Abstract
In eukaryotes, box H/ACA small nucleolar RNAs (snoRNAs) guide sites of pseudouridine (Ψ) formation in rRNA. These snoRNAs reside in RNP complexes containing the putative Ψ synthase, Cbf5p. In this study we have identified Cbf5p-associated RNAs in Euglena gracilis, an early diverging eukaryote, by immunoprecipitating Cbf5p-containing complexes from cellular extracts. We characterized one box H/ACA-like RNA which, however, does not appear to guide Ψ formation in rRNA. We also identified four single Ψ-guide box AGA RNAs. We determined target sites for these putative Ψ-guide RNAs and confirmed that the predicted Ψ modifications do, in fact, occur at these positions in Euglena rRNA. The Cbf5p-associated snoRNAs appear to be encoded by multicopy genes, some of which are clustered in the genome together with methylation-guide snoRNA genes. These modification-guide snoRNAs and snoRNA genes are the first ones to be reported in euglenid protists, the evolutionary sister group to the kinetoplastid protozoa. Unexpectedly, we also found and have partially characterized a selenocysteine tRNA homolog in the anti-Cbf5p-immunoprecipitated sample.
Keywords: rRNA, snoRNA, pseudouridine, methylation, Cbf5p, selenocysteine tRNA
INTRODUCTION
During the process of ribosome biogenesis, the ribosomal RNA (rRNA) acquires site-specific posttranscriptional nucleoside modifications, the two most common of which are isomerization of uridine (U) to pseudouridine (Ψ) and methylation of ribose at the 2′ hydroxyl (O2′-methylation). In eukaryotic and archaeal organisms, modification-guide RNAs help to specify the positions of most of the sites of rRNA modification (for review, see Bachellerie et al. 2002; Kiss 2002; Omer et al. 2003). The first identified guide RNAs were shown to reside predominantly in the nucleolus of yeast and vertebrate cells and were thus termed small nucleolar (sno) RNAs. More recently, this class of RNAs has also been identified in several plant (for review, see Brown et al. 2003), trypanosomatid (for review, see Uliel et al. 2004), and archaeal species. The RNA types targeted for modification by these guide RNAs have expanded to include the small nuclear (sn) RNAs, archaeal tRNAs, the spliced leader RNA of trypanosomes (X.-h. Liang et al. 2002), and possibly even mRNA (Cavaillé et al. 2000). Some of the vertebrate guide RNAs that act on nonribosomal RNA species have been shown to reside in the Cajal bodies within the nucleoplasm (Darzacq et al. 2002; Richard et al. 2003). The modification-guide RNAs are grouped into two classes depending, in part, on whether they target sites of O2′-methylation or Ψ formation.
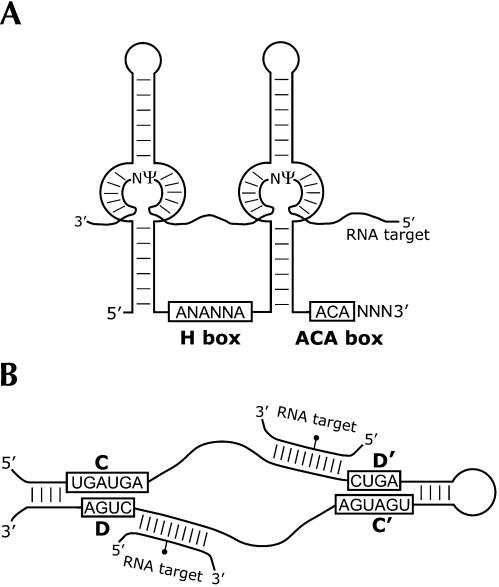
In yeast and vertebrates, the RNAs that guide Ψ formation are termed the box H/ACA RNAs. This class is characterized by the conserved H box sequence (ANANNA) within the hinge region of the RNA, and the ACA box sequence that is located invariantly 3 nt from the 3′ end of the RNA (Fig. 1A ▶). Box H/ACA snoRNAs have a bipartite structure consisting of two extended helices, each interrupted by internal bulge regions. The site of Ψ formation is specified through base-pairing interactions between the single-stranded bulge regions and the RNA targeted for modification (Ganot et al. 1997a; Ni et al. 1997). An additional hairpin structure following the hinge region and preceding the 3′-terminal helix is sometimes present in these RNAs (Ganot et al. 1997b). Interestingly, the few identified trypanosome Ψ-guide RNAs are smaller than those identified to date in other eukaryotes, having only one extended helical stem structure (i.e., they are “single-guide” snoRNAs). Whereas most box H/ACA RNAs are involved in specifying sites of Ψ formation, a few such as yeast snR30 mediate nucleolytic cleavage events that occur during maturation of the primary rRNA transcript.
FIGURE 1.
Structure and function of the two classes of modification-guide RNA. (A) A eukaryotic box H/ACA RNA. The two extended helices are interrupted by internal bulge regions that base pair to sequences flanking the unpaired uridine in the substrate RNA, specifying its conversion to Ψ. In this example, the box H/ACA guide RNA is depicted as specifying two sites of pseudouridine formation in the same target RNA species. (B) A eukaryotic box C/D RNA. The conserved sequence motifs are shown boxed and are juxtaposed to form part of the K-turn structural element (Watkins et al. 2000; Klein et al. 2001). Helical elements found in some eukaryotic box C/D RNAs are shown. The region immediately 5′ to the D and/or D′ box pairs with the target RNA. The nucleotide in the target RNA that is O2′-methylated, indicated by the black circle, is paired to the residue exactly 5 nt from the D or D′ box.
The box C/D RNAs are the guide RNAs that specify sites of O2′-methylation. Compared to the Ψ-guide RNAs, box C/D snoRNAs display a higher degree of structural similarity between archaea and eukaryotes. These RNAs contain conserved sequence elements, the C box (UGAUGA) and the D box (CUGA) near their 5′ and 3′ ends, respectively (Fig. 1B ▶). Less well conserved internal copies of these box elements, the C′ and D′ boxes, are also present. The box C/D snoRNAs base pair to their modification-target RNAs using the sequences upstream and adjacent to the D and/or D′ boxes (Cavaillé et al. 1996; Kiss-László et al. 1996; Nicoloso et al. 1996). In many eukaryotic box C/D RNAs, the 5′ and 3′ ends are able to base pair to form a terminal helix. The modification-guide RNAs are found in RNP complexes with well defined core protein components. Eukaryotic box H/ACA RNAs are associated with Gar1p, Nop10p, Nhp2p, and the putative Ψ synthase, Cbf5p (dyskerin; Watkins et al. 1998). These proteins are evolutionarily conserved, and homologs have been identified in many eukaryotic and archaeal species. The eukaryotic box C/D RNAs are present in distinct RNP complexes containing the proteins Snu13p (human 15.5-kDa protein), Nop56p, Nop58p, and Nop1p (fibrillarin). A strategy used successfully to isolate box C/D RNAs from Typanosoma brucei (Dunbar et al. 2000a) and archaea (Omer et al. 2000) and box H/ACA RNAs from yeast (Balakin et al. 1996) entails immunoprecipitation of guide RNAs residing in RNPs, using antibodies raised against protein components of the complexes.
The protist Euglena gracilis is a member of the phylum Euglenozoa, which includes the distantly but specifically related kinetoplastid protozoa, including genera such as Crithidia, Leishmania, and Trypanosoma. E. gracilis is therefore a phylogenetically key organism in which to examine the properties and evolution of modification-guide RNP complexes. Additionally, the ribosome assembly pathway of this protist exhibits several unusual and interesting features, including a naturally and extensively fragmented cytoplasmic large subunit (LSU) rRNA that is initially transcribed as a continuous precursor from an extrachromosomal circular DNA (Schnare and Gray 1990; Greenwood et al. 2001). Mature E. gracilis LSU rRNA comprises 14 discrete species (including the 5.8S rRNA), in contrast to what is seen in most other eukaryotic organisms, whose LSU rRNA consists of only two (5.8S + 25–28S) species. Ongoing research in our laboratory indicates that the E. gracilis cytoplasmic rRNAs are extensively modified with both O2′-methyl (Nm) and Ψ residues: >150 Nm and >70 Ψ have so far been identified in mapping studies of modification sites in E. gracilis rRNAs (M.N. Schnare, unpubl.). Therefore, we predicted that E. gracilis will contain a large collection of modification-guide RNAs, many of which may well be unique to this organism. For these reasons and because the collection of characterized Ψ-guide RNAs is largely confined to a narrow phylogenetic group of eukaryotes (the “crown” group), we set out to identify Ψ-guide RNAs from this organism.
