chimeric SRP RNA molecules, which are equipped with a MjSR helix 8 to bind SRP54 in the absence of SRP19, is explained by the fact that the three-dimensional structures of SRP19-bound MjSR is very similar to hSR in the ternary complex. Although A194 pairs with U224 in the 1LNG structure (Fig. 1 ▶; Hainzl et al. 2002), this residue probably participates in the formation of a looped-out AACC stack, thus generating essentially identical structures of hSR and MjSR helix 8 (Fig. 1 ▶, cf. 1MFQ and pr). Also, SRP54 is unlikely to bind unless AACC extrudes and forms a continuous stack. In agreement with the idea that the conformation of the asymmetric loop of an archaeal SRP RNA helix 8 remains unchanged upon binding of SRP54, similar chemical modification patterns generated by probing of A. fulgidus SRP RNA with diethylpyrocarbonate in the absence or presence of SRP54 have been described (Diener and Wilson 2000).
For a more precise determination of the residues in helix 8 that are responsible for the SRP19-dependency of SRP54 binding, MjSR-derived residues were introduced into helix 8 of Ch5 at the locations described in Table 1 ▶. Although the parental Ch5 RNA binds SRP19 and SRP54M completely, the binding of SRP54M to the Ch6, Ch7, and Ch10 mutant RNAs is significantly impaired or completely abolished even when SRP19 is present. The diminished SRP54M binding activities are not due to the misfolding of the mutant RNAs because SRP19 binds completely to all the SRP RNAs investigated here (data not shown). Significant amounts (63±13%) of SRP54M bind to the Ch8 RNA in the absence of SRP19, and nearly complete binding (94±7%) of SRP54M is achieved in the presence of SRP19, indicating that a moderate SRP19 independency may be mediated by the distal portion of helix 8. It is concluded that the binding of SRP54M is reduced or completely lost when the vicinity of the asymmetric loop is composed of residues originating from both hSR and MjSR. This result demonstrates that only a finely tuned combination of residues surrounding the asymmetric loop is able to provide a proper binding site for SRP54.
Binding to SRP54M is almost completely abolished in Ch11, where the two MjSR base pairs positioned adjacent to the asymmetric loop are replaced with hSR-derived residues. In contrast, two mutations placed further away from the asymmetric loop in the Ch12 mutant RNA retain complete (97±4%) binding of SRP54M. Interestingly, binding of SRP54 to the Ch12 RNA is dependent of SRP19, although only three nucleotides (C198, G209, and G210 of hSR) are different from the SRP19-independent Ch5 RNA. Binding of SRP54M is abolished when 2 bp located proximal to the asymmetric loop are changed to the corresponding hSR residues (mutant RNA Ch13). In contrast, changing the three most proximal base pairs of helix 8 into hSR-derived residues (mutant RNA Ch14) retains the activity of Ch5. Binding of SRP19 was impaired in mutant RNAs Ch11 and Ch13, but only in the presence of SRP54, possibly due to steric hindrance between the two bound proteins.
The binding of SRP54M to Ch12 is entirely dependent on SRP19, although this RNA contains only three hSR-derived residues in an otherwise MjSR-like helix 8, demonstrating that C189–G210 and C190–G209 (shown in gray in Fig. 2 ▶) are crucial in conveying SRP19 dependency. As evidenced by the results obtained with Ch15, the transversion of the C189–G210 base pair of mutant RNA Ch12 to a G–C base pair is insufficient to grant SRP19 dependency, although a slight reduction in the SRP54M binding activity of the Ch15 mutant RNA (85±11% in the presence of SRP19) is apparent (Table 1 ▶). In Ch16, changing G209 to an adenosine and thus converting the C190–G209 Watson-Crick base pair to a noncanonical MjSR-like C–A pair is also insufficient for conveying the SRP19-dependent assembly behavior of hSR. These results demonstrate that the three residues C190, G209, and G210 are necessary for the SRP19-dependent binding of SRP54.
Comparing the crystal structures of the binary (1L9A) and the ternary complex (1MFQ) with respect to C189, G209, and G210 shows that the C189–G210 base pair is arranged in essentially the same way. C190–G209, however, is sheared in the binary complex but forms a standard Watson-Crick base pair in the ternary complex with SRP54. The deviation from a Watson-Crick pair by ~1.5Å in 1L9A (data not shown) represents a subtle but apparently functionally significant difference.
