Abstract
In Salmonella enterica serovar Typhimurium five of the eight family codon boxes are decoded by a tRNA having the modified nucleoside uridine-5-oxyacetic acid (cmo5U) as a wobble nucleoside present in position 34 of the tRNA. In the proline family codon box, one (tRNAProcmo5UGG) of the three tRNAs that reads the four proline codons has cmo5U34. According to theoretical predictions and several results obtained in vitro, cmo5U34 should base pair with A, G, and U in the third position of the codon but not with C. To analyze the function of cmo5U34 in tRNAProcmo5UGG in vivo, we first identified two genes (cmoA and cmoB) involved in the synthesis of cmo5U34. The null mutation cmoB2 results in tRNA having 5-hydroxyuridine (ho5U34) instead of cmo5U34, whereas the null mutation cmoA1 results in the accumulation of 5-methoxyuridine (mo5U34) and ho5U34 in tRNA. The results suggest that the synthesis of cmo5U34 occurs as follows: U34 →? ho5U →CmoB mo5U →CmoA? cmo5U. We introduced the cmoA1 or the cmoB2 null mutations into a strain that only had tRNAProcmo5UGG and thus lacked the other two proline-specific tRNAs normally present in the cell. From analysis of growth rates of various strains and of the frequency of +1 frameshifting at a CCC-U site we conclude: (1) unexpectedly, tRNAProcmo5UGG is able to read all four proline codons; (2) the presence of ho5U34 instead of cmo5U34 in this tRNA reduces the efficiency with which it reads all four codons; and (3) the fully modified nucleoside is especially important for reading proline codons ending with U or C.
Keywords: tRNA, modified nucleoside, wobble, family codon box, uridine-5-oxyacetic acid, synthesis
INTRODUCTION
When the ribosome translates a genetic message, correct aminoacyl-tRNAs are selected one at a time through recognition of the anticodon that fit the A-site triplet in mRNA. The genetic code is composed of 64 triplets, of which three are recognized as stop codons. This leaves 61 codons as sense codons, all of which represents an amino acid in the final protein (Fig. 1A ▶). Triplets with the same first two letters represent a codon box, resulting in 16 codon boxes. All four codons in eight of the codon boxes code for a single amino acid; these boxes are denoted family codon boxes (fourfold degenerate codon box). The remaining eight codon boxes are denoted mixed codon boxes (twofold degenerate codon box). Because each codon does not have a complementary tRNA, this requires that one tRNA be able to read more than one codon triplet that differs only in the third letter of the triplet as explained by the wobble hypothesis (Crick 1966). According to this hypothesis, uridine present in the first position (denoted position 34) of the anticodon (U34), base pairs with adenosine (A) and guanosine (G) but not with uridine (U) or cytidine (C), because the latter base pairs would be too short. However, not long after the wobble hypothesis was proposed, Nishimura and colleagues discovered that some tRNAs with modified nucleosides in the wobble position have decoding capacities that are more extensive than the wobble hypothesis would allow for (for review, see Nishimura 1979). One of these modified nucleosides is uridine-5-oxyacetic acid (cmo5U34, earlier called V nucleoside), which base pairs not only with A and G as unmodified U would do as predicted by the wobble hypothesis, but also with U. In Escherichia coli and in Salmonella enterica serovar Typhimurium, tRNAs with genetically encoded U34 reading codons in the family boxes specific for valine, alanine, threonine, proline, and serine have cmo5U34 or, at least in the alanine- and serine-specific tRNAs (Pope et al. 1978), its methylester mcmo5U34 as a wobble nucleoside. The extended wobble capacity is caused by the ability of the cmo5-side chain to influence the puckering equilibrium of the ribose ring in such a way that the majority of the cmo5U molecules are in the C2′-endo conformation, whereas the conformation of the unmodified U is equally distributed between the C2′- and C3′-endo conformations. cmo5U34 in the C2′-endo conformation base pairs not only with A and G, but also with U, thus explaining the codon binding capacities of a cmo5U34-containing tRNA (Yokoyama et al. 1985; Agris et al. 1992).
FIGURE 1.
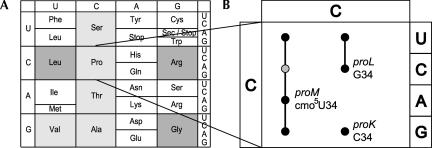
(A) The genetic code. The eight codon boxes with shaded background are the family codon boxes, containing four codons representing one amino acid (fourfold degenerate). The five family codon boxes in lighter shade contain tRNA having cmo5U as a wobble nucleoside. The codon boxes with white background are the mixed codon boxes. (B) The proline family codon box (CCN). proK, L, and M denote the genes encoding tRNAProCGG, tRNAProGGG and tRNAProcmo5UGG, respectively, and the wobble nucleosides, which are present in position 34, are indicated. A circle corresponds to a codon read by a tRNA and a line connecting two or more circles indicates that the same tRNA is able to read those codons (e.g., the proL tRNA contains G34 and reads the CCU and CCC codons). The gray circle for tRNAProcmo5UGG (codon CCC) indicates that this tRNA reads the CCC codon (results presented in this article), and the black circles show the codon reading abilities predicted by the wobble hypothesis and the revised wobble rules.
According to the revised wobble rules, cmo5U34, which is present only in tRNAs reading family codon boxes, should read A, G, and U, but not C. Triplet-dependent binding and in vitro protein synthesis have shown that this is indeed the case (Oda et al. 1969; Ishikura et al. 1971; Mitra et al. 1979; Samuelsson et al. 1980). The presence of cmo5U34 in the anticodon stem and loop construct (ASL) of tRNAValcmo5UAC enables this ASL to bind to A- and P-sites programmed with the A-, G-, and U- ending Val codons and cmo5U34 is required for translocation from the A- to the P-site at these codons (Phelps et al. 2004; for review, see Agris 2004). Whereas a completely unmodified tRNASerUGA reads UCA well and UCU poorly, introduction of 5-methoxy-uridine (mo5U34) in the wobble position of an otherwise unmodified tRNASerUGA enhances reading of UCU and UCG at the expense of less efficient reading of UCA (Takai et al. 1999). However, no ability to base pair with C(III) was observed (N34 denotes the nucleoside in the first position of the anticodon [wobble position] and N(III) denotes the third nucleoside in the codon). Thus, in vitro experiments support the theoretical considerations that xmo5U34 derivatives extend the binding capacities of U from only base pairing with A(III) and G(III) to base pairing with U(III) as well. Accordingly, one would expect that a bacterium having only a cmo5U34-containing tRNA to read the codons in a family box would not be viable because such a tRNA would not be able to read all four codons in such a box. However, in vivo there is evidence that some cmo5U34-containing tRNAs may base pair with C(III). First, a strain containing only the tRNAAlacmo5UGC is viable, demonstrating that tRNAAlacmo5UGC can read all four Ala codons (Gabriel et al. 1996). Second, according to the model that explains how an aberrant tRNA may induce frameshifting (Qian et al. 1998), a near cognate tRNA may out-compete the cognate tRNA, provided that the latter is defective in some manner. Following a 3-nt translocation, this near cognate tRNA resides in the P-site. If for some reason the ribosome pauses at this site (e.g., caused by low concentration of the next aminoacyl-tRNA, a rare codon, or a stop codon), the near cognate tRNA in the P-site may shift into the +1 frame. In the proline family codon box, three Salmonella tRNAs, encoded by the proK, proL, and proM genes, read the proline codons (Fig. 1B ▶). The CCC codon is, according to the above theory, only read by the proL tRNAProGGG, because the proM tRNAProcmo5UGG should not read the CCC codon. However, a frameshifting event at a CCC codon is dependent on tRNAProcmo5UGG, suggesting that this tRNA may read the CCC codon (Qian et al. 1998). It is not clear from this observation how efficient the cmo5U34-containing proline-specific tRNA reads the CCC codon. Third, a strain lacking the proL tRNAProGGG, which reads CCC and CCU codons, is viable, supporting the notion that the proM tRNAProcmo5UGG is able to read the CCC codon (Chen et al. 2002), unless the proK tRNAProCGG contributes to the CCC reading (Qian et al. 1998). Thus in vivo, at least tRNAAlacmo5UGC and perhaps tRNAProcmo5UGG are able to read C-ending codons contrary to the theory and to several results obtained in vitro. Because the impact of some modified nucleosides is tRNA species dependent (Li et al. 1997), results from an analysis of decoding capacities for one tRNA, like tRNAAlacmo5UGC, cannot be extrapolated to another tRNA, for example, tRNAProcmo5UGG. Moreover, the role of cmo5U34 was not addressed in these studies, as well-defined mutants defective in the synthesis of this modified nucleoside were not available.
