Abstract
Ubiquitously expressed interferon regulatory factor 3 (IRF-3) is directly activated after virus infection and functions as a key activator of the immediate-early alpha/beta interferon (IFN) genes, as well as the RANTES chemokine gene. In the present study, a tetracycline-inducible expression system expressing a constitutively active form of IRF-3 (IRF-3 5D) was combined with DNA microarray analysis to identify target genes regulated by IRF-3. Changes in mRNA expression profiles of 8,556 genes were monitored after Tet-inducible expression of IRF-3 5D. Among the genes upregulated by IRF-3 were transcripts for several known IFN-stimulated genes (ISGs). Subsequent analysis revealed that IRF-3 directly induced the expression of ISG56 in an IFN-independent manner through the IFN-stimulated responsive elements (ISREs) of the ISG56 promoter. These results demonstrate that, in addition to its role in the formation of a functional immediate-early IFN-β enhanceosome, IRF-3 is able to discriminate among ISRE-containing genes involved in the establishment of the antiviral state as a direct response to virus infection.
Virus infection of susceptible host cells induces the transcription of multiple cellular genes, including interferons (IFNs), cytokines, and chemokines involved in the establishment of an antiviral state, in cell growth regulation, and in immune activation (33, 38). The subsequent secretion of the newly synthesized IFNs and their binding to cell surface receptors of neighboring cells result in the induction of a set of IFN-stimulated genes (ISGs) which are integral components in the development of the antiviral state. Control over ISGs is exerted, via the JAK/STAT signaling pathway, by an IFN-α/β-activated transcription factor complex, termed ISGF3 [ISGF3γ (IRF-9)/STAT1/STAT2] which binds to a common enhancer element referred to as the ISRE (IFN-stimulated responsive element) (40). This process causes a delayed response to virus infection, since it requires the synthesis of IFN-α/β in order to produce a cellular antiviral response. However, evidence exists for alternative pathways that regulate the transcription of ISGs under the control of the ISRE enhancer in a more timely fashion, without the need for prior de novo synthesis of IFNs and bypassing the JAK-STAT pathway (15, 25, 28, 31, 45, 46).
IFN-α/β genes are induced through the coordinate activation of transcriptional regulatory proteins including the interferon regulatory factors (IRFs) and NF-κB. IRF-3 is a 55-kDa protein that is expressed constitutively in a variety of tissues and maintained in a latent conformation in the cytoplasm. Virus-induced C-terminal phosphorylation of IRF-3 represents an important posttranslational modification, leading to dimerization, cytoplasm-to-nucleus translocation, association with CBP/p300 coactivators, and stimulation of DNA binding and transcriptional activities through binding to ISRE sites (22, 30, 35, 47, 50). Transient-coexpression assays have demonstrated that IRF-3 is a key component in the regulation of human IFN-β and IFN-α1 (murine α4) promoter (17, 25, 35, 36). However, IRF-3 alone is not sufficient to induce expression of endogenous human IFN-α1 and IFN-β (16, 48). After virus infection, IRF-3 cooperates with NF-κB and ATF-2/c-Jun to form a transcriptionally active enhanceosome complex on the IFN-β promoter (18, 26, 45).
Secretion of newly synthesized IFNs and binding to cognate receptors on the surface of surrounding cells activate the JAK/STAT pathway, which in turn induces the expression of another member of the IRF family, IRF-7. Activation of IRF-7 by virus-induced phosphorylation mediates the induction of delayed IFN-α (20, 25, 34). In addition to its involvement in the transcriptional induction of immediate-early IFN genes, IRF-3 also directly controls the expression of the CC chemokine RANTES in response to paramyxovirus infection (13, 21). Furthermore, human cytomegalovirus (HCMV)-induced activation of the ISG54 gene has been shown to be mediated by a transcriptional activator complex that contains IRF-3 (30).
Replacement of the serine and threonine residues in the C-terminal domain of IRF-3 that are phosphorylation targets with the phosphomimetic aspartic acid creates a constitutively active form of IRF-3 (IRF-3 5D) that is able to stimulate transcription in the absence of virus infection (1, 21, 22, 46). To date, several studies using DNA microarray analysis have identified genes whose expression is altered in response to virus infection (5, 6, 27, 51). In the present study, we used a molecular approach to profile genes that are directly responsive to IRF-3 without the requirement for virus infection; a tetracycline-inducible system was used to regulate the expression of the constitutively active form of IRF-3 in Jurkat T cells and coupled with DNA microarray analysis to profile IRF-3 target genes. Genes activated by IRF-3 5D included only a subset of the ISGs, i.e., ISG54, ISG56, and ISG60. Subsequent molecular analysis of endogenous ISG56 expression demonstrated high level transcriptional induction by IRF-3, mediated through the ISRE elements of the ISG56 promoter. Together, these results imply that IRF-3 activation in response to virus infection leads to selective regulation of IFN-responsive genes involved in the establishment of the antiviral state.
MATERIALS AND METHODS
Plasmid constructs and mutagenesis.
The 561-pGL3 luciferase reporter construct contains the −3 to −654 nucleotides of the ISG56 promoter (44) cloned into the SacI/HindIII restriction sites of the pGL3 basic vector. Point mutations of the ISRE sites in the promoter were generated by overlap PCR mutagenesis with Vent DNA polymerase, and the mutations were confirmed by sequencing. The IRF-3, IRF-3 5D, and IRF-3ΔN expression plasmids were described previously (22).
Cell culture, transfection, and luciferase assays.
rtTA-Neo and rtTA-IRF-3 5D Jurkat cells (16) were grown in RPMI 1640 medium containing 10% heat-inactivated calf serum, glutamine, antibiotics, 2.5 μg of puromycin/ml, and 400 μg of G418 (Gibco)/ml. Cells were induced with doxycycline (DOX) at 1 μg/ml for the indicated time in the presence of neutralizing antibodies against IFN-α/β (Sigma). HEC1B cells (American Type Culture Collection) were grown in minimal essential medium supplemented with 10% heat-inactivated fetal bovine serum, nonessential amino acids, sodium pyruvate, glutamine, and antibiotics and then transfected with Fugene reagent according to the manufacturer's instructions (Roche). For luciferase assays, subconfluent cells in 24-well plates were transfected with 10 ng of pRLTK reporter (Renilla luciferase, internal control), 100 ng of pGL3 reporter and the indicated amounts of expression plasmids. Cells were assayed for reporter gene activities after 24 h. For immunoblot analysis, cells in 60-mm plates were transfected with a total of 10 μg of DNA constructs and collected after 36 to 48 h. Where indicated, cells were treated with Sendai virus (40 hemagglutinating units [HAU]/ml) for 2 h in serum-free medium and further cultured for the indicated time in complete medium.