In the Ch18 mutant RNA, the C189–G210 and C190–G209 base pairs of hSR were changed into their equivalent MjSR-like G–C and C–A base pairs in an effort to convert hSR into a molecule that might bind SRP54 independently of SRP19. Ch18 binds significant amounts (44±8%) of SRP54M, but only when SRP19 is added, demonstrating that the context into which the two crucial base pairs are embedded may be important. The Ch19 mutant RNA contains an additional base pair toward a more MjSR-like helix 8 and reveals that a predominantly hSR-like mutant RNA can assume a low level (26±9%) of SRP19-independent binding of SRP54.
Conclusions
The investigation of chimeric SRP RNA molecules composed of portions derived from MjSR and hSR shows that the destruction of the A213 and A214 minor motifs has no effect on the SRP19-dependent binding of SRP54. Therefore, this interhelical RNA–RNA interaction likely plays a stabilizing role in a later stage of the assembly. Profound effects on the binding of SRP54 are observed with mutant RNAs possessing a chimeric character in the vicinity of the asymmetric loop. Because the structures of hSR, MjSR, and the mutant helix 8 RNAs are expected to be very similar, this finding indicates that minor structural differences are of paramount importance for the stability of the SRP54–RNA interaction.
Crucial for the SRP19 dependency are 2 bp corresponding to C189–G210 and C190–G209. These residues are positioned strategically between the conserved major binding site of SRP54 and the asymmetric loop of human hSR helix 8. The slightly but significantly altered geometry of the C190–G209 base pair in the ternary complex with SRP54 provides a striking example that a minor structural alteration is of critical importance for SRP assembly.
MATERIALS AND METHODS
Construction and synthesis of chimeric mutant SRP RNAs
PCR site-directed mutagenesis (Nelson and Long 1989) was used to obtain Ch10, Ch11, and Ch12, with oligonucleotides 5′-CTTT GACCTGCTCCGTTTCCGACCTGGGCCGGTTC-3′, 5′-CTTTGA GTTGCTCCCTTCCGGGCCTGGCCCGGTTC-3′, and 5′-CCTT TCCCCTGCTCCCTTCCGGGCCTGGGGGGGTT-3′ (base changes relative to Ch5 are shown underlined) in amplification reactions with Ch5 plasmid DNA as the template and 5′-GGGGTACTAG TAACCCGGGCAGGCACCCCAGGCTTTACACT-3′ as the upstream primer. Reactions were carried out in 50 μL of 50 mM Tris-Cl (pH 8.3), 2 mM MgCl2, 250 μg/mL BSA, 2% (w/v) sucrose, and 2 U Taq polymerase (Invitrogen) in a Rapid Cycler (Idaho Technology) using 35 cycles of 94°C, 60°C, and 5 sec at 74°C. Samples were diluted 10-fold with water, and 1 μL aliquots were mixed with 10 ng of Ch5 DNA in 48 μL PCR buffer containing 2 U of Taq polymerase. One-step extensions were carried out in a Perkin Elmer 4800 thermocycler by incubation for 1 min at 94°C, 1 min at 45°C, and 10 min at 72°C. Primers 5′-GGGGTACTAG TAACCCGGGC-3′ and 5′-CGCCAGGGTTTTCCCAGTCACGAC3′ were added, and samples were transferred to glass capillaries for amplification in a Rapid Cycler for 40 cycles of 94°C, 2 sec at 50°C, and 5 sec at 74°C. Samples were loaded onto a 1.5% agarose gel, and DNA fragments corresponding in size to 531 bp were cut from the gel. DNAs were extracted and digested with restriction endonucleases BamHI and EcoRI (New England Biolabs). Fragments corresponding to 328 bp were isolated from 1.5% agarose gels, extracted, and ligated to purified EcoRI- and BamHI-digested pΔ35 vector DNA (Zwieb 1991). The ligation mixture was used to transform competent E. coli DH5-α cells (Life Technologies) followed by selection on LB plates containing 100 μg/mL ampicillin. Clones were screened by isolating plasmid DNAs on a small scale, followed by restriction mapping with EcoRI and BamHI.