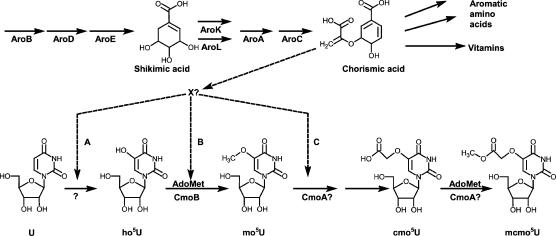
Mutants defective in the synthesis of the aromatic amino acids are also deficient in cmo5U and mcmo5U in their tRNA (Björk 1980). The presence of shikimic acid in the growth medium restores the synthesis of these modified nucleosides in an aroD mutant but not in an aroC mutant (distal to shikimic acid but prior to chorismic acid; Fig. 2 ▶), demonstrating that there is a metabolic link between the synthesis of chorismic acid and the synthesis of cmo5U and mcmo5U (Björk 1980). One carbon atom in the –O–CH2–COO− side chain of cmo5U originates from the methyl group of L-methionine whereas the other originates neither from carbonate nor from the C1 pool (Hagervall et al. 1990). Because no extra compound, except an occasional presence of ho5U (at 0%–15% of the level of cmo5U), was detected in tRNA from the Aro− mutants, it was suggested that chorismic acid or a metabolic derivative of it (X in Fig. 2 ▶) is required for the first step in the synthesis of cmo5U (Hagervall et al. 1990). As the occasional presence of ho5U might be unevenly distributed among the various tRNA species, it was not possible to state conclusively that a specific tRNA has an unmodified U34 or not. Clearly, the nonexistence of well-defined mutants defective in the biosynthesis of cmo5U34 has hampered the in vivo analysis of the function of this modified wobble nucleoside. Moreover, to unravel the molecular mechanism of the metabolic link between the synthesis of aromatic amino acids and tRNA modification, it is necessary to identify the enzymes required for the synthesis of cmo5U. Here, we report the identification of two genes encoding proteins required for the synthesis of cmo5U. As one of the mutants (cmoA) accumulates mo5U in tRNA whereas the other mutant (cmoB) accumulates ho5U, the biosynthetic pathway can be schematized as shown in Figure 2 ▶. Using these well-defined mutants, we also show that the presence of cmo5U34 in tRNAProcmo5UGG is required for efficient reading of the U- and C-ending proline codons.
FIGURE 2.
The proposed biosynthetic pathway for the synthesis of cmo5U and mcmo5U. The dashed reaction arrows indicate the link between the synthesis of cmo5U and chorismic acid. The arrow denoted A is according to Hagervall et al. (1990) and the arrows denoted B and C are the suggested link between chorismic acid and the synthesis of cmo5U34 according to present results. (X?) A possible unknown derivative of chorismic acid. (U) Uridine, (ho5U) 5-hydroxyuridine, (mo5U) 5-methoxyuridine (cmo5U) uridine-5-oxyacetic acid, (mcmo5U) uridine-5-oxyacetic acid methyl ester.
RESULTS
Isolation of a mutant lacking cmo5U34
At the +1 frameshift site -NNN-CCC-UGA- (the triplets are in zero frame and one of the Cs in the sequence CCC is the inserted nucleoside resulting in the +1 frameshift mutation) the near cognate tRNAProcmo5UGG, encoded by the proM gene, may read the codon CCC provided either that the cognate CCC-reading tRNAProGGG is defective (Qian et al. 1998) or absent (this article). Following a normal 3-nt translocation, the P-site-located near cognate tRNAProcmo5UGG slips forward 1 nt provided that the ribosome is stalled, which may happen if the codon in the A-site is a stop codon (Qian et al. 1998). If such a frameshift mutation is in the hisD gene, and the strain is also deleted for the proL gene, the strain will be phenotypically His+, because the +1 frameshift mutation in the hisD gene will be suppressed according to the model presented above. However, the presence of the cmo5U34 wobble nucleoside is a prerequisite for the His+ phenotype (Qian et al. 1998). Therefore, we predict that a mutation in an enzyme required for the synthesis of cmo5U34 would induce a His− phenotype of the His+ strain having the mutations ΔproL and a frameshift mutation in the his-operon.
We have devised a screening method based on these observations. The parent strain GT6606 (pTHF14/ΔproL, hisO1242, hisD3749) contains the hisD3749 allele, which is a +1 frameshift mutation resulting in the sequence -CCC-UGA in the hisD gene. This strain lacks tRNAProGGG, caused by the ΔproL deletion, which induces suppression of the hisD3749 mutation, and therefore the strain GT6606 is His+. Mutations were induced in strain GT6604, which is a pool of EZ::TN insertions, by overexpression of DinB, which induces single-base deletions and base substitutions at a ratio of 2:1 (Wagner and Nohmi 2000). Phage P22 was grown on such mutagenized cells and the resulting phage stock was used to infect the parent strain GT6606 (pTHF14/ΔproL, hisO1242, hisD3749). Clones resistant to kanamycin (KmR) were selected on medium E plates containing 0.2% glucose and “low histidine” (0.1 μM histidine). Using this concentration of histidine, 3 to 4 days of incubation at 37°C results in a clear difference in colony size between the parent strain GT6606, which is His+, and strain GT6607, which is His− due to lack of cmo5U34 in tRNAProcmo5UGG caused by the aroD::Tn10 mutation (Björk 1980). The smaller colony size of the latter strain is a result of less frameshift suppression caused by the cmo5U34 deficiency of the tRNAProcmo5UGG. Among approximately 45,000 KmR colonies, 534 tiny colonies were found that potentially were defective in suppression of the hisD3749 mutation. Histidinol is the substrate for the HisD enzyme (for review, see Winkler 1996). A mutant of interest should grow poorly on a histidinol-containing plate but normally on a histidine plate. Such a screen should exclude all mutations causing slow growth for reasons other than poor frameshift suppression of the hisD3749 mutation. Ninety-five of the KmR transductants that grew poorly or not at all on histidinol but grew normally or nearly normally on histidine containing plates were saved for further testing.
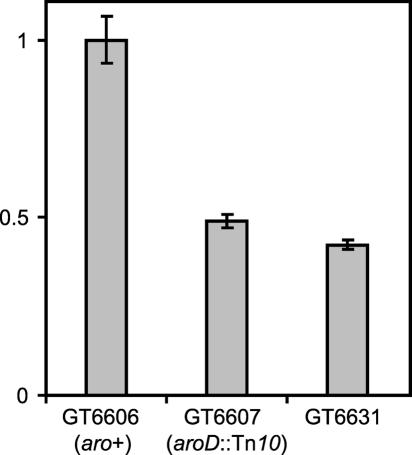
The parent strain GT6606 (pTHF14/ΔproL, hisO1242, hisD3749) also contains the plasmid pTHF14, which harbors a lacZ gene with a similar frameshift mutation as hisD3749 in the beginning of the lacZ gene (Hagervall et al. 1993). These cells are Lac+ because of suppression of this frameshift mutation in the lacZ gene in a way similar to the suppression of the hisD3749 mutation. To identify true frameshift antisuppressors from other mutations of no interest, we measured the level of suppression of the frame-shift mutation in the lacZ gene. Of the 95 His− mutants isolated above, one mutant, strain GT6631, has a significant decrease in β-galactosidase activity (Fig. 3 ▶). Apparently a low but significant suppression of the frameshift mutation in the lacZ gene still occurs in strain GT6631. Analysis of modified nucleosides in total tRNA from this mutant showed a complete loss of cmo5U34 (data not shown).
FIGURE 3.
