Abstract
We present a fast and simple protocol for large-scale preparation and purification of RNA oligonucleotides. RNA oligonucleotides are prepared by in vitro transcription with T7 RNA polymerase from linearized plasmid DNA templates constructed by PCR. In place of denaturing polyacrylamide gel electrophoresis (PAGE), size-exclusion chromatography is employed to purify the RNA oligonucleotide from the transcription mixture yielding >99% pure RNA product. In contrast to PAGE-based purification, the gel filtration method does not require denaturation of the RNA oligonucleotide, which is desirable for larger RNAs, and the product is free of low-molecular-weight acrylamide contaminants, which greatly benefits NMR, crystallographic, and other biophysical studies of large RNAs and RNA–protein complexes.
Keywords: RNA, NMR, in vitro transcription, size-exclusion chromatography, polyacrylamide
INTRODUCTION
Many biophysical and structural methods require milligram quantities of pure RNA. For example, structure determination of RNAs in solution using nuclear magnetic resonance (NMR) spectroscopy (Lukavsky and Puglisi 2001; Lukavsky et al. 2003) is performed on milligram quantities of unlabeled and isotopically 13C and/or 15N-labeled RNA oligonucleotides, which are efficiently prepared by in vitro transcription from DNA templates using T7 RNA polymerase (Milligan et al. 1987; Puglisi and Wyatt 1995). Purification of the desired RNA product from the transcription reactions is commonly achieved by denaturing polyacrylamide gel electrophoresis (PAGE) and subsequent elution from the gel matrix (Wyatt et al. 1991). Finally, the pure RNA product is equilibrated to the desired buffer by either microdialysis over several days or using centrifugal filtration devices with appropriate molecular weight cut-off (MWCO).
This lengthy purification protocol suffers from several disadvantages, which become even more apparent when larger RNA oligonucleotides are prepared (>40 nt). Denaturing PAGE purification of RNA always leaves highly water-soluble acrylamide oligomers in the desired RNA product, which cannot be completely removed (P. Lukavsky, unpubl.). These oligomers, apparently products of incomplete polymerization and/or partial hydrolysis of the gel during electrophoresis, can bind RNA. This leads to an increase in molecular size of the RNA and might catalyze base pair exchange through groove interactions, both of which reduce the information content of NMR spectroscopic experiments, especially for larger RNAs with several helical segments (P. Lukavsky, unpubl.). In addition, protons of the acrylamide oligomers give rise to strong signals in both the aromatic and aliphatic region of proteins and can therefore compromise the interpretation of NMR data from RNA–protein complexes.
Another major disadvantage of PAGE purification of larger RNAs is that the RNA is precipitated after the transcription reaction and purified under denaturing conditions. Precipitation of larger and highly helical RNAs can lead to irreversible formation of aggregates, whose denaturation during electrophoresis can be incomplete. In these cases denaturing PAGE fails to yield a discrete gel band of the desired RNA, and up to 90% of the RNA can be lost due to aggregation in the well or slowly migrating aggregate species, which smear over the entire gel (P. Lukavsky, unpubl.). Incomplete denaturation of large RNAs also hampered the development of robust high-performance liquid chromatography (HPLC) purification protocols (Shields et al. 1999), and aggregation of partially unfolded RNAs might interfere with refolding of the RNA into the native conformation.
To avoid these common disadvantages of denaturing PAGE purification of RNA, we present here a robust and quick RNA purification protocol using size-exclusion chromatography. The method does not require denaturation of the RNA oligonucleotide, and yields >99% pure, acryl-amide-free product suitable for NMR structural studies. Another benefit of the method is the high recovery of isotopically labeled nucleoside triphosphates (NTPs), which significantly reduces the cost of preparing labeled RNA oligonucleotides.