Ch13 was constructed by first introducing a two-nucleotide change into Ch5 with 5′-GGTTCACCAGTCCTTAG-3′. The resulting plasmid was used as the template in PCR reactions with 5′-CAGCACCCTGGTTTTCCG-3′. For Ch14, three nucleotides were changed in Ch5 by using 5′-CAGCACCGGACCTTTCC-3′ before the intermediate was used as a template with 5′-CTAAGGAGGGC CGAAC-3′.
PCR overlap extension mutagenesis (Higuchi et al. 1988) was carried out to obtain mutants Ch15 to Ch19. For Ch15, Ch5 was the template DNA in two separate PCR reactions with the primer pairs 5′-GCCTTTCCGCTGCTCCCT-3′ and 5′-GGGGTACTAG TAACCCGGGCAGGCACCCCAGGCTTTACACT-3′, and with 5′AGGGAGCAGCGGAAAGGC-3′ and 5′-CGCCAGGGTTTTCCCA GTCACGAC-3′ using 35 PCR cycles in an Indy thermocycler (Idaho Technology) for 5 sec at 94°C, 2 sec at 50°C, and 10 sec at 74°C with a temperature slope of 2°C/sec. The DNA fragments were purified and used in amplification reactions with flanking primers. The 528-bp PCR product was digested with EcoRI and BamHI, purified by agarose electrophoresis. Essentially the same method was used to obtain Ch16, except that Ch12 was the template and the mutagenic primers 5′-GCCTTTCCCTTGCTCCCT-3′ and 5′-AGGGAGCAAG GGAAAGGC-3′ were used at an annealing temperature of 60°C. For Ch17, two PCR reactions contained 5′-AGCACGGGAGTTAGA CCTGCTC-3′ and 5′-GGGGTACTAGTAACCCGGGCAGGCACC CCAGGCTTTACACT-3′, and 5′-AACGGAGCAGGTCTAACTCC3′ and 5′-CGCCAGGGTTTTCCCAGTCACGAC-3′, respectively, were used with the hSR plasmid as the template. To obtain Ch18, 5′-AG GTCGGAAACGGAGCAACTCAA-3′ and 5′-TCCGTTTCCGACCTG GCCCGGTTCAC-3′ were the mutagenic primers. 5′-TCGGAAACG GAGCAACGCAAAAC-3′ and 5′-TCCGTTTCCGACCTGGCGCGGT TCAC-3′ were used to synthesize Ch19. Plasmid DNAs of all mutant constructs were purified by CsCl density gradient centrifugation, and sequences were verified by using commercial providers.
RNAs were synthesized by run-off transcription in a volume of 20 μL for 2 h at 37°C containing 4 μg of DraI-linearized plasmid DNA and components of the T7-MEGAshortscript kit (Ambion). RNA concentrations were determined from a standard curve obtained with known amounts of E. coli 5S ribosomal RNA (Sigma) by electrophoresis of sample aliquots on 2% agarose gels, followed by staining with ethidium bromide and densitometry by using ImageJ available at http://rsb.info.nih.gov/ij/. Typically, yields were ~200 μg of RNA per reaction.
Purification of human SRP19 and SRP54M
Human SRP19 (molecular weight, 16,145 D) was purified by using the previously described protocol (Walker et al. 1995) with minor modifications. Briefly, competent E. coli BL21-DE3 cells were transformed with the expression plasmid pET23d-19X (Chittenden et al. 1994), incubated overnight at 37°C, and used to seed a 20-L fermenter (Bioflo IV, New Brunswick Scientific). Protein expression was induced by the addition of IPTG (Gold Biotechnologies) to 1 mM, followed by continued growth for 2 h. Cells were harvested by centrifugation, lysed by passing through a French press, subjected to differential centrifugation, and loaded onto a Biorex 70 (Bio-Rad) cation exchange column. SRP19-containing fractions eluted at ~300 mM salt were concentrated by using a Amicon Centricon 10 device, and purified on a Superdex 75 (Pharmacia) gel filtration column equilibrated in 25 mM Tris-Cl (pH 7.5), 150 mM NaCl, and 5 mM DTT. The protein sample was adjusted to 50% glycerol and stored at −20°C.