RESULTS
Isolation of E. gracilis Ψ-guide RNAs
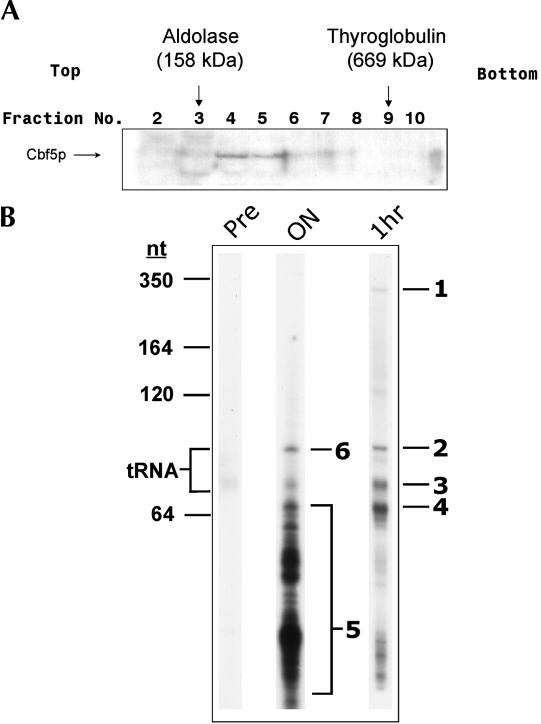
The Cbf5p homolog from E. gracilis was previously identified and described (Watanabe and Gray 2000). The predicted protein sequence contains a conserved PUA domain, C-terminal KKE repeats, and a TruB (Ψ synthase-like) domain, and is expected to be a central component in E. gracilis Ψ-guide RNP complexes. We employed the E. graci-lis Cbf5p cDNA sequence as a tool to isolate E. gracilis Ψ-guide RNAs via immunoprecipitation of Cbf5p-containing RNP complexes. Recombinant Cbf5p was used to stimulate the production of rabbit polyclonal antibodies (αCbf5p) that were in turn used to detect Cbf5p-containing complexes in partially purified E. gracilis cell extracts fractionated on glycerol gradients. Cbf5p-containing complexes were detected in the 200–500-kDa size range of the gradient (Fig. 2A ▶). Appropriate fractions were pooled, and Cbf5p complexes were isolated by incubating the pool with purified αCbf5p for 1 h, followed by extraction of immunoprecipitated RNAs with phenol. The RNAs were then radiola-beled at their 3′ ends, separated by electrophoresis in an 8% denaturing polyacrylamide gel, and visualized by autoradiography (Fig. 2B ▶). Most of the immunoprecipitated RNAs were of similar size (55–70 nt; bands 3 and 4, Fig. 2B ▶), slightly smaller than E. gracilis tRNAs. Two larger species (bands 1 and 2, Fig. 2B ▶) were also detected.
FIGURE 2.
Purification and visualization of E. gracilis Cbf5p-associated RNAs. E. gracilis crude cell extracts were fractionated on glycerol gradients, and Cbf5p-containing complexes were immunoprecipi-tated. (A) Western blot analysis of glycerol gradient fractions. Protein components from equivalent volumes of each gradient fraction were resolved by SDS-PAGE and probed with αCbf5p. Protein size markers were run on a parallel gradient, and their migration positions are indicated at the top. (B) Visualization of immunoprecipitated RNAs. αCbf5p RNAs immunoprecipitated from the concentrated glycerol gradient fraction were radioactively 3′ end-labeled and resolved by electrophoresis in an 8% denaturing polyacrylamide gel. The position of migration of size markers is indicated at the left, as is the position of migration of tRNAs. Immunoprecipitation was performed overnight (“ON”) or for 1 h (“1hr”). Immunoprecipitation with pre-immune serum (“Pre”) was performed for 1 h. RNA species for which sequencing reactions were performed are labeled as bands 1–4 and 6. Distinct RNA species were also isolated from region 5 of the overnight immunoprecipitation sample and sequenced.
Determination of the sequences of Cbf5p-associated RNAs
To determine the sequence of some of the immunoprecipi-tated RNAs, chemical sequencing of 3′ end-labeled species was performed. The sequence obtained for band 2/6 (Fig. 2B ▶) indicated that it was likely a tRNA species, based on the presence of a 3′-terminal CCA sequence. This species was subsequently identified as selenocysteine tRNA (tRNASec; see below).
Sequences obtained from bands 3 and 4 (Fig. 2B ▶) revealed the presence of many different comigrating RNA species. Interestingly, whereas the sequence from band 4 was heterogeneous, the motif ‘AGAU‘ was clearly distinguishable near the 3′ ends of these RNAs and was therefore a sequence common to many of these RNAs (data not shown). When crude αCbf5p was used for immunoprecipitation in conjunction with longer incubation times, smaller immunoprecipitated RNA species became prominent (lane ON, Fig. 2B ▶). This result presumably reflects internal cleavage of the larger immunoprecipitated RNA species evident after shorter incubations. Because the majority of the RNAs immunoprecipitated with the purified Cbf5p antibodies (conditions favoring negligible RNA degradation) were located in bands 3 and 4 and not resolvable into unique RNA sequences, longer incubations with crude αCbf5p proved to be an effective strategy to reduce the sizes of immunoprecipitated RNAs such that they could be readily resolved for RNA sequence analysis.
Some of the immunoprecipitated RNA species (region 5, Fig. 2B ▶) were further resolved in 10% denaturing polyacryl-amide gels into distinct homogenous bands that yielded a unique RNA sequence. Four of these RNAs had an AGA motif (AGA box) 3 nt from their 3′ ends. The fifth RNA had ACA instead of AGA at this position. The partial, 3′ end sequences of these RNAs were used in the design of oligo-nucleotide primers for 5′ RACE experiments, to obtain full-length cDNA sequences for these E. gracilis Cbf5p-associated RNAs.
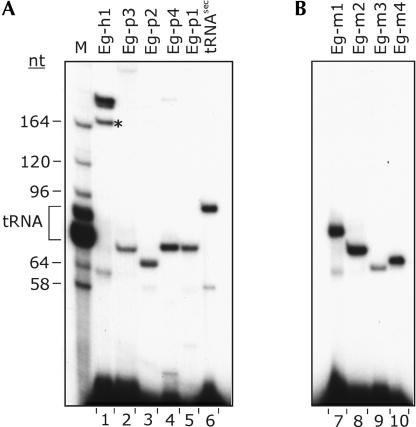
Primer extension experiments clearly detected all of the RNAs in question in total E. gracilis RNA (Fig. 3A ▶). The primer extension products generated from the four AGA box RNAs were of similar size to the majority of the Cbf5p-associated RNAs seen in the 1-h immunoprecipitation experiment (cf. Figs. 2B ▶ and 3A ▶). We designated these RNA species E. gracilis pseudouridine-guide RNAs (Eg-p) 1–4. In contrast, the RNA species containing the ACA box sequence, designated Eg-h1 for E. gracilis box H/ACA-like RNA, primed synthesis of a product corresponding to a much larger species in total E. gracilis RNA. Because only one major RNA species significantly larger than tRNA was detected in the 1-h immunoprecipitated sample, we postulated that the Eg-h1 RNA species isolated from region 5 (Fig. 2B ▶) might correspond to a 3′ end fragment of the RNA species visualized as band 1 in the 1-h immunoprecipitation sample (Fig. 2B ▶). We obtained 3′ end RNA sequence for band 1 and confirmed that this sequence was identical to the 3′ end sequence obtained for the fragment of Eg-h1 isolated from region 5 (data not shown).
FIGURE 3.
Detection by RT primer extension of small RNAs in E. gracilis total RNA. The name of each RNA analyzed is indicated at the top of each lane. (A) Detection of RNAs found associated with E. gracilis Cbf5p. Lane M is a size marker generated by 3′ end-labeling E. gracilis total RNA. Sizes (in nt) of some of the LSU rRNA species and the position of migration of tRNAs are indicated on the left. Because the primers used contained an 11-nt 5′ anchor sequence and the complementary region was not always exactly at the 3′ end (see Figs. 4 ▶, 6 ▶, 7 ▶), the primer extension products are expected to be longer than the mature RNA by the following number of nt: Eg-h1, +9; Eg-p1, +11; Eg-p2, +1; Eg-p3, +8; Eg-p4, +10; tRNASec, +7. The band marked with * in lane 1 was also characterized (see text). (B) Detection of box C/D RNAs predicted from genomic sequences. These primers also contained the 11-nt anchor, and in each case the complementarity started at the 3′ end of the box-D sequence.
