Abstract
Ovine betaretroviruses include Jaagsiekte sheep retrovirus (JSRV) and enzootic nasal tumor virus (ENTV). JSRV and ENTV represent a unique class of oncogenic retroviruses that induce tumors of the respiratory tract. JSRV and ENTV are highly related but induce different diseases. Expression of the JSRV envelope (Env) induces transformation of rodent fibroblasts in vitro and phosphorylation of Akt, a central player in the phosphatidylinositol 3-kinase (PI-3K)/Akt signal transduction pathway. However, little information is available on the molecular biology of ENTV. In this study, we initially assessed whether the ENTV Env has the same properties as the homologous JSRV protein. We performed entry and interference assays using retroviral vectors pseudotyped with either the JSRV or the ENTV Env and sheep choroid plexus cells, choroid plexus cells stably expressing the JSRV Env protein, human 293T cells, mouse NIH 3T3 cells, or NIH 3T3 cells expressing human hyaluronidase 2 (HYAL2), the cellular receptor for JSRV. The results obtained indicated that ENTV and JSRV share the same receptor in sheep cells and that they can use human HYAL2 as a cellular receptor in mouse cells. The ENTV Env induces transformation of rodent fibroblasts in vitro. As with the JSRV Env, the tyrosine at position 590 is critical for ENTV Env-induced cell transformation, and Akt is phosphorylated in ENTV Env-transformed cells but not in the parental cell lines. Thus, ovine betaretroviruses share a common mechanism of cell transformation. We further investigated the relevance of Akt activation in cells transformed by ovine betaretroviruses. A PI-3K inhibitor blocked Akt phosphorylation in JSRV Env-transformed cells, suggesting a possible involvement of PI-3K in JSRV and ENTV Env-induced cell transformation. In addition, phosphorylated Akt was detected in a cell line derived from a lung tumor of a sheep with naturally occurring ovine pulmonary adenocarcinoma.
The ovine betaretroviruses Jaagsiekte sheep retrovirus (JSRV) and enzootic nasal tumor virus (ENTV) are the causes of naturally occurring neoplasms of the respiratory tracts of sheep and goats (12, 28, 29). JSRV causes a lung adenocarcinoma (ovine pulmonary adenocarcinoma [OPA]) (33) originating from type II pneumocytes and Clara cells, whereas ENTV causes an adenocarcinoma of secretory cells of the olfactory mucosa (10, 11). These viruses are unique among retroviruses in the capacity to induce transformation of secretory epithelial cells of the respiratory tract.
The mechanism of JSRV-induced cell transformation has recently been investigated, since it offers a new paradigm of retroviral transformation (39). JSRV is a replication-competent retrovirus with no cell-derived oncogenes. Expression of JSRV Env induces transformation of rodent fibroblasts in classical transformation assays (21, 37). The cytoplasmic tail in the carboxy-terminal portion of the transmembrane region (TM) of JSRV Env is a major determinant of cell transformation, possibly via activation of the phosphatidylinositol 3-kinase (PI-3K)/Akt cell signaling pathway (32). Hyaluronidase 2 (HYAL2), a glycosylphosphatidylinositol-anchored protein, has recently been discovered to be the cellular receptor for JSRV (36, 37).
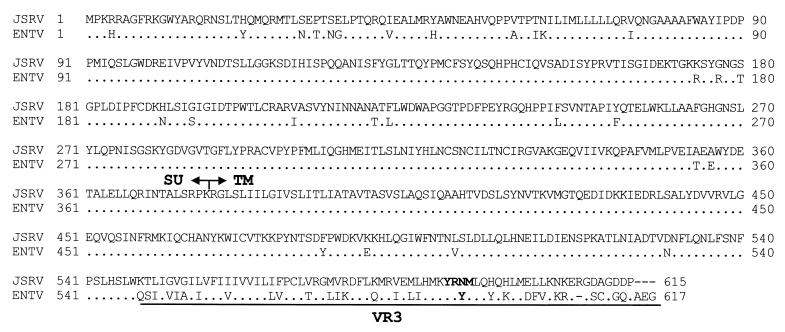
The molecular biology of ENTV and the mechanisms of ENTV-induced cell transformation have not been investigated in detail to date. ENTV is closely related to JSRV (6, 7). ENTV and JSRV are 88.8% identical at the nucleotide level. Interestingly, the main areas of divergence are the U3 in the long terminal repeat (LTR) and the carboxy-terminal portion of the TM in the envelope. The differences in the TM are clustered in the carboxy-terminal portion of the protein in a region known as VR3 (3, 6, 31), where major differences between the exogenous and the endogenous ovine betaretroviruses have been observed. In the VR3 region of JSRV Env, there is a Y-X-X-M motif (positions 590 to 593), a putative docking site for PI-3K, which has been found to be absolutely necessary for virus-induced transformation of rodent fibroblasts (32). Interestingly, the JSRV-related endogenous loci sequenced to date lack this motif, while it is conserved in ENTV (3, 6, 31). Studies on ENTV-induced cell-transformation might be useful, because they would further support a novel mechanism of cell transformation used by ovine betaretroviruses.