3-Galactosidase assay showing suppression of the +1 frameshift mutation in lacZ of pTHF14. GT6631 is a mutant of the parental strain GT6606 that grew poorly on plates containing histidi-nol, but grew normally on plates containing histidine. All values are averages of three independent cultures and relative to the values in strain GT6606 (set to 1.0, which equals 1345 ± 88 miller units). A Student’s t-test (two-tailed; two sample equal variance) shows that both GT6607 and GT6631 are significantly lower than GT6606 (p < 0.001).
The EZ::TN transposon is inserted in yecO, a gene of unknown function
The transposon EZ::TN used to make the pool of randomly inserted transposons contains the plasmid origin R6Kγori. Upon digestion of DNA containing this transposon, plasmids can therefore be generated that can only replicate in an E. coli strain containing the λ pir protein, which is required for R6Kγori to be functional. To clone the EZ::TN insertion point, chromosomal DNA prepared from strain GT6631 was digested, ligated, and transformed into E. coli strain GRB1369 (λ pir+). Plasmids were isolated from three different transformants and the DNA next to the transposon was sequenced. All three plasmids had the transposon inserted in the proQ gene. Various P22 crosses to different strains revealed a genetic inconsistency and it was shown that the original mutant GT6631 unexpectedly contained two transposons, one inserted in proQ and the other in the yecO gene (data not shown). A strain having only a transposon in proQ is still His+, whereas a strain [GT6811 (yecO::EZ)] with only a transposon inserted in the yecO gene is His−. When a phage lysate was grown on the latter strain and used as donor in a cross with strain GT6787 (ΔproL, hisO1242, hisD3749) as recipient, 100% (300 tested) of the KmR transductants were also His−, implying that the His− phenotype is due to either the insertion in yecO itself or a mutation in a gene very close to it.
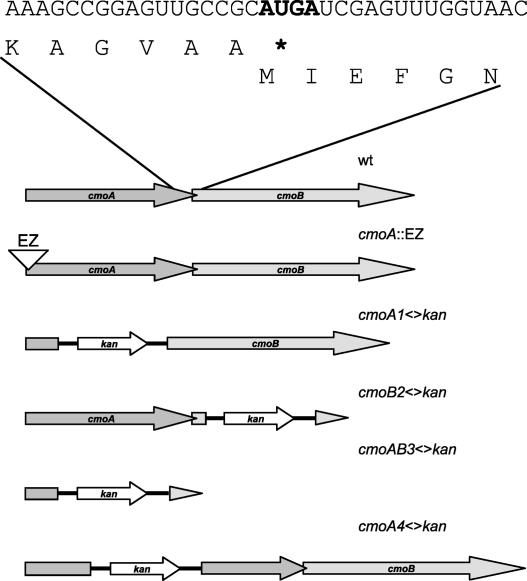
The yecO gene is the first gene in a potential two-cistron operon, predicted in RegulonDB (Salgado et al. 2004), containing another gene, yecP, downstream from yecO. We have denoted these genes cmoA (yecO) and cmoB (yecP), respectively (Fig. 4 ▶). Both CmoA and CmoB are predicted S-adenosyl-L-methionine (SAM)-dependent methyltransferases, making them likely candidates for the two methyl transfer reactions potentially involved in the synthesis of cmo5U34 and mcmo5U34 (see Fig. 2 ▶ and Discussion).
FIGURE 4.
The gene organization of the cmoAB operon. (Top) The translational overlap between cmoA and cmoB is indicated with the RNA sequence and the corresponding peptide sequences. The start (AUG) codon for cmoB and the stop (UGA) codon for cmoA are indicated in bold. (Bottom) Schematic representation of the different mutants constructed in this study. The transposon EZ::TN 〈R6Kγori/KAN-2〉 is abbreviated EZ, the white triangle indicates the insertion point within the first few codons of the cmoA gene; kan is the kana-mycin resistance cassette from plasmid pKD4.
CmoB and CmoA mediate the conversion of hydroxyuridine (ho5U) to 5-methoxyuridine (mo5U) and of mo5U to cmo5U, respectively
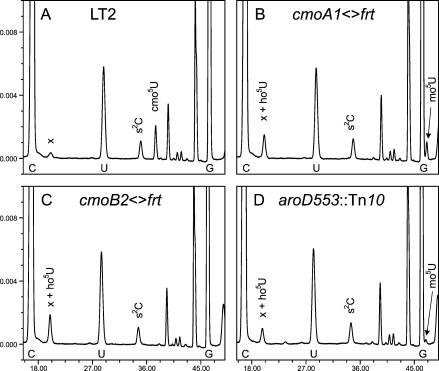
Strains carrying a replacement of cmoA, cmoB, or both genes by a KmR cassette (Fig. 4 ▶) were generated according to the method devised by Datsenko and Wanner (2000). HPLC analysis of modified nucleosides in tRNA from these mutants, still containing the KmR cassettes, revealed that they all lack cmo5U. The cmoB2<>kan mutant contained instead of cmo5U an extra compound identified as ho5U (data not shown) and the same results were obtained when the KmR cassette had been removed (Fig. 5C ▶). These results suggest that the CmoB peptide is involved in the conversion of ho5U to mo5U. The cmoA1<>kan mutant contained two additional compounds identified as ho5U and mo5U. After FLP-mediated removal of the resistance markers the results were the same (Fig. 5B ▶). Thus, the cmoA deletion mutant contained both ho5U and mo5U in tRNA prepared from exponentially growing cells. However, preparing tRNA from cells in stationary phase resulted in the accumulation of mo5U and almost no ho5U (Table 1 ▶, “24 h”). These results suggest that the insertions (the KmR cassette or the “scar” sequence, see Materials and Methods) in the cmoA gene, which is located upstream of the cmoB gene, is polar on the expression of the latter gene, consistent with the suggestion that the CmoB peptide is involved in the conversion of ho5U to mo5U. To further test this suggestion, we replaced the DNA sequence corresponding to amino acids 60–68 in CmoA, which contains the AdoMet binding site, with the scar sequence. This resulted in an even lower level of mo5U than the level observed in the former deletion (cmoA1; Table 1 ▶, compare strains GT6945 (cmoA4<>frt) and GT6854 (cmoA1<>frt)). Apparently, this cmoA construct was even more polar on the expression of cmoB than the first construct, consistent with the suggestion that insertions in the cmoA gene may be polar to different degree on the expression of the downstream gene cmoB. Thus, whereas the cmoA mutations did not induce one specific block in the synthesis of cmo5U, the cmoB mutations did, making the latter mutation an excellent tool to study the influence of hypomodification of cmo5U on translation efficiency.
FIGURE 5.
HPLC chromatograms monitored at 290 nm. The positions of cmo5U, ho5U, and mo5U are indicated above the corresponding peaks. The three major nucleosides C, U, and G are indicated below the corresponding peaks. “x” indicates an unidentified compound with a spectrum that is not similar to ho5U, comigrating with ho5U in all analyses. About 25 μg of total tRNA was digested to nucleosides and analyzed. The degradation procedure used, the treatment by alkaline phosphatase at pH 8.3, hydrolyzes most of mcmo5U to cmo5U.
TABLE 1.
Quantification of cmo5U, ho5U, and mo5U compared to Ψ in tRNA from different strains
| Strain | Relevant genotype | Growth conditions | ho5U/Ψa | mo5U/Ψ | cmo5U/Ψ |
| LT2 | Wild type | 100 klett | 0 | N/Db | 0.082 |
| LT2 | Wild type | 24 h | 0 | N/D | 0.079 |
| GT6854 | cmoA1<>frt | 100 klett | 0.041 | 0.036 | N/D |
| 24 h | 0.0017 | 0.087 | N/D | ||
| GT6945 | cmoA4<>frt (ΔSAM) | 100 klett | 0.084 | N/D | N/D |
| GT6856 | cmoB2<>frt | 100 klett | 0.072 | N/D | N/D |
| GT6858 | cmoAB3<>frt | 100 klett | 0.072 | N/D | N/D |
| GT6909 | aroD553::Tn10 | 100 klett | 0.014 | 0.0081 | N/D |
| 24 h | 0.011 | 0.023 | N/D |
The area of each compound monitored at 290 nm was divided with that of pseudouridine (Ψ) at 254 nm. Cells were either harvested at 100 klett units (about 4 × 108 cells/mL) or after incubation of the culture for 24 h.
aAn unknown compound, comigrating with ho5U, was present in all samples, so all values for ho5U are corrected by subtracting the value found in tRNA from strain LT2 at the same conditions.
b(N/D) Not detected.