RESULTS AND DISCUSSION
Design of the novel RNA purification protocol
All methods for in vitro enzymatic RNA oligonucleotide synthesis using T7 RNA polymerase use reactions with the same basic components. The DNA template can consist of either two synthetic DNA oligonucleotides, a top strand DNA comprising the 17-nt T7 promoter sequence and a bottom strand complementary to the T7 promoter and the desired RNA product, or a linearized plasmid DNA containing both the T7 promoter sequence, RNA coding sequence, and an appropriate restriction enzyme cutting site to linearize the plasmid DNA (Fig. 1A ▶). The template concentration varies from 150 to 600 nM for DNA oligonucleotides and from 30 to 50 nM for linearized plasmid DNA. Beside the DNA template, the in vitro transcription mixture usually contains 40 mM Tris-HCl (pH 8.1 at 37°C), 1 mM spermidine, 5 mM dithiothreitol, 0.1% Triton-X 100, 4 mM each NTP, 12–24 mM magnesium chloride, and 1200 U/mL T7 RNA polymerase (Wyatt et al. 1991; Puglisi and Wyatt 1995). By the end of the 1–2-h incubation at 37°C, this mixture usually also contains the desired RNA product and some abortive RNA oligonucleotides smaller than 10 nt. Purification of the RNA is then typically achieved by first removing the T7 RNA polymerase from the mixture by phenol/chloroform extraction, subsequent ethanol precipitation of the oligonucleotides (DNA and RNA), and purification using preparative, denaturing PAGE.
FIGURE 1.
Sequence, design, and PCR construction of DNA templates for in vitro transcription. (A) Synthetic DNA template and design of plasmid DNA template for in vitro transcription of HCV IRES domain IIId (Lukavsky et al. 2000). (B) PCR amplification of DNA template for in vitro transcription of HCV IRES domain IIId (Lukavsky et al. 2001). (C) PCR strategy using overlapping primers for construction of DNA template for in vitro transcription of HCV IRES domain IIId (Lukavsky et al. 2000).
Using a linearized plasmid DNA as a template for in vitro transcription has major advantages: The number of small abortive RNA products produced during transcription is generally lower, and the size of the template itself is about 100 times larger than the RNA product compared to synthetic DNA templates. Besides the plasmid DNA and RNA product, all other components of the transcription mixture are small molecules, at least 10 times smaller than the desired RNA product. This size distribution of the reaction mixture simplifies subsequent RNA purification to separation of a medium-molecular-weight RNA product from a very-large-molecular-weight DNA and low-molecular-weight buffer components, all of which differ in size from each other by factors of 10–100. This advantageous size difference suggests that size-exclusion chromatography can be used for purification of RNA oligonucleotides. A detailed description of our plasmid DNA-templated RNA transcription and the entire RNA purification by size-exclusion chromatography is given in the following sections.
Design and preparation of linearized plasmid DNA for in vitro transcription
The high-copy-number plasmid DNA pUC18 is used as template for in vitro transcription of RNA. To prepare a plasmid coding for a given RNA, polymerase chain reaction (PCR) is used. A fragment containing a 4-nt overhang and HindIII restriction site, the T7 promoter sequence, followed by the desired RNA coding sequence and two spacer nucleotides, followed by BbsI and EcoRI restriction sites and a 4-nt overhang is prepared by PCR (Fig. 1B,C). Instead of BbsI, which cuts two and six nucleotides upstream of the restriction site, BsaI can be used, which cuts one and five nucleotides upstream of the restriction site. Corresponding DNA oligonucleotides up to a length of 80 nt are usually amplified by PCR using primers corresponding to the 4-nt overhang/ HindIII site/T7 promoter and BbsI/ EcoRI/4-nt overhang sites (Fig. 1B ▶). Longer DNA oligonucleotides can be prepared by PCR using overlapping primers (Fig. 1C ▶). DNA fragments are then digested with HindIII and EcoRI and sub-cloned into pUC18 vector digested with the same enzymes. To prepare sufficient plasmid for preparation of milligram quantities of RNA, a 2-L culture usually yields 4–8 mg of DNA plasmid, which can be purified using QIAfilter plasmid GIGA kits. The purified DNA plasmid (700 μg/mL) is then linearized with the appropriate restriction enzyme, and the reaction mixture containing the linearized plasmid can be used directly for the RNA transcription reaction without further purification.