In this study, we examined the properties of ENTV Env and further investigated the activation of the PI-3K/Akt pathway in rodent fibroblasts transformed by the ovine betaretroviruses. Initially, we looked at receptor usage and at the transforming potential of ENTV Env. By entry and interference assays, we determined that ENTV and JSRV use the same cellular receptor. ENTV Env induces transformation of NIH 3T3 mouse and 208F rat cells, and Y590 is a major determinant of transformation. In addition, phosphorylated Akt was detected in ENTV Env-transformed cells but not in the parental NIH 3T3 cells. Thus, phosphorylation of Akt is a common event in ovine betaretrovirus transformation of rodent fibroblasts. We then further investigated the relevance of Akt phosphorylation in cells transformed by ovine betaretroviruses. PI-3K inhibitors blocked Akt phosphorylation in JSRV Env-transformed cells, and the JSRV Y590 Env mutant did not induce Akt activation. In addition, phosphorylated Akt was detected in a cell line derived from an OPA tumor.
MATERIALS AND METHODS
Plasmids.
The pCMV3JS21ΔGP plasmid expresses the JSRV21 Env protein and has been described previously (21, 32). To construct pCMV3ENTVΔGP, the JSRV Env coding region of pCMV3JS21ΔGP was replaced with the ENTV Env coding region by using the BseAI restriction site in the peptide leader of JSRV Env and the BlnI site in the U3 region of the downstream LTR. ENTV env was derived by PCR from tumor DNA obtained from a sheep with a naturally occurring nasal tumor.
Alternatively, ENTV env was expressed in pSX2 (23) by replacing the 10A1 env of pSX2 with the ENTV env and adding a gene encoding neomycin phosphotransferase to produce pSX2neo.Eenv. ENTV Env mutants were constructed in pSX2neo.Eenv by changing the tyrosine codons at positions 590, 592, and 596 into phenylalanine codons by site-directed mutagenesis. The resulting plasmids pSX2neo.EenvY590F, pSX2neo.EenvY592F, and pSX2neo.EenvY596F were sequenced in the region containing the mutation to confirm the accuracy of the mutagenesis.
Plasmids pSUxTMxNruIen and pCMV3JS21ΔGPY590D have been described previously (32). pSUxTMxNruIen has the SU and most of the TM from JSRV21 Env and the last portion of the VR3 region from the endogenous JSRV-related virus enJS56A1. Plasmid pCMV3JS21ΔGPY590D expresses the JSRV21 Env with a point mutation, Y to D at position 590 (32). The envelope regions including the splice donors and splice acceptors from pSUxTMxNruIen, pCMV3JS21ΔGP, and pCMV3JS21ΔGPY590D were amplified by PCR and inserted between the SalI and ClaI sites of the retroviral vector pLNCX2 (Clontech) to produce pLNCSUxTMxNruIen, pLNCJSE, and pLNCJSEY590D, respectively.
Cells.
All the cells used in this study were grown at 37°C with 5% CO2 and 95% humidity. 293T cells (17), sheep choroid plexus (SCP) cells (ATCC no. CRL-1700), 293-GP cells, 293GP-luc cells, 293-ecopack cells (Clontech), 208F rat cells (35), and 208F.ENTV and 208F.JSRV cells were grown in Dulbecco's modified Eagle medium (DMEM; American Type Culture Collection) with 10% fetal bovine serum (FBS). 293-GP is a 293-based cell line expressing Moloney murine leukemia virus (MoMLV) Gag and Pol. 293GP-luc cells express an MoMLV-based vector containing a firefly luciferase gene besides MoMLV Gag and Pol. 293-ecopack is a 293-based packaging cell line expressing MoMLV Gag, Pol, and Env. 208F.ENTV and 208F.JSRV are cell lines derived from foci of 208F cells transformed, respectively, by ENTV or JSRV Env. NIH 3T3, NIH 3T3-HYAL2, NIH 3T3.JSRV, NIH 3T3.ENTV, NIH 3T3-JSENV, and NIH 3T3-JSENVY590D cells were grown in DMEM with 10% calf serum. NIH 3T3-HYAL2 is an NIH 3T3-based cell line stably expressing human HYAL2 (32). NIH 3T3.JSRV is a cell line derived from a focus of JSRV-transformed NIH 3T3 cells that was previously called MP1 (32). NIH 3T3.ENTV cells were derived in a similar fashion from a focus of ENTV Env-transformed NIH 3T3 cells. NIH 3T3-JSENV and NIH 3T3-JSENVY590D were derived by infection of NIH 3T3 cells with the retroviral vectors LNCJSE and LNCΔGPY590D, respectively (obtained by transiently transfecting 293-ecopack cells), and subsequent selection with G418 (Invitrogen). Two weeks postinfection, the G418-resistant cells were pooled to generate the cell lines. SPC-JSENVchim cells were derived in a similar way by infection of SPC cells with the retroviral vector LNCSUxTMxNruIen and subsequent selection with G418 as above. The JS-8 cell line is derived from a lung tumor of a sheep with OPA (15) and was grown in F12-K medium (Invitrogen) supplemented with 10% fetal bovine serum.
Transformation assays.
Transformation assays were performed essentially as previously described for NIH 3T3 (21) or rat 208F (24) cells. Briefly, NIH 3T3 cells (3 × 105 per 10-cm-diameter dish) were transfected with 28 μg of plasmid DNA by using the CalPhos mammalian transfection kit (Clontech) as recommended by the manufacturer. Cells were left to reach confluence, and the medium was replaced every 3 days. Transformed foci were counted approximately 4 weeks after transfection. 208F cells (5 × 105 per 6-cm-diameter dish) were transfected with 10 μg of plasmid. One day after transfection, the cells were trypsinized and split into five 6-cm-diameter dishes. After the cells became confluent, the medium was replaced with DMEM plus 5% FBS and 1 μM dexamethasone every 3 days, and foci of transformed cells were counted 2 weeks after transfection.