E. gracilis box AGA Ψ-guide RNAs
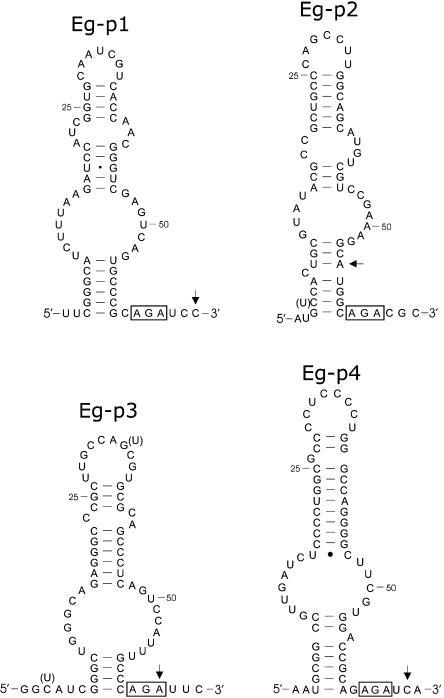
Following gel purification of the primer extension products, 5′ RACE was performed and several clones were sequenced for each Cbf5p-associated RNA. The complete sequences and predicted secondary structures of the AGA box-containing RNAs are illustrated in Figure 4 ▶. The RNAs are ~65 nt in length and have extended helical structures interrupted by internal bulges. Some sequence heterogeneity was detected for Eg-p2 and Eg-p3, suggesting that they exist as multiple isoforms. None of the variations in sequence altered the predicted secondary structures.
FIGURE 4.
Secondary structures of E. gracilis box AGA modification-guide RNAs. In the secondary structures the conserved AGA motif is boxed. Sites of sequence heterogeneity in the RNAs are indicated in parentheses. For each RNA, the arrow indicates the 3′-most nucleotide that anneals to the RT primer used for the experiments of Figure 3 ▶. GenBank accession nos. are AY572245, AY572242, AY572244, and AY572243 for the Eg-p1 to Eg-p4 RNAs, respectively.
In other eukaryotes, the base-pairing interaction between the box H/ACA Ψ-guide RNA and the target RNA occurs within the internal bulge region of the guide RNA. As a result, the uridine targeted for isomerization is positioned 14–16 nt from the H or ACA box sequence. Therefore, we searched the completely determined E. gracilis rRNA sequences for complementarity to the single-stranded bulged regions of the box AGA RNAs. In every case, the box AGA RNA exhibited a unique and extensive sequence complementarity to a specific region in the E. gracilis cytoplasmic LSU rRNA (Fig. 5A ▶). Three of these sequence complementarities map to regions within LSU species 8 of the naturally fragmented LSU rRNA. In all instances, the interaction between the box AGA guide and rRNA substrate positions the target U 14 or 15 nt from the AGA box sequence. The U selected for isomerization is also located in a 2-nucleotide single-stranded “pseudouridylation pocket” analogous to the structures generated between yeast box H/ACA RNAs (Ganot et al. 1997a) and archaeal Ψ-guide RNAs (Rozhdestvensky et al. 2003) and their respective target RNAs. Each of these U residues has been shown (or is predicted) to be modified to Ψ in other eukaryotes (Bakin et al. 1994; Ganot et al. 1997a; Ni et al. 1997; Ofengand and Bakin 1997; Giordano et al. 1999; Marker et al. 2002; Yuan et al. 2003). Box H/ACA RNAs have been identified that have the potential to target modification of three of these sites (Ganot et al. 1997a; Ni et al. 1997; Marker et al. 2002; Yuan et al. 2003), but Eg-p1 RNA represents the first example of a guide RNA targeting LSU rRNA position 2754.
FIGURE 5.
Target sites of pseudouridine formation in rRNA guided by E. gracilis box AGA RNAs. (A) Bipartite base-pairing interactions of the box AGA guide RNAs (top strand) to their predicted rRNA target site (bottom strand). The hairpin structural element in the guide RNA that interrupts the base-pairing interaction is shown schematically. Sites of pseudouridine formation are indicated by “Ψ”. Numbering of nucleotides in the mature LSU rRNA begins at the 5′ end of the 5.8S rRNA as position 1. The LSU species in which a Ψ target site resides is indicated in parentheses; that is, LSU2754 (sp. 8) is nucleotide 2754, located in LSU species 8. In each case, the position marked by * is identically marked in the corresponding autoradiogram shown in (B). (B) Autoradiograms of chemical sequencing gels of 3′ end-labeled E. gracilis 5.8S rRNA and an internal fragment of LSU rRNA species 8. Blanks in the U-specific ladders indicate the positions of Ψ residues, with those predicted from guide RNA sequences denoted by filled triangles.
To determine whether these E. gracilis rRNA positions are modified, chemical sequencing of E. gracilis rRNA fragments was performed. The expected positions in the U-specific chemical cleavage ladder were blank, indicative of modified uridines (Fig. 5B ▶). The presence of Ψ at position 68 in 5.8S rRNA was confirmed by thin layer chromatography (M.N. Schnare, unpubl.). LSU rRNA position 2802 was identified by a reverse transcription (RT)-based method as Ψ in animals (human, mouse, Drosophila; Ofengand and Bakin 1997); however, this residue was already known to be Ψm in human and Xenopus laevis rRNA (Maden 1988). This position in LSU rRNA species 8 is cleaved normally by alkali (data not shown), indicating that Ψ2802 is not O2′-methylated in Euglena.
E. gracilis box H/ACA RNA
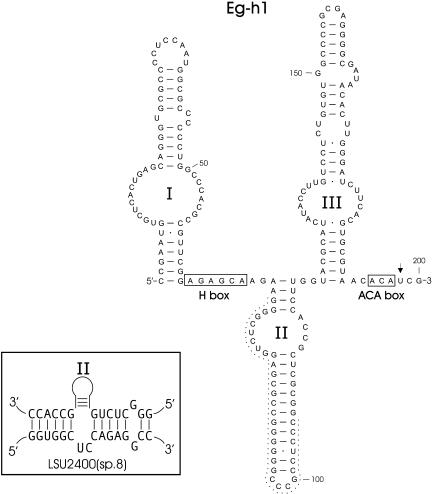
Primer extension, immunoprecipitation, and RNA sequencing provided evidence of a single large (200-nt) Cbf5p-associated RNA. The complete sequence and predicted secondary structure for the Eg-h1 RNA are shown in Figure 6 ▶. In addition to its ACA box sequence, this RNA has a canonical H-box sequence located in the single-stranded hinge region between stem structures I and II. Eg-h1 most closely resembles the box H/ACA RNAs seen in yeast and vertebrate species. A search of the E. gracilis rRNA sequences for sequence complementarity to each of the extended stem structures in Eg-h1 revealed significant base-pairing potential only to the bulge region of stem structure II (Fig. 6 ▶). We examined this site, position 2400 in E. gracilis LSU species 8, and our analysis indicates that this position is not modified to Ψ (data not shown). Eg-h1 appears to be more abundant than the box AGA RNAs as evidenced by primer extension experiments. The smaller RT primer extension product (* in lane Eg-h1, Fig. 3A ▶) had the same sequence as Eg-h1 but contained an internal deletion encompassing positions 75 to 106 (Fig. 6 ▶). This truncated product appears to be an RT artifact attributable to template switching (Johnson et al. 2000; Zaphiropoulos 2002) and resulting from sequence similarity at the boundaries of the deleted segment. This inference is supported by the observation that whereas it was possible to generate a PCR product from E. gracilis genomic DNA that contained the longer version of the Eg-h1 sequence using PCR primers annealing near the 5′ and 3′ end of the gene, a smaller PCR product containing the internal deletion was not seen (data not shown).
FIGURE 6.
Structure of an E. gracilis box H/ACA RNA (GenBank accession no. AY572240). The conserved H and ACA motifs are shown boxed. The inset depicts a potential base-pairing interaction between stem structure II and LSU species 8. The dashed line highlights nucleotides absent from the smaller RT primer extension product generated from this RNA (see text). The arrow indicates the 3′-most nucleotide that anneals to the RT primer.
E. gracilis selenocysteine tRNASec
Analysis of the complete sequence of the tRNA species identified in the Cbf5p-immunoprecipitated sample indicates it is an E. gracilis tRNASec homolog. This tRNA is 90 nt long, and its sequence can be folded into a secondary structure containing nine pairs in the acceptor stem and four pairs in the TΨC stem (Fig. 7A ▶): the so-called 9/4 representation (for review, see Commans and Böck 1999). The sequence also contains the conserved noncanonical U·U pairing in the acceptor stem, characteristic of the other known eukary-otic selenocysteine tRNAs (Rao et al. 2003). High conservation of the sequence in the anticodon stem and loop is also evident (Fig. 7B ▶). Notably, the well conserved UG (*, Fig. 7B ▶) in most eukaryotic and eubacterial tRNASec sequences (Rao et al. 2003) is instead CU in the E. gracilis sequence (positions 64 and 65, Fig. 7A ▶). In the inferred secondary structure, compensatory base changes in the 3′ portion of the TΨC stem maintain the helical structure despite the changes in the 5′ half of the stem. The partial 3′ end RNA sequence obtained for this tRNA featured two ambiguous positions: a weak A at position 71 and a blank at position 68 (positions 58 and 55, respectively, in the standard tRNA numbering scheme). These results likely reflect the presence of m1A at position 71 and Ψ at position 68, conserved modifications seen in most eukaryotic selenocysteine tRNAs and in many other tRNA species. This is only the second reported protist tRNASec sequence, the other being from Chlamydomonas reinhardtii (Rao et al. 2003). The presence of modifications and a 3′ CCA end, as well as the degree of structural conservation, all suggest that this species is a functional tRNASec in E. gracilis.