We have shown earlier the occasional occurrence (0%–15% of the level of cmo5U) of ho5U in tRNA prepared from an aroD mutant (Hagervall et al. 1990). Analysis of tRNA from strain GT6909 (aroD553::Tn10) confirmed the absence of cmo5U and a low level of ho5U but we also observed a low level of mo5U (Fig. 5D ▶). Analysis of tRNA from strain GT6909 (aroD553::Tn10) grown into the stationary phase revealed an increased level of mo5U at the expense of ho5U and still no detectable level of cmo5U (Table 1 ▶, aroD, “24 h”). Apparently, the aroD mutation does not induce a specific block in the synthesis of cmo5U, and chorismic acid or a derivative of it participates in all steps known leading to cmo5U, although the degree of requirement seems to be quite different in different biosynthetic steps. Because of this heterogeneous accumulation of various intermediates in the synthesis of cmo5U in an aroD mutant, an aroD mutation is less suited as a tool to study the functional aspect of cmo5U.
ho5U and mo5U in proM tRNA influences +1 frameshift suppression differently
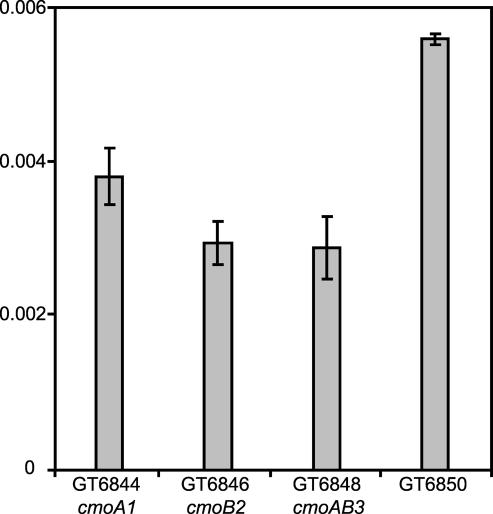
To investigate the effect on +1 frameshifting by the partially modified tRNAProho5UGG and tRNAPromo5UGG we constructed a series of strains (GT6844–GT6851) having ΔproL, hisO1242, hisD2504::mudK with or without the hisD3749 +1 frameshift mutation, and the three deletions cmoA1<>frt, cmoB2<>frt, and cmoAB3<>frt. The mudK is inserted after the 71st codon of the hisD gene, so that an in-frame fusion between hisD and lacZ is produced downstream from the mutation in hisD3749 (the extra C in hisD3749 is inserted in codon 14). The strains having the hisD3749 mutation require a +1 frameshift to produce β-galactosidase, whereas strains without the hisD3749 mutation were used as in-frame controls. Therefore, the β-galactosidase activity is a measurement of the level of the HisD–LacZ fusion protein and thereby a direct measurement of the frequency of frameshifts that correct the hisD3749 frameshift mutation. In the wild-type strain, GT6850, frameshifting is about twice that observed in the cmoB2<>frt and cmoAB3<>frt mutants, which both have ho5U34 instead of cmo5U (Fig. 6 ▶). However, in the cmoA1<>frt mutant, which in addition to ho5U also has mo5U (Table 1 ▶), the level of frameshifting was reduced by 30% compared to that observed in the wild type. Apparently, the presence of mo5U is less detrimental to the ability of proM tRNA to induce frameshifting compared to the presence of ho5U. We conclude that the presence of ho5U instead of cmo5U34 in proM tRNA induces frameshifting less efficiently and thus decodes CCC less well compared to the proM tRNAProcmo5UGG containing cmo5U.
FIGURE 6.
Relative frameshifting frequencies at the CCC-UGA frameshifting site in hisD3749. All strains are ΔproL, hisO1242, hisD3749, hisD2504::MudK, and the values are relative to the corresponding in-frame construct (same genotype except without hisD3749) and are averages of three cultures. The cells were grown in LB + 100 mg/L kanamycin at 37°C. A Student’s t-test (two-tailed; two sample equal variance) shows that cmoA1<>frt, cmoB2<>frt, and cmoAB3<>frt significantly differ from GT6850 (wild type; p ≤ 0.001). cmoA1<>frt is also different from cmoB2<>frt and cmoAB3<>frt (p< 0.05).
Presence of cmo5U34 in proM tRNAProcmo5UGG is required for efficient reading of all four proline codons, including the CCU and CCC codons
Introduction of the cmoA1<>frt or the cmoB2<>frt mutations into the wild-type strain did not influence the growth rate in any of the three growth media tested (Table 2 ▶, part A). Because the cmo5U-containing proM tRNAProcmo5UGG, which should read CCA, CCG, and CCU but not CCC, is only one of three proline specific tRNAs, such an experiment may be too insensitive to reveal a small decrease in the efficiency of this tRNA in the presence of the other two proline-specific tRNAs. Therefore, we deleted the genes (proK and proL) for tRNAProCGG reading CCG and tRNAProGGG reading CCU/C, leaving the proM tRNAProcmo5UGG as the only tRNA reading the proline codons (Fig. 1B ▶). Surprisingly, such a strain is viable, showing that the proM tRNAProcmo5UGG reads all four proline codons. In fact, such a strain grew as well as the wild-type strain in Rich-MOPS medium and showed only about 10% reduction in growth rate in MOPS-glucose and MOPS-acetate media, caused by the lack of the proL and proK tRNAs (Table 2 ▶, compare strain LT2 with strain GT6902 (proK<>frt, ΔproL)). Such a strain, which only has one tRNA (proM tRNAProcmo5UGG) reading the four proline codons, is ideal to study the effect of hypomodification of tRNAProcmo5UGG. We therefore introduced into strain GT6902 (proK<>frt, ΔproL), the cmoA1<>frt, cmoB2<>frt, or the cmoAB3<>frt alleles. In the three media tested, there was a 16%–27% reduction in growth rate for all mutants compared to the control strain GT6902 (proK<>frt, ΔproL) (Table 2 ▶, part B). Thus, the presence of ho5U or mo5U instead of cmo5U in proM tRNA reduced the efficiency of this tRNA to read the four proline codons. According to the theory (Crick 1966; Nishimura 1979) and to in vitro results (Oda et al. 1969; Ishikura et al. 1971; Mitra et al. 1979; Samuelsson et al. 1980; Takai et al. 1999; Agris 2004; Phelps et al. 2004), tRNAProcmo5UGG should not read the CCC codon, which is normally decoded by the proL tRNAProGGG. Therefore, we expected that the reduction caused by the hypomodification was mainly due to an inefficient reading of the CCC codon. If so, by combining the cmoAB3<>frt mutation, which results in the presence of ho5U instead of cmo5U in the proM tRNA, with either proK<>frt or ΔproL mutations, a differential effect would be observed, that is, a larger decrease in growth rate would be observed when the proL tRNAProGGG was lacking than when proK tRNAProCGG was absent in conjunction with the cmoAB3<>frt mutation (Fig. 1B ▶). Indeed, this was the case, because strain GT6912 (cmoAB3<>frt, ΔproL) grew 14% slower than the corresponding control strain whereas strain GT6899 (cmoAB3<>frt, proK<>frt) grew as its control strain (Table 2 ▶, parts C and D). Therefore, ho5U can only partially substitute for the cmo5U in base pairing with U and C. We conclude that the proM tRNAProcmo5UGG is able to read all four proline codons, including the CCC codon, but requires the presence of cmo5U for an efficient reading.