RNA isolation, preparation of cDNA, microarray hybridization, scanning, and data analysis.
Total RNA was isolated by lysing cells in Trizol reagent (Gibco) and poly(A) mRNAs were affinity purified on Oligotex column (Qiagen). Generation of cDNA, fluorescence labeling, hybridization to Incyte's UniGEM (v.2.0) Gene Expression Microarray, and data analysis were performed by Incyte Pharmaceuticals, Inc., Palo Alto, Calif. Briefly, poly(A) mRNA samples from rtTA-Neo and rtTA-IRF-3 5D Jurkat cells induced with DOX for 36 h were labeled with Cy3 and Cy5 fluorescence dyes, respectively, by reverse transcription. The probes were then used for competitive hybridization to the array, which contains a total of 9,182 elements representing 8,556 unique human genes and expressed sequence tags. The results were analyzed by using GEM Tool 2.4 software.
Northern blot analysis.
Poly(A) mRNAs (2 μg) were resolved by 1% agarose formaldehyde gel electrophoresis and transferred to Hybond N+ membrane (Amersham) in 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). The membrane was hybridized with cDNA probes labeled with [α32-P]dCTP (ICN) by random priming (High Prime kit; Roche). A 1.3-kb PCR product derived from ISG56 cDNA was used as a probe (provided by K. Mossman). The arginase II probe was obtained by PCR amplification of the arginase II cDNA (provided by M. Mori). A phospholipase C (PLC)-γ2-specific probe was generated by extracting the PLC-γ2 cDNA from a pFlag-CMV2 construct (provided by P. G. Suh). A cDNA probe for GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as a control. Hybridizations were perfomed overnight at 42°C in hybridization solution (0.1% sodium dodecyl sulfate [SDS]; 50% formamide, 5× SSC, 5× Denhardt solution). Membranes were washed two times in 2× SSC-0.1% SDS at 25°C for 15 min and one time in 1× SSC-0.1% SDS at 65°C for 30 min. Membranes were stripped in boiling stripping solution (0.1× SSC-0.1% SDS).
Immunoblot analysis.
rtTA-Neo and rtTA-IRF-3 5D Jurkat cells or transfected HEC1B cells were washed twice in phosphate-buffered saline (PBS), lysed in 50 mM Tris-HCl (pH 7.4)-1% NP-40-0.25% sodium deoxycholate-150 mM NaCl-1 mM EDTA supplemented with 1 mM phenylmethylsulfonyl fluoride, 5 μg of aprotinin/ml, and 5 μg of leupeptin/ml for 15 min on ice. Whole-cell extracts (50 μg) were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred on nitrocellulose membrane (Bio-Rad). The membrane was blocked in PBS containing 0.05% Tween 20 and 5% nonfat dry milk for 1 h and incubated with primary antibody, anti-IRF-3 (2 μg/ml; Santa-Cruz), anti-ISG56 (1:2,000) (15), or anti-α-actin (Chemicon) in blocking solution. After four 10-min washes in PBS-0.05% Tween 20, the membranes were incubated for 1 h with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse immunoglobulin G (1:5,000 to 1:10,000) in blocking solution. Immunoreactive proteins were visualized by enhanced chemiluminescence.
RESULTS
Tet-inducible expression of IRF-3 5D and DNA microarray.
The constitutively active form of IRF-3, IRF-3 5D, mimics the virus-activated phosphorylated form of IRF-3 with the capacity to dimerize, translocate to the nucleus, associate with CBP/p300, bind to DNA, and activate the transcription of target genes in the absence of virus infection (16, 21-23). To identify novel genes whose expression is under the control of IRF-3, a Jurkat cell line inducibly expressing IRF-3 5D in response to DOX stimulation was used, together with DNA microarray analysis. rtTA-Neo (control cells) and rtTA-IRF-3 5D Jurkat cells induced with DOX for 36 h were used. At this time of induction, IRF-3 5D was expressed at high levels, whereas the proapoptotic effects of IRF-3 5D were not detected (16). Although expression of IRF-3 5D alone does not induce the synthesis of endogenous IFNs (16, 48), DOX-induced cells were treated with neutralizing antibodies against IFN-α/β (16, 21) to rule out autocrine and/or paracrine effects of undetectable levels of IFNs. Poly(A) mRNAs isolated from DOX-induced rtTA-Neo and rtTA-IRF-3 5D Jurkat cells were used to generate fluorescent dye (Cy3 and Cy5, respectively)-labeled cDNAs. These fluorescent cDNAs were simultaneously hybridized to human UniGEM v.2.0 Gene Expression Microarray, and the differential expression of each clone was calculated from the relative intensities of the Cy3 and Cy5 fluorescent signals. Of the 9,182 elements monitored, 14 showed an expression increase of >1.5-fold (Table 1). Of those 14, 3 clones corresponding to ISG54, ISG56, and ISG60 were strongly induced. On the other hand, in the same analysis, 2.8% of the total elements were found to be repressed by ≥2-fold. A partial gene list of downregulated genes is provided in Table 1.
TABLE 1.