Entry assays.
Entry mediated by JSRV or ENTV was assessed by the ability of the JSRV envelope to pseudotype MoMLV-based vectors (36). 293GP-luc cells (Clontech) were transfected with either pCMV3JS21ΔGP or pCMV3ENTVΔGP. Supernatants were collected and stored at −70°C. Subsequently, serial dilutions of the vector supernatants were used to infect NIH 3T3, NIH 3T3-HYAL2, 293T, SPC, and SPC-JSENVchim cells. Experiments were performed twice, with duplicate determinations for each dilution. Cells were lysed after 72 h, and luciferase assays were performed by using the Luciferase assay kit (Promega) as recommended by the manufacturer.
Western blotting.
For detection of Akt, NIH 3T3, NIH 3T3.JSRV, NIH 3T3.ENTV, 208F, 208F.JSRV, 208F.ENTV, and JS-8 cells were grown at 37°C under 5% CO2 in DMEM (or F12-K medium for JS-8 cells) and 10% of the appropriate serum as indicated above until they reached approximately 80% confluence. Cells were then washed twice with phosphate-buffered saline and grown for another 24 h in a medium lacking calf or fetal bovine serum. In some experiments to assess the activity of PI-3K inhibitors, LY294002 (Sigma) (3.125 to 100 μM) was added to the serum-starved cells for 30 min before preparation of cell lysates. Cells were then lysed in a buffer containing 0.5% NP-40, 50 mM HEPES buffer (pH 7.8), 100 mM sodium fluoride, 10 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, and 1 protease inhibitor cocktail tablet (Roche) per 50 ml of lysis buffer. Five to 10 μg of cell lysates was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting by standard procedures (2). Filters were incubated with polyclonal rabbit antisera to either Akt or Akt phosphorylated at serine 473 (both from Cell Signaling), and detection was carried out by using a donkey anti-rabbit immunoglobulin labeled with horseradish peroxidase (Amersham) followed by an enhanced chemiluminescence detection system (Supersignal; Pierce) as recommended by the manufacturers.
RESULTS
ENTV uses HYAL2 as a cellular receptor.
JSRV and ENTV are highly related viruses. The Env proteins of ENTV and JSRV are 90% identical; in the SU the identity is 93% (96% from the signal peptide to the SU-TM boundary) (Fig. 1). Given the high homology between these proteins, we hypothesized that these two viruses most probably use the same cellular receptor. Using retroviral vectors pseudotyped with either ENTV or JSRV Env, we found that the two viruses behave identically (Fig. 2). The ENTV vector, like the JSRV vector, cannot transduce mouse cell lines such as NIH 3T3 but enters NIH 3T3 cells expressing human HYAL2, the cellular receptor for JSRV (37). Both ENTV and JSRV enter human cells such as 293T. To prove that JSRV and ENTV use the same cellular receptor in sheep cells also, we performed interference experiments. Both JSRV and ENTV vectors were able to enter SCP cells, but entry was blocked in SPC cells stably expressing JSRV Env. Thus, JSRV and ENTV use the same cellular receptor in sheep cells, probably sheep HYAL2, although this has not been formally demonstrated by our experiments.
FIG. 1.
Amino acid alignment of the JSRV and ENTV Env proteins (GenBank accession no. AF105220 and Y16627). The identity of the JSRV and ENTV Env proteins at the amino acid level is 84%, but in the VR3 region they are only approximately 50% identical. The Y-X-X-M motif (positions 590 to 593) required for JSRV Env transformation is conserved in the ENTV Env.
FIG. 2.
Entry assays with MoMLV-luciferase vectors pseudotyped with the JSRV or ENTV Env protein. Values are expressed as relative light units (RLU) per milliliter; threefold dilutions were used in this experiment. Both JSRV and ENTV vectors enter SCP cells (CP), but their entry is inhibited in CP-ENVchim cells, which stably express JSRV Env, indicating that the two viruses use the same cellular receptor. Likewise, JSRV Env and ENTV Env can enter human (293) cells but cannot enter mouse (NIH 3T3) cells unless they express human HYAL2. Values are averages from duplicate experiments for each dilution. The experiments were repeated at least once with a different DNA preparation and gave essentially the same results.
ENTV Env induces transformation of NIH 3T3 and rat 208F cells.
