Abstract
HIV-1 uses a programmed -1 ribosomal frameshift to produce the precursor of its enzymes. This frameshift occurs at a specific slippery sequence followed by a stimulatory signal, which was recently shown to be a two-stem helix, for which a three-purine bulge separates the upper and lower stems. In the present study, we investigated the response of the bacterial ribosome to this signal, using a translation system specialized for the expression of a firefly luciferase reporter. The HIV-1 frameshift region was inserted at the beginning of the coding sequence of the luciferase gene, such that its expression requires a −1 frameshift. Mutations that disrupt the upper or the lower stem of the frameshift stimulatory signal or replace the purine bulge with pyrimidines decreased the frameshift efficiency, whereas compensatory mutations that re-form both stems restored the frame-shift efficiency to near wild-type level. These mutations had the same effect in a eukaryotic translation system, which shows that the bacterial ribosome responds like the eukaryote ribosome to the HIV-1 frameshift stimulatory signal. Also, we observed, in contrast to a previous report, that a stop codon immediately 3′ to the slippery sequence does not decrease the frameshift efficiency, ruling out a proposal that the frameshift involves the deacylated-tRNA and the peptidyl-tRNA in the E and P sites of the ribosome, rather than the peptidyl-tRNA and the aminoacyl-tRNA in the P and A sites, as commonly assumed. Finally, mutations in 16S ribosomal RNA that facilitate the accommodation of the incoming aminoacyl-tRNA in the A site decreased the frameshift efficiency, which supports a previous suggestion that the frameshift occurs when the aminoacyl-tRNA occupies the A/T entry site.
Keywords: bacterial ribosome, HIV-1 programmed −1 ribosomal frameshift, ribosomal RNA mutagenesis
INTRODUCTION
The human immunodeficiency virus type 1 (HIV-1) uses a programmed −1 ribosomal frameshift to produce the Gag-Pol polyprotein, the precursor of its enzymes, when ribosomes translate the full-length viral messenger RNA (mRNA; Jacks et al. 1988a; for review, see Brierley and Pennell 2001). A programmed −1 frameshift requires two cis-acting elements in the mRNA: a slippery sequence (U UUU UUA for HIV-1, with the spaces indicating the initial reading frame), where the change in the reading frame takes place, and a frameshift stimulatory signal, located downstream from the slippery sequence. It had been proposed that the frameshift stimulatory signal makes the ribosomes pause when they encounter the slippery sequence, increasing the probability of alternate events (Tu et al. 1992; Somogyi et al. 1993; Lopinski et al. 2000). However, no correlation was found between pausing and frameshift efficiency (Kontos et al. 2001), and it is now proposed that a specific interaction between the ribosomes and the frameshift stimulatory signal promotes frameshifting through an unknown mechanism.
The HIV-1 frameshift stimulatory signal was initially found to be a simple stem–loop of 11 base pairs (the classic frameshift stimulatory signal), separated from the slippery sequence by a spacer of 8 nt (Fig. 1A; Jacks et al. 1988a; Kang 1998). Recently, it was shown by our group, with a eukaryotic reporter system, that the HIV-1 frameshift stimulatory signal is an extended helix made of two stems separated by a purine bulge (Dulude et al. 2002). The upper stem–loop corresponds to the classic signal, and the lower stem results from the pairing of the spacer with a complementary region downstream of the upper stem–loop (Fig. 1B). This pairing was demonstrated by mutagenesis and probing studies with HIV-1 group M subtype B, the subtype that prevails in North America and Western Europe. It was subsequently found that the frameshift stimulatory signal of all other subtypes of group M can be folded into the same structure as in subtype B, despite sequence variations in the stimulatory region (Baril et al. 2003). We will refer to the frameshift stimulatory signal characterized by Dulude et al. (2002) as the complete HIV-1 frameshift stimulatory signal.
FIGURE 1.
The HIV-1 frameshift region. The slippery site is underlined and the spaces indicate the initial reading frame. (A) HIV-1 frameshift region with the classic frameshift stimulatory signal (11 bp stem–loop; Jacks et al. 1988a) separated from the slippery sequence by a spacer of 8 nt. (B) HIV-1 frameshift region with the complete frameshift stimulatory signal characterized by Dulude et al. (2002). This frameshift stimulatory signal is a two-stem helix interrupted by a purine-rich bulge. The upper stem–loop corresponds to the stem–loop showed in A and the lower stem is made by pairing the spacer to a complementary sequence downstream of the upper stem–loop.
The model proposed to describe programmed −1 frame-shifts in eukaryotes is the two-tRNA simultaneous slippage model (Jacks et al. 1988b), which is supported by mutagenesis and protein sequencing studies (Jacks et al. 1988b; Dinman et al. 1991; Brierley et al. 1992; Brierley and Pennell 2001). In this model, the change in the reading frame occurs before peptide bond formation. The peptidyl-tRNA (p-tRNA) in the P site and the aminoacyl-tRNA (aa-tRNA) in the A site unpair simultaneously from the mRNA, slip together with the ribosome by one base in the 5′ direction of the mRNA and re-pair in the −1 reading frame. This model also applies to bacteria in several cases, but there is more diversity in the mechanisms accounting for programmed −1 frameshifts in bacteria (Farabaugh 1997a; Atkins et al. 2001; Baranov et al. 2002; Napthine et al. 2003).
Studies with a reporter gene had previously shown that the frameshift of HIV-1 can be reproduced in bacteria by inserting either the HIV-1 classic frameshift region or only the slippery sequence at the beginning of the coding sequence of a reporter gene (Weiss et al. 1989; Yelverton et al. 1994; Horsfield et al. 1995; Brunelle et al. 1999). Yelverton et al. (1994), from protein sequencing studies, suggested that the HIV-1 slippery sequence is prone to frame-shifting in bacteria by both the two-tRNA slippage mechanism of Jacks et al. (1988b) and by p-tRNA slippage before the binding of the incoming aa-tRNA, which pairs to the A-site codon in the −1 frame. Two of the studies with bacteria suggested that the shift in the reading frame occurs after peptide bond formation, either when the deacylated-tRNA and the p-tRNA occupy, respectively, the P/E and A/P hybrid states (Weiss et al. 1989) or when they are fully translocated in the E and P sites (Horsfield et al. 1995). However, the study of Brunelle et al. (1999) supported that the slippage of the tRNAs occurs prior to peptide bond formation, in showing that the frameshift efficiency was increased in the presence of a peptide bond inhibitor but unaffected by a translocation inhibitor. Similar observations on the effect of translation inhibitors were made when studying programmed −1 frameshifts in yeast (Dinman et al. 1997; Tumer et al. 1998; Harger et al. 2002; Meskauskas et al. 2003). Thus, the model from Jacks et al. (1988b) seems to be most appropriate to describe the HIV-1 programmed −1 frameshift. However, if the programmed −1 ribosomal frameshift occurs when an aa-tRNA occupies the A site, a problem is encountered in that the rate of peptide bond formation is extremely fast (Pape et al. 1998; Rodnina and Wintermeyer 2001; Gromadski and Rodnina 2004), and this does not give much time for the frameshift to take place. To solve this problem, it was suggested that the change in the reading frame occurs when the aa-tRNA occupies the A/T entry site, before being accommodated in the A site (Farabaugh 1997b; Brunelle et al. 1999). At this stage, the aa-tRNA is complexed with the elongation factor Tu (EF-Tu) bound to GTP, and it is only after GTP hydrolysis and release of EF-T·GDP from the ribosome that the aa-tRNA can be accommodated in the A site.