FIGURE 7.
E. gracilis selenocysteine tRNASec. (A) Cloverleaf secondary structural model of E. gracilis tRNASec (GenBank accession no. AY572241) in the 9/4 representation. The CU dinucleotide sequence, indicated by * in (B), is bolded. The gray box highlights the residues of the TΨC stem that are discussed in the text. The arrow marks the 3′-most nucleotide that anneals to the RT primer. (B) Multiple sequence alignment of some eukaryotic Sec tRNAs. RNA sequences shown are inferred from the reported gene sequences. Identical positions have a black background; conserved positions have a gray background. The most highly conserved region, the anticodon stem and loop, is delineated. * indicates two nucleotides uniquely variant in the E. gracilis sequence. Sources of the sequences are: Eg, Euglena gracilis (this study); Cr, Chlamydomonas reinhardtii (Rao et al. 2003); Dm, Drosophila melanogaster (Lowe and Eddy 1997; Genomic tRNA database [GtRDB]); Ce, Caenorhabditis elegans (Lowe and Eddy 1997; GtRDB).
Organization of E. gracilis box AGA RNA genes
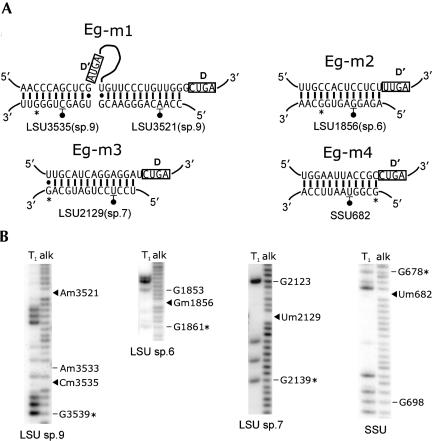
In plants and trypanosomes, modification-guide snoRNA genes are often clustered and arranged in tandem repeats within the genome. Therefore, it was of interest to determine whether similar arrangements exist in the E. gracilis genome. Based on the E. gracilis modification-guide RNA sequences, PCR primers were designed to anneal near the 5′ and 3′ ends of guide RNA genes in order to amplify sequences between any tandem copies. PCR products were obtained when using primers for the Eg-p1, Eg-p2, Eg-p3, and Eg-h1 genes. The sequences obtained and positions of the primers used for Eg-p (1–3) are shown in Figure 8 ▶. The sequences immediately upstream or downstream of the primer binding sites were identical to those obtained by the RNA sequencing and 5′ RACE experiments, verifying that specific amplification between copies of modification-guide RNA genes had occurred. Analysis of the intergenic regions revealed sequence motifs indicative of eukaryotic box C/D methylation-guide RNA genes. The spacing and organization of the box sequences and the presence of extensive sequence complementarities between the predicted box C/D RNA and E. gracilis rRNA suggest strongly that these species function as methylation-guide RNAs. We have designated these RNAs E. gracilis methylation-guide RNAs (Eg-m)1–4. The predicted base-pairing interactions between each box C/D RNA and regions of E. gracilis rRNA are shown in Figure 9A ▶. Based on the invariant n+5 rule for O2′-methyl site selection, nucleotide positions in E. gracilis rRNA predicted to be modified are indicated. All of the base-pairing inter-actions are extensive (>10 bp) and uninterrupted. Interestingly, Eg-m1 is predicted to be a double-guide RNA, able to base pair to a 24-nt contiguous stretch from positions 3518 to 3541 in LSU species 9, within the peptidyltransferase region of the E. gracilis rRNA. To verify that these predicted methylation-guide RNAs are expressed, RT primer extension analysis was performed on total E. gracilis RNA using primers designed in each case to anneal to the entire D box sequence and upstream of it. All of the predicted RNAs could be readily detected (Fig. 3B ▶). From the RT-primer extension products, 5′ RACE experiments were used to map the 5′ ends of the RNAs. These products had the same sequences as the genomic sequences, with the 5′ ends of the expressed RNAs positioned within a few nucleotides of the predicted C-box sequences (Fig. 8 ▶). The 3′ ends of these RNAs would be predicted to be a few nucleotides downstream of the D box sequence (CUGA), as is the case in all other box C/D RNAs characterized to date. The E. gracilis box H/ACA RNA (Eg-h1) gene was also found to be organized in tandem repeats; however, no modification-guide RNAs were evident in the intergenic region between Eg-h1 genes (data not shown). No products were obtained using a similar PCR strategy for the Eg-p4 or the tRNASec genes.
FIGURE 8.
Organization of E. gracilis modification-guide RNA genes. Genomic sequences between copies of Ψ-guide RNA genes for Eg-p2, accession no. AY572246 (A), Eg-p3, accession no. AY572247 (B) and Eg-p1, accession no. AY572248 (C). Capitalized sequence (shown in blue) marks the 5′ and 3′ end sequences of the box AGA RNA genes. The arrows indicate sequences to which the primers anneal. In the intergenic sequences (set in lowercase), regions encoding box C/D RNAs are shown in red and bolded, with the characteristic C- and D-box motifs shown boxed. Regions with extensive complementarity to E. gracilis rRNA are underlined. In B, copies of an imperfect direct repeat are shown in bold and italics.
FIGURE 9.
Target sites for O2′-methylation in rRNA guided by E. gracilis box C/D RNAs. (A) Predicted base-pairing interactions between the box C/D RNAs and sites of E. gracilis rRNA O2′-methylation. The relevant portion of the box C/D guide RNA (top strand) is shown paired to the rRNA (bottom strand), and the nucleotide position of modification is underscored and indicated with a filled circle. Numbering of rRNA positions is as described in Figure 5 ▶. In each case, the position marked by * is identically marked in the corresponding autoradiogram shown in B. (B) Autoradiograms of sequencing gels containing RNase T1 (G-specific) and alkali (alk) ladders of 3′ end-labeled E. gracilis LSU rRNA species 6, 7, 9 and an internal fragment of SSU rRNA. O2′-methyl residues are blank in the alkali ladders, with those predicted from guide RNA sequences indicated by filled triangles.
To determine whether E. gracilis rRNA is O2′-methylated at the predicted positions, E. gracilis rRNA fragments were 3′ end-labeled and subjected to partial alkaline hydrolysis (Fig. 9B ▶). The expected nucleotide positions were blank, indicative of the presence of the O2′-methyl group. The presence of Um2129 in LSU rRNA species 7 was verified by thin layer chromatography (M.N. Schnare, unpubl.).
Whereas the Gm1856 and Um2129 modifications are specific to E. gracilis LSU rRNA, the Um682 in SSU rRNA is highly conserved among eukaryotes, and the corresponding box C/D guide RNAs have been identified (Cavaillé and Bachellerie 1998; Lowe and Eddy 1999; Qu et al. 1999; Dunbar et al. 2000a; Brown et al. 2001; Hüttenhofer et al. 2001; D. Liang et al. 2002; Chen et al. 2003). Both Am3521 and Cm3535, specified by Eg-m1 RNA in E. gracilis, have been found in plant LSU rRNA, but are targeted by two different snoRNAs (Barneche et al. 2001; Brown et al. 2001; D. Liang et al. 2002; Li et al. 2003). One or the other of these two Nm residues is present in other eukaryotes, but in each case the corresponding snoRNA does not have the potential to target the other site (Lowe and Eddy 1999; Xu et al. 2001; Higa et al. 2002; Morales et al. 2002).
DISCUSSION
In this study we identified the first modification-guide RNAs from the protist Euglena gracilis. We isolated and characterized several RNA species that are associated with Cbf5p in this organism and found that many of these have predicted structures consistent with their function as Ψ-guide RNAs. We used these sequences to obtain information about the genomic organization of some of the Ψ-guide RNA genes. In turn, these results led to the identification of several box C/D RNA genes.
Most of the Cbf5p-associated RNAs reported here are single Ψ-guide RNAs; that is, they contain only one extended helical structure and lack an H-box sequence. To date, all of the reported Ψ-guide RNAs from trypanosomatid protozoa (nine from Leptomonas collosoma, three from Trypanosoma brucei, one each from Leishmania seymouri, Leishmania tarentolae, Trypanosoma cruzi, and Crithidia fasciculata; Liang et al. 2001; X.-h. Liang et al. 2002; Uliel et al. 2004; Liang et al. 2004) are also single Ψ-guide RNAs. Based on the E. gracilis αCbf5p immunoprecipitation profile (1 h lane, Fig. 2B ▶), it appears that a large fraction of the Cbf5p-associated RNAs in this organism will be single-guide RNAs, because most of the RNA species are only 55–70 nt in length. Like their trypanosomatid counterparts, the E. gracilis single Ψ-guide RNAs all have an AGA box sequence located 3 nt from their 3′ end. This appears to be an important sequence motif in eukaryotic single Ψ-guide RNAs that distinguishes them from the box H/ACA RNAs. At this position in the identified eukaryotic box H/ACA RNAs, the sequence ACA, AUA, or AAA is present; in contrast, AGA is rarely, if ever, observed (we have not found one instance of AGA in this position in our examination of documented eukaryotic box H/ACA RNAs). In an in vivo expression study of yeast snR11, a change in the box sequence from ACA to AGA blocked accumulation of the mutated box H/ACA RNA (Balakin et al. 1996). In contrast, snR11 mutants containing either AUA or AAA at this position were readily detected in vivo. This observation raises interesting questions as to the potential differences in the assembly and/or structure of these two different types of Ψ-guide RNP complex. Furthermore, one can postulate that a box AGA single Ψ-guide RNA may be the functional equivalent of the 5′ half of a box H/ACA RNA, especially considering that the sequence AGANNA is frequently seen as an H-box sequence within box H/ACA RNAs.