TABLE 2.
cmo5U34 in proM tRNA is required for efficient reading of proline codons
| Rich MOPS | MOPS glucose | MOPS acetate | ||||||
| Strain | Genotype | ka | (km− kc)/kcb | k | (km− kc)/kc | k | (km− kc)/kc | |
| A | LT2 | Wild type (control) | 1.36 | 0.00 | 0.95 | 0.00 | 0.33 | 0.00 |
| GT6854 | cmoA1<>frt | 1.36 | 0.00 | 0.93 | −0.02 | 0.34 | 0.02 | |
| GT6856 | cmoB2<>frt | 1.36 | 0.01 | 0.95 | −0.01 | 0.34 | 0.02 | |
| GT6858 | cmoAB3<>frt | 1.36 | 0.00 | 0.93 | −0.02 | 0.34 | 0.02 | |
| B | GT6902 | ΔproL, proK<>frt(control) | 1.35 | 0.00 (−0.01) | 0.86 | 0.00 (−0.09) | 0.29 | 0.00 (−0.12) |
| GT6913 | cmoA1<>frt, ΔproL, proK<>frt | 1.09 | −0.20 | 0.68 | −0.21 | 0.24 | −0.16 | |
| GT6914 | cmoB2<>frt, ΔproL, proK<>frt | 1.01 | −0.25 | 0.63 | −0.27 | 0.22 | −0.22 | |
| GT6915 | cmoAB3<>frt, ΔproL, proK<>frt | 1.01 | −0.25 | 0.63 | −0.26 | 0.22 | −0.25 | |
| C | GT6877 | proK<>frt (control) | N.D.c | N.D. | 0.96 | 0.00 (0.01) | N.D. | N.D. |
| GT6899 | cmoAB3<>frt, proK<>frt | N.D. | N.D. | 0.93 | −0.03 | N.D. | N.D. | |
| D | GT6879 | ΔproL (control) | N.D. | N.D. | 0.94 | 0.00 (−0.01) | N.D. | N.D. |
| GT6912 | cmoAB3<>frt, ΔproL | N.D. | N.D. | 0.81 | −0.14 | N.D. | N.D. |
Numbers in bold indicate those that we consider significantly different from the relevant control. Numbers within parentheses are the differences in growth rates of the control strains compared to LT2.
aGrowth rate (h−1), k = ln 2/g, where g (h) is the generation time.
b(km) Growth rate of mutant, (kc) growth rate of control strain (A: LT2, B: GT6902, C: GT6877, D: GT6879).
c(N/D) Not determined.
DISCUSSION
We know from earlier work that chorismic acid is required at an early step in the synthesis of cmo5U (Björk 1980). However, how and at what step chorismic acid is required in the synthesis of cmo5U is not known. One of the two carbon atoms of the side chain –O–CH2–COOH originates from the methyl group of L-methionine, suggesting that at least one step in the synthesis of cmo5U is an AdoMet-dependent transmethylation reaction. We describe here the identification of two genes (cmoA and B) involved in the synthesis of cmo5U. Both proteins contain an AdoMet-binding motif and accordingly two biosynthetic steps in the synthesis of cmo5U should be AdoMet transmethylation reactions. One such step is the conversion of ho5U to mo5U in which CmoB is involved. Even though CmoA, which apparently is involved in the conversion of mo5U to cmo5U, has an AdoMet-binding motif, it is not obvious how a methylation would be involved in this step, because the following carbon atom of the side chain –O–CH2–COOH apparently does not originate from AdoMet (Hagervall et al. 1990). However, the CmoA peptide may be in a complex that, in addition to the conversion of mo5U to cmo5U, also participates in the methylation of cmo5U to mcmo5U. If so, the CmoA peptide is the AdoMet-binding peptide in the tRNA(mcmo5U34)methyltransferase partially purified by Pope and Reeves (1978) that catalyzes the formation of mcmo5U in at least the tRNAs specific for alanine and serine (Pope et al. 1978). Clearly, purification of the CmoA and CmoB peptides and analysis of the in vitro reactions must be performed before the reaction in which these peptides participate can be unraveled.
Present results confirm our earlier observation that the presence of chorismic acid or a derivative of it (X in Fig. 2 ▶) is critical in the formation of cmo5U (Table 1 ▶; Björk 1980; Hagervall et al. 1990). As demonstrated earlier (Hagervall et al. 1990), we also observed accumulation of a small amount of ho5U, but also of mo5U, in tRNA from cells lacking chorismic acid as in an aroD mutant (Table 1 ▶). Upon prolonged incubation of the culture, some of the ho5U was converted to mo5U (Table 1 ▶, “24 h”). Still, no cmo5U was observed, suggesting that chorismic acid or a derivative thereof (X in Fig. 2 ▶) is required for the conversion of mo5U to cmo5U. If so, the chorismic acid requirement would be at the same step(s) in which the CmoA peptide participates. We noticed that upon prolonged incubation of cells in stationary phase in the absence of chorismic acid the amount of mo5U increased at the expense of ho5U, suggesting that this conversion can proceed without chorismic acid although at a low efficiency. Still, the level of mo5U was much less than that observed in the cmoA1 mutant under similar conditions (Table 1 ▶). We can not rule out the possibility that chorismic acid is also required earlier than at the mo5U to cmo5U conversion step, because ho5U also accumulates under these conditions. The level of ho5U accumulated in the absence of chorismic acid never reached the level observed in the cmoB mutant (Table 1 ▶), suggesting that this metabolite also stimulates the U to ho5U conversion. Therefore, chorismic acid may play an integral role in the conversion of U to ho5U, of ho5U to mo5U, and of mo5U to cmo5U. The requirement in the mo5U to cmo5U conversion seems to be stricter than in the earlier steps, as no cmo5U was formed in the absence of chorismic acid.
We have earlier shown that m1G37, which is present 3′ and next to the anticodon in all three proline tRNAs, improves reading frame maintenance (Björk et al. 1989). Deficiency of the wobble nucleoside cmo5U34 may influence the synthesis of m1G37. However, a base substitution in the wobble position does not influence the synthesis of m1G37 (Qian and Björk 1997). Even if a deficiency of cmo5U34 would cause a reduced level of m1G37, such a deficiency would increase frameshifting rather than the decrease we observed (Fig. 6 ▶). It is also unlikely that cmo5U34 deficiency would cause less charging of proM tRNAProcmo5UGG, because alterations in the anticodon loop and stem do not influence the prolinylation of tRNA (Qian and Björk 1997). Accordingly, the anticodon is not an important recognition determinant for prolyl-tRNA synthetase (McClain et al. 1994). Therefore, the observed effect on frameshifting and growth rates is caused by anticodon–codon aberrations and not from a reduced level of prolyl-tRNA.
We demonstrate here the viability of a cell having only the proM tRNAProcmo5UGG, thus showing that this tRNA can read all four proline codons including the C-ending codon. This was unexpected considering the stereochemical theory (Yokoyama et al. 1985) and several in vitro results (Oda et al. 1969; Ishikura et al. 1971; Mitra et al. 1979; Samuelsson et al. 1980; Takai et al. 1999; Agris 2004; Phelps et al. 2004), which suggest that the cmo5U modification extends the wobble capacity of U to pair with A(III), G(III), and U(III) but not with C(III). However, this observation is similar to the report by Gabriel et al. (1996), who have shown that a strain having only the cmo5U34-containing tRNAAlacmo5UGC is viable, suggesting that the tRNAAlacmo5UGC reads all four alanine codons in vivo. Moreover, Lim and Curran (2001) suggested, from theoretical considerations, that xo5U derivatives may misread C(III). We also have to remember that the in vitro results were obtained using a tRNA devoid of all other modified nucleosides except for mo5U (Takai et al. 1999) or an anticodon loop and stem derivative (Phelps et al. 2004), whereas our in vivo results were obtained with a tRNA lacking only the cmo5U34. These differences in tRNA may partly explain the difference obtained between in vitro and in vivo. Although the mutant lacking the proL and proK tRNAs grew as wild type in one medium, it showed a clear growth disadvantage in the other two media tested (Table 2 ▶). Thus, under some physiological conditions cells containing proL and proK tRNAs have a selective advantage.
Hypomodification in conjunction with lack of proK tRNAProCGG did not influence the growth rate, whereas hypomodification in conjunction with lack of proL tRNAProGGG did (Table 2 ▶). We therefore attribute the decrease in growth rate caused by the hypomodification in the proL, proK double mutant to the inefficient reading of the CCC and CCU codons, because these codons are normally read by the proL tRNAProGGG (Fig. 1B ▶). These results suggest that the cmo5U modification is able to interact with C(III) and U(III). Therefore, the codon reading of the proM tRNAProcmo5UGG is not the outcome of a “two-out-of-three” reading, which implies that only the two first nucleosides of the anticodon base pair with C(I) and C(II) and the third nucleoside does not interact with the third nucleoside in the codon (Lagerkvist 1978). We observed a larger decrease in growth rate in the ΔproL, proK<>frt double mutant than that observed in the ΔproL single mutant. This difference may be attributed to less hypomodified proM tRNAProho5UGG available to read the CCC and CCU codons, when it also has to read CCG codons, in the double mutant compared to that available in the single mutant.