RNA transcription using plasmid DNA templates
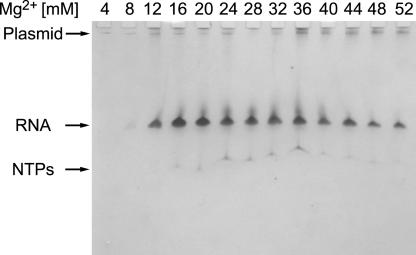
The magnesium chloride concentration of in vitro transcription reactions should be optimized to maximize the yield of the RNA oligonucleotide. Routinely, we perform 13 small-scale (25-μL) transcription reactions with different magnesium concentrations from 4–52 mM at a nucleotide concentration of 4 mM each NTP, and we analyze the yield by denaturing PAGE (Fig. 2 ▶). Maximum yield of RNA, as judged by UV-shadowing of the polyacrylamide gel, is usually obtained at Mg2+ concentrations between 12 and 24 mM and is dependent on plasmid concentration. As seen in Figure 2 ▶, no abortive RNA products are observed, and the transcribed RNA is stable under the transcription conditions as judged by a single visible RNA band. In some cases, RNAs might cleave at specific sites under the transcription conditions in the presence of Mg2+ (P. Lukavsky, unpubl.). These sites usually coincide with UpA or CpA steps in unstructured regions of the RNA, such as hairpin loops or internal loops. As structural studies require stable RNA constructs to perform longer NMR experiments, we usually try to replace the adenine in such regions with a cytosine. These changes in sequence should not affect the biological relevance of the RNA construct, and this should be tested independently.
FIGURE 2.
Denaturing PAGE analysis of RNA yield from in vitro transcriptions at different magnesium concentrations. RNA, plasmid DNA and NTPs are visualized by staining with 0.1% toluidine blue.
RNA purification using size-exclusion chromatography
To prepare milligram quantities of RNA, typically a 20 mL in vitro transcription reaction is performed, which yields up to four 250 μL RNA NMR samples (1–2 mM). To obtain a high RNA yield from transcriptions, the standard transcription protocol was altered. During transcription, there is a buildup of pyrophosphate, a reaction product that inhibits the rate of transcription by sequestering Mg2+ (Kern and Davis 1997). After 1 h of incubation at 37°C, pyrophosphate precipitate is spun down and, depending on the size of the pellet, additional magnesium chloride (up to 20 mM final concentration) is added to the reaction. This procedure can increase the yield by up to twofold. The reaction is usually stopped after 2 h at 37°C upon addition of EDTA. The restriction enzyme, which is carried over with the linearized plasmid DNA, and the T7 RNA polymerase are removed from the reaction mixture by phenol/chloroform extraction, and the mixture is concentrated to about 1 mL using centrifugal devices with appropriate MWCO. In the case of a transcription reaction of an isotopically labeled RNA, collection of the flow-through from the centrifugal devices yields high recovery of the expensive, labeled NTPs, which can then be purified following published procedures (Batey et al. 1992).
The RNA product is isolated from the partially purified reaction mixture by gel filtration chromatography. Because the main purpose of the size-exclusion chromatography protocol for RNA purification is to separate plasmid DNA from the desired RNA oligonucleotide after the removal of proteins by phenol extraction from the transcription reaction, the matrix for the size-exclusion chromatography was chosen to achieve maximum separation between the plas-mid DNA and the RNA oligonucleotide. In principle, the plasmid DNA could also be removed by digestion using DNAse, but this would require prolonged incubation of the transcription reaction at 37°C in the presence of Mg2+, which could lead to partial degradation of the RNA oligonucleotide. Bio-Gel A 50m gel (Bio-Rad), an agarose-based size-exclusion matrix, has an ideal exclusion limit (350 base pairs) for the separation of plasmid DNA from RNA. The purification protocol is described below.