IRF-3 5D-responsive genes identified by microarray analysis
| Protein group and accession no. | Fold induction | Gene or gene product |
|---|---|---|
| Known IFN-induced proteins | ||
| M14660 | +18.3 | ISG54/IFI54 |
| X03557 | +8.4 | ISG56/IFI56 |
| AF026939 | +3.4 | IFN-induced protein with TPR 4/IFI60/ISG60 |
| D28915 | +1.9 | Hepatitis C virus-associated microtubular aggregate 44-kDa protein |
| M55542 | +1.7 | GBP1 |
| M87284 | +1.5 | 2′-5′ OAS |
| A1739106 | +1.5 | ISG15 |
| Metabolism, D86724 | +3.6 | Arginase type II |
| Phospholipases and related proteins | ||
| X14034 | +3.4 | PLC-γ2 |
| M80899 | +1.8 | AHNAK nucleoprotein |
| Nuclear receptor, X03225 | +2 | α-Glucocorticoid receptor |
| Posttranslational modification, AB004550 | +1.8 | UDP-Gal:βGlcNAc-β-1,4-galactosyltransferase, polypeptide 5 |
| Unknown function | ||
| AF026941 | +3.5 | CIG5a |
| D90070 | +2.1 | PMA-induced protein 1 |
| Cell signaling | ||
| Z46973 | −4 | Phosphoinositide-3-kinase, class 3 |
| L07590 | −3.3 | PP2A regulatory subunit B α isoform (PR72) and β isoform (PR130) |
| NM_002890 | −2.7 | RAS p21 protein activator 1 |
| AA203389 | −3.1 | Phenylalanine hydroxylase |
| AW297828 | −2.7 | GTP-binding protein overexpressed in skeletal muscle |
| AW172382 | −2.7 | Serine/threonine 17b (apoptosis inducing) |
| Cell surface proteins and ligand | ||
| Y00636 | −3 | CD58 antigen |
| D78152 | −3 | Annexin IV |
| NM_001797 | −2.8 | Cadherin 11 |
| NM_003105 | −2.8 | Sortilin-related receptor |
| AW027713 | −2.7 | CD1B antigen, b polypeptide |
| X59065 | −2.7 | Fibroblast growth factor 1 |
| Enzymes and related proteins | ||
| M64554 | 3.5 | Coagulation factor XIII, B polypeptide |
| NM_003816 | −3.3 | Disintegrin and metalloproteinase domain 9 |
| AI920897 | −3.1 | Tissue inhibitor of metalloproteinase 3 |
| X51420 | −3 | Tyrosinase-related protein 1 |
| Oncogenes, U65002 | −3.1 | Pleiomorphic adenoma gene 1 (PLAG1) |
| Extracellular matrix-related proteins, AI802950 | −3.1 | Microfibril-associated glycoprotein 2 |
| Nuclear proteins, U18271 | −2.8 | Thymopoietin beta/LAP2 |
| Translation-related proteins, AA315872 | −2.7 | Ribosomal protein S7 |
CIG, CMV-induced proteins identified by differential display (52).
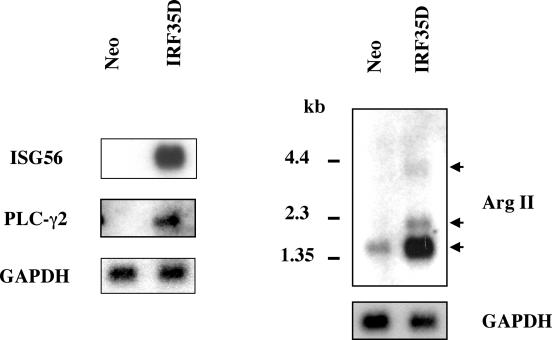
To confirm that genes whose expression was increased in the microarray analysis were also induced when examined biochemically, poly(A) mRNA isolated from rtTA-Neo and rtTA-IRF-3 5D Jurkat cells induced with DOX for 36 h were analyzed by Northern blotting. As illustrated in Fig. 1, 3 of the 14 upregulated genes, namely, ISG56, PLC-γ2, and arginase II, were strongly induced in rtTA-IRF-3 5D Jurkat cells versus rtTA-Neo Jurkat cells. Moreover, ISG54 has also been reported to be induced in response to IRF-3 5D in IFN-unresponsive cells (46).
FIG. 1.
IRF-3 5D-inducible expression of ISG56 gene. rtTA-Neo and rtTA-IRF-3 5D Jurkat cells were induced with DOX for 36 h in the presence of IFN-neutralizing antibodies. Poly(A) mRNA (2 μg/lane) was subjected to Northern blot analysis with 32P-labeled probes. After hybridization with ISG56 probe, the membrane was stripped and rehybridized with a PLC-γ2 probe, followed by treatment with GAPDH probe for normalization. A second membrane was hybridized with arginase II probe, stripped, and rehybridized with a GAPDH probe.
IRF-3-inducible expression of ISG56.
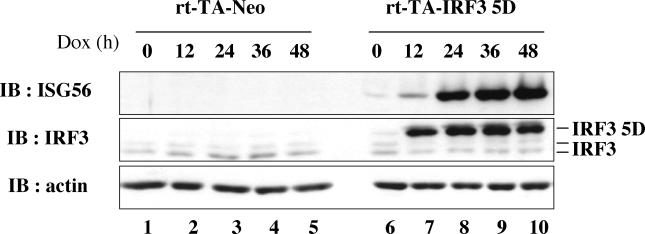
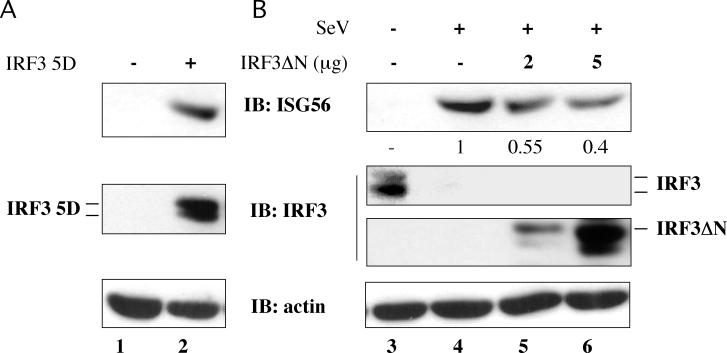
A subset of the genes known to be regulated by the IFN pathway dominated the expression profile of stimulated genes. Among these genes, HCMV-induced activation of the ISG54 gene has been reported to be mediated by a transcriptional complex that contains IRF-3 (30). To further investigate the potential regulation of ISG56 by IRF-3, whole-cell extracts obtained from rtTA-Neo and rtTA-IRF-3 5D Jurkat cells treated with DOX for various times were analyzed by immunoblotting. A strong induction of ISG56 was observed in rtTA-IRF-3 5D Jurkat cells beginning at 12 h (Fig. 2, lane 7) and was sustained throughout DOX treatment (Fig. 2, lanes 8 to 10), with the same kinetic profile as IRF-3 5D expression. The expression of ISG56 in response to virus infection was further examined in HEC1B cells, which do not respond to treatment with IFN-α/β (11). ISG56 protein expression was induced in HEC1B cells either by ectopic IRF-3 5D expression (Fig. 3A) or by Sendai virus stimulation (Fig. 3B, lane 4). Furthermore, virus induction was inhibited in a concentration-dependent manner by expression of a dominant-negative form of IRF-3, IRF-3ΔN (Fig. 3B, lanes 5 and 6), which lacks the N-terminal DNA-binding domain (22). These results demonstrate that IRF-3 is sufficient to induce ISG56 in response to virus infection via an IFN-independent pathway.