The stability of the C3′- and C2′-endo conformations of pU is almost the same, whereas the C2′-endo form of pcmo5U and pmo5U is more stable than the C3′-endo conformation (enthalpy differences between C2′- and C3′-endo are 0.1 (pU), -0.72 (pmo5U), and -0.67 (pcmo5U) kcal/mole (Yokoyama et al. 1985). Interestingly, pho5U favors the C2′-endo conformation (−0.28 kcal/mole difference) less than either pmo5U or pcmo5U but more than pU. Thus, from these considerations we would expect the largest difference in growth rate between the fully modified tRNA containing cmo5U34 and tRNA containing ho5U34. This was also the case when we analyzed the antisuppressor activity (Fig. 6 ▶, cf. wild type and cmoB mutant) and the reduction in growth rate caused by the cmoB2<>frt deletion, which results in only ho5U being present in its tRNA (Table 2 ▶). The stability of the C2′-endo conformation for pmo5U and pcmo5U are almost the same, but we still observed a significant difference between cmoA1<>frt, which contains mo5U in its tRNA, and the wild type, both in the antisuppressor assay (Fig. 6 ▶) and in growth rate determinations (Table 2 ▶). However, the cmoA1<>frt mutant contains a mixture of ho5U and mo5U, but we do not know how these two intermediates are distributed in the proM tRNA pool. Therefore, the phenotypic differences we observed between the wild type and the cmoA1<>frt mutant might be attributed to the presence of ho5U in a fraction of the proM tRNA. We conclude that although our results are not as expected from the stereochemical theory (the fact that the wild-type proM tRNAProcmo5UGG in fact reads the CCC codon), they are consistent with the observed effect caused by the presence of ho5U instead of cmo5U in the proM tRNA.
The UGA suppressor supK, was originally thought to be the structural gene for the tRNA(mcmo5U)methyl-transferase (Atkins and Ryce 1974; Reeves and Roth 1975), because mutations in the supK gene reduce the activity of this enzyme in extracts (Reeves and Roth 1975). However, we know now that supK is allelic to prfB, which encodes release factor 2 (RF2), which recognizes the UGA and UAA stop codons (Kawakami et al. 1988). The translational stop codon for cmoA, which is UGA, overlaps the start codon AUG for cmoB (Fig. 4 ▶). Thus, a defective RF2 may allow read-through at the cmoA stop codon, resulting in less CmoB and a 45-amino-acid elongated CmoA, which may be inactive. Thus, a defective RF2 may result in less CmoA activity, consistent with the suggestion that the CmoA peptide is part of the tRNA(mcmo5U)methyltransferase. Further work on the function of CmoA may solve the conundrum of how a defective RF2 can reduce the activity of the tRNA(mcmo5U)methyltransferase in extracts.
MATERIALS AND METHODS
Bacteria and growth conditions
All strains are derivatives of Salmonella enterica serovar Typhimurium strain LT2 (Table 3 ▶). As rich liquid medium, either LB (Bertani 1951) or NB (0.8% Difco nutrient broth; Difco Laboratories) supplemented with the aromatic amino acids, aromatic vitamins, and adenine at concentrations as described previously (Davis et al. 1980) were used. MOPS (morpholinepropanesulfonic acid) medium supplemented with 0.4% (w/v) glucose or 0.4% (w/v) sodium acetate (Neidhardt et al. 1974) and rich MOPS (Neidhardt et al. 1977) were used as defined liquid media for growth rate determinations. As solid rich medium, TYS agar (10 g of Trypticase peptone, 5 g of yeast extract, 5 g of NaCl, and 15 g of agar/L) was used. Medium E (Vogel and Bonner 1956) containing 15 g agar/L and 0.2% glucose was used as solid minimal medium. All growth experiments were done at 37°C. To determine the growth rates, an overnight culture of the different strains were diluted to 0.05–0.1 OD420 units in prewarmed medium, pregrown to at least a fivefold increase in optical densities, never exceeding an OD420 = 0.8 during this pregrowth period. The cell suspension was then diluted to an OD420 of 0.05, and growth was monitored with a Shimadzu UV-1601 spectrophotometer at 420 nm during at least a 10-fold increase in cell density. Growth rate is expressed as k where k = ln 2/mass doubling time in hours. Concentrations of the antibiotics used were, unless otherwise stated, Carbenicillin (Cb): 50 mg/L; Kanamycin (Km): 100 mg/L; Chloramphenicol (Cm): 12.5 mg/L.
TABLE 3.
Salmonella enterica serovar Typhimurium and Escherichia coli strains and plasmids
| Strain | Relevant genotype | Source |
| Salmonella enterica serovar Typhimurium | ||
| GT6589 | pSMP24/ΔproL, hisO1242, hisD3749, zef-201::Tn10dCm | This study |
| GT6604 | Pool of EZa-insertions in GT6589 | This study |
| GT6606 | ΔproL, hisP1242, hisD3749, zef-201::Tn10dCm/pTHF14 | This study |
| GT6607 | aroD553::Tn10, ΔproL, hisO1242, hisD3749, zef-201::Tn10dCm/pTHF14 | This study |
| GT6631 | proQ::EZa, cmoA::EZa, ΔproL, hisO1242, hisD3749, zef-201::Tn10dCm/pTHF14 | This study |
| GT6787 | ΔproL, hisO1242, hisD3749 | This study |
| GT6811 | cmoA::EZa, ΔproL, hisO1242, hisD3749 | This study |
| GT6831 | cmoA1<>kan | This study |
| GT6833 | cmoB2<>kan | This study |
| GT6835 | cmoAB3<>kan | This study |
| GT6844 | cmoA1<>frt, ΔproL, hisO1242, hisD3749, hisD2504::MudK | This study |
| GT6845 | cmoA1<>frt, ΔproL, hisO1242, hisD2504::MudK | This study |
| GT6846 | cmoB2<>frt, ΔproL, hisO1242, hisD3749, hisD2504::MudK | This study |
| GT6847 | cmoB2<>frt, ΔproL, hisO1242, hisD2504::MudK | This study |
| GT6848 | cmoAB3<>frt, ΔproL, hisO1242, hisD3749, hisD2504::MudK | This study |
| GT6849 | cmoAB3<>frt, ΔproL, hisO1242, hisD2504::MudK | This study |
| GT6850 | ΔproL, hisO1242, hisD3749, hisD2504::MudK | This study |
| GT6851 | ΔproL, hisO1242, hisD2504::MudK | This study |
| GT6854 | cmoA1<>frt | This study |
| GT6856 | cmoB2<>frt | This study |
| GT6858 | cmoAB3<>frt | This study |
| GT6877 | proK<>frt | This study |
| GT6879 | ΔproL | This study |
| GT6899 | cmoAB3<>frt, proK<>frt | This study |
| GT6902 | ΔproL, proK<>frt | This study |
| GT6909 | aroD553::Tn10 | This study |
| GT6912 | cmoAB3<>frt, ΔproL | This study |
| GT6913 | cmoA1<>frt, ΔproL, proK<>frt | This study |
| GT6914 | cmoB2<>frt, ΔproL, proK<>frt | This study |
| GT6915 | cmoAB3<>frt, ΔproL, proK<>frt | This study |
| GT6944 | cmoA4<>kan (ΔSAM) | This study |
| GT6945 | cmoA4<>frt (ΔSAM) | This study |
| Escherichia coli | ||
| GRB1369 | λ pir | Miller and Mekalanos 1988 |
| Plasmids | ||
| pCP20 | FLP recombinase helper plasmid | Datsenko and Wanner 2000 |
| pKD4 | Template plasmid for amplification of kanR-cassette | Datsenko and Wanner 2000 |
| pKD46 | λ-Red recombinase helper plasmid | Datsenko and Wanner 2000 |
| pSMP24 | dinB (AmpR) | D. Andersson, pers. comm. |
| pTHF14 | +1 frameshift site (suppressible at CCC-UGA) in lacZ | Hagervall et al. 1993 |
aEZ is short for the transposon EZ::TN™ 〈R6Kγori/KAN-2〉
Genetic procedures
Transduction with phage P22 HT105/1 (int-201; Schmieger 1972) was performed as described previously (Davis et al. 1980). Mutagenesis was performed as follows: A pool of transposon insertions was generated in strain GT6589 (pSMP24/ΔproL, hisO1242, hisD3749, zef-201::Tn10dCm) using the EZ::TN 〈R6Kγori/KAN-2〉Tnp Transposome Kit (Epicentre), producing a pool of approximately 40,000 KmR transposants. Colonies were pooled and pregrown in LB + Cb for 30 min at 37°C. To introduce point mutations, the pool was further mutagenized by inducing expression of DinB (DNA-polymerase IV, an error prone polymerase; Wagner et al. 1999; Wagner and Nohmi 2000) from the plasmid pSMP24, by diluting the culture into 10 tubes containing 1 ml LB + Cb + 0.04% L-arabinose. After overnight growth, phage P22 was added to make phage lysates on each of these 10 1-ml cultures. By transduction using these doubly mutagenized phage lysates and screening for a phenotype among the KmR transductants, one may find mutants whose phenotype is either due to insertion of the transposon in a target gene or a point mutation within cotransduction distance from the transposon.