In this study we identified and characterized a larger Cbf5p-associated RNA (Eg-h1), which possesses many of the features typical of other eukaryotic box H/ACA RNAs. Unlike the E. gracilis single Ψ-guide RNAs, Eg-h1 has an ACA box sequence near its 3′ end. In the archaeon, Archaeoglobus fulgidis, a large (~230-nt) box H/ACA-like RNA and several small, putative single Ψ-guide RNAs were detected (Tang et al. 2002). Both types of RNA also appear to be present in several species of Pyrococcus (Rozhdestvensky et al. 2003). The identification of the two types of box H/ACA-like RNAs in organisms as phylogenetically distant as E. gracilis and these archaeal species suggests to us that single Ψ-guide RNAs will be phylogenetically widespread, may make up a significant fraction of all Ψ-guide RNAs, and are not a highly derived characteristic unique to trypanosome species. Indeed, the data presented here indicate that the common ancestor of trypanosomatid and euglenid protists likely contained single Ψ-guide RNAs. It will be interesting to determine whether single Ψ-guide RNAs are present in higher eukaryotes and, if so, whether they account for some of the Ψ sites for which targeting snoRNAs have not yet been identified.
In phylogenetic trees based on rRNA sequence comparisons, Euglena and trypanosomatids appear as early diverging eukaryotes (Sogin et al. 1986; Cavalier-Smith 1993). Although the validity of this early placement has been questioned (e.g., Cavalier-Smith 1998), there is little doubt that the phylum Euglenozoa separated from the main line of eukaryotic evolution prior to the emergence of the eukaryotic crown taxa (animals, fungi, and plants). In that context, it is reasonable to suggest that single Ψ-guide RNAs might have become fused during evolution to generate the double-guide RNAs prominent in the later diverging crown groups. According to this scenario, Eg-p2 RNA would be homologous to the 5′-guide portion of human U69 snoRNA (Ganot et al. 1997a,b), whereas Eg-p3 RNA would be the homolog of the 3′-guide portion of yeast snR32 (Ganot et al. 1997a; Ni et al. 1997). This proposal is problematic in that putative homologs of Eg-p4 are located in the 3′-half of Drosophila melanogaster Dm-3 RNA (Yuan et al. 2003) but in the 5′-half of Arabidopsis thaliana AtsnoR83 (clone Ath-220, Marker et al. 2002) RNA. Thus, for this “fusion” hypothesis to be correct, such fusion events would have had to occur independently in different lineages; alternatively, reshuffling of guide domains might have occurred in some lineages. Analysis of additional single Ψ-guide RNAs and their genes in E. gracilis and other protists should be helpful in evaluating some of these possibilities.
We observed that the E. gracilis Eg-h1 box H/ACA RNA is more abundant than the single Ψ-guide RNAs, and we have not been able to find Ψ target sites in cytoplasmic rRNA for any of its extended helices. These properties are reminiscent of eukaryotic box H/ACA RNAs that have functions other than specifying sites of Ψ formation. Examples are yeast snR30, which is involved in pre-rRNA cleavage reactions (Morrisey and Tollervey 1993), and vertebrate telomerase RNAs, which possess box H/ACA-like domains at their 3′ ends (for review, see Terns and Terns 2002). Alternatively, this E. gracilis RNA may target sites of Ψ formation within RNA species other than cytoplasmic rRNA.
The recent identification of an apparent yeast snR30 (human U17) homolog RNA in Tetrahymena thermophila (Atzorn et al. 2004) suggested the possibility that the E. gracilis Eg-h1 RNA might be a homolog of this RNA. However, the highly conserved short sequence motifs m1 (AUAUUCCUA) and m2 (AAACCAU) that are present in the same relative positions in the Tetrahymena, yeast, and animal RNAs (within the 3′-most extended helical structure) are not present in the E. gracilis RNA. It is possible that these sequences have diverged significantly in E. gracilis.
We have provided evidence that several of the E. gracilis Cbf5p-associated RNAs are encoded by multicopy genes. Some of these genes are found clustered with other modification-guide RNA genes. The genomic arrangements are most similar to the organization of modification-guide RNA genes in trypanosome (Dunbar et al. 2000b; Liang et al. 2001, 2004; Xu et al. 2001) and plant species (Leader et al. 1997; Barneche et al. 2001; Brown et al. 2001; Qu et al. 2001; Liang et al. 2002), where the genes are found clustered and the clusters are sometimes arrayed as tandem repeats. In between copies of Ψ-guide RNA genes, we have identified four methylation-guide RNA genes. Of these, three are predicted to be single methylation-guide RNAs, each specifying single sites of O2′-methylation in cytoplasmic rRNA using either their D or D′ adjacent sequences. All four RNAs utilize the n+5 rule for methylation site selection. The Eg-m1 RNA is particularly interesting because it is predicted to be a double-guide RNA that specifies two nearby sites of O2′-methylation, separated by only 13 nt, within the peptidyltransferase region of the LSU rRNA.
Finding an E. gracilis selenocysteine tRNA in the αCbf5p-immunoprecipitated RNA sample was unexpected. Repeatedly, we have seen this tRNA species become significantly enriched relative to background tRNA levels during immunoprecipitation experiments using this antibody (Fig. 2B ▶, cf. pre-immune sera lane and the 1-h lane). It is interesting to find this tRNA species in immunoprecipitations targeted at Cbf5p because this protein is evolutionarily related to the eubacterial tRNA Ψ55 synthase, TruB (Nurse et al. 1995; Koonin 1996). The tRNASec species has structural features that distinguish it from most other tRNA species and permit specific recognition by a dedicated elongation factor, eEFsec (Fagegaltier et al. 2000; Tujebajeva et al. 2000). It is possible that nucleoside modification within this tRNA species or perhaps even intracellular localization is somehow mediated through a Ψ-guide RNP component. In this regard, we note that Lin and Momany (2003) proposed additional cellular function(s) for the homolog of Cbf5p in Aspergillus nidulans, based on their characterization of a mutation in the conserved RNA-binding domain (PUA) of the protein. This mutation affected growth of the fungus but not overall pseudouridylation of rRNA.
Although we cannot currently rule out the possibility that antibodies directed at the E. gracilis Cbf5p cross-react with another protein having similar sequence and/or structural properties, the tantalizing possibility exists that there is a specific interaction between tRNASec and a component of E. gracilis Ψ-guide RNPs.
MATERIALS AND METHODS
Protein expression and antibody production
Total E. gracilis cDNA was generated from isolated E. gracilis poly(A)+ RNA (Promega PolyATract) by reverse transcription at 55°C using Thermoscript Reverse Transcriptase (Gibco-BRL) and the Thermoscript oligo(dT) primer. The E. gracilis Cbf5 ORF was then specifically amplified by PCR with Pfu DNA polymerase (Stratagene) using oligonucleotides oAR1 (ggcgcggatccGCCATG GCAAAGGCTCAGTGGG; sense +69 to +90 of E. gracilis Cbf5 cDNA) and oAR2 (cgcgggaattcctgcagAGCTATGCCGTTTTCTC CGCCTTC; antisense +1454 to +1477 of Cbf5 cDNA); uppercase letters denote sense or antisense Cbf5 sequence (Watanabe and Gray 2000). The PCR product was cloned between the BamHI and EcoRI sites of pBluescript™IIKS+ (Stratagene), and then the Cbf5 ORF was subcloned into the BamHI and XhoI sites in pET32Ek/ LIC (Novagen). The NdeI fragment (containing a thioredoxin tag) was removed. Recombinant Cbf5p expression was induced with 1 mM IPTG overnight at room temperature in BL21 (DE3) cells. Cells were lysed by sonication, and inclusion bodies were resuspended in solubilization buffer (50 mM NaH2PO4 pH 7.0, 150 mM NaCl, 6 M guanidine-HCl). Recombinant Cbf5p was purified via the N-terminal His tag on Talon Metal Affinity Resin (Clontech) under denaturing conditions, in the solubilization buffer, according to the manufacturer’s protocol. The protein was eluted from the resin in 50 mM NaOAc pH 5.0, 200 mM NaCl and concentrated by ammonium sulfate precipitation. The protein sample was further purified by SDS-PAGE fractionation. Gel slices containing recombinant Cbf5p were crushed, lyophilized, emulsified in Freund’s adjuvant, and injected subcutaneously into a female New Zealand White rabbit. Approximately 200 μg recombinant Cbf5p was injected five times at intervals of 3 wks. Serum was prepared according to standard procedures (Harlow and Lane 1988).