In this study, our aim was to investigate the response of the bacterial ribosome to the complete HIV-1 frameshift stimulatory signal and to reassess the mechanism of programmed −1 frameshifting. Experiments with bacteria benefit from the advantages that the structure of the bacterial ribosome has been characterized to near atomic resolution (Ban et al. 2000; Schluenzen et al. 2000; Wimberly et al. 2000; Yusupov et al. 2001) and that it readily lends itself to genetic manipulations. We inserted the complete HIV-1 frameshift region at the beginning of a reporter gene encoded by a bacterial plasmid that also contains a ribosomal RNA (rRNA) operon from Escherichia coli. With this plas-mid, the reporter mRNA is exclusively translated by a subset of ribosomes that contain plasmid-encoded 16S rRNA (Lee et al. 1997; Morosyuk et al. 2000, 2001; Bélanger et al. 2002; for review, see Brakier-Gingras et al. 2003). When various mutations were introduced in the complete HIV-1 frame-shift stimulatory signal, we observed that the bacterial ribosome responds to the perturbations caused by these mutations exactly like the eukaryote ribosome. Also, insertion of a stop codon immediately 3′ to the slippery sequence did not decrease the frameshift efficiency, in contrast to a previous report showing that a stop codon at this position severely reduces frameshifting. Moreover, we found that the HIV-1 programmed −1 frameshift was decreased by 16S rRNA mutations that facilitate the accommodation of the aa-tRNA in the A site, supporting the suggestion that the frameshift occurs before the aa-tRNA occupies the A site.
RESULTS
Response of the bacterial ribosome to the complete HIV-1 frameshift stimulatory signal
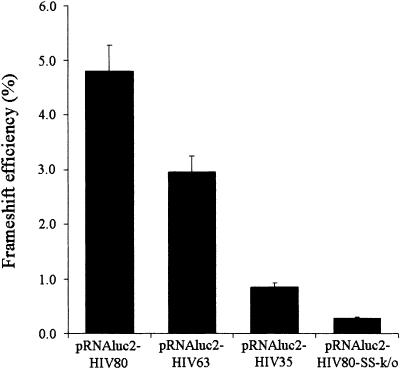
The HIV-1 programmed −1 ribosomal frameshift has already been reproduced in bacteria, using reporter genes with the HIV-1 frameshift region inserted at the beginning of their coding sequence (Weiss et al. 1989; Yelverton et al. 1994; Horsfield et al. 1995; Brunelle et al. 1999). However, in these studies, the frameshift region was limited to either the slippery sequence or the classic frameshift region, encompassing the slippery sequence plus an 11 bp stem–loop (Fig. 1A). In the present study, we first investigated how the bacterial ribosome responds to the complete frameshift region of HIV-1 defined by Dulude et al. (2002; Fig. 1B). To this end, we used a specialized ribosome system (Lee et al. 1997; Morosyuk et al. 2000, 2001; Bélanger et al. 2002), for which translation of a plasmid-encoded reporter mRNA is carried out by a specific subset of ribosomes containing 16S rRNA encoded by the same plasmid. With this system, mutations in 16S rRNA only affect the expression of the reporter gene. The complete HIV-1 frameshift region was amplified by PCR from the eukaryote vector pLuc/HIV/B (−1) (Baril et al. 2003), which contains the frameshift region of HIV-1 group M subtype B inserted at the beginning of the coding sequence of the luciferase gene. The resulting PCR fragment was inserted between the NsiI and Pfl23II restriction sites of the firefly luciferase gene of pRNAluc2 (Bélanger et al. 2002), a plasmid coding for the specialized ribosome system. This generated pRNAluc2-HIV80 (−1) (Fig. 2A), in which the complete HIV-1 frameshift region is inserted after the initiator AUG codon, such that a −1 frameshift is required for the expression of luciferase. An in-frame (0) control construct was derived from pRNAluc2-HIV80 (−1), in which an adenine was added immediately after the slippery sequence, generating pRNAluc2-HIV80 (0). With this construct, luciferase is produced by ribosomes that do not change the reading frame and the frameshift efficiency can be assessed by dividing the luciferase activity of the (−1) construct by the sum of the luciferase activity of the (0) and (−1) constructs. Starting with pRNAluc2-HIV80, a series of pRNAluc2-HIV constructs were generated (Fig. 2B) with a (0) and a (−1) version for each construct. We deleted by PCR the sequence downstream from the upper stem–loop of the complete frameshift region, generating a construct that contains the classic frameshift region (pRNAluc2-HIV63). We also constructed a plasmid that contains the slippery sequence of the HIV-1 frameshift region without a stimulatory signal (pRNAluc2-HIV35), and, finally, we inactivated the slippery sequence in the complete HIV-1 frameshift region (pRNAluc2-HIV80-SS-k/o). The frameshift efficiencies measured with these constructs are presented in Figure 3. With plasmid pRNAluc2-HIV63, which contains the classic frameshift region, the frameshift efficiency was about 3.0%, a value comparable to that obtained with a eukaryotic reporter in cultured mammalian cells (2.1 to 5.5%; Reil et al. 1993; Cassan et al. 1994; Dulude et al. 2002; Baril et al. 2003). When the inserted frameshift region contains the slippery sequence and the complete frameshift stimulatory signal (pRNAluc2-HIV80), the frameshift efficiency rose to 4.8%, an increase comparable to that observed in studies using a eukaryotic reporter (Dulude et al. 2002; Baril et al. 2003). When the inserted frameshift region contains only the slippery sequence (pRNAluc2-HIV35), the frameshift efficiency decreased to 0.8%, and when the slippery sequence is inactivated (pRNAluc2-HIV80-SS-k/o), the frameshift efficiency dropped to 0.3%.
FIGURE 2.
Description of the luciferase expression vectors of the pRNAluc2-HIV series. (A) The complete HIV-1 frameshift region from plasmid pLuc/HIV/B (−1) (Baril et al. 2003) was amplified by PCR between the NsiI and Pfl23II restriction sites (bold) and inserted at the beginning of the coding sequence of luciferase (gray) in plasmid pRNAluc2 (Bélanger et al. 2002), generating pRNAluc2-HIV80 (−1). Position +1 corresponds to the first base of the translation initiation codon. The HIV-1 frameshift region begins at nt +19 and ends at nt +72. The slippery sequence is underlined. In the (−1) construct, the luciferase coding sequence is in the −1 reading frame relative to the initiator AUG codon, so that a −1 frameshift is required to produce luciferase. For the (0) construct, an adenine was added immediately after the slippery sequence, so that ribosomes produce luciferase through conventional translation. (B) Sequences of the HIV-1 frameshift region in all the constructs used in this study. Nucleotides that were substituted or deleted are, respectively, underlined or represented by dashed lines. The numbers in the names of the plasmids (35, 63, and 80) refer to the length of the HIV-1 frameshift region inserted at the beginning of the luciferase coding sequence. Plasmid pRNAluc2-HIV80-SS-k/o, altered slippery sequence; pRNAluc2-HIV80-US1, upper stem destabilization (the 3′ strand is identical to the 5′ strand); pRNAluc2-HIV80-US12, inversion of the strands of the upper stem, pRNAluc2-HIV80-LS1, lower stem destabilization (the 5′ strand is mutated); pRNAluc2-HIV80-LS2, lower stem destabilization (the 3′ strand is mutated); pRNAluc2-HIV-LS12, combination of the mutations in the lower stem (the 5′and 3′ strands are mutated simultaneously); pRNAluc2-HIV80-YB, substitution of the purine bulge with pyrimidines; pRNAluc2-HIV80-UAA, -UAG and -UGA, replacement of the codon located immediately 3′ of the slippery sequence with a stop codon.