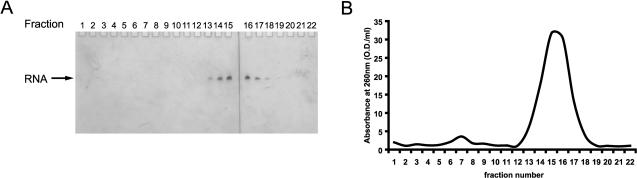
The concentrated, protein-free transcription reaction mixture is applied to an equilibrated size-exclusion column, and chromatography is performed at a low flow-rate overnight, collecting 5-mL fractions. A typical elution profile shows two major peaks, the first one containing the plasmid DNA and the second major peak, which contains the RNA and carried over NTPs and/or small abortive transcripts (see Fig. 3 ▶). The fractions containing the RNA are combined, washed several times with the appropriate NMR buffer, and concentrated appropriately (final RNA concentration of 1–2 mM) using centrifugal devices. This procedure yields >99% pure RNA and removal of NTPs, small abortive transcripts, and Triton-X 100.
FIGURE 3.
RNA purification by size-exclusion chromatography. (A) Denaturing PAGE analysis of individual 5-mL fractions collected from size-exclusion chromatography performed on in vitro transcribed RNA. RNA is visualized by staining with 0.1% toluidine blue. (B) Elution profile obtained from the same size-exclusion chromatography. The optical absorbance of each 5-mL fraction was measured at 260 nm.
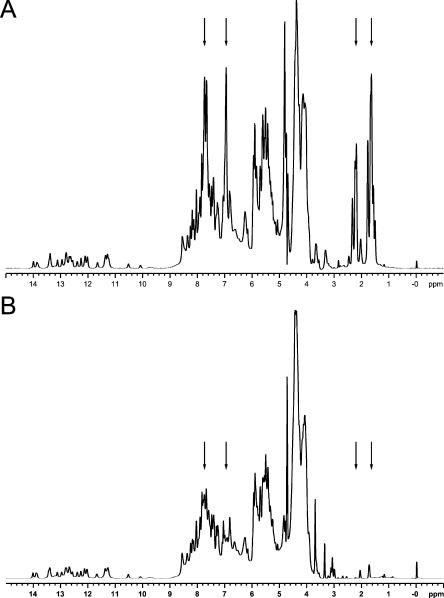
One-dimensional (1D) NMR spectra of domain II of the hepatitis C viral internal ribosome entry site (HCV IRES) RNA (Lukavsky et al. 2003) purified by either denaturing PAGE or size-exclusion chromatography are shown in Figure 4 ▶. The 1D spectrum of the PAGE-purified RNA sample shows significant contamination with acrylamide oligomers even after extensive microdialysis against NMR-buffer over several days. Characteristic resonances arising from the acrylamide oligomers are observed between 1.5–2.5 and 6.5–8.0 ppm, respectively (Fig. 4A ▶). The new purification protocol yields highly pure RNA without any acrylamide contamination, as seen in Figure 4B ▶. Resonances in the imino “fingerprint” region (10–14 ppm) display the same pattern, indicating that both RNAs are folded in the same conformation, which was also confirmed by collection of 2D NOESY spectra (data not shown).
FIGURE 4.
1D NMR spectra of HCV IRES domain II (Lukavsky et al. 2003). (A) 1D NMR spectrum of HCV IRES domain II purified by denaturing PAGE. The proton resonance lines arising from the acryl-amide oligomers are indicated by arrows. (B) 1D NMR spectrum of HCV IRES domain II purified by size-exclusion chromatography. The arrows in the same positions as in Figure 1A ▶ indicate the absence of acrylamide oligomers.
The purification protocol presented here yields >99% pure RNA for NMR spectroscopic studies in less than 2 d, and is therefore ideal for rapid screening of different constructs for NMR structural studies. In addition, samples are not contaminated with acrylamide, which is advantageous for the interpretation of NMR data collected on RNA–protein complexes. Although these are clear advantages of the new purification method, it will not yield RNAs with homogeneous ends, which is commonly required for x-ray crystallographic studies of small RNAs. The method described above could be combined with ribozyme cleavage to generate homogeneous ends, but requires that the RNA product be significantly different in size than the ribozyme strand to achieve purification. Nonetheless, this method should have applications to larger RNAs or RNA–protein assemblies that might not require homogeneous 3′-ends for proper biophysical analysis. The benefits of rapidly synthesized, pure RNAs for NMR, crystallographic, spectroscopic, and calorimetric studies are manifold.