Identification of Cbf5p-associated RNAs
Extract preparation
Euglena gracilis strain Z (1.0 L; photosynthetic) was grown to mid-log phase at pH 5.5 with aeration and a fluorescent light source. The modified salts growth medium was based on conditions described by Eisenstadt and Brawerman (1967) with the following modifications: substitution of CaCl2 (0.02 g/L) for CaCO3, FeSO4 (6 mg/L) for FeCl3, MnCl2 (1.8 mg/L) for MnSO4. Sodium citrate (2.7 mM), CoCl2 (1.6 mg/L), and sodium molyb-date (0.2 mg/L) were added to the medium, which was then titrated to pH 5.5 with phosphoric acid. Ethanol (0.2% v/v) was added as a carbon source.
Harvested cells were resuspended in ~20 mL lysis buffer (50 mM Tris-HCl pH 7.5, 150 mM NaCl, 2 mM MgCl2) and lysed by two passes through a French Pressure cell at 15,000 psi. Cell debris was removed by centrifugation (10,000g for 5 min), and the supernatant (~12 mL) was loaded onto four 30-mL, 10%–30% (v/v) glycerol gradients containing 20 mM Tris-HCl pH 7.5, 100 mM NaCl, and 2 mM MgCl2. Gradients were centrifuged at 6°C for 16 h at 26,000 rpm in an SW27Ti rotor. Fractions were collected (17 × 2 mL) and analyzed by Western blotting using αCbf5p (1:5000 dilution). Those fractions containing Cbf5p were pooled and concentrated in Amicon Ultra concentrators (Millipore) with exchange to the immunoprecipitation buffer (50 mM Tris-HCl pH 8.5, 150 mM NaCl, 2 mM MgCl2, 0.1% (v/v) NP-40). All procedures were carried out at 4°C unless otherwise indicated.
Immunoprecipitation
The concentrated glycerol gradient fraction was incubated overnight (16 h) at 4°C with crude anti-Cbf5p sera (100 μL) or for 1 h at 4°C with an equivalent amount of purified antibodies (HPLC, Hi-Trap Protein A HP) in immunoprecipitation buffer with gentle mixing. Protein A Sepharose Cl-4B (5 mg per immunoprecipitation; Amersham) was then added and the sample was mixed for an additional 1 h. Immunoprecipitates were recovered by centrifugation and washed six times by resuspension in 1 mL of immunoprecipitation buffer followed by centrifugation. Immunoprecipitated RNAs were extracted with phenol, with incubation for 5 min at 65°C in the 0.05% SDS, 0.3 M NaOAc, phenol mix. RNAs were then precipitated and washed in ethanol.
Determination of the sequence of Cbf5p-associated RNAs
RNA sequencing
Immunoprecipitated RNAs were 3′ end-labeled with [5′-32P] pCp and RNA ligase and separated by electrophoresis in an 8% denaturing polyacrylamide gel. Bands of interest were excised, eluted, and subjected to chemical sequencing (Peattie 1979). Based on the sequences obtained, oligonucleotides were designed for 5′ RACE experiments in order to obtain the full-length RNA sequences present in Euglena gracilis total RNA prepared as described in Schnare and Gray (1990). These primers contained the anchor sequence GGCGCGAATTC at their 5′ ends.
5′ RACE
5′ End-labeled, gel-purified oligonucleotide primers (1 pmole per reaction) were annealed to 15 μg total E. gracilis RNA at 65°C for 5 min, 47°C for 10 min, followed by room temperature for 5 min. cDNA was synthesized using SuperscriptII RT (Invitrogen) at 47°C for 45 min, otherwise following the manufacturer’s protocol. Samples were then treated with RNase H and gel-purified to remove excess primer. The cDNAs were 3′ end-tailed with dG using terminal deoxynucleotidyl transferase (Invitrogen) according to the manufacturer’s protocol (using 20 μM dGTP). Samples were then heat-inactivated at 90°C for 3 min, and 10% of the reaction mix (3 μL) was used as template for PCR amplification with Taq DNA polymerase (Amersham). PCR was performed using oAR8 (CTCCCGCTTCCAGATCTCGAG(C15)G/A/T) and the RNA-specific primer that had been employed in the cDNA synthesis step. Cycling parameters were: 94°C (4 min); five cycles of 94°C, 50°C, 72°C (30 sec each); five cycles of 94°C, 55°C, 72°C (30 sec each); 25 cycles of 94°C, 60°C, 72°C (30 sec each); 72°C (7 min). PCR products were cloned into the pCR2.1 Topo vector (Invitrogen) using the Topo TA Cloning Kit (Invitrogen) according to the manufacturer’s protocol. Sequencing of clones was performed using an automated ABI Prism 377 DNA sequencer.
Modification-guide RNA targets and gene organizations
Guide RNA-targeted sites for Ψ or O2′-methylation in E. gracilis rRNAs were determined using SPIN v1.1 of Staden 2002 to search the E. gracilis rRNA sequences (Sogin et al. 1986; Schnare et al. 1990; Greenwood et al. 2001) for significant sequence complementarity to a guide RNA. RNA structures were drawn using XRNA (Beta html version; B.Weiser, UCLA).
Modified nucleotide mapping
3′ end-labeled RNA fragments were subjected to chemical sequencing reactions (Peattie 1979) and/or partial digestion with RNase T1 (Donis-Keller et al. 1977) and alkali (Schnare and Gray 1982). In order to generate discrete fragments of the larger E. gracilis rRNAs we adapted an RNase protection approach (Casey and Davidson 1977; Van Stolk and Noller 1984), using PCR products spanning the regions of interest. Taq polymerase was removed from the PCR products by digestion with proteinase K, phenol extraction, and ethanol precipitation (Crowe et al. 1991; Barnes 1992).
RNA (15 μg) and an excess of gel-purified PCR product were dissolved in 10 μL of 70% formamide (deionized), 0.36 M NaCl, 20 mM HEPES, pH 7. The samples were denatured at 90°C for 5 min and hybridized at 46°C for 4 h. Unhybridized RNA was digested with RNase T1, and the sample was phenol-extracted and precipitated (Van Stolk and Noller 1984). 3′ phosphates were removed by treatment with bacterial alkaline phosphatase, and the protected RNA fragments were purified away from RNase T1 digestion products by precipitation on ice with an equal volume of 30% PEG 3350, 1.5 M NaCl (Barnes 1992) in the presence of 20 μg linear polyacrylamide (Gaillard and Strauss 1990). The PEG precipitate was washed with 80% ethanol, dried, redissolved, phenol-extracted (3×), ethanol-precipitated (2×), and washed again with 80% ethanol prior to 3′ end-labeling for sequencing.
Gene organization
PCR was performed using Taq DNA polymerase (Amersham) and appropriate primer pairs under standard PCR conditions with 20 ng E. gracilis genomic DNA as template. The Eg-p1 genomic repeat was amplified more efficiently when 5%–10% DMSO was added to the PCR reaction. PCR products were cloned into the pCR2.1 Topo vector (Invitrogen) and sequenced as described above.
Acknowledgments
We thank Amanda Lohan for helpful discussions concerning the identification of the E. gracilis tRNASec. David Spencer provided valuable technical advice for growing the E. gracilis cultures and for the computer analyses. We also thank Lesley Davis and Marlena Dlutek for performing the automated sequencing reactions. A.G.R. was supported by a Nova Scotia Health Research Foundation Post Doctoral Fellowship. This research is supported by operating grant MOP-11212 from the Canadian Institutes for Health Research to M.W.G, with salary support provided by the Canada Research Chairs Program and Canadian Institute for Advanced Research (Program in Evolutionary Biology).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.rnajournal.org/cgi/doi/10.1261/rna.7300804.