FIGURE 3.
Frameshift efficiencies for insertions of different lengths of the HIV-1 frameshift region. The constructs are as described in the legend to Figure 2B. The frameshift efficiencies were calculated from luciferase activities in the bacterial lysates as described in the text. The frameshift efficiencies are the means of three independent experiments, with the bars representing the standard error of the means.
Effect of mutations in the upper and lower stems and in the bulge of the complete frameshift stimulatory signal of HIV-1
To further investigate the response of the bacterial ribosome to the structural features of the complete HIV-1 frameshift stimulatory signal, we introduced mutations that impaired the formation of the upper or the lower stem or that changed the sequence of the bulge (Fig. 4). Using pRNAluc2-HIV80, which contains the complete HIV-1 frameshift region, we analyzed the contribution of the upper stem to frameshift efficiency by destabilizing (pRNAluc2-HIV80-US1) or by re-forming this stem (pRNAluc2-HIV80-US12). The destabilization of the upper stem dramatically reduced the HIV-1 frameshift efficiency (0.6%), a value similar to that obtained with the construct containing only the slippery sequence (0.8%), whereas re-formation of the upper stem restored frameshift efficiency to near wild-type level (5.1%). These results underscore the importance of the upper stem for frameshift stimulation, as shown with a eukaryotic system (Parkin et al. 1992).
FIGURE 4.
Contribution to the frameshift efficiency of the upper stem, the lower stem, and the bulge of the HIV-1 complete frameshift stimulatory signal. (A) Schematic description of mutations (blue) of the HIV-1 frameshift region in pRNAluc2-HIV80 (−1) and (0); see description in Figure 2B. The slippery sequence is underlined. (B) Frameshift efficiencies calculated from luciferase activities in the bacterial lysates as described in the text, with the pRNAluc2-HIV80 derivatives described in A. Plasmid pRNAluc2-HIV80 (black) is set as a reference for comparison with the mutant constructs (gray). The frameshift efficiencies are the means of three independent experiments, with the bars representing the standard error of the means.
A similar set of mutations was performed in the lower stem, mutating either the 5′ strand (pRNAluc2-HIV80-LS1) or the 3′ strand (pRNAluc2-HIV80-LS2), or re-forming the stem by combining the complementary mutations in both strands (pRNAluc2-HIV80-LS12). Impairing the formation of the lower stem decreased the frameshift efficiency from 4.8 to about 3.0%, the value observed when only the classic stem–loop was present, whereas re-formation of the lower stem increased the frameshift efficiency to a near wild-type level (4.3%). This shows that the bacterial ribosome responds to the formation of the lower stem, the effect on frameshift efficiency being, however, small compared to the effect of the upper stem. A similar observation was made with a eukaryotic system. Finally, we analyzed the effect on frameshift efficiency of substituting the purine residues of the bulge with pyrimidines (pRNAluc2-HIV80-YB). With the substituted bulge, the frameshift efficiency was decreased to 3.3%, a value similar to that obtained with the classic HIV-1 frameshift region, in the absence of the lower stem (3.0%). Again, the same situation was observed with a eukaryotic system, when substituting the purine bulge.
Effect of a stop codon located immediately after the HIV-1 slippery sequence
Horsfield et al. (1995) observed that the presence of a stop codon immediately after the slippery sequence of HIV-1 decreased the frameshift efficiency 5- to 10-fold when the HIV-1 frameshift was investigated in bacteria. To account for this observation, they proposed that the frameshift occurs after peptide bond formation and translocation, when the two tRNAs that shift the reading frame occupy the P and E sites and the stop codon occupies the A site. The results of Horsfield et al. (1995) were obtained with a reporter gene in which the inserted frameshift region contained only the HIV-1 slippery sequence, without any stimulatory signal. We decided to investigate the effect of a stop codon located after the slippery sequence, either alone or followed by the complete frameshift stimulatory signal. The GGG codon that immediately follows the slippery sequence in the HIV-1 frameshift region was replaced with a nonsense codon (UAA, UAG, or UGA). Contrasting with Horsfield et al. (1995), we found that the presence of a stop codon after the slippery sequence did not decrease the frameshift efficiency, whether the frameshift stimulatory signal was present or not (Table 1). Moreover, we observed in our system that the frameshift efficiency was not affected by the presence of sublethal doses of spectinomycin, an inhibitor of translocation (data not shown). This also supports that the frameshift occurs before translocation; otherwise it would have been affected by spectinomycin. The reason for the discrepancy between our results and those of Horsfield et al. is not clear. It is worth mentioning here that a detailed study by Bertrand et al. (2002), who investigated the effect of the sequence following a variety of slippery sequences, also showed that the presence of a stop codon did not decrease the frameshift efficiency.
TABLE 1.
Effect on HIV-1 programmed −1 frameshift of a stop codon located immediately 3′ of the slippery sequence
| Frameshift efficiency (%) | ||||
| HIV constructs | Wild-type (GGG) | UAA | UAG | UGA |
| pRNAluc2-HIV35 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 |
| pRNAluc2-HIV80 | 4.8 ± 0.4 | 5.4 ± 0.6 | 5.4 ± 0.6 | 5.2 ± 0.5 |
Plasmid pRNAluc2-HIV35 contains only the slippery sequence and pRNAluc2-HIV80 contains the slippery sequence plus the complete frameshift stimulatory signal. The wild-type GGG codon that immediately follows the slippery sequence was substituted with a stop codon (UAA, UAG, or UGA). The frameshift efficiencies were calculated from the luciferase activities in the bacterial lysates as described in the text. The values are the means ± standard error of three independent experiments.