FIG. 2.
IRF-3 5D-inducible expression of ISG56 gene. rtTA-Neo (lanes 1 to 5) and rtTA-IRF-3 5D (lanes 6 to 10) Jurkat cells were exposed to DOX (1 μg/ml) and anti-IFN antibodies for the indicated times. Whole-cell extracts (50 μg) were subjected to SDS-PAGE and analyzed by immunoblotting with anti-ISG56 antibodies. Membranes were stripped and reprobed with anti-IRF-3 and anti-actin antibodies.
FIG. 3.
IRF-3-induced expression of ISG56 is IFN independent. (A) IFN-unresponsive HEC1B cells were transfected with an empty vector (lane 1) or expression plasmid encoding IRF-3 5D (5 μg) (lane 2) and harvested 36 h posttransfection. (B) HEC1B cells were transfected either with an empty vector (lanes 3 and 4) or with the indicated amount of IRF 3ΔN expression plasmid (lanes 5 and 6). At 36 h posttransfection, cells were infected with Sendai virus (+) and further cultured for 10 h. In panels A and B, whole-cell extracts (50 μg) were analyzed by immunoblotting with anti-ISG56, anti-IRF-3, and anti-actin antibodies. In panel B, the relative levels of ISG56 expression are indicated.
Transactivation of ISG56 promoter by IRF-3.
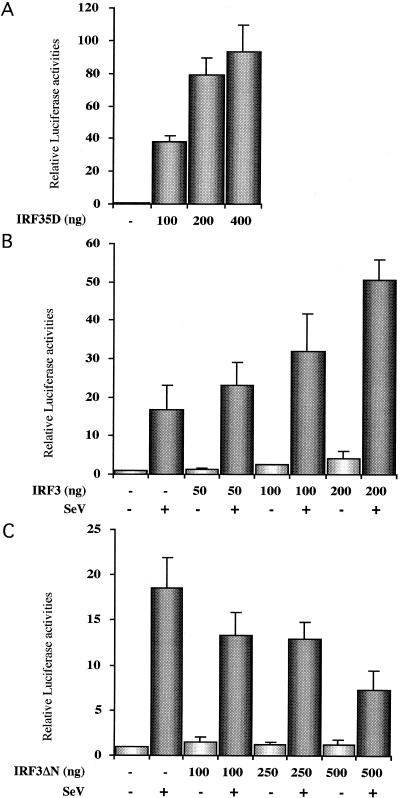
The ability of IRF-3 to directly regulate the ISG56 promoter (44) was further assayed. Cotransfection of an IRF-3 5D expressing plasmid into HEC1B cells with the 561-luciferase construct resulted in a dose-dependent increase in promoter activity (Fig. 4A). Sendai virus infection of HEC1B resulted in a 17-fold induction of promoter activity, and this induction was increased in a dose-dependent manner by cotransfection of an IRF-3-expressing plasmid (Fig. 4B). To further evaluate the dependence of the ISG56 promoter on activated IRF-3, cells were transfected with increasing amounts of the dominant-negative IRF-3ΔN construct. Induction of the ISG56 promoter after Sendai virus infection was inhibited in a dose-dependent manner with >50% inhibition at the highest concentration of IRF-3ΔN (Fig. 4C).
FIG. 4.
Transactivation of the ISG56 promoter by IRF-3. HEC1B cells were cotransfected with the 561-luciferase reporter construct containing the ISG56 promoter and increasing amounts of IRF-3 5D (A), IRF-3 (B), and IRF-3ΔN (C) expression plasmids. At 8 h posttransfection, cells were left untreated or infected with Sendai virus as indicated. The relative luciferase activities were measured at 24 h posttransfection and are expressed as the fold activation relative to the basal level in the presence of the control vector. The results were normalized by using Renilla luciferase. Each value represents the mean ± the standard error of triplicate independent samples. The data are representative of at least two different experiments with similar results.
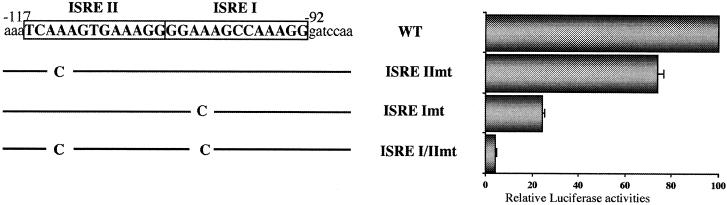
Involvement of ISRE sites in the IRF-3-dependent regulation of the 561-luciferase promoter.
Two ISRE consensus sites are present in the promoter of ISG56, at positions −92 to −104 (ISRE I) and positions −105 to −117 (ISRE II) (44). To test the direct involvement of these sites in the IRF-3-mediated activation of the ISG56 promoter, point mutations were introduced in the ISRE sites of the 561-luciferase construct. The ISRE I and ISRE II sites were mutated either independently (ISRE Imt and ISRE IImt constructs, respectively) or together (ISRE I/IImt) to differentiate the contribution of each site to IRF-3 activation (Fig. 5). An IRF-3 5D-expressing plasmid was cotransfected with the wild-type or mutated 561-luciferase constructs into HEC1B cells. Analysis of the promoter activity demonstrated that mutation of the ISRE II site slightly decreased the activation of the promoter (26%), whereas mutation of the ISRE I site resulted in a strong inhibition (76%) (Fig. 5). Mutation of both sites completely abrogated the induction of the promoter (Fig. 5). These results demonstrate that IRF-3-dependent activation of the ISG56 promoter is mediated through both ISRE sites, with a major contribution by the ISRE I.