REFERENCES
- Atzorn, V., Fragapane, P., and Kiss, T. 2004. U17/snR30 is a ubiquitous snoRNA with two conserved sequence motifs essential for 18S rRNA production. Mol. Cell. Biol. 24: 1769–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachellerie, J.-P., Cavaillé, J., and Hüttenhofer, A. 2002. The expanding snoRNA world. Biochimie 84: 775–790. [DOI] [PubMed] [Google Scholar]
- Bakin, A., Lane, B.G., and Ofengand, J. 1994. Clustering of pseudouridine residues around the peptidyltransferase center of yeast cytoplasmic and mitochondrial ribosomes. Biochemistry 33: 13475–13483. [DOI] [PubMed] [Google Scholar]
- Balakin, AG., Smith, L., and Fournier, M.J. 1996. The RNA world of the nucleolus: Two major families of small RNAs defined by different box elements with related functions. Cell 86: 823–834. [DOI] [PubMed] [Google Scholar]
- Barneche, F., Gaspin, C., Guyot R., and Echeverria, M. 2001. Identification of 66 box C/D snoRNAs in Arabidopsis thaliana: Extensive gene duplications generated multiple isoforms predicting new ribosomal RNA 2′-O-methylation sites. J. Mol. Biol. 311: 57–73. [DOI] [PubMed] [Google Scholar]
- Barnes, W.M. 1992. The fidelity of Taq polymerase catalyzing PCR is improved by an N-terminal deletion. Gene 112: 29–35. [DOI] [PubMed] [Google Scholar]
- Brown, J.W.S., Clark, G.P., Leader, D.J., Simpson, C.G., and Lowe, T. 2001. Multiple snoRNA gene clusters from Arabidopsis.RNA 7: 1817–1832. [PMC free article] [PubMed] [Google Scholar]
- Brown, J.W.S., Echeverria, M., and Qu, L.-H. 2003. Plant snoRNAs: Functional evolution and new modes of gene expression. Trends Plant Sci. 8: 42–49. [DOI] [PubMed] [Google Scholar]
- Casey, J. and Davidson, N. 1977. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 4: 1539–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillé, J. and Bachellerie, J.-P. 1998. SnoRNA-guided ribose methylation of rRNA: Structural features of the guide RNA duplex influencing the extent of the reaction. Nucleic Acids Res. 26: 1576–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillé, J., Nicoloso, M., and Bachellerie, J.-P. 1996. Targeted ribose methylation of RNA in vivo directed by tailored antisense RNA guides. Nature 383: 732–735. [DOI] [PubMed] [Google Scholar]
- Cavaillé, J., Buiting, K., Kiefmann, M., Lalande, M., Brannan, C.I., Horsthemke, B., Bachellerie, J.-P., Brosius, J., and Hüttenhofer, A. 2000. Identification of brain-specific and imprinted small nucleolar RNA genes exhibiting an unusual genomic organization. Proc. Natl. Acad. Sci. 97: 14311–14316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier-Smith, T. 1993. Kingdom Protozoa and its 18 phyla. Microbiol. Rev. 57: 953–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 1998. A revised six-kingdom system of life. Biol. Rev. 73: 203–266. [DOI] [PubMed] [Google Scholar]
- Chen, C.-L., Liang, D., Zhou, H., Zhuo, M., Chen, Y.-Q., and Qu, L.-H. 2003. The high diversity of snoRNAs in plants: Identification and comparative study of 120 snoRNA genes from Oryza sativa. Nucleic Acids Res. 31: 2601–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commans, S. and Böck, A. 1999. Selenocysteine inserting tRNAs: An overview. FEMS Microbiol. Rev. 23: 335–351. [DOI] [PubMed] [Google Scholar]
- Crowe, J.S., Cooper, H.J., Smith, M.A., Sims, M.J., Parker, D., and Gewert, D. 1991. Improved cloning efficiency of polymerase chain reaction (PCR) products after proteinase K digestion. Nucleic Acids Res. 19: 184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzacq, X., Jády, B.E., Verheggen, C., Kiss, A.M., Bertrand, E., and Kiss, T. 2002. Cajal body-specific small nuclear RNAs: A novel class of 2′-O-methylation and pseudouridylation guide RNAs. EMBO J. 21: 2746–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller, H., Maxam, A.M., and Gilbert, W. 1977. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 4: 2527–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar, D.A., Wormsley, S., Lowe, T.M., and Baserga, S.J. 2000a. Fibrillarin-associated box C/D small nucleolar RNAs in Trypanosoma brucei. J. Biol. Chem. 275: 14767–14776. [DOI] [PubMed] [Google Scholar]
- Dunbar, D.A., Chen, A.A., Wormsley, S., and Baserga, S.J. 2000b. The genes for small nucleolar RNAs in Trypanosoma brucei are organized in clusters and are transcribed as a polycistronic RNA. Nucleic Acids Res. 28: 2855–2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstadt, J.M. and Brawerman, G. 1967. Isolation of chloroplasts from Euglena gracilis. Methods Enzymol. 12: 476–478. [Google Scholar]
- Fagegaltier, D., Hubert, N., Yamada, K., Mizutani, T., Carbon, P., and Krol, A. 2000. Characterization of mSelB, a novel mammalian elongation factor for selenoprotein translation. EMBO J. 19: 4796–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard, C. and Strauss, F. 1990. Ethanol precipitation of DNA with linear polyacrylamide as carrier. Nucleic Acids Res. 18: 378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganot, P., Bortolin, M.-L., and Kiss, T. 1997a. Site-specific pseudouridine formation in preribosomal RNA is guided by small nucleolar RNAs. Cell 89: 799–809. [DOI] [PubMed] [Google Scholar]
- Ganot, P., Caizergues-Ferrer, M., and Kiss, T. 1997b. The family of box ACA small nucleolar RNAs is defined by an evolutionarily conserved secondary structure and ubiquitous sequence elements essential for RNA accumulation. Genes & Dev. 11: 941–956. [DOI] [PubMed] [Google Scholar]
- Giordano, E., Peluso, I., Senger, S., and Furia, M. 1999. minifly, a Drosophila gene required for ribosome biogenesis. J. Cell Biol. 144: 1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood, S.J., Schnare, M.N., Cook, J.R., and Gray, M.W. 2001. Analysis of intergenic spacer transcripts suggests ‘read-around’ transcription of the extrachromosomal circular rDNA in Euglena gracilis. Nucleic Acids Res. 29: 2191–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow, E. and Lane, D. 1988. Antibodies: A laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- Higa, S., Maeda, N., Kenmochi, N., and Tanaka, T. 2002. Location of 2′-O-methyl nucleotides in 26S rRNA and methylation guide snoRNAs in Caenorhabditis elegans. Biochem. Biophys. Res. Comm. 297: 1344–1349. [DOI] [PubMed] [Google Scholar]
- Hüttenhofer, A., Kiefmann, M., Meier-Ewert, S., O’Brien, J., Lehrach, H., Bachellerie, J.-P., and Brosius, J. 2001. RNomics: An experimental approach that identifies 201 candidates for novel, small, non-messenger RNAs in mouse. EMBO J. 20: 2943–2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, K.N., Gordon, K.H., and Hanzlik, T.N. 2000. Reverse transcription of a naturally occurring nonretroviral RNA produces a precise deletion in the majority of its cDNA products. IUBMB Life 49: 223–227. [DOI] [PubMed] [Google Scholar]
- Kiss, T. 2002. Small nucleolar RNAs: An abundant group of noncoding RNAs with diverse cellular functions. Cell 109: 145–148. [DOI] [PubMed] [Google Scholar]
- Kiss-László, Z., Henry, Y., Bachellerie, J.-P., Caizergues-Ferrer, M., and Kiss, T. 1996. Site-specific ribose methylation of preribosomal RNA: A novel function for small nucleolar RNAs. Cell 85: 1077–1088. [DOI] [PubMed] [Google Scholar]
- Klein, D.J., Schmeing, T.M., Moore, P.B., and Steitz, T.A. 2001. The kink-turn: A new RNA secondary structure motif. EMBO J. 20: 4214–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin, E.V. 1996. Pseudouridine synthases: Four families of enzymes containing a putative uridine-binding motif also conserved in dUTPases and dCTP deaminases. Nucleic Acids Res. 24: 2411–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leader, D.J., Clark, G.P., Watters, J., Beven, A.F., Shaw, P.J., and Brown, J.W.S. 1997. Clusters of multiple different small nucleolar RNA genes in plants are expressed as and processed from poly-cistronic pre-snoRNAs. EMBO J. 16: 5742–5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, X.-h., Liu, L., and Michaeli, S. 2001. Identification of the first trypanosome H/ACA RNA that guides pseudouridine formation on rRNA. J. Biol. Chem. 276: 40313–40318. [DOI] [PubMed] [Google Scholar]