MATERIALS AND METHODS
Construction and preparation of plasmid templates for in vitro transcription
Oligodeoxynucleotides were purchased from Sigma Genosys, desalted, and lyophilized in 40 nmole synthesis scale. The appropriate DNA template (see Fig. 1B ▶) was amplified by PCR or constructed from overlapping primers by PCR (see Fig. 1C ▶). Primers were designed with melting temperatures from 60°–65°C. A typical 50 μL PCR reaction contained 20–40 pmoles of each primer (15 μL total) and 35 μL of PCR Supermix (Invitrogen). Thirty cycles were performed with 2 min each at 94°C, 60°C, and 72°C, followed by a final 12-min extension at 72°C. PCR products were purified using the QIAquick PCR purification kit (QIAGEN), and then dissolved in 50 μL of deionized water (Millipore) and digested with HindIII-EcoRI overnight at 37°C. Digested fragments were purified using the QIAquick PCR purification kit (QIAGEN), dissolved in 50 μL of deionized water, and inserted into pUC18 digested with the same enzymes. Ligation of inserts and isolation of correct plasmids followed standard protocols.
Sequenced plasmids were transformed into DH5α, and 2 L of culture (2xTY media) were grown overnight at 37°C. Plasmid DNA was isolated using the QIAfilter plasmid GIGA kit and re-suspended in deionized water at a concentration of 700 μg/mL. A typical plasmid yield from a 2-L culture was in the range of 4–8 mg. The purified DNA plasmid was then linearized with the appropriate restriction enzyme within 24 h at 37°C using 1 unit (1U) of enzyme per 50 μg plasmid DNA. In the case of BsaI, two products are obtained, because pUC18 contains an internal BsaI site. The reaction mixture containing only fully linearized plasmid can be used directly without removal of the restriction enzyme for the RNA transcription reaction.
In vitro transcription of RNA from linearized plasmid DNA templates
All procedures used deionized water (Millipore) and chemicals purchased from Sigma. His-tagged T7 RNA polymerase was prepared in-house (Grodberg and Dunn 1988). Transcribed RNAs were analyzed by denaturing PAGE (8% and 8 M urea) and visualized by UV-shadowing and/or staining with 0.1% toluidine blue. The RNA yield from in vitro transcriptions was optimized for each individual DNA template in 25 μL trial reactions by varying the magnesium concentration from 4 to 52 mM. A typical large-scale 20 mL transcription reaction mixture contained 40 mM Tris-HCl (pH 8.1 at 37°C), 1 mM spermidine, 5 mM dithiothreitol, 0.1% Triton-X 100, 4 mM each NTP, 12–24 mM magnesium chloride, and 1200 U/mL T7 RNA polymerase (Wyatt et al. 1991; Puglisi and Wyatt 1995). After 1 h of incubation at 37°C, pyrophosphate, which forms during the in vitro transcription reaction, was pelleted by centrifugation, and additional magnesium chloride added to the reaction. The optimal amount of additional magnesium chloride depends on the size of the pyrophosphate pellet and can be determined in small-scale reactions (see above). The reaction is stopped after 1 additional h at 37°C upon addition of EDTA to a final concentration of 50 mM. Three consecutive phenol/chloroform extractions (20 mL each) are performed to completely remove the restriction enzyme and the T7 RNA polymerase from the reaction mixture. Another three extractions with chloroform (20 mL each) are performed to remove traces of phenol. Then the mixture is concentrated to about 1 mL using Centriprep centrifugal devices with appropriate MWCO. The flow-through from transcription reaction of isotopically labeled RNA samples can be collected and purified following published procedures (Batey et al. 1992).