Effect of 16S rRNA mutations on HIV-1 programmed −1 ribosomal frameshift
To further investigate the mechanism underlying the HIV-1 programmed −1 frameshift, we examined how 16S rRNA mutations that either speed up or slow down the accommodation of an aa-tRNA in the A site affect this frameshift. A single mutation (A900U) and a double mutation (C899A/A900G) in the 900 tetraloop capping helix 27 of 16S rRNA (Bélanger et al. 2002) make the ribosomes error-prone, whereas the C912U mutation, located at the bottom of helix 27 (Leclerc et al. 1991) increases translational fidelity. From an analysis of the crystal structure of the 30S ribosomal subunit, Ogle et al. (2002, 2003) showed that a transition of the 30S subunit from an open to a closed conformation is required for the accommodation of the aminoacyl-tRNA in the A site, and they inferred from this analysis that mutations at the bottom of helix 27 that make the ribosomes hyperaccurate, such as the C912U mutation, disfavor this transition of the 30S subunit. From the same type of analysis, we concluded that the mutations in the 900 tetraloop capping helix 27 that make the ribosomes error-prone, such as the A900U and the C899A/A900G mutations, facilitate this transition (Bélanger et al. 2004). We introduced each of the mutations mentioned above (the C912U, the A900U, and the C899A/A900G mutations) into the 16S rRNA of different pRNAluc2-HIV constructs that contain the slippery sequence alone (pRNAluc2-HIV35), or with the classic frameshift stimulatory signal (pRNAluc2-HIV63), or with the complete frameshift stimulatory signal (pRNAluc2-HIV80). The effects of the 16S rRNA mutations on HIV-1 programmed −1 frameshift are presented in Table 2. The results show that with the two errorprone mutants, the frameshift efficiency was decreased in the presence of the complete frameshift stimulatory signal (from 4.8 to 3.8 and 2.3%) or in the presence of the classic frameshift stimulatory signal (from 3.0 to 2.3 and 1.9%). In the absence of a stimulatory signal, the effects are within experimental variations (from 0.8 to 0.7 and 0.6%). However, the C912U mutation, which makes the ribosomes hyperaccurate, did not change the frameshift efficiency, whether a frameshift stimulatory signal was present or not.
TABLE 2.
Effect of 16S rRNA mutations on HIV-1 programmed −1 frameshift
| Frameshift efficiency (%) | ||||
| HIV constructs | Wild-type 16S rRNA | A900U | C899A/A900G | C912U |
| pRNAluc2-HIV35 | 0.8 ± 0.1 | 0.7 ± 0.2 | 0.6 ± 0.1 | 0.9 ± 0.2 |
| pRNAluc2-HIV63 | 3.0 ± 0.4 | 2.3 ± 0.2 | 1.9 ± 0.2 | 3.3 ± 0.6 |
| pRNAluc2-HIV80 | 4.8 ± 0.4 | 3.8 ± 0.4 | 2.3 ± 0.3 | 5.0 ± 0.6 |
Plasmids pRNAluc2-HIV35, pRNAluc2-HIV63, and pRNAluc2-HIV80 contain, respectively, only the slippery sequence, the slippery sequence plus the classic stem-loop, and the slippery sequence plus the complete frameshift stimulatory signal. The A900U, C899A/A900G, and C912U mutations are located in 16S rRNA. The A900U and the C899A/A900G mutations make the ribosomes errorprone (Bélanger et al. 2002), whereas the C912U mutation makes the ribosomes hyperaccurate (Leclerc et al. 1991). The frameshift efficiencies were calculated from luciferase activities in bacterial lysates as described in the text. The values are the means ± standard error of three independent experiments.
DISCUSSION
In this study, we investigated the HIV-1 programmed −1 ribosomal frameshift with a bacterial system, using the complete HIV-1 frameshift region defined by Dulude et al. (2002). This region was inserted at the beginning of the coding sequence of a luciferase reporter gene, such that only the ribosomes that shift the reading frame produce luciferase. The frameshift region contains a slippery sequence and an extended bulged helix composed of two stems, located immediately downstream of the slippery sequence. As was the case with a eukaryotic system, we found with the bacterial system that the presence of the extended helix increased the frameshift efficiency, compared to the classic frameshift region. We also found that mutations that disrupt the formation of the upper or the lower stem of the frameshift stimulatory signal or substitute the purine bulge with pyrimidines decreased the frameshift efficiency, exactly as they do in a eukaryotic system. In both systems, disruption of the upper stem had a major effect, whereas a smaller effect was observed when disrupting the lower stem. This shows that the bacterial ribosome responds like the eukaryote ribosome to the HIV-1 frameshift stimulatory signal, which makes it an appropriate tool to further investigate the frameshift mechanism of HIV-1.
As mentioned in the Introduction, it is currently assumed that the change in the reading frame in a programmed −1 frameshift occurs with a ribosome bearing a p-tRNA in the P site and an aa-tRNA in the A site, prior to peptide bond formation. A refinement of this model suggests that the aa-tRNA is in the A/T site and not in the A site, which would give more time for the shift to take place. Horsfield et al. (1995) had proposed that the shift in the reading frame occurs with two tRNAs that occupy the E and P sites of the ribosome, based on their observations that a stop codon introduced immediately after the HIV-1 slippery sequence dramatically decreased frameshifting. Our results contradict their observations in showing that the HIV-1 frameshift efficiency was not decreased by the presence of a stop codon after the slippery sequence in constructs containing the slippery sequence, either alone or in the presence of the complete frameshift stimulatory signal. As to whether the shift occurs when the aa-tRNA is in the A or in the A/T site, we reasoned that if the aa-tRNA is in the A/T site, mutations that speed up the occupancy of the A site should decrease the frameshift efficiency. Conversely, mutations that slow down the accommodation step should increase the frame-shift efficiency. The A900U and the C899A/A900G mutations in the 16S rRNA, which make the ribosomes error-prone, facilitate a transition of the 30S subunit to a closed conformation that promotes the accommodation of the aa-tRNA in the A site. These mutations were found to decrease the frameshift efficiency, which provides an experimental support to the suggestion that frameshifting occurs when the aa-tRNA is in the A/T site. However, the C912U mutation, which makes the ribosomes hyperaccurate, did not change the frameshift efficiency, although it disfavors the transition of the 30S subunit to the closed conformation, thus slowing down the accommodation of the aa-tRNA in the A site. In that case, as explained below, the increased control of translational accuracy could counteract frame-shifting.