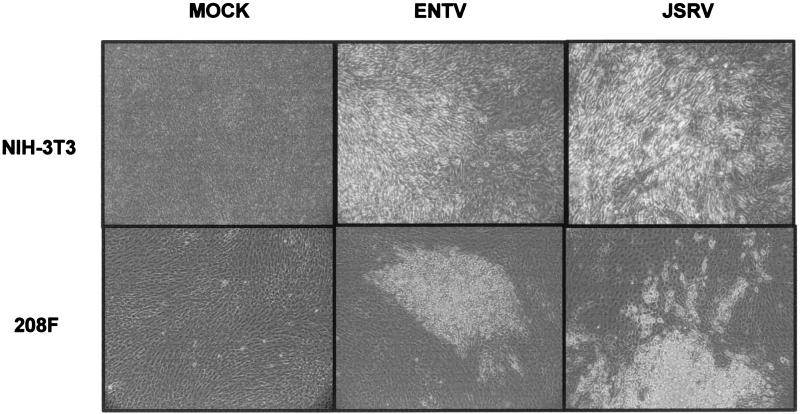
The JSRV Env functions essentially as a viral oncogene, and the cytoplasmic tail of the JSRV TM is a major determinant of cell transformation. Because JSRV and ENTV are rather divergent in this region (21, 37), we asked whether ENTV Env also was able to induce transformation of rodent fibroblasts. By performing classical transformation assays with an expression plasmid for ENTV Env (pCMV3ENTVΔGP), we were able to induce foci of transformed cells both in NIH 3T3 cells, where they appear 2 to 4 weeks posttransfection, and in rat 208F cells, where the foci start to be visible after only 7 to 8 days. The sizes and numbers of the foci induced were similar for JSRV and ENTV Env proteins (Fig. 3), indicating that the relative efficiencies of transformation of the two viruses are the same.
FIG. 3.
Transformation assays in NIH 3T3 and 208F cells. NIH 3T3 and 208F cells were transfected with pCMV3JS21ΔGP or pCMV3ENTVΔGP as described in the text. Micrographs show the typical appearance of transformed foci after 3 weeks (NIH 3T3 cells) and 12 days (208F cells).
The cytoplasmic tail of ENTV TM is a major determinant of transformation.
The TM of JSRV Env is a major determinant of transformation (32). The identity at the amino acid level between the TM of JSRV and that of ENTV is 84%, but in the VR3 region, which includes the cytoplasmic tail, the identity is only 50%. Differences in VR3 determined the inability of the JSRV-related endogenous retroviruses (enJSRVs) to induce cell transformation (32). The Y-X-X-M motif at positions 590 to 593 in JSRV Env was found to be absolutely necessary for JSRV transformation (32) and is conserved in ENTV also (see Fig. 1), while it is lost in the known enJSRV sequences (31). In addition, the ENTV Env has two other tyrosines, at positions 592 and 596. To determine the importance of these tyrosines, we performed transformation assays in 208F cells with pSX2neo.Eenv and with the ENTV Env mutants pSX2neo.EenvY590F, pSX2neo.EenvY592F, and pSX2neo.EenvY596F (Table 1). We found that, as with the JSRV Env, Y590 in the ENTV Env was critical for ENTV transformation. The Y592 and Y596 mutants were still able to induce cell transformation, although with reduced efficiency (approximately 5 times) relative to wild-type ENTV Env. This reduction in the transformation efficiency suggests that Y592 and Y596 contribute to cell transformation, through the same or possibly a different pathway than does Y590, or might merely influence the expression or correct conformation of the ENTV Env.
TABLE 1.
Transformation assays with ENTV Env mutants in rat 208F cellsa
| Plasmid | No. of foci per μg of plasmid DNA |
|---|---|
| pSX2neo.Eenv | 148 ± 19 |
| pSX2neo.EenvY590F | 1.4 ± 0.6 |
| pSX2neo.EenvY592F | 33 ± 9 |
| pSX2neo.EenvY596F | 27 ± 8 |
| None | <0.2 |
208F cells were transfected with the indicated plasmids and were trypsinized and divided into several dishes the next day. After the cells reached confluence, the medium was replaced with DMEM containing 5% FBS and 1 μM dexamethasone and the cells were fed every 3 days. Foci were counted 14 days after transfection, and results are means from two to three experiments ± standard deviations.
Phosphorylated Akt is detectable in ENTV Env-transformed cells.
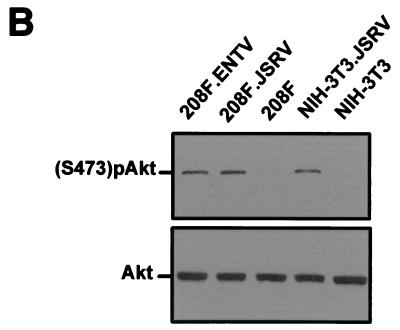
Y-X-X-M (when the tyrosine is phosphorylated) is a putative docking site for PI-3K. One of the major downstream effectors of PI-3K is Akt, which is activated by phosphorylation at serine 473 and threonine 308 (9). We previously demonstrated that Akt is phosphorylated in JSRV Env-transformed NIH 3T3 cells (32). Here we expanded ENTV Env-transformed foci to establish the NIH 3T3.ENTV and 208F.ENTV cell lines. By Western blot analysis, we detected phosphorylated Akt in serum-starved NIH 3T3.ENTV and 208F.ENTV cells but not in the parental NIH 3T3 or 208F cell lines (Fig. 4). Thus, Akt is phosphorylated in both JSRV and ENTV Env-transformed cells in vitro.
FIG. 4.
Akt activation in ENTV Env-transformed cells. Serum-starved cells transformed by ENTV or JSRV Env protein and the parental cell lines were photographed (A) and analyzed by SDS-PAGE and Western blotting (B) as described in Materials and Methods. Akt phosphorylated in serine is visible in the transformed cells but not in the parental cell lines. The same lysates were analyzed by using an antiserum against Akt as a loading control.
Akt phosphorylation is inhibited by PI-3K-specific inhibitors.