Size-exclusion chromatographic purification of in vitro transcribed RNA
A 1.5 × 50-cm glass column (Bio-Rad) is filled up to 45 cm with Bio-Gel A 50-m (coarse grade) gel filtration matrix (Bio-Rad) and equilibrated in a cold room (4°C) with several column volumes of gel filtration buffer (150 mM sodium chloride, 50 mM sodium phosphate buffer at pH 6.5, 0.1 mM EDTA) at a flow rate of 10–12 mL per h using a peristaltic pump. The concentrated, protein-free transcription reaction mixture (see above) is loaded onto the equilibrated size-exclusion column, and the chromatography is performed at the same flow rate overnight, collecting 5–6-mL fractions. Fractions are analyzed by denaturing PAGE. RNA-containing fractions are combined and concentrated to 1 mL in Centriprep centrifugal devices, washed twice with the buffer in which the NMR experiments will be performed (e.g., 10 mM sodium phosphate, pH 6.40), concentrated again to 1 mL, and then washed three times more with the NMR buffer. This procedure yields >99% pure RNA and removal of NTPs, small abortive transcripts, and Triton-X 100. The final concentration step to the desired NMR concentration is performed in a 2-mL Centricon centrifugal device. As a general rule, the MWCO should be 3000 D for RNA oligonucleotides below 30 nt and 10,000 D for larger constructs, but it is advisable to check for the presence of RNA in the first filtrate from the Centriprep device. This is best done by gel filtration on an HPLC system using an SEC-125 column (Bio-Rad) and the same gel filtration buffer.
Acknowledgments
We thank L. Easton for in vitro transcription of RNA and gel analysis and I. Kim for helpful discussions. This work was supported by NIH grant AI47365, the Hutchison Foundation, and Eli Lilly.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.rnajournal.org/cgi/doi/10.1261/rna.5264804.
REFERENCES
- Batey, R.T., Inada, M., Kujawinski, E., Puglisi, J.D., and Williamson, J.R. 1992. Preparation of isotopically labeled ribonucleotides for multidimensional NMR spectroscopy of RNA. Nucleic Acids Res. 20: 4515–4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodberg, J. and Dunn, J.J. 1988. ompT encodes the Escherichia coli outer membrane protease that cleaves T7 RNA polymerase during purification. J. Bacteriol. 170: 1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern, J.A. and Davis, R.H. 1997. Application of solution equilibrium analysis of in vitro RNA transcription. Biotechnol. Prog. 13: 747–756. [DOI] [PubMed] [Google Scholar]
- Lukavsky, P.J. and Puglisi, J.D. 2001. RNAPack: An integrated NMR approach to RNA structure determination. Methods 25: 316–332. [DOI] [PubMed] [Google Scholar]
- Lukavsky, P.J., Otto, G.A., Lancaster, A.M., Sarnow, P., and Puglisi, J.D. 2000. Structures of two essential RNA domains for Hepatitis C virus internal ribosome entry site function. Nat. Struct. Biol. 7: 1105–1110. [DOI] [PubMed] [Google Scholar]
- Lukavsky, P.J., Kim, I., Otto, G.A., and Puglisi, J.D. 2003. Structure of HCV IRES domain II determined by NMR. Nat. Struct. Biol. 10: 1033–1038. [DOI] [PubMed] [Google Scholar]
- Milligan, J.F., Groebe, D.R., Witherell, G.W., and Uhlenbeck, O.C. 1987. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 15: 8783–8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi, J.D. and Wyatt, J.R. 1995. Biochemical and NMR studies of RNA conformation with an emphasis on RNA pseudoknots. Methods Enzymol. 261: 323–350. [DOI] [PubMed] [Google Scholar]
- Shields, T.P., Mollova, E., Marie, S.L., Hansen, M.R., and Pardi, A. 1999. High-performance liquid chromatography purification of homogenous-length RNA produced by trans cleavage with a hammerhead ribozyme. RNA 5: 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt, J.R., Chastain, M., and Puglisi, J.D. 1991. Synthesis and purification of large amounts of RNA oligonucleotides. Biotechniques 11: 764–769. [PubMed] [Google Scholar]