SRP54M (molecular weight, 23,310 Da; residues 297–504 of full-length human SRP54; Gowda et al. 1998), representing the methionine-rich domain of human SRP54, was expressed in E. coli BL21-DE3 cells and purified by chromatography on Biorex 70 as described (Gowda et al. 1999; Yin et al. 2001). Protein concentrations were determined by SDS-PAGE, using Coomassie blue R250 stained sample aliquots and known amounts of lysozyme (Bio-world) as standards.
Protein binding activities of the SRP RNAs
The protein binding activities of the RNAs were measured by using a modification of the procedure described by Lingelbach et al. (1988). Each reaction contained 50 μL of 50 mM Tris-HCl (pH 7.9), 300 mM KOAc, 5 mM MgCl2, 1 mM DTT, 10% glycerol, 15 μg of wild-type or mutant RNA, and 0.75 μg of purified human SRP54M protein with or without 0.9 μg human SRP19. Samples were incubated for 10 min at 37°C and loaded onto 40 μL-bed volume DEAE-Sepharose Fast Flow (Pharmacia) columns prepared in aerosol-barrier tips (Continental Laboratory Products) equilibrated in 50 mM Tris-HCl (pH 7.9), 300 mM KOAc, 5 mM MgCl2, and 1mM DTT. The columns were washed with 150 μL of 300 mM KOAc buffer, and the unbound materials were collected as flowthrough (F). RNA and bound proteins were eluted (E) with 200 μL of the same buffer but containing 1 M KOAc. One microgram of BSA (NEB) was added to each sample, followed by the addition of 40 μL TCA and incubation on ice for 30 min. Proteins were collected by a 10-min centrifugation at 4°C, resuspended in SDS loading buffer, and analyzed by SDS PAGE on 15% gels followed by staining with Commassie blue R250. Pictures of the stained gels were recorded, and the number of pixels in each band was measured by using Quantity One software (BioRad).
Acknowledgments
This work was supported by National Institutes of Health grant GM-49034 to C.Z.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.rnajournal.org/cgi/doi/10.1261/rna.5232404.
REFERENCES
- Batey, R.T., Rambo, R.P., Lucast, L., Rha, B., and Doudna, J.A. 2000. Crystal structure of the ribonucleoprotein core of the signal recognition particle. Science 287: 1232–1239. [DOI] [PubMed] [Google Scholar]
- Chittenden, K., Black, S., and Zwieb, C. 1994. Systematic site-directed mutagenesis of protein SRP19: Identification of the residues essential for binding to signal recognition particle RNA. J. Biol. Chem. 269: 20497–20502. [PubMed] [Google Scholar]
- Diener, J.L. and Wilson, C. 2000. Role of SRP19 in assembly of the Archaeoglobus fulgidus signal recognition particle. Biochemistry 39: 12862–12874. [DOI] [PubMed] [Google Scholar]
- Gowda, K., Black, S., Moeller, I., Sakakibara, Y., Liu, M-C., and Zwieb, C. 1998. Protein SRP54 of human signal recognition particle: Cloning, expression, and comparative analysis of functional sites. Gene 207: 197–207. [DOI] [PubMed] [Google Scholar]
- Gowda, K., Clemons, W.J., Zwieb, C., and Black, S. 1999. Expression, purification, and crystallization of the conserved methionine-rich domain of human signal recognition particle 54-kD protein. Protein Sci. 8: 1144–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainzl, T., Huang, S., and Sauer-Eriksson, A.E. 2002. Structure of the SRP19 RNA complex and implications for signal recognition particle assembly. Nature 417: 767–771. [DOI] [PubMed] [Google Scholar]
- Higuchi, R., Krummel, B., and Saiki, R.K. 1988. A general method of in vitro preparation and specific mutagenesis of DNA fragments: Study of protein and DNA interactions. Nucleic Acids Res. 16: 7351–7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, S.N., Hunt, H.D., Horton, R.M., Pullen, J.K., and Pease, L.R. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77: 51–59. [DOI] [PubMed] [Google Scholar]