The phosphorylation of Akt in both JSRV and ENTV Env-transformed cells suggests that these two viruses might induce cell transformation, at least in rodent fibroblasts, by similar, if not identical, mechanisms. Akt is a downstream effector of PI-3K; however, in our previous studies we did not establish if the phosphorylation of Akt by JSRV Env was indeed PI-3K dependent (32). Here, we cultured NIH 3T3.JSRV cells (a JSRV Env-transformed cell line) (32) in the presence of increasing doses of the PI-3K-specific inhibitor LY294002 and monitored the phosphorylation of Akt. Dose-dependent inhibition of Akt phosphorylation was detected by Western blot analysis (Fig. 5A). Thus, we demonstrated that Akt S473 phosphorylation in ovine betaretrovirus-transformed rodent fibroblasts is PI-3K dependent.
FIG. 5.
Akt activation in JSRV Env-transformed cells is PI-3K dependent. (A) Lysates of serum-starved NIH 3T3.JSRV cells (NIH 3T3 cells transformed by JSRV Env) were obtained after 30 min of incubation with different amounts of LY294002, a specific PI-3K inhibitor, and were analyzed by SDS-PAGE and Western blotting for the presence of phosphorylated Akt. (Top) Activation of Akt is inversely related to the quantity of LY294002 added to the medium. A 12.5 μM concentration of LY294002 is sufficient to inhibit Akt phosphorylation in JSRV Env-transformed cells. (Bottom) The same lysates were analyzed by using an antiserum against Akt as a loading control. (B) Akt activation is shown in NIH 3T3-JSENV cells, which stably express JSRV Env, but not in NIH 3T3-JSENVY590D cells, which express JSRV Env with a mutation of Y590. As above, the lysates were also analyzed for the presence of Akt as a loading control. (C) Lysates of serum-starved JS-8 cells were analyzed by SDS-PAGE and Western blotting for the presence of phosphorylated Akt. (Top) JS-8 shows constitutive activation of Akt, as does the JSRV-transformed cell line NIH 3T3.JSRV. (Bottom) Presence of Akt in the cell lysates as a loading control.
Akt phosphorylation is dependent on Y590.
In previous work, we have established by use of a JSRV Env mutant with a single point mutation (Y590D) that Y590 is essential for JSRV- and ENTV-induced cell transformation (32). We indirectly linked Akt phosphorylation to Y590 by generating NIH 3T3 cell lines stably expressing JSRV Env or the JSRV ENVY590D mutant by infection of NIH 3T3 cells with the retroviral vector pLNCJSE or pLNCJSEY590D and selection of transduced cells with G418. The resulting cell lines (NIH 3T3-JSENV and NIH 3T3-JSENVY590D) were tested for the presence of phosphorylated Akt. As expected, we did not detect phosphorylated Akt in NIH 3T3-JSENVY590D cells (Fig. 5B), but we did detect phosphorylated Akt in the control NIH 3T3-JSENV cell line. These experiments link the Y590 of the ovine betaretrovirus Env proteins with PI-3K/Akt phosphorylation and cell transformation in vitro.
Evidence of PI-3K/Akt phosphorylation in ovine betaretrovirus-induced tumors in vivo.
Cancer is a multistep process. Expression of JSRV or ENTV Env is sufficient to transform immortalized rodent fibroblasts in vitro and appears to activate, directly or indirectly, the PI-3K/Akt pathway (21, 32, 37). These events might not be significant for induction of cell transformation in vivo.
As a first step to investigate whether the PI-3K/Akt pathway is also activated in vivo, we tested the JS-8 cell line. JS-8 originated from the tumor of a sheep with naturally occurring OPA (15). We could detect phosphorylated (S473) Akt in serum-starved JS-8 cells (Fig. 5C), and phosphorylation was inhibited by addition of the PI-3K inhibitor LY294002 (data not shown). This result would argue that the PI-3K/Akt pathway might also play a role in naturally occurring tumors in vivo, although the lack of a sheep-derived type II pneumocyte cell line precludes the use of proper controls.
DISCUSSION
JSRV and ENTV are unique retroviruses that induce malignancies of secretory cells of the respiratory tract. Much attention has been given to JSRV because expression of the Env protein is sufficient to induce transformation of rodent fibroblasts in vitro (21, 37). The cytoplasmic tail of JSRV Env is a major determinant of cell transformation, and a putative PI-3K docking site in this region is absolutely necessary for cell transformation (32). Akt, a downstream effector of PI-3K, is phosphorylated in JSRV-transformed cells.
In this study, we showed that, like JSRV Env, ENTV Env is able to induce transformation of rodent fibroblasts in vitro. Tyrosine 590 in the cytoplasmic tail of ENTV Env is a major determinant of cell transformation, and in ENTV Env-transformed cells it is possible to detect phosphorylated Akt. Thus, the possible activation of the PI-3K/Akt pathway mediated by the viral transmembrane protein is a common event in JSRV and ENTV Env-induced cell transformation. In a previous study, we had shown Akt phosphorylation in NIH 3T3 cells transformed by JSRV (32). Here we have extended those results, showing that rat 208F cells also show Akt phosphorylation when transformed by either JSRV or ENTV Env. The available data point to the activation of the PI-3K/Akt pathway as a main event in ovine betaretrovirus-induced cell transformation, at least in immortalized rodent fibroblasts, and they rule out an activation of this pathway only in NIH 3T3 cells. Obviously, the mechanisms of transformation of immortalized cell lines do not necessarily reflect the mechanisms of cell transformation in vivo. Different or additional mechanisms might be necessary for pulmonary oncogenesis to occur. However, we have shown in this study that the JS-8 cell line, derived from a naturally occurring OPA tumor, shows constitutively phosphorylated Akt. In addition, phosphorylated Akt is detectable in tumor cells of a proportion of naturally occurring ENT tumors (M. De las Heras and M. Palmarini, unpublished data).