DNA sequencing was performed on chromosomal DNA, plasmid DNA, or PCR products as described in the manual for the Applied Biosystems ABI Prism cycle-sequencing BigDye Ready Reaction kit. Gene replacements were generated as described earlier by transforming with PCR products containing resistance markers flanked by the DNA sequence surrounding the sequence to be deleted (Datsenko and Wanner 2000). The resistance cassettes were later eliminated using FLP recombinase expressed from plasmid pCP20. This method leaves a “scar” sequence (denoted “frt”) of 84 nt, containing stop codons in all reading frames and a Shine–Dalgarno site and a start codon at one end of the scar sequence. The deletion of cmoA (cmoA1) replaces nt 71–743 (of a total of 744 nt) of the cmoA gene with the scar sequence, so that the start codon of cmoB is replaced by the start codon of the scar sequence. This was done to try to avoid polar effects on the expression of cmoB. In cmoA4 (ΔSAM) nt 177–204 (corresponding to the sequence encoding the SAM-binding site (VYDLGCSLG) were replaced. The deletion of cmoB (cmoB2) replaces nt 58–907 (of a total of 965 nt). The cmoAB3 deletion replaces the region from nt 71 of cmoA to nt 907 of cmoB. The deletion of proK replaces the sequence corresponding to 3 nt before the 5′ end of the mature tRNA to 10 nt after the CCA end. Primers for amplifying the KmR cassette from pKD4 were designed with 35–55 nt 5′-tails homologous to the sequences next to the sequence to be replaced. For PCR verification of the insertions, primers binding 100–350 bp away from the ends of the sequence to be replaced were used in combination with primers binding inside the KmR cassette. The deletion of proL (Chen et al. 2002) was generated using the suicide plasmid pMAK705 (Hamilton et al. 1989), containing a temperature-sensitive replicon, carrying a 1.5-kb fragment containing a deletion from 26 bp before proL to 5 bp after proL.
Nomenclature of mutants: an allele number followed by <> and “kan” indicates that the gene is replaced by the KmR cassette (e.g., cmoB2<>kan). After FLP-mediated removal of the cassette, the mutation is referred to with the same allele number, but with “frt” as description of the replacing scar sequence (e.g., cmoB2<>frt).
Analysis of modified nucleosides in tRNA
Bacterial strains were grown overnight in medium LB, diluted 100 times in 100 mL of the same medium and grown to 100 klett units (approximately 4 × 108 cells/mL) or in 50 mL and grown into the stationary phase for 24 h. Cells were harvested by centrifugation, and the pellets were resuspended in 5 mL TE buffer (10 mM Tris, 1 mM Na2EDTA at pH 8.0). Three milliliters TRIzol (Invitrogen Life Technologies) reagent were added and the suspension was incubated on ice during 1.5–2 h. Cell debris was pelleted by centrifugation and the aqueous phase was extracted with chloroform until no white interphase was formed. RNA was precipitated by 2.5 volumes of cold 99.5% ethanol containing 1% (w/v) potassium acetate. After washing the pellet twice with 70% ethanol, the pellet was dissolved in 2 mL buffer R200 (10 mM Tris-H3PO4 at pH 6.3, 15% ethanol, 200 mM KCl) and applied to a Nucleobond AX500 column (Macherey-Nagel Gmbh & Co.), preequilibrated with the same buffer. The column was washed once with 6 mL R200 and once with 2.5 mL R650 (same composition as R200, except with 650 mM KCl). Finally, tRNA was eluted with 7 mL R650. tRNA was precipitated by 0.7 volumes isopropanol, washed once with 70% ethanol, and dissolved in water. tRNA was digested to nucleosides by nuclease P1 followed by treatment with bacterial alkaline phosphatase at pH 8.3 (Gehrke et al. 1982). The mcmo5U is alkili labile, why most mcmo5U is converted to cmo5U under the conditions used.
The hydrolysate was analyzed by HPLC as described earlier (Gehrke and Kuo 1990) except that a Develosil 5μ RP-AQUEOUS C-30 column (Phenomenex) was used and the gradient was slightly modified to obtain optimal separations of cmo5U, ho5U, and mo5U. The first buffer (A) had 0.5% instead of 2.5% methanol, and the time for the isocratic part of the gradient was extended from 0–12 min to 0–22 min. To identify mo5U and ho5U, synthetic markers were added to the digested tRNA from cmoA1<>frt and cmoB2<>frt mutants, respectively, and when these spiked samples were analyzed, areas of the compounds identified as mo5U and ho5U increased compared to the nonspiked digested tRNA (data not shown).
β-Galactosidase assays
β-Galactosidase assays were performed as described earlier (Hagervall et al. 1993). During the screening procedure cultures for β-galactosidase assays were grown in 2.2-ml 96-well polypropylene blocks (Marsh Biomedical Products) and assays were performed essentially as described by Griffith and Wolf (2002).
Acknowledgments
This work was supported by grants from the Swedish Cancer Foundation (Project 680) and Swedish Science Research council (Project BU-2930). We thank Kerstin Jacobsson for excellent technical assistance in performing HPLC analysis, Dan Andersson for the plasmid pSMP24 prior to its publication, and Barry Wanner for the plasmids pKD4, pKD46, and pCP20. We are grateful for the generous gifts of synthetic samples of cmo5U from S. Nishimura, Banyu Tsukuba Research Institute, Tsukuba, Japan and mo5U and cmo5U from K. Murao, Tochigi-Kan, Japan. We thank G. Roberts, who suggested using TRIzol to prepare tRNA. The critical reading of the manuscript by A. Byström, T. Hagervall, M. Pollard, and M. Wikström, Umeå is gratefully acknowledged.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.rnajournal.org/cgi/doi/10.1261/rna.7106404.