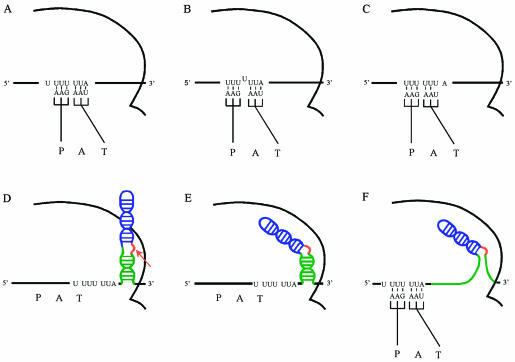
Although the description of programmed −1 frameshifts traditionally considers that the two tRNAs move along with the ribosome relative to the mRNA, it is the mRNA that likely slides relative to the two ribosome-bound tRNAs. Indeed, the two tRNAs have several points of contact with the ribosome in addition to their interaction with the mRNA (Yusupov et al. 2001; Stark et al. 2002; Valle et al. 2003), and these contacts contribute to maintain them in their position on the ribosome, even when the interaction between their anticodons and the codons of the mRNA is disrupted. Recently, Baranov et al. (2004) proposed that there is no simultaneous slippage of the two tRNAs in programmed −1 frameshifts, as this would have required a coordination of the dissociation of these tRNAs from the mRNA, which does not appear to be supported by contemporary structural data. Baranov et al. proposed that repositioning of the p-tRNA in the −1 frame precedes the slippage of the aa-tRNA and that the efficiency of frameshifting depends on the difference in the stability of the codon–anticodon interaction in the new frame versus the initial frame. Adapting the model of Baranov et al. and taking into account the fact that it is the mRNA that slides relative to the ribosome and the tRNAs and that the aa-tRNA is in the A/T site when the frameshift occurs, we describe the programmed −1 frameshift directed by the HIV-1 frameshift region as follows (Fig. 5A–C): Codon–anticodon interactions at the A and P sites are dynamic, and they break and re-form independently. Disruption of the codon–anticodon interaction at the P site allows a segment of the mRNA in the P site to move toward the 3′ direction, and the HIV-1 slippery sequence is such that the p-tRNA can re-pair in the −1 frame. The displacement of the mRNA leaves an unpaired base between the A-site and P-site codons, a situation that is incompatible with the geometry of the decoding center sensing the codon–anticodon interaction in the A site for the incoming aa-tRNA in the A/T site (Ogle et al. 2001; Ramakrishnan 2002). To remedy this situation, one possibility is a subsequent movement of the segment of mRNA in the A site after disruption of the codon–anticodon interaction, so that the aa-tRNA can re-pair in the −1 frame. If this occurs, both tRNAs contribute to maintain the mRNA in the new frame and translation continues in this −1 frame. If, however, the subsequent movement of the mRNA segment in the A site does not occur rapidly enough, the aa-tRNA is expected to be rejected, which decreases the chance of frameshifting because the segment of mRNA in the P site can move back to its initial position, with re-pairing of the p-tRNA in the initial frame. Alternatively, if this aa-tRNA is rejected, another aa-tRNA can pair to the mRNA in the −1 frame before the segment of mRNA in the P site moves back to the initial frame. The latter situation, in which the incoming aa-tRNA binds in the −1 frame after a shift of mRNA segment relative to the p-tRNA, would correspond to a mechanism proposed by Yelverton et al. (1994) to account in part for frameshifting, as indicated in the Introduction. As underscored by Baranov et al. (2004), it is the disruption of the codon–anticodon interaction at the P site that triggers the frameshift. Disruption of the codon–anti-codon interaction at the A site cannot be followed by a shift of the mRNA in the −1 frame when the adjacent upstream triplet in the P site is paired to the p-tRNA. With HIV-1 slippery sequence, there is no difference in the stability of the codon–anticodon interaction in the P site in the initial and in the −1 frames, and the presence of an aa-tRNA in the −1 frame is essential to maintain the new frame.
FIGURE 5.
Description of the movement of the mRNA resulting in the programmed −1 frameshift (A,B,C) and hypothetical model for the interaction between the HIV-1 frameshift stimulatory signal and the ribosome (D,E,F). The P, A, and T sites and the HIV-1 slippery sequence are indicated. In A–C, the frameshift stimulatory signal is not represented. (A) The slippery sequence of the mRNA is in the decoding center. The p-tRNA and the aa-tRNA occupy, respectively, the P and A/T site. (B) Disruption of the codon–anticodon interaction in the P site is followed by a realignment of the mRNA and re-pairing of the p-tRNA in the −1 frame, leaving an unpaired base in the mRNA between the P-site and the A-site codons. (C) Disruption of the codon–anticodon interaction in the A site is followed by a displacement of the mRNA and re-pairing of the aa-tRNA in the −1 frame. In D–F, the stimulatory signal is represented in color: upper stem–loop, blue; bulge, red, and lower stem, green. The p-tRNA and the aa-tRNA are indicated only in F. (D) Encounter between the ribosome and the HIV-1 frameshift stimulatory signal, before the slippery sequence reaches the P and A sites. The bulge of the frameshift stimulatory signal, which is pointed out by a red arrow, anchors to the ribosome. (E) This anchoring facilitates a specific interaction between the upper stem–loop and the ribosome. (F) The ribosome unwinds the lower stem and progresses along the mRNA, until the slippery sequence reaches the P and A sites. The frameshift occurs as described in A–C.
However, because an aa-tRNA is submitted to the control of the pairing of its anticodon with the codon of the mRNA before being accommodated in the A site, this raises one argument against a shift prior to this accommodation. Indeed, under these conditions, the realignment of the mRNA segment in the A site should trigger rejection of the aa-tRNA because the third base pair formed between the codon of the HIV-1 slippery sequence and the anticodon of this aa-tRNA is now a mismatch (U-U). Again, this rejection would decrease the chance of frameshifting by making it possible for the mRNA to come back to its initial position. One appealing hypothesis to reconcile the occurrence of slippage before the accommodation of the aa-tRNA in the A site and the antagonistic control of translational accuracy is that the frameshift stimulatory signal induces a structural perturbation in the ribosome that specifically reduces this control. Error-prone ribosomes are less likely to reject incorrect tRNAs, but the aa-tRNA reaches rapidly the A site, limiting the time available for the shift to occur, and the frameshift efficiency can be expected to decrease, which was observed experimentally. In contrast, with hyperaccurate ribosomes, the aa-tRNA spends more time in the A/T site, but the control of accuracy is more stringent, increasing the probability of rejection of an incorrect aa-tRNA and thus counteracting the frameshift. The frameshift efficiency was not changed with the hyperaccurate ribosomes that we investigated. However, interestingly, it has been observed that the efficiency of a programmed −1 frameshift was increased with yeast eEF1A (the eukaryotic homolog of factor EF-Tu) mutants, that were altered such that the residence time of the complex between the aa-tRNA and this factor in the A/T site of the ribosome was increased (Dinman and Kinzy 1997; Harger et al. 2002). Another model for programmed −1 frameshifts was proposed by Plant et al. (2003), in which the −1 frameshift occurs concurrently with the movement of the aa-tRNA from the A/T to the A site. During this movement, the anticodon of the tRNA moves by 9 Å and pulls along the mRNA in the 5′ direction (Noller et al. 2002). The presence of frameshift stimulatory signals that resist to unwinding would interfere with the movement of the mRNA and create a tension, which can be relieved by a displacement of the mRNA in the 3′ direction, requiring disruption of the codon–anticodon interactions in the A and P sites and re-pairing in the −1 frame. However, if this model were correct, frameshifting would depend on the rate of transition of the aa-tRNA from the A/T to the A site but be independent of the residence time of the complex between the elongation factor and the aa-tRNA on the ribosome, which does not appear to be the case.
How the frameshift stimulatory signal interacts with the ribosome and exerts its role is still unknown. We previously suggested that the bulged helix acting as the frameshift stimulatory signal of HIV-1 could be engulfed without melting or re-form inside the ribosome and, subsequently, interact with the ribosome so as to promote the frameshift when the slippery sequence occupies the A and P sites (Dulude et al. 2002). The narrow dimensions of the channel through which the mRNA passes, as defined by X-ray crystallographic studies of the bacterial ribosome complexed with a short mRNA (Yusupova et al. 2001), do not favor this possibility, unless we assume that the channel is transiently distorted and enlarged. An alternative view (Fig. 5D–F) is that a first contact occurs when the frameshift stimulatory signal encounters the surface of the ribosome, before the slippery sequence occupies the A and P sites. This encounter can lead to a specific interaction between the upper stem–loop and the ribosome. We propose that the purine bulge of the signal favors this interaction by anchoring to a specific position, increasing the likelihood that the upper stem–loop finds its site of interaction on the ribosome. The ribosome subsequently unwinds the lower stem of the frame-shift stimulatory signal while progressing along the mRNA until the slippery sequence occupies the A and P sites. For most ribosomes, translation of this sequence proceeds according to standard rules, but for a minority of ribosomes, there is a relative movement of the mRNA such that the p-tRNA in the P site and the aa-tRNA in the A/T site re-pair in a −1 frame, resulting in a mismatch for this aa-tRNA. The interaction between the ribosome and the upper stem–loop would decrease the probability of rejection of the mismatched aa-tRNA, preventing the return of the mRNA to the initial reading frame, and the aa-tRNA could be accommodated in the A site. This step would be followed by peptide bond formation and translation would resume in the −1 reading frame according to conventional rules.