Our model proposes that the JSRV or ENTV Y590 is phosphorylated and initiates the recruitment of PI-3K to the membrane and the subsequent activation of downstream effectors including Akt. However, the possible activation of the PI-3K/Akt pathway could be an indirect consequence of JSRV or ENTV Env expression. Coimmunoprecipitation studies will establish this important point. We have not yet succeeded in generating a suitable Env antiserum or a functional Env tagged with an immune epitope to perform these experiments (A. Alberti, C. Murgia, and M. Palmarini, unpublished data).
Both PI-3K and Akt have been described as retrovirus-transduced oncogenes. The catalytic subunit of PI-3K has been transduced by avian sarcoma virus 16 (ASV 16) (5), whereas Akt has been transduced by an ecotropic murine leukemia virus (4, 42). In addition, the PI-3K/Akt pathway has recently been found to be involved in induction of erythropoietin independence of erythroid cells following infection with Friend spleen focus-forming virus (26) and in transformation of Rat-1 cells by human T-cell leukemia virus type I Tax (20), further demonstrating that activation of the PI-3K pathway can lead to or participate in cell transformation. Indeed, the PI-3K/Akt pathway has been shown to be activated in several human malignancies including lung cancer (16, 25, 34, 40).
The exogenous ovine betaretroviruses can be considered a new class of transforming retroviruses. They behave like acutely transforming retroviruses, but they are replication competent, and the viral envelope is able to induce cell transformation. Another retrovirus with similar characteristics is avian hemangioma virus, whose envelope induces cell proliferation in vitro (1).
Although the mechanisms of carcinogenesis might be very similar, ENTV and JSRV induce tumors of different cell types. ENTV induces an adenocarcinoma of the gland cells of the nasal turbinates, whereas JSRV induces an adenocarcinoma arising from the differentiated epithelial cells of the distal airways. This difference is explainable by the different tropisms exhibited by the two viruses, which most probably reflect the different transcriptional activities of the viral LTRs. The JSRV U3 is a main determinant of tropism for type II pneumocytes and Clara cells (27), the transformed cells in OPA. The JSRV U3 has enhancer-binding motifs that interact with lung-specific transcription factors such as HNF-3β (22, 27, 44). The differences in this region between JSRV and ENTV most likely determine the different tropisms for these two viruses. For instance, the ENTV LTRs do not respond to HNF-3β (C. Murgia, M. Palmarini, and H. Fan, unpublished data). The U3, along the carboxy-terminal region of the TM, is the region of highest divergence between JSRV and ENTV. Interestingly, the LTRs of enJSRVs, which are closely related to JSRV and ENTV, also have several differences from the exogenous betaretroviruses in the U3 region. Indeed, enJSRVs show a tropism for the genital tract of the ewe rather than for the respiratory tract and are influenced by progesterone (30, 31, 41).
In this study, we also showed that ENTV, like JSRV, can use human HYAL2 as a cellular receptor, in agreement with recently published results (13). HYAL2 is expressed in many cell types (18, 43); thus, its distribution does not govern the in vivo tropism of JSRV. HYAL2 has a putative tumor suppressor function, as it has been shown that some human lung and breast cancers have deletions in the region on human chromosome 3 that contains the HYAL2 gene (3p21.3) (8, 19). Loss of heterozygosity in tumors is often a signature for loss of a growth-inhibitory (tumor suppressor) gene (14, 38). The interaction between JSRV and HYAL2 might still be important for transformation, although HYAL2 might not necessarily work as a tumor suppressor gene. Future studies are required in order to understand the role of HYAL2 in cell transformation and to understand whether Y590 is indeed phosphorylated and if the JSRV and ENTV Env proteins interact directly with PI-3K.
Acknowledgments
This work was supported by the Georgia Cancer Coalition, a biotechnology grant from the University of Georgia, and grants DK47754, HL54881, CA95706-01, and CA09437 (training grant support for S.-L.L.) from the National Institutes of Health.