FIG. 5.
Involvement of ISRE sites in IRF-3 5D-dependent induction of the ISG56 promoter. A schematic representation of the ISG56 promoter mutants generated by overlap PCR is provided. HEC1B cells were cotransfected with the wild-type (WT) or mutated 561-luciferase reporter constructs and 200 ng of IRF-3 5D expression plasmid. The relative luciferase activities were measured at 24 h posttransfection. After normalization for the basal level in the presence of the reporter construct alone and Renilla luciferase, the luciferase activities were expressed as percentages of the activation observed with the wild-type 561-luciferase reporter. Each value represents the mean ± the standard error of triplicate independent samples. The data are representative of at least two different experiments with similar results.
DISCUSSION
In this study, we report the first systematic analysis of genes regulated by IRF-3, by using a combination of both Tet-inducible expression of a constitutively active form of IRF-3 and DNA microarray analysis. A total of 14 genes were upregulated, and 257 were downregulated in Jurkat T cells. Seven of the genes exhibiting an increased transcriptional response to IRF-3 5D expression belong to the family of known IFN-induced genes: ISG54, ISG56, ISG60, hepatitis C-associated microtubular aggregate 44-kDa protein, guanylate binding protein 1 (GBP1), 2′-5′ oligoadenylate synthase (2′-5′ OAS), and ISG15. In comparison with other microarray studies with herpes simplex virus (HSV)-, HCMV-, and human papillomavirus type 16 (HPV16)-infected cells, a striking overlap in the profile of activated genes was observed, thus supporting the concept that many viruses elicit the production of IFN antiviral genes directly via IRF-3 activation (Table 2). In HSV- and/or HCMV-infected cells, in which IRF-3 is activated, ISG54, ISG56, ISG15, 2′-5′ OAS, GBP1, and CIG5 were found to be upregulated (5, 27, 41, 51). On the other hand, in HPV-infected cells in which IRF-3 is inhibited via physical interaction with HPV16 E6 protein, ISG54, ISG56, 2′-5′ OAS, GBP1, and hepatitis C virus-associated microtubular aggregate 44-kDa protein gene expression was found to be downregulated (6, 32). This partial overlap of the target gene profiles also demonstrates that the constitutively active form of IRF-3 in combination with microarray technology is a powerful molecular tool to explore early events in the host response to viral infection.
TABLE 2.
Comparison of microarray experiments with IRF-3 5D-expressing cells, virus-infected cells, or IFN-stimulated cellsa
| Gene | Upregulation (+) or downregulation (−) with:
|
|||||
|---|---|---|---|---|---|---|
| IRF-3 5D | HSV-1b | HCMVc | HPV31d | IFNe
|
||
| α | β | |||||
| ISG54 | + | + | + | − | + | + |
| ISG56 | + | + | + | − | + | + |
| ISG60 | + | ND | + | ND | ND | ND |
| CIG5 | + | + | + | ND | + | ND |
| PMA-induced protein 1 | + | + | + | ND | + | + |
| Microtubular aggregate protein 44 | <2 | ND | ND | − | + | + |
| 2′-5′ OAS | <2 | + | + | − | + | + |
| ISG15 | <2 | + | + | ND | + | + |
| GBP1 | <2 | ND | + | − | + | + |
This study represents the first demonstration that the phosphomimetic form of IRF-3 is sufficient to stimulate a subset of the IFN-stimulated genes. The physiological relevance of the ISG56 induction was demonstrated in Sendai virus-infected, IFN-nonresponsive cells in the presence or absence of a dominant-negative form of IRF-3, IRF-3ΔN. It has also previously been reported that double-stranded RNA (dsRNA)-dependent activation of the ISG56 gene requires IRF-1 (2). It is therefore possible that, in the context of virus infection, both IRF-3 and IRF-1 cooperate in the regulation of ISG56, which would explain why ISG56 induction was not completely abolished by IRF-3ΔN expression in response to Sendai virus infection. Using gene reporter assays and point mutations of the ISREs of the ISG56 promoter, we demonstrated that IRF-3 is sufficient to induce high-level activation, mediated through the ISRE I and to a lesser extent through the ISRE II of the ISG56 promoter.
The response of cells to virus infection includes activation of IRF-3, which cooperates with other transcriptional activators to induce IFN-α/β genes via the ISRE enhancer (18, 26, 45). Secretion of newly synthesized IFNs and binding to cognate receptors on the surface of surrounding cells activate ISGF3, which in turn contributes to ISG regulation through ISRE elements. Based on the similarity of the target sequence and on data that describe expression of ISRE-containing genes directly by the virus infection pathway (15, 25, 28, 31, 45, 46), one would have expected that most of the ISGs were also regulated directly by IRF3. However, in this study, we demonstrate that, in vivo, activation of IRF-3 is sufficient to activate only a subset of ISGs, namely, ISG54, ISG56, ISG60, CIG5, and phorbol myristate acetate (PMA)-induced protein 1 and, to a lesser extent, the hepatitis C-associated microtubular aggregate 44-kDa protein, GBP1, 2′-5′ OAS, and ISG15. Other IFN-inducible genes present on the microarray gene panel, including PKR, were not upregulated (Table 3), whereas other microarray studies demonstrated that the ISGs listed in Table 3 were upregulated in response to IFN-α and/or IFN-β (8, 9, 27). These results support the idea that IRF-3 can discriminate among ISRE-containing genes. Using mouse embryonic fibroblast (MEF) cells with a targeted disruption of the IRF-3 gene, Taniguchi's group also showed that in the context of Newcastle disease virus infection, the ISGs were also differentially regulated by IRF-3. Indeed, whereas ISG54 and GBP1 were activated by IRF-3, full-level induction of ISG15 required not only IRF3, but ISGF3 as well, and PKR and 2′-5′ OAS gene expression was totally dependent on the IFN-ISGF3 pathway (28). The molecular mechanism underlying this differential regulation remains undefined, but a recent study demonstrated that IRF-3 possesses a very restricted ISRE DNA-binding site specificity [GAAA(C/G)(C/G)GAAAN(T/C)]. IRF-7, on the other hand, has a broader DNA-binding specificity [GAA(A/T)N(C/T)GAAAN(T/C)] that contributes to its capacity to stimulate delayed-type ISGs after IFN synthesis (20). Furthermore, the nucleotide sequences surrounding the ISRE site may also be critical for IRF-3 binding (7, 28).