Modeling studies of the HIV-1 frameshift stimulatory signal on the structure of the bacterial ribosome characterized by X-ray studies (Yusupov et al. 2001) could identify potential sites of interaction between the frameshift stimulatory signal and the ribosome. Because the bacterial ribosome responds like the eukaryote ribosome to this signal, it constitutes a convenient tool to investigate these sites, using not only the expanding knowledge of its structure, but also the wealth of bacterial genetics. The characterization of the interaction between the HIV-1 frameshift stimulatory signal and the ribosome will provide useful information for the development of agents that interfere with this interaction.
MATERIALS AND METHODS
Reagents
Vent DNA Polymerase was from New England Biolabs. Restriction enzymes were from Fermentas and Amersham Biosciences. T4 DNA ligase, calf intestine alkaline phosphatase, and bacteriophage T4 polynucleotide kinase were from Fermentas. DNA sequencing reactions were carried out with the T7 sequenase v2.0 and the T7 Sequencing kit from Amersham Biosciences. Primers were purchased from Biocorp Inc. and are identified as follows: primer 1: 5′-GCTACCATGCATGGTCGAAGCGC-3′, primer 2: 5′-GCAACCAACCGAACGGACATTTCG-3′, primer 3: 5′-CCGCAAATGCATGGTCGAAGCGCTAATTTTTTAAGGGAAGATCTGG-3′, primer4: 5′-GCCAACCGAACGGACATTTCGAAGTATTCCGCGTACG TG-3′, primer 5: 5′-CCGCTTGTGCGGGCCCCCGTCAATTCATT TAAGTTTTAACCTTGCGGCC-3′ and primer 6: 5′-GGCACAACA ACTGGCGGGCAAACAGTCG-3′.
Plasmids
All plasmids were maintained and expressed in E. coli DH5α. Plasmid pRNAluc2, in which the HIV-1 frameshift region was inserted, contains a copy of the E. coli rrnB operon, under control of the lacUV5 promoter and a reporter gene coding for the firefly luciferase. The ribosomal binding site of the luciferase messenger and the messenger binding site of the 16S rRNA have been mutated, respectively, to 5′-AUCCC and to 5′-GGGAU, such that the luciferase reporter mRNA is exclusively translated by ribosomes containing the plasmid-encoded 16S rRNA. An NsiI restriction site has been created between the initiator AUG codon and the second codon of the luciferase coding sequence in pRNAluc2 (Bélanger et al. 2002). The complete HIV-1 frameshift region plus a portion of the luciferase gene, flanked by an NsiI and a Pfl23II restriction site, was amplified from plasmid pLuc/HIV/B (−1) (Baril et al. 2003), using primers 1 and 2 for the forward and reverse reactions, respectively. The resulting PCR fragment was inserted into pRNAluc2, generating pRNAluc2-HIV80 (−1) (see Fig. 2A), in which the insertion is such that the luciferase coding sequence is in the −1 reading-frame relative to the initiator AUG codon. To measure the HIV-1 frameshift efficiency, a control construct, pRNAluc2-HIV80 (0), was derived from pRNAluc2-HIV80 (−1), by adding an adenine in the frameshift region immediately downstream of the slippery sequence, such that the luciferase coding sequence is in-frame with the initiator AUG codon. This was performed by PCR, using primers 3 and 4, respectively, for the forward and reverse reactions. Derivatives of pRNAluc2-HIV80 (−1) and (0) were as follows: deletion of the sequence downstream from the upper stem (pRNAluc2-HIV63), deletion of the complete frameshift stimulatory signal (pRNAluc2-HIV35), mutations in the slippery sequence (pRNAluc2-HIV80-SS-k/o), mutations in the lower stem (pRNAluc2-HIV80-LS1), and mutations in the bulge (pRNAluc2-HIV80-YB). These derivatives were created by PCR, by amplifying mutated DNA fragments from pRNAluc2-HIV80 (−1) and (0) and subcloning the different PCR fragments between the NsiI and Pfl23II restriction sites in pRNAluc2. Additional derivatives of pRNAluc2-HIV80 (−1) and (0) contained mutations in the upper stem (pRNAluc2-HIV80-US1 and pRNAluc2-HIV80-US12) or in the lower stem (pRNAluc2-HIV80-LS2 and pRNAluc2-HIV80-LS12). They were created by PCR with four primers (Ho et al. 1989), by amplifying mutated DNA fragments from pRNAluc2-HIV80 (−1) and (0), and sub-cloning the different PCR fragments into pRNAluc2, as described above. To investigate the effects of mutations in 16S rRNA on frameshifting, the C912U mutation was introduced between the two ApaI–ApaI restriction sites in pRNAluc2-HIV80 (−1) and (0), in pRNAluc2-HIV63 (−1) and (0), and in pRNAluc2-HIV35 (−1) and (0) by PCR, by amplifying a mutated DNA fragment from pRNAluc2. The PCR was performed with, respectively, primers 5 and 6 for the forward and the reverse reactions. An ApaI–ApaI fragment containing the A900U mutation or the C899A/A900G mutation of the 900 tetraloop capping helix 27 from 16S rRNA was obtained from plasmids pRNAluc2-GCUA and pRNAluc2-GAGA (Bélanger et al. 2002), respectively, and was introduced in the same plasmids as for the assays with the C912U mutation. Derivatives of pRNAluc2-HIV-80 (−1) and pRNAluc2-HIV35 (−1), in which the GGG codon following the slippery sequence was replaced with UAA, UAG, or UGA, were obtained by a standard PCR approach. For these constructs, frameshift efficiencies were calculated, using as a (0) construct pRNAluc2-HIV80 (0) and pRNAluc2-HIV35 (0).
Luciferase assays
Overnight cultures of E. coli DH5α transformed with the appropriate plasmids were grown in LB medium containing 100 μg/mL of ampicillin (LB-Ap100) at 37°C. The cultures were diluted to an absorbance of 0.1 at 600 nm in 1 mL of LB-Ap100 and incubated for 1 h at 37°C. The expression of plasmid-encoded rRNA was induced by addition of IPTG to a final concentration of 1 mM and the cultures were incubated for another 3 h at 37°C. A sample of 45 μL was assayed for luciferase activity as described in Bélanger et al. (2002). Luminescence was measured for 10 sec using an EG&G Berthold Lumat LB 9507 luminometer. The values were normalized for an equal number of cells.
Acknowledgments
This study was supported by a grant from the Canadian Institutes for Health Research to Léa Brakier-Gingras. We are grateful to François Bélanger, Dominic Dulude, Pascale Legault, Stephen Michnick, and Sergey Steinberg for critical reading of the manuscript, and to Martin Baril and Matthieu Gagnon for stimulating discussions. We thank Phil Cunningham for advice and comments for the use of the specialized ribosome system.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
aa-tRNA, aminoacyl-tRNA
EF-Tu, elongation factor Tu
HIV-1, human immunodeficiency virus type 1
IPTG, isopropyl-β-d-thiogalactopyranoside
p-tRNA, peptidyl-tRNA
rRNA, ribosomal RNA
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.7670704.