REFERENCES
- 1.Alian, A., D. Sela-Donenfeld, A. Panet, and A. Eldor. 2000. Avian hemangioma retrovirus induces cell proliferation via the envelope (env) gene. Virology 276:161-168. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 2000. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 3.Bai, J., J. V. Bishop, J. O. Carlson, and J. C. DeMartini. 1999. Sequence comparison of JSRV with endogenous proviruses: envelope genotypes and a novel ORF with similarity to a G-protein-coupled receptor. Virology 258:333-343. [DOI] [PubMed] [Google Scholar]
- 4.Bellacosa, A., J. R. Testa, S. P. Staal, and P. N. Tsichlis. 1991. A retroviral oncogene, akt, encoding a serine-threonine kinase containing an SH2-like region. Science 254:274-277. [DOI] [PubMed] [Google Scholar]
- 5.Chang, H. W., M. Aoki, D. Fruman, K. R. Auger, A. Bellacosa, P. N. Tsichlis, L. C. Cantley, T. M. Roberts, and P. K. Vogt. 1997. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science 276:1848-1850. [DOI] [PubMed] [Google Scholar]
- 6.Cousens, C., E. Minguijon, R. G. Dalziel, A. Ortin, M. Garcia, J. Park, L. Gonzalez, J. M. Sharp, and M. de las Heras. 1999. Complete sequence of enzootic nasal tumor virus, a retrovirus associated with transmissible intranasal tumors of sheep. J. Virol. 73:3986-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cousens, C., E. Minguijon, M. Garcia, L. M. Ferrer, R. G. Dalziel, M. Palmarini, M. De las Heras, and J. M. Sharp. 1996. PCR-based detection and partial characterization of a retrovirus associated with contagious intranasal tumors of sheep and goats. J. Virol. 70:7580-7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daly, M. C., R. H. Xiang, D. Buchhagen, C. H. Hensel, D. K. Garcia, A. M. Killary, J. D. Minna, and S. L. Naylor. 1993. A homozygous deletion on chromosome 3 in a small cell lung cancer cell line correlates with a region of tumor suppressor activity. Oncogene 8:1721-1729. [PubMed] [Google Scholar]
- 9.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 10.De las Heras, M., J. A. Garcia de Jalon, and J. M. Sharp. 1991. Pathology of enzootic intranasal tumour in thirty-eight goats. Vet. Pathol. 28:474-481. [DOI] [PubMed] [Google Scholar]
- 11.De las Heras, M., J. A. Garcia de Jalon, E. Minguijon, E. W. Gray, P. Dewar, and J. M. Sharp. 1995. Experimental transmission of enzootic nasal intranasal tumours of goats. Vet. Pathol. 32:19-23. [DOI] [PubMed] [Google Scholar]
- 12.DeMartini, J. C., and D. F. York. 1997. Retrovirus-associated neoplasms of the respiratory system of sheep and goats. Ovine pulmonary carcinoma and enzootic nasal tumor. Vet. Clin. North Am. Food Anim. Pract. 13:55-70. [DOI] [PubMed] [Google Scholar]
- 13.Dirks, C., F.-M. Duh, S. K. Rai, M. I. Lerman, and A. D. Miller. 2002. Mechanism of cell entry and transformation by enzootic nasal tumor virus. J. Virol. 76:2141-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray, J. W., and C. Collins. 2000. Genome changes and gene expression in human solid tumors. Carcinogenesis 21:443-452. [DOI] [PubMed] [Google Scholar]
- 15.Jassim, F. A. 1988. Identification and characterization of transformed cells in Jaagsiekte, a contagious lung tumour of sheep. Ph.D. thesis. University of Edinburgh, Edinburgh, United Kingdom.
- 16.Kobayashi, M., S. Nagata, T. Iwasaki, K. Yanagihara, I. Saitoh, Y. Karouji, S. Ihara, and Y. Fukui. 1999. Dedifferentiation of adenocarcinomas by activation of phosphatidylinositol 3-kinase. Proc. Natl. Acad. Sci. USA 96:4874-4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lebkowsky, J. S., S. Clancy, and M. P. Calos. 1985. Simian virus 40 replication in adenovirus-transformed human cells antagonizes gene expression. Nature 317:169-171. [DOI] [PubMed] [Google Scholar]
- 18.Lepperdinger, G., B. Strobl, and G. Kreil. 1998. HYAL2, a human gene expressed in many cells, encodes a lysosomal hyaluronidase with a novel type of specificity. J. Biol. Chem. 273:22466-22470. [DOI] [PubMed] [Google Scholar]
- 19.Lerman, M. I., J. D. Minna, et al. 2000. The 630-kb lung cancer homozygous deletion region on human chromosome 3p21.3: identification and evaluation of the resident candidate tumor suppressor genes. Cancer Res. 60:6116-6133. [PubMed] [Google Scholar]
- 20.Liu, Y., Y. Wang, M. Yamakuchi, S. Masuda, T. Tokioka, S. Yamaoka, I. Maruyama, and I. Kitajima. 2001. Phosphoinositide-3 kinase-PKB/Akt pathway activation is involved in fibroblast Rat-1 transformation by human T-cell leukemia virus type I tax. Oncogene 20:2514-2526. [DOI] [PubMed] [Google Scholar]
- 21.Maeda, N., M. Palmarini, C. Murgia, and H. Fan. 2001. Direct transformation of rodent fibroblasts by Jaagsiekte sheep retrovirus DNA. Proc. Natl. Acad. Sci. USA 98:4449-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGee-Estrada, K., M. Palmarini, and H. Fan. 2001. HNF-3β is a critical factor for the expression of the Jaagsiekte sheep retrovirus (JSRV) long terminal repeat in type II pneumocytes but not in Clara cells. Virology 292:87-97. [DOI] [PubMed] [Google Scholar]
- 23.Miller, A. D., and F. Chen. 1996. Retrovirus packaging cells based on 10A1 murine leukemia virus for production of vectors that use multiple receptors for cell entry. J. Virol. 70:5564-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller, A. D., T. Curran, and I. M. Verma. 1984. c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell 36:51-60. [DOI] [PubMed] [Google Scholar]