TABLE 3.
IFN-stimulated genes that are unresponsive to IRF-3 5D expression
| Accession no. | Gene | Expression upon stimulation witha:
|
|
|---|---|---|---|
| IFN-α | IFN-β | ||
| M87503 | ISGF3γ/p48/IRF-9 | + | + |
| AA478534 | STAT1 | + | + |
| BC007922 | ISG20 | ND | ND |
| AF135187 | MxA | + | + |
| M30818 | MxB | + | + |
| NM_021034 | IFN-induced transmembrane protein 3 (IFITM3) | ND | ND |
| NM_008326 | IFN-induced protein 1 (IFI1) | ND | ND |
| U72882 | IFI35 | + | + |
| BC015603 | IFI6-16 | + | + |
| J04164 | IFI9-27 | + | + |
| X14454 | IRF-1 | + | + |
| NM_001572 | IRF-7 | + | + |
| AW270525 | dsRNA-dependent protein kinase (PKR) | + | + |
| NM_001111 | dsRNA adenosine deaminase (ADAR1) | + | + |
| NM_004120 | GBP2 | + | + |
In addition to IFN treatment, ISG54 and ISG56 are also induced by other agents, including viral infection, dsRNA, and lipopolysaccharides (37). These latter stimuli are also known to activate IRF-3 (29, 47). HCMV, vesicular stomatitis virus, Newcastle disease virus, HSV, measles virus, and Sendai virus have all been shown to induce activation through IRF-3 phosphorylation (22, 29, 30, 39, 50, 52). Thus, the IRF-3-dependent induction described in this study explains the IFN-independent regulation of these ISGs in response to different stimuli.
Three of the ISGs that are IRF-3 5D responsive—ISG54, ISG56, and ISG60—encode highly homologous tetratricopeptide repeat (TPR)-containing proteins which belong to the same gene family (10, 43). The TPR repeat motifs are involved in protein-protein interactions and represent a recurring structural motif in many proteins involved in the IFN antiviral response. Recently, a novel posttranslational regulation of human IRF-4 by the TPR-containing immunophilin FKBP52 has been described (24).
The specific functions of many IFN-induced proteins remain to be determined. Interestingly, ISG56 has recently been shown to downregulate protein synthesis through interaction with the p48 subunit of eIF-3 (14) and to act as a strong negative regulator of cell proliferation (12). IRF-3 5D has previously been shown to induce apoptosis when overexpressed in cell lines (16), although the exact mechanism mediating this process remains unknown. Thus, the translation inhibitory function of ISG56 may provide, at least in part, a mechanistic explanation for the ability of IRF-3 5D to mediate apoptosis. It will also be interesting to analyze the involvement of the other genes identified in this study as new IRF-3 target genes, in the apoptosis process.
Intriguingly, a large number of cellular genes were also significantly downregulated by IRF-3 5D expression. This result is in contrast to previous data demonstrating that IRF-3 functions as a strong activator of gene transcription through its association with the coactivator CBP/p300 (19, 22, 49). The inhibitory effect of IRF-3 may be explained by sequestration of CBP/p300 as a consequence of its interaction with highly expressed IRF-3 5D. This mechanism has previously been shown to be involved in the repression of AP-1-dependent transcription by the adenovirus E1A 12S gene product (3). Thus, the downregulation of genes observed in our study may not be physiologically relevant, since the endogenous phosphorylated form of IRF-3 has a very short half-life and does not accumulate in cells.
The profile of genes modulated by IRF-3 5D was independently evaluated by using 32P-labeled RNA from rtTA-Neo and rtTA-IRF-3 5D Jurkat cells, together with cDNA macroarray (Atlas Human 1.2; Clontech) analysis (data not shown). A total of 1,176 genes were analyzed by this method, and the overall results obtained were similar to the results obtained by microarray, thus decreasing the possibility that a systematic error in Cy3 and Cy5 labeling (4, 42) may be responsible for the profile observed in our study.
In conclusion, these results demonstrate that IRF-3 is sufficient to induce a specific protein synthesis-independent antiviral response. Indeed, IRF-3 acts not only by contributing to the induction of the immediate early IFN-α/β genes but also through direct activation of a subset of the antiviral IFN-stimulated genes.
Acknowledgments
We thank K Mossman, P. G. Suh, and M. Mori for reagents used in this study and members of the Molecular Oncology Group, Lady Davis Institute, for helpful discussions.
This research was supported by grants from the Canadian Institutes for Health Research, the National Cancer Institute of Canada, and the CANVAC Network Centers of Excellence. N.G. was supported by ARC and FRSQ postdoctoral fellowships, M.J.S. was supported by a CIHR postdoctoral fellowship, and J.H. was supported by a CIHR Senior Scientist award.