REFERENCES
- Atkins, J.F., Baranov, P.V., Fayet, O., Herr, A.J., Howard, M.T., Ivanov, I.P., Matsufuji, S., Miller, W.A., Moore, B., Prère, M.F., et al. 2001. Overriding standard decoding: Implications of recoding for ribosome function and enrichment of gene expression. Cold Spring Harbor Symp. Quant. Biol. 66: 217–232. [DOI] [PubMed] [Google Scholar]
- Ban, N., Nissen, P., Hansen, J., Moore, P.B., and Steitz, T.A. 2000. The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science 289: 905–920. [DOI] [PubMed] [Google Scholar]
- Baranov, P.V., Gesteland, R.F., and Atkins, J.F. 2002. Recoding: Translational bifurcations in gene expression. Gene 286: 187–201. [DOI] [PubMed] [Google Scholar]
- ———. 2004. P-site tRNA is a crucial initiator of ribosomal frame-shifting. RNA 10: 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baril, M., Dulude, D., Gendron, K., Lemay, G., and Brakier-Gingras, L. 2003. Efficiency of a programmed −1 ribosomal frameshift in the different subtypes of the human immunodeficiency virus type 1 group M. RNA 9: 1246–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger, F., Léger, M., Saraiya, A.A., Cunningham, P.R., and Brakier-Gingras, L. 2002. Functional studies of the 900 tetraloop capping helix 27 of 16S ribosomal RNA. J. Mol. Biol. 320: 979–989. [DOI] [PubMed] [Google Scholar]
- Bélanger, F., Gagnon, M.G., Steinberg, S.V., Cunningham, P.R., and Brakier-Gingras, L. 2004. Study of the functional interaction of the 900 tetraloop of 16S ribosomal RNA with helix 24 within the bacterial ribosome. J. Mol. Biol. 338: 683–693. [DOI] [PubMed] [Google Scholar]
- Bertrand, C., Prère, M.F., Gesteland, R.F., Atkins, J.F., and Fayet, O. 2002. Influence of the stacking potential of the base 3′ of tandem shift codons on −1 ribosomal frameshifting used for gene expression. RNA 8: 16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakier-Gingras, L., Bélanger, F., and O’Connor, M. 2003. Probing the role of ribosomal RNA in protein synthesis through mutagenesis. In Translation mechanisms (eds. J. Lapointe and L. Brakier-Gingras), pp. 247–263. Landes Bioscience, Georgetown, TX, and Kluwer Academic/Plenum Publishers, New York.
- Brierley, I. and Pennell, S. 2001. Structure and function of the stimulatory RNAs involved in programmed eukaryotic −1 ribosomal frameshifting. Cold Spring Harbor Symp. Quant. Biol. 66: 233–248. [DOI] [PubMed] [Google Scholar]
- Brierley, I., Jenner, A.J., and Inglis, S.C. 1992. Mutational analysis of the “slippery-sequence” component of a coronavirus ribosomal frameshifting signal. J. Mol. Biol. 227: 463–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunelle, M.N., Payant, C., Lemay, G., and Brakier-Gingras, L. 1999. Expression of the human immunodeficiency virus frameshift signal in a bacterial cell-free system: Influence of an interaction between the ribosome and a stem-loop structure downstream from the slippery site. Nucleic Acids Res. 27: 4783–4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassan, M., Delaunay, N., Vaquero, C., and Rousset, J.P. 1994. Translational frameshifting at the gagpol junction of human immunodeficiency virus type 1 is not increased in infected T-lymphoid cells. J. Virol. 68: 1501–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinman, J.D. and Kinzy, T.G. 1997. Translational misreading: Mutations in translation elongation factor 1α differentially affect programmed ribosomal frameshifting and drug sensitivity. RNA 3: 870–881. [PMC free article] [PubMed] [Google Scholar]
- Dinman, J.D., Icho, T., and Wickner, R.B. 1991. A −1 ribosomal frameshift in a double-stranded RNA virus of yeast forms a gagpol fusion protein. Proc. Natl. Acad. Sci. 88: 174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinman, J.D., Ruiz-Echevarria, M.J., Czaplinski, K., and Peltz, S.W. 1997. Peptidyl-transferase inhibitors have antiviral properties by altering programmed −1 ribosomal frameshifting efficiencies: Development of model systems. Proc. Natl. Acad. Sci. 94: 6606–6611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulude, D., Baril, M., and Brakier-Gingras, L. 2002. Characterization of the frameshift stimulatory signal controlling a programmed −1 ribosomal frameshift in the human immunodeficiency virus type 1. Nucleic Acids Res. 30: 5094–5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh, P.J. 1997a. Programmed −1 frameshift sites in prokaryotes. In Programmed alternative reading of the genetic code, pp. 103–134. Landes Bioscience, Austin, Texas and Springer-Verlag, Heidelberg, Germany.
- ———. 1997b. Programmed −1 frameshifting in eukaryotes. In Programmed alternative reading of the genetic code, pp. 69–102. Landes Bioscience, Austin, TX, and Springer-Verlag, Heidelberg, Germany.