- 25.Moore, S. M., R. C. Rintoul, T. R. Walker, E. R. Chilvers, C. Haslett, and T. Sethi. 1998. The presence of a constitutively active phosphoinositide 3-kinase in small cell lung cancer cells mediates anchorage-independent proliferation via a protein kinase B and p70s6k-dependent pathway. Cancer Res. 58:5239-5247. [PubMed] [Google Scholar]
- 26.Nishigaki, K., C. Hanson, T. Ohashi, D. Thompson, K. Muszynski, and S. Ruscetti. 2000. Erythroid cells rendered erythropoietin independent by infection with Friend spleen focus-forming virus show constitutive activation of phosphatidylinositol 3-kinase and Akt kinase: involvement of insulin receptor substrate-related adapter proteins. J. Virol. 74:3037-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmarini, M., S. Datta, R. Omid, C. Murgia, and H. Fan. 2000. The long terminal repeat of Jaagsiekte sheep retrovirus is preferentially active in differentiated epithelial cells of the lungs. J. Virol. 74:5776-5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palmarini, M., and H. Fan. 2001. Retrovirus-induced ovine pulmonary adenocarcinoma, an animal model for lung cancer. J. Natl. Cancer Inst. 93:1603-1614. [DOI] [PubMed] [Google Scholar]
- 29.Palmarini, M., H. Fan, and J. M. Sharp. 1997. Sheep pulmonary adenomatosis: a unique model of retrovirus-associated lung cancer. Trends Microbiol. 5:478-483. [DOI] [PubMed] [Google Scholar]
- 30.Palmarini, M., C. A. Gray, K. Carpenter, H. Fan, F. W. Bazer, and T. Spencer. 2001. Expression of endogenous betaretroviruses in the ovine uterus: effects of neonatal age, estrous cycle, pregnancy, and progesterone. J. Virol. 75:11319-11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmarini, M., C. Hallwirth, D. York, C. Murgia, T. de Oliveira, T. Spencer, and H. Fan. 2000. Molecular cloning and functional analysis of three type D endogenous retroviruses of sheep reveal a different cell tropism from that of the highly related exogenous Jaagsiekte sheep retrovirus. J. Virol. 74:8065-8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palmarini, M., N. Maeda, C. Murgia, C. De-Fraja, A. Hofacre, and H. Fan. 2001. A phosphatidylinositol 3-kinase docking site in the cytoplasmic tail of the Jaagsiekte sheep retrovirus transmembrane protein is essential for envelope-induced transformation of NIH 3T3 cells. J. Virol. 75:11002-11009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmarini, M., J. M. Sharp, M. De las Heras, and H. Fan. 1999. Jaagsiekte sheep retrovirus is necessary and sufficient to induce a contagious lung cancer in sheep. J. Virol. 73:6964-6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillips, W. A., F. St. Clair, A. D. Munday, R. J. Thomas, and C. A. Mitchell. 1998. Increased levels of phosphatidylinositol 3-kinase activity in colorectal tumors. Cancer 83:41-47. [DOI] [PubMed] [Google Scholar]
- 35.Quade, K. 1979. Transformation of mammalian cells by avian myelocytomatosis virus and avian erythroblastosis virus. Virology 98:461-465. [DOI] [PubMed] [Google Scholar]
- 36.Rai, S. K., J. C. DeMartini, and A. D. Miller. 2000. Retrovirus vectors bearing Jaagsiekte sheep retrovirus Env transduce human cells by using a new receptor localized to chromosome 3p21.3. J. Virol. 74:4698-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rai, S. K., F. M. Duh, V. Vigdorovich, A. Danilkovitch-Miagkova, M. I. Lerman, and A. D. Miller. 2001. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for Jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc. Natl. Acad. Sci. USA 98:4443-4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robertson, G. P., H. J. Huang, and W. K. Cavenee. 1999. Identification and validation of tumor suppressor genes. Mol. Cell Biol. Res. Commun. 2:1-10. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg, N. 2001. New transformation tricks from a barnyard retrovirus: implications for human lung cancer. Proc. Natl. Acad. Sci. USA 98:4285-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shayesteh, L., Y. Lu, W. L. Kuo, R. Baldocchi, T. Godfrey, C. Collins, D. Pinkel, B. Powell, G. B. Mills, and J. W. Gray. 1999. PIK3CA is implicated as an oncogene in ovarian cancer. Nat. Genet. 21:99-102. [DOI] [PubMed] [Google Scholar]
- 41.Spencer, T. E., A. G. Stagg, M. M. Joyce, G. Jenster, C. G. Wood, F. W. Bazer, A. A. Wiley, and F. F. Bartol. 1999. Discovery and characterization of endometrial epithelial messenger ribonucleic acids using the ovine uterine gland knockout model. Endocrinology 140:4070-4080. [DOI] [PubMed] [Google Scholar]
- 42.Staal, S. P., J. W. Hartley, and W. P. Rowe. 1977. Isolation of transforming murine leukemia viruses from mice with a high incidence of spontaneous lymphoma. Proc. Natl. Acad. Sci. USA 74:3065-3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strobl, B., C. Wechselberger, D. R. Beier, and G. Lepperdinger. 1998. Structural organization and chromosomal localization of Hyal2, a gene encoding a lysosomal hyaluronidase. Genomics 53:214-219. [DOI] [PubMed] [Google Scholar]
- 44.Whitsett, J. A., and S. W. Glasser. 1998. Regulation of surfactant protein gene transcription. Biochim. Biophys. Acta 1408:303-311. [DOI] [PubMed] [Google Scholar]