REFERENCES
- 1.Azimi, N., Y. Tagaya, J. Mariner, and T. A. Waldmann. 2000. Viral activation of interleukin-15 (IL-15): characterization of a virus-inducible element in the IL-15 promoter region. J. Virol. 74:7338-7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandyopadhyay, S. K., G. T. Leonard, Jr., T. Bandyopadhyay, G. R. Stark, and G. C. Sen. 1995. Transcriptional induction by double-stranded RNA is mediated by interferon-stimulated response elements without activation of interferon-stimulated gene factor 3. J. Biol. Chem. 270:19624-19629. [DOI] [PubMed] [Google Scholar]
- 3.Bannister, A. J., and T. Kouzarides. 1995. CBP-induced stimulation of c-Fos activity is abrogated by E1A. EMBO J. 14:4758-4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartosiewicz, M., M. Trounstine, D. Barker, R. Johnston, and A. Buckpitt. 2000. Development of a toxicological gene array and quantitative assessment of this technology. Arch. Biochem. Biophys. 376:66-73. [DOI] [PubMed] [Google Scholar]
- 5.Browne, E. P., B. Wing, D. Coleman, and T. Shenk. 2001. Altered cellular mRNA levels in human cytomegalovirus-infected fibroblasts: viral block to the accumulation of antiviral mRNAs. J. Virol. 75:12319-12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, Y. E., and L. A. Laimins. 2000. Microarray analysis identifies interferon-inducible genes and Stat-1 as major transcriptional targets of human papillomavirus type 31. J. Virol. 74:4174-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daly, C., and N. C. Reich. 1995. Characterization of specific DNA-binding factors activated by double-stranded RNA as positive regulators of interferon alpha/beta-stimulated genes. J. Biol. Chem. 270:23739-23746. [DOI] [PubMed] [Google Scholar]
- 8.Der, S. D., A. Zhou, B. R. G. Williams, and R. H. Silverman. 1998. Identification of genes differentially regulated by interferon α, β, or γ using oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:15623-15628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Veer, M. J., M. Holko, M. Frevel, E. Walker, S. Der, J. M. Paranjape, R. H. Silverman, and B. R. Williams. 2001. Functional classification of interferon-stimulated genes identified using microarrays. J. Leukoc. Biol. 69:912-920. [PubMed] [Google Scholar]
- 10.de Veer, M. J., H. Sim, J. C. Whisstock, R. J. Devenish, and S. J. Ralph. 1998. IFI60/ISG60/IFIT4, a new member of the human IFI54/IFIT2 family of interferon-stimulated genes. Genomics 54:267-277. [DOI] [PubMed] [Google Scholar]
- 11.Fuse, A., H. Ashino-Fuse, and T. Kuwata. 1984. Binding of 125I-labeled human interferon to cell lines with low sensitivity to interferon. Gann 75:379-384. [PubMed] [Google Scholar]
- 12.Geiss, G., G. Jin, J. Guo, R. Bumgarner, M. G. Katze, and G. C. Sen. 2001. A comprehensive view of regulation of gene expression by double-stranded RNA-mediated cell signaling. J. Biol. Chem. 32:30173-30182. [DOI] [PubMed] [Google Scholar]
- 13.Genin, P., M. Algarte, P. Roof, R. Lin, and J. Hiscott. 2000. Regulation of RANTES chemokine gene expression requires cooperativity between NF-κB and IFN-regulatory factor transcription factors. J. Immunol. 164:5352-5361. [DOI] [PubMed] [Google Scholar]
- 14.Guo, J., D. J. Hui, W. C. Merrick, and G. C. Sen. 2000. A new pathway of translational regulation mediated by eukaryotic initiation factor 3. EMBO J. 19:6891-6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo, J., K. L. Peters, and G. C. Sen. 2000. Induction of the human protein P56 by interferon, double-stranded RNA, or virus infection. Virology 267:209-219. [DOI] [PubMed] [Google Scholar]
- 16.Heylbroeck, C., S. Balachandran, M. J. Servant, C. DeLuca, G. N. Barber, R. Lin, and J. Hiscott. 2000. The IRF-3 transcription factor mediates Sendai virus-induced apoptosis. J. Virol. 74:3781-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juang, Y. T., W. Lowther, M. Kellum, W. C. Au, R. Lin, J. Hiscott, and P. M. Pitha. 1998. Primary activation of interferon α and interferon β gene transcription by interferon regulatory factory-3. Proc. Natl. Acad. Sci. USA 95:9837-9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim, T. K., and T. Maniatis. 1997. The mechanism of transcriptional synergy of an in vitro assembled interferon-beta enhanceosome. Mol. Cell 1:119-129. [DOI] [PubMed] [Google Scholar]
- 19.Kumar, K. P., K. M. McBride, B. K. Weaver, C. Dingwall, and N. C. Reich. 2000. Regulated nuclear-cytoplasmic localization of interferon regulatory factor 3, a subunit of double-stranded RNA-activated factor 1. Mol. Cell. Biol. 20:4159-4168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin, R., P. Genin, Y. Mamane, and J. Hiscott. 2000. Selective DNA binding and association with the CREB binding protein coactivator contribute to differential activation of alpha/beta interferon genes by interferon regulatory factors 3 and 7. Mol. Cell. Biol. 20:6342-6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin, R., C. Heylbroeck, P. Genin, P. M. Pitha, and J. Hiscott. 1999. Essential role of interferon regulatory factor 3 in direct activation of RANTES chemokine transcription. Mol. Cell. Biol. 19:959-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin, R., C. Heylbroeck, P. M. Pitha, and J. Hiscott. 1998. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 18:2986-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin, R., Y. Mamane, and J. Hiscott. 1999. Structural and functional analysis of interferon regulatory factor 3: localization of the transactivation and autoinhibitory domains. Mol. Cell. Biol. 19:2465-2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mamane, Y., S. Sharma, L. Petropoulos, R. Lin, and J. Hiscott. 2000. Posttranslational regulation of IRF-4 activity by the immunophilin FKBP52. Immunity 12:129-140. [DOI] [PubMed] [Google Scholar]