- Gromadski, K.B. and Rodnina, M.V. 2004. Kinetic determinants of high-fidelity tRNA discrimination on the ribosome. Mol. Cell 13: 191–200. [DOI] [PubMed] [Google Scholar]
- Harger, J.W., Meskauskas, A., and Dinman, J.D. 2002. An “integrated model” of programmed ribosomal frameshifting. Trends Biochem. Sci. 27: 448–454. [DOI] [PubMed] [Google Scholar]
- Ho, S.N., Hunt, H.D., Horton, R.M., Pullen, J.K., and Pease, L.R. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77: 51–59. [DOI] [PubMed] [Google Scholar]
- Horsfield, J.A., Wilson, D.N., Mannering, S.A., Adamski, F.M., and Tate, W.P. 1995. Prokaryotic ribosomes recode the HIV-1 gagpol −1 frameshift sequence by an E/P site post-translocation simultaneous slippage mechanism. Nucleic Acids Res. 23: 1487–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacks, T., Power, M.D., Masiarz, F.R., Luciw, P.A., Barr, P.J., and Varmus, H.E. 1988a. Characterization of ribosomal frameshifting in HIV-1 gagpol expression. Nature 331: 280–283. [DOI] [PubMed] [Google Scholar]
- Jacks, T., Madhani, H.D., Masiarz, F.R., and Varmus, H.E. 1988b. Signals for ribosomal frameshifting in the Rous sarcoma virus gagpol region. Cell 55: 447–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, H. 1998. Direct structural evidence for formation of a stem-loop structure involved in ribosomal frameshifting in human immunodeficiency virus type 1. Biochim. Biophys. Acta 1397: 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontos, H., Napthine, S., and Brierley, I. 2001. Ribosomal pausing at a frameshifter RNA pseudoknot is sensitive to reading phase but shows little correlation with frameshift efficiency. Mol. Cell. Biol. 21: 8657–8670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc, D., Melançon, P., and Brakier-Gingras, L. 1991. Mutations in the 915 region of Escherichia coli 16S ribosomal RNA reduce the binding of streptomycin to the ribosome. Nucleic Acids Res. 19: 3973–3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K., Varma, S., SantaLucia Jr., J., and Cunningham, P.R. 1997. In vivo determination of RNA structure-function relationships: analysis of the 790 loop in ribosomal RNA. J. Mol. Biol. 269: 732–743. [DOI] [PubMed] [Google Scholar]
- Lopinski, J.D., Dinman, J.D., and Bruenn, J.A. 2000. Kinetics of ribosomal pausing during programmed −1 translational frameshifting. Mol. Cell. Biol. 20: 1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meskauskas, A., Harger, J.W., Jacobs, K.L., and Dinman, J.D. 2003. Decreased peptidyltransferase activity correlates with increased programmed −1 ribosomal frameshifting and viral maintenance defects in the yeast Saccharomyces cerevisiae. RNA 9: 982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morosyuk, S.V., Lee, K., SantaLucia Jr., J., and Cunningham, P.R. 2000. Structure and function of the conserved 690 hairpin in Escherichia coli 16S ribosomal RNA: Analysis of the stem nucleotides. J. Mol. Biol. 300: 113–126. [DOI] [PubMed] [Google Scholar]
- Morosyuk, S.V., SantaLucia Jr., J., and Cunningham, P.R. 2001. Structure and function of the conserved 690 hairpin in Escherichia coli 16S ribosomal RNA. III. Functional analysis of the 690 loop. J. Mol. Biol. 307: 213–228. [DOI] [PubMed] [Google Scholar]
- Napthine, S., Vidakovic, M., Girnary, R., Namy, O., and Brierley, I. 2003. Prokaryotic-style frameshifting in a plant translation system: Conservation of an unusual single-tRNA slippage event. EMBO J. 22: 3941–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noller, H.F., Yusupov, M.M., Yusupova, G.Z., Baucom, A., and Cate, J.H. 2002. Translocation of tRNA during protein synthesis. FEBS Lett. 514: 11–16. [DOI] [PubMed] [Google Scholar]
- Ogle, J.M., Brodersen, D.E., Clemons Jr., W.M., Tarry, M.J., Carter, A.P., and Ramakrishnan, V. 2001. Recognition of cognate transfer RNA by the 30S ribosomal subunit. Science 292: 897–902. [DOI] [PubMed] [Google Scholar]
- Ogle, J.M., Murphy, F.V., Tarry, M.J., and Ramakrishnan, V. 2002. Selection of tRNA by the ribosome requires a transition from an open to a closed form. Cell 111: 721–732. [DOI] [PubMed] [Google Scholar]
- Ogle, J.M., Carter, A.P., and Ramakrishnan, V. 2003. Insights into the decoding mechanism from recent ribosome structures. Trends Biochem. Sci. 28: 259–266. [DOI] [PubMed] [Google Scholar]
- Pape, T., Wintermeyer, W., and Rodnina, M.V. 1998. Complete kinetic mechanism of elongation factor Tudependent binding of aminoacyl-tRNA to the A site of the E. coli ribosome. EMBO J. 17: 7490–7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin, N.T., Chamorro, M., and Varmus, H.E. 1992. Human immunodeficiency virus type 1 gagpol frameshifting is dependent on downstream mRNA secondary structure: Demonstration by expression in vivo. J. Virol. 66: 5147–5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant, E.P., Jacobs, K.L., Harger, J.W., Meskauskas, A., Jacobs, J.L., Baxter, J.L., Petrov, A.N., and Dinman, J.D. 2003. The 9-Å solution: How mRNA pseudoknots promote efficient programmed −1 ribosomal frameshifting. RNA 9: 168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishnan, V. 2002. Ribosome structure and the mechanism of translation. Cell 108: 557–572. [DOI] [PubMed] [Google Scholar]
- Reil, H., Kollmus, H., Weidle, U.H., and Hauser, H. 1993. A hepta-nucleotide sequence mediates ribosomal frameshifting in mammalian cells. J. Virol. 67: 5579–5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodnina, M.V. and Wintermeyer, W. 2001. Fidelity of aminoacyl-tRNA selection on the ribosome: Kinetic and structural mechanisms. Annu. Rev. Biochem. 70: 415–435. [DOI] [PubMed] [Google Scholar]
- Schluenzen, F., Tocilj, A., Zarivach, R., Harms, J., Gluehmann, M., Janell, D., Bashan, A., Bartels, H., Agmon, I., Franceschi, F., et al. 2000. Structure of functionally activated small ribosomal subunit at 3.3 Å resolution. Cell 102: 615–623. [DOI] [PubMed] [Google Scholar]
- Somogyi, P., Jenner, A.J., Brierley, I., and Inglis, S.C. 1993. Ribosomal pausing during translation of an RNA pseudoknot. Mol. Cell. Biol. 13: 6931–6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark, H., Rodnina, M.V., Wieden, H.J., Zemlin, F., Wintermeyer, W., and van Heel, M. 2002. Ribosome interactions of aminoacyl-tRNA and elongation factor Tu in the codonrecognition complex. Nat. Struct. Biol. 9: 849–854. [DOI] [PubMed] [Google Scholar]
- Tu, C., Tzeng, T.H., and Bruenn, J.A. 1992. Ribosomal movement impeded at a pseudoknot required for frameshifting. Proc. Natl. Acad. Sci. 89: 8636–8640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumer, N.E., Parikh, B.A., Li, P., and Dinman, J.D. 1998. The pokeweed antiviral protein specifically inhibits Ty1-directed +1 ribosomal frameshifting and retrotransposition in Saccharomyces cerevisiae. J. Virol. 72: 1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle, M., Zavialov, A., Li, W., Stagg, S.M., Sengupta, J., Nielsen, R.C., Nissen, P., Harvey, S.C., Ehrenberg, M., and Frank, J. 2003. Incorporation of aminoacyl-tRNA into the ribosome as seen by cryo-electron microscopy. Nat. Struct. Biol. 10: 899–906. [DOI] [PubMed] [Google Scholar]
- Weiss, R.B., Dunn, D.M., Shuh, M., Atkins, J.F., and Gesteland, R.F. 1989. E. coli ribosomes re-phase on retroviral frameshift signals at rates ranging from 2 to 50 percent. New Biol. 1: 159–169. [PubMed] [Google Scholar]
- Wimberly, B.T., Brodersen, D.E., Clemons Jr., W.M., Morgan-Warren, R.J., Carter, A.P., Vonrhein, C., Hartsch, T., and Ramakrishnan, V. 2000. Structure of the 30S ribosomal subunit. Nature 407: 327–339. [DOI] [PubMed] [Google Scholar]
- Yelverton, E., Lindsley, D., Yamauchi, P., and Gallant, J.A. 1994. The function of a ribosomal frameshifting signal from human immunodeficiency virus −1 in Escherichia coli. Mol. Microbiol. 11: 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusupov, M.M., Yusupova, G.Z., Baucom, A., Lieberman, K., Earnest, T.N., Cate, J.H., and Noller, H.F. 2001. Crystal structure of the ribosome at 5.5 Å resolution. Science 292: 883–896. [DOI] [PubMed] [Google Scholar]
- Yusupova, G.Z., Yusupov, M.M., Cate, J.H., and Noller, H.F. 2001. The path of messenger RNA through the ribosome. Cell 106: 233–241. [DOI] [PubMed] [Google Scholar]