- Liang, D., Zhou, H., Zhang, P., Chen, Y.-Q., Chen, X., Chen, C.-L., and Qu, L.-H. 2002. A novel gene organization: Intronic snoRNA gene clusters from Oryza sativa. Nucleic Acids Res. 30: 3262–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, X.-h., Xu, Y.-X., and Michaeli, S. 2002. The spliced leader-associated RNA is a trypanosome-specific sn(o) RNA that has the potential to guide pseudouridine formation on the SL RNA. RNA 8: 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, X.-h., Ochaion, A., Xu, Y.-x., Liu, Q., and Michaeli, S. 2004. Small nucleolar RNA clusters in trypanosomatid Leptomonas collosoma. J. Biol. Chem. 279: 5100–5109. [DOI] [PubMed] [Google Scholar]
- Li, W., Jiang, G., Jin, Y.-X., and Wang, D.-B. 2003. Detection of 2′-O-ribose methylation sites on rice 25 S rRNA. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 35: 289–295. [PubMed] [Google Scholar]
- Lin, X. and Momany, M. 2003. The Aspergillus nidulans swoC1 mutant shows defects in growth and development. Genetics 165: 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe, T.M. and Eddy, S.R. 1997. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 25: 955–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 1999. A computational screen for methylation guide snoRNAs in yeast. Science 283: 1168–1171. [DOI] [PubMed] [Google Scholar]
- Maden, B.E.H. 1988. Locations of methyl groups in 28S rRNA of Xenopus laevis and man. Clustering in the conserved core of molecule. J. Mol. Biol. 201: 289–314. [DOI] [PubMed] [Google Scholar]
- Marker, C., Zemann, A., Terhorst, T., Kiefmann, M., Kastenmayer, J.P., Green, P., Bachellerie, J.-P., Brosius, J., and Hüttenhofer, A. 2002. Experimental RNomics: Identification of 140 candidates for small non-messenger RNAs in the plant Arabidopsis thaliana. Curr. Biol. 12: 2002–2013. [DOI] [PubMed] [Google Scholar]
- Morales, L., Romero, I., Diez, H., Del Portillo, P., Montilla, M., Ni-cholls, S., and Puerta, C. 2002. Characterization of a candidate Trypanosoma rangeli small nucleolar RNA gene and its application in a PCR-based parasite detection. Experimental Parasitol. 102: 72–80. [DOI] [PubMed] [Google Scholar]
- Morrissey, J.P. and Tollervey, D. 1993. Yeast snR30 is a small nucleolar RNA required for 18S rRNA synthesis. Mol. Cell Biol. 13: 2469–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni, J., Tien, A.L., and Fournier, M.J. 1997. Small nucleolar RNAs direct site-specific synthesis of pseudouridine in ribosomal RNA. Cell 89: 565–573. [DOI] [PubMed] [Google Scholar]
- Nicoloso, M., Qu, L.-H., Michot, B., and Bachellerie, J.-P. 1996. Intron-encoded, antisense small nucleolar RNAs: The characterization of nine novel species points to their direct role as guides for the 2′-O-ribose methylation of rRNAs. J. Mol. Biol. 260: 178–195. [DOI] [PubMed] [Google Scholar]
- Nurse, K., Wrzesinski, J., Bakin, A., Lane, B.G., and Ofengand, J. 1995. Purification, cloning, and properties of the tRNA Ψ55 synthase from Escherichia coli. RNA 1: 102–112. [PMC free article] [PubMed] [Google Scholar]
- Ofengand, J. and Bakin, A. 1997. Mapping to nucleotide resolution of pseudouridine residues in large subunit ribosomal RNAs from representative eukaryotes, prokaryotes, archaebacteria, mitochondria and chloroplasts. J. Mol. Biol. 266: 246–268. [DOI] [PubMed] [Google Scholar]
- Omer, A.D., Lowe, T.M., Russell, A.G., Ebhardt, H., Eddy, S.R., and Dennis, P.P. 2000. Homologs of small nucleolar RNAs in Archaea. Science 288: 517–522. [DOI] [PubMed] [Google Scholar]
- Omer, A.D., Ziesche, S., Decatur, W.A., Fournier, M.J., and Dennis, P.P. 2003. RNA-modifying machines in archaea. Mol. Microbiol. 48: 617–629. [DOI] [PubMed] [Google Scholar]
- Peattie, D.A. 1979. Direct chemical method for sequencing RNA. Proc. Natl. Acad. Sci. 76: 1760–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, L.H., Henras, A., Lu, Y.-J., Zhou, H., Zhou, W.-X., Zhu, Y.Q., Zhao, J., Henry, Y., Caizergues-Ferrer, M., and Bachellerie, J.-P. 1999. Seven novel methylation guide small nucleolar RNAs are processed from a common polycistronic transcript by Rat1p and RNase III in yeast. Mol. Cell. Biol. 19: 1144–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, L.H., Meng, Q., Zhou, H., and Chen, Y.-Q. 2001. Identification of 10 novel snoRNA gene clusters from Arabidopsis thaliana. Nucleic Acids Res. 29: 1623–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, M., Carlson, B.A., Novoselov, S.V., Weeks, D.P., Gladyshev, V. N., and Hatfield, D.L. 2003. Chlamydomonas reinhardtii selenocysteine tRNA[Ser]Sec. RNA 9: 923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard, P., Darzacq, X., Bertrand, E., Jády, B.E., Verheggen, C., and Kiss, T. 2003. A common sequence motif determines the Cajal body-specific localization of box H/ACA scaRNAs. EMBO J. 22: 4283–4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozhdestvensky, T.S., Tang, T.H., Tchirkova, I.V., Brosius, J., Bachellerie, J.-P., and Hüttenhofer, A. 2003. Binding of L7Ae protein to the K-turn of archaeal snoRNAs: A shared RNA binding motif for C/D and H/ACA box snoRNAs in Archaea. Nucleic Acids Res. 31: 869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnare, M.N. and Gray, M.W. 1982. Nucleotide sequence of an exceptionally long 5.8S ribosomal RNA from Crithidia fasciculata. Nucleic Acids Res. 10: 2085–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 1990. Sixteen discrete RNA components in the cytoplasmic ribosome of Euglena gracilis. J. Mol. Biol. 215: 73–83. [DOI] [PubMed] [Google Scholar]
- Schnare, M.N., Cook, J.R., and Gray, M.W. 1990. Fourteen internal transcribed spacers in the circular ribosomal DNA of Euglena gracilis. J. Mol. Biol. 215: 85–91. [DOI] [PubMed] [Google Scholar]
- Sogin, M.L., Elwood, H.J., and Gunderson, J.H. 1986. Evolutionary diversity of eukaryotic small-subunit rRNA genes. Proc. Natl. Acad. Sci. 83: 1383–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, T-H., Bachellerie, J.-P., Rozhdestvensky, T., Bortolin, M.-L., Huber, H., Drungowski, M., Elge, T., Brosius, J., and Hüttenhofer, A. 2002. Identification of 86 candidates for small non-messenger RNAs from the archaeon Archaeoglobus fulgidis. Proc. Natl. Acad. Sci. 99: 7536–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terns, M.P. and Terns, R.M. 2002. Small nucleolar RNAs: Versatile transacting molecules of ancient evolutionary origin. Gene Expr. 10: 17–39. [PMC free article] [PubMed] [Google Scholar]
- Tujebajeva, R.M., Copeland, P.R., Xu, X.-M., Carlson, B.A., Harney, J.W., Driscoll, D.M., Hatfield, D.L., and Berry, M.J. 2000. Decoding apparatus for eukaryotic selenocysteine insertion. EMBO Rep. 1: 158–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uliel, S., Liang, X.-h., Unger, R., and Michaeli, S. 2004. Small nucleolar RNAs that guide modification in trypanosomatids: Repertoire, targets, genome organisation, and unique functions. Intl. J. Parasitol. 34: 445–454. [DOI] [PubMed] [Google Scholar]
- Van Stolk, B.J. and Noller, H.F. 1984. Chemical probing of conformation in large RNA molecules. Analysis of 16 S ribosomal RNA using diethylpyrocarbonate. J. Mol. Biol. 180: 151–177. [DOI] [PubMed] [Google Scholar]
- Watanabe, Y.-i. and Gray, M.W. 2000. Evolutionary appearance of genes encoding proteins associated with box H/ACA snoRNAs: Cbf5p in Euglena gracilis, an early diverging eukaryote, and candidate Gar1p and Nop10p homologs in archaebacteria. Nucleic Acids Res. 28: 2342–2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins, N.J., Gottschalk, A., Neubauer, G., Kastner, B., Fabrizio, P., Mann, M., and Lührmann, R. 1998. Cbf5p, a potential pseudouridine synthase, and Nhp2p, a putative RNA-binding protein, are present together with Gar1p in all H BOX/ACA-motif snoRNPs and constitute a common bipartite structure. RNA 4: 1549–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins, N.J., Ségault, V., Charpentier, B., Nottrott, S., Fabrizio, P., Bachi, A., Wilm, M., Rosbash, M., Branlant, C., and Lührmann, R. 2000. A common core RNP structure shared between the small nucleolar box C/D RNPs and the spliceosomal U4 snRNP. Cell 103: 457–466. [DOI] [PubMed] [Google Scholar]
- Xu, Y.-X., Liu, L., Lopez-Estraño, C., and Michaeli, S. 2001. Expression studies on clustered trypanosomatid box C/D small nucleolar RNAs. J. Biol. Chem. 276: 14289–14298. [DOI] [PubMed] [Google Scholar]
- Yuan, G., Klämbt, C., Bachellerie, J.-P., Brosius, J., and Hüttenhofer, A. 2003. RNomics in Drosophila melanogaster: Identification of 66 candidates for novel non-messenger RNAs. Nucleic Acids Res. 31: 2495–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaphiropoulos, P.G. 2002. Template switching generated during reverse transcription? FEBS Lett. 527: 326. [DOI] [PubMed] [Google Scholar]