- 25.Marie, I., J. E. Durbin, and D. E. Levy. 1998. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 17:6660-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merika, M., A. J. Williams, G. Chen, T. Collins, and D. Thanos. 1998. Recruitment of CBP/p300 by the IFNβ enhanceosome is required for synergistic activation of transcription. Mol. Cell 1:277-287. [DOI] [PubMed] [Google Scholar]
- 27.Mossman, K. L., P. F. MacGregor, J. J. Rozmus, A. B. Goryachev, A. M. Edwards, and J. R. Smiley. 2001. Herpes simplex virus triggers and then disarms a host antiviral response. J. Virol. 75:750-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakaya, T., M. Sato, N. Hata, M. Asagiri, H. Suemori, S. Noguchi, N. Tanaka, and T. Taniguchi. 2001. Gene induction pathways mediated by distinct IRFs during viral infection. Biochem. Biophys. Res. Commun. 283:1150-1156. [DOI] [PubMed] [Google Scholar]
- 29.Navarro, L., and M. David. 1999. p38-dependent activation of interferon regulatory factor 3 by lipopolysaccharide. J. Biol. Chem. 274:35535-35538. [DOI] [PubMed] [Google Scholar]
- 30.Navarro, L., K. Mowen, S. Rodems, B. Weaver, N. Reich, D. Spector, and M. David. 1998. Cytomegalovirus activates interferon immediate-early response gene expression and an interferon regulatory factor 3-containing interferon-stimulated response element-binding complex. Mol. Cell. Biol. 18:3796-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholl, M. J., L. H. Robinson, and C. M. Preston. 2000. Activation of cellular interferon-responsive genes after infection of human cells with herpes simplex virus type 1. J. Gen. Virol. 81:2215-2218. [DOI] [PubMed] [Google Scholar]
- 32.Ronco, L. V., A. Y. Karpova, M. Vidal, and P. M. Howley. 1998. Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity. Genes Dev. 12:2061-2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato, M., H. Suemori, N. Hata, M. Asagiri, K. Ogasawara, K. Nakao, T. Nakaya, M. Katsuki, S. Noguchi, N. Tanaka, and T. Taniguchi. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-α/β gene induction. Immunity 13:539-548. [DOI] [PubMed] [Google Scholar]
- 35.Sato, M., N. Tanaka, N. Hata, E. Oda, and T. Taniguchi. 1998. Involvement of IRF family transcription factor IRF-3 in virus-induced activation of IFN-β gene. FEBS Lett. 425:112-116. [DOI] [PubMed] [Google Scholar]
- 36.Schafer, S. L., R. Lin, P. A. Moore, J. Hiscott, and P. M. Pitha. 1998. Regulation of type 1 interferon gene expression by interferon regulatory factor 3. J. Biol. Chem. 273:2714-2720. [DOI] [PubMed] [Google Scholar]
- 37.Sen, G. C. 2000. Novel functions of interferon-induced proteins. Semin. Cancer Biol. 10:93-101. [DOI] [PubMed] [Google Scholar]
- 38.Sen, G. C. 2001. Viruses and interferons. Annu. Rev. Microbiol. 55:255-281. [DOI] [PubMed] [Google Scholar]
- 39.Servant, M. J., B. ten Oever, C. LePage, L. Conti, S. Gessani, I. Julkunen, R. Lin, and J. Hiscott. 2001. Identification of distinct signaling pathways leading to the phosphorylation of interferon regulatory factor 3. J. Biol. Chem. 276:355-363. [DOI] [PubMed] [Google Scholar]
- 40.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 41.Stingley, S. W., J. J. Ramirez, S. A. Aguilar, K. Simmen, R. M. Sandri-Goldin, P. Ghazal, and E. K. Wagner. 2000. Global analysis of herpes simplex virus type 1 transcription using an oligonucleotide-based DNA microarray. J. Virol. 74:9916-9927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taniguchi, M., K. Miura, H. Iwao, and S. Yamanaka. 2001. Quantitative assessment of DNA microarrays: comparison with Northern blot analyses. Genomics 71:34-39. [DOI] [PubMed] [Google Scholar]
- 43.Wathelet, M. G., I. M. Clauss, J. Content, and G. A. Huez. 1988. The IFI-56K and IFI-54K interferon-inducible human genes belong to the same gene family. FEBS Lett. 231:164-171. [DOI] [PubMed] [Google Scholar]
- 44.Wathelet, M. G., I. M. Clauss, C. B. Nols, J. Content, and G. A. Huez. 1987. New inducers revealed by the promoter sequence analysis of two interferon-activated human genes. Eur. J. Biochem. 169:313-321. [DOI] [PubMed] [Google Scholar]
- 45.Wathelet, M. G., C. H. Lin, B. S. Parekh, L. V. Ronco, P. M. Howley, and T. Maniatis. 1998. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-β enhancer in vivo. Mol. Cell 1:507-518. [DOI] [PubMed] [Google Scholar]
- 46.Weaver, B. K., O. Ando, K. P. Kumar, and N. C. Reich. 2001. Apoptosis is promoted by the dsRNA-activated factor (DRAF1) during viral infection independent of the action of interferon or p53. FASEB J. 15:501-515. [DOI] [PubMed] [Google Scholar]
- 47.Weaver, B. K., K. P. Kumar, and N. C. Reich. 1998. Interferon regulatory factor 3 and CREB-binding protein/p300 are subunits of double-stranded RNA-activated transcription factor DRAF1. Mol. Cell. Biol. 18:1359-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yeow, W. S., W. C. Au, Y. T. Juang, C. D. Fields, C. L. Dent, D. R. Gewert, and P. M. Pitha. 2000. Reconstitution of virus-mediated expression of interferon alpha genes in human fibroblast cells by ectopic interferon regulatory factor-7. J. Biol. Chem. 275:6313-6320. [DOI] [PubMed] [Google Scholar]
- 49.Yoneyama, M., W. Suhara, Y. Fukuhara, and T. Fujita. 1997. Direct activation of a factor complex composed of IRF-3 and CBP/p300 by virus infection. J. Interferon Cytokine Res. 17:S53. [Google Scholar]
- 50.Yoneyama, M., W. Suhara, Y. Fukuhara, M. Fukada, E. Nishida, and T. Fujita. 1998. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 17:1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu, H., J. P. Cong, G. Mamtora, T. Gingeras, and T. Shenk. 1998. Cellular gene expression altered by human cytomegalovirus: global monitoring with oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 95:14470-14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu, H., J. P. Cong, and T. Shenk. 1997. Use of differential display analysis to assess the effect of human cytomegalovirus infection on the accumulation of cellular RNAs: induction of interferon-responsive RNAs. Proc. Natl. Acad. Sci. USA 94:13985-13990. [DOI] [PMC free article] [PubMed] [Google Scholar]