- Huang, Q., Abdulrahman, S., Yin, J., and Zwieb, C. 2002. Systematic site-directed mutagenesis of human protein SRP54: Interactions with signal recognition particle RNA and modes of signal peptide recognition. Biochemistry 41: 11362–11371. [DOI] [PubMed] [Google Scholar]
- Keenan, R.J., Freymann, D.M., Stroud, R.M., and Walter, P. 2001. The signal recognition particle. Annu. Rev. Biochem. 70: 755–775. [DOI] [PubMed] [Google Scholar]
- Kuglstatter, A., Oubridge, C., and Nagai, K. 2002. Induced structural changes of 7SL RNA during the assembly of human signal recognition particle. Nat. Struct. Biol. 9: 740–744. [DOI] [PubMed] [Google Scholar]
- Larsen, N. and Zwieb, C. 1991. SRP-RNA sequence alignment and secondary structure. Nucleic Acids Res. 19: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingelbach, K., Zwieb, C., Webb, J.R., Marshallsay, C., Hoben, P.J., Walter, P., and Dobberstein, B. 1988. Isolation and characterization of a cDNA clone encoding the 19 kDa protein of signal recognition particle (SRP): Expression and binding to 7SL RNA. Nucleic Acids Res. 16: 9431–9442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai, K., Oubridge, C., Kuglstatter, A., Menichelli, E., Isel, C., and Jovine, L. 2003. Structure, function and evolution of the signal recognition particle. EMBO J. 22: 3479–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, R.M. and Long, G.L. 1989. A general method of site-specific mutagenesis using a modification of the Thermus aquaticus polymerase. Anal. Biochem. 180: 147–151. [DOI] [PubMed] [Google Scholar]
- Oubridge, C., Kuglstatter, A., Jovine, L., and Nagai, K. 2002. Crystal structure of SRP19 in complex with the S domain of SRP RNA and its implication for the assembly of the signal recognition particle. Mol. Cell 9: 1251–1261. [DOI] [PubMed] [Google Scholar]
- Pakhomova, O.N., Deep, S., Huang, Q., Zwieb, C., and Hinck, A.P. 2002. Solution structure of protein SRP19 of Archaeoglobus fulgidus signal recognition particle. J. Mol. Biol. 317: 145–158. [DOI] [PubMed] [Google Scholar]
- Rose, M.A. and Weeks, K.M. 2001. Visualizing induced fit in early assembly of the human signal recognition particle. Nat. Struct. Biol. 8: 515–520. [DOI] [PubMed] [Google Scholar]
- Rosenblad, M.A., Gorodkin, J., Knudsen, B., Zwieb, C., and Samuelsson, T. 2003. SRPDB: Signal Recognition Particle Database. Nucleic Acids Res. 31: 363–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, P., Black, S., and Zwieb, C. 1995. Cooperative assembly of signal recognition particle RNA with protein SRP19. Biochemistry 34: 11989–11997. [DOI] [PubMed] [Google Scholar]
- Walter, P. and Blobel, G. 1983. Disassembly and reconstitution of the signal recognition particle. Cell 34: 525–533. [DOI] [PubMed] [Google Scholar]
- Wild, K., Sinning, I., and Cusack, S. 2001. Crystal structure of an early protein–RNA assembly complex of the signal recognition particle. Science 294: 598–601. [DOI] [PubMed] [Google Scholar]
- Wild, K., Weichenrieder, O., Strub, K., Sinning, I., and Cusack, S. 2002. Towards the structure of the mammalian signal recognition particle. Curr. Opin. Struct. Biol. 12: 72–81. [DOI] [PubMed] [Google Scholar]
- Yin, J., Yang, C., and Zwieb, C. 2001. Assembly of human signal recognition particle (SRP): Overlap of regions required for binding of protein SRP54 and assembly control. RNA 7: 1389–1396. [PMC free article] [PubMed] [Google Scholar]
- Zwieb, C. 1991. Interaction of protein SRP19 with signal recognition particle RNA lacking individual RNA-helices. Nucleic Acids Res. 19: 2955–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwieb, C. and Eichler J. 2002. Getting on target: The archaeal signal recognition particle. Archaea 1: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]