REFERENCES
- Agris, P.F. 2004. Decoding the genome: A modified view. Nucleic Acids Res. 32: 223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agris, P.F., Sierzputowska-Gracz, H., Smith, W., Malkiewicz, A., Sochacka, E., and Nawrot, B. 1992. Thiolation of uridine carbon-2 restricts the motional dynamics of the transfer RNA wobble position nucleoside. J. Am. Chem. Soc. 114: 2652–2656. [Google Scholar]
- Atkins, J.F. and Ryce, S. 1974. UGA and non-triplet suppressor reading of the genetic code. Nature 249: 527–530. [DOI] [PubMed] [Google Scholar]
- Bertani, G. 1951. Studies on Lysogenesis. J. Bacteriol. 62: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk, G.R. 1980. A novel link between the biosynthesis of aromatic amino acids and transfer RNA modification in Escherichia coli. J.Mol. Biol. 140: 391–410. [DOI] [PubMed] [Google Scholar]
- Björk, G.R., Wikström, P.M., and Byström, A.S. 1989. Prevention of translational frameshifting by the modified nucleoside 1-methyl-guanosine. Science 244: 986–989. [DOI] [PubMed] [Google Scholar]
- Chen, P., Qian, Q., Zhang, S., Isaksson, L.A., and Björk, G.R. 2002. A cytosolic tRNA with an unmodified adenosine in the wobble position reads a codon ending with the non-complementary nucleoside cytidine. J. Mol. Biol. 317: 481–492. [DOI] [PubMed] [Google Scholar]
- Crick, F.H.C. 1966. Codonanticodon pairing. The wobble hypothesis. J. Mol. Biol. 19: 548–555. [DOI] [PubMed] [Google Scholar]
- Datsenko, K.A. and Wanner, B.L. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. 97: 6640–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, W., Botstein, D., and Roth, J.R. 1980. A manual for genetic engineering: Advanced bacterial genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- Gabriel, K., Schneider, J., and McClain, W.H. 1996. Functional evidence for indirect recognition of G.U in tRNA(Ala) by alanyl-tRNA synthetase. Science 271: 195–197. [DOI] [PubMed] [Google Scholar]
- Gehrke, C.W. and Kuo, K.C. 1990. Ribonucleoside analysis by reversed-phase high performance liquid chromatography. In Chromatography and modification of nucleosides. Part A. Analytical methods for major and modified nucleosides (eds. C.W. Gehrke et al.), vol. 45A, pp. A3–A71. Elsevier, Amsterdam.
- Gehrke, C.W., Kuo, K.C., McCune, R.A., Gerhardt, K.O., and Agris, P.F. 1982. Quantitative enzymatic hydrolysis of tRNAs: Reversed-phase high-performance liquid chromatography of tRNA nucleosides. J. Chromatogr. 230: 297–308. [PubMed] [Google Scholar]
- Griffith, K.L. and Wolf, R.E. 2002. Measuring β-galactosidase activity in bacteria: Cell growth, permeabilization, and enzyme assays in 96-well arrays. Biochem. Biophys. Res. Commun. 290: 397–402. [DOI] [PubMed] [Google Scholar]
- Hagervall, T.G., Jönsson, Y.H., Edmonds, C.G., McCloskey, J.A., and Björk, G.R. 1990. Chorismic acid, a key metabolite in modification of tRNA. J. Bacteriol. 172: 252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagervall, T.G., Tuohy, T.M., Atkins, J.F., and Björk, G.R. 1993. Deficiency of 1-methylguanosine in tRNA from Salmonella typhimurium induces frameshifting by quadruplet translocation. J. Mol. Biol. 232: 756–765. [DOI] [PubMed] [Google Scholar]
- Hamilton, C.M., Aldea, M., Washburn, B.K., Babitzke, P., and Kushner, S.R. 1989. New method for generating deletions and gene replacements in Escherichia coli. J. Bacteriol. 171: 4617–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikura, H., Yamada, Y., and Nishimura, S. 1971. Structure of serine tRNA from Escherichia coli. I. Purification of serine tRNA’s with different codon responses. Biochim. Biophys. Acta 228: 471–481. [DOI] [PubMed] [Google Scholar]
- Kawakami, K., Inada, T., and Nakamura, Y. 1988. Conditionally lethal and recessive UGA-suppressor mutations in the prfB gene encoding peptide chain release factor 2 of Escherichia coli. J. Bacteriol. 170: 5378–5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagerkvist, U. 1978. “Two out of three”: An alternative method for codon reading. Proc. Natl. Acad. Sci. 75: 1759–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J.N., Esberg, B., Curran, J.F., and Björk, G.R. 1997. Three modified nucleosides present in the anticodon stem and loop influence the in vivo aa-tRNA selection in a tRNA-dependent manner. J. Mol. Biol. 271: 209–221. [DOI] [PubMed] [Google Scholar]
- Lim, V.I. and Curran, J.F. 2001. Analysis of codon: Anticodon interactions within the ribosome provides new insights into codon reading and the genetic code structure. RNA 7: 942–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain, W.H., Schneider, J., and Gabriel, K. 1994. Distinctive acceptor-end structure and other determinants of Escherichia coli tRNAPro identity. Nucleic Acids Res. 22: 522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, V.L. and Mekalanos, J.J. 1988. A novel suicide vector and its use in construction of insertion mutations: Osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170: 2575–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra, S.K., Lustig, F., Akesson, B., Axberg, T., Elias, P., and Lagerkvist, U. 1979. Relative efficiency of anticodons in reading the valine codons during protein synthesis in vitro. J. Biol. Chem. 254: 6397–6401. [PubMed] [Google Scholar]
- Neidhardt, F.C., Bloch, P.L., and Smith, D.F. 1974. Culture medium for enterobacteria. J. Bacteriol. 119: 736–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidhardt, F.C., Bloch, P.L., Pedersen, S., and Reeh, S. 1977. Chemical measurement of steady-state levels of ten aminoacyl-transfer ribonucleic acid synthetases in Escherichia coli. J. Bacteriol. 129: 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, S. 1979. Modified nucleosides in tRNA. In Transfer RNA: Structure, properties, and recognition (eds. P.R. Schimmel et al.), pp. 59–79. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- Oda, K., Kimura, F., Harada, F., and Nishimura, S. 1969. Restoration of valine acceptor activity by combining oligonucleotide fragments derived from a Bacillus subtilis ribonuclease digest of Escherichia coli valine transfer RNA. Biochim. Biophys. Acta 179: 97–105. [DOI] [PubMed] [Google Scholar]
- Phelps, S.S., Malkiewicz, A., Agris, P.F., and Joseph, S. 2004. Modified nucleotides in tRNALys and tRNAVal are important for translocation. J. Mol. Biol. 338: 439–444. [DOI] [PubMed] [Google Scholar]
- Pope, W.T. and Reeves, R.H. 1978. Purification and characterization of a tRNA methylase from Salmonella typhimurium. J. Bacteriol. 136: 191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope, W.T., Brown, A., and Reeves, R.H. 1978. The identification of the tRNA substrates for the supK tRNA methylase. Nucleic Acids Res. 5: 1041–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, Q. and Björk, G.R. 1997. Structural requirements for the formation of 1-methylguanosine in vivo in tRNAProGGG of Salmonella typhimurium. J. Mol. Biol. 266: 283–296. [DOI] [PubMed] [Google Scholar]
- Qian, Q., Li, J.N., Zhao, H., Hagervall, T.G., Farabaugh, P.J., and Björk, G.R. 1998. A new model for phenotypic suppression of frameshift mutations by mutant tRNAs. Mol. Cell 1: 471–482. [DOI] [PubMed] [Google Scholar]
- Reeves, R.H. and Roth, J.R. 1975. Transfer ribonucleic acid methylase deficiency found in UGA supressor strains. J. Bacteriol. 124: 332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado, H., Gama-Castro, S., Martínez-Antonio, A., Díaz-Peredo, E., Sánchez-Solano, F., Peralta-Gil, M., Garcia-Alonso, D., Jiménez-Jacinto, V., Santos-Zavaleta, A., Bonavides-Martínez, C., et al. 2004. RegulonDB (version 4.0): Transcriptional regulation, operon organization and growth conditions in Escherichia coli K-12. Nucleic Acids Res. 32 Database issue: D303–D306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson, T., Elias, P., Lustig, F., Axberg, T., Fölsch, G., Åkesson, B., and Lagerkvist, U. 1980. Aberrations of the classic codon reading scheme during protein synthesis in vitro. J. Biol. Chem. 255: 4583–4588. [PubMed] [Google Scholar]
- Schmieger, H. 1972. Phage P22-mutants with increased or decreased transduction abilities. Mol. Gen. Genet. 119: 75–88. [DOI] [PubMed] [Google Scholar]
- Takai, K., Okumura, S., Hosono, K., Yokoyama, S., and Takaku, H. 1999. A single uridine modification at the wobble position of an artificial tRNA enhances wobbling in an Escherichia coli cell-free translation system. FEBS Lett. 447: 1–4. [DOI] [PubMed] [Google Scholar]
- Vogel, H.J. and Bonner, D.M. 1956. Acetylornithinase of Escherichia coli: Partial purification and some properties. J. Biol. Chem. 218: 97–106. [PubMed] [Google Scholar]
- Wagner, J. and Nohmi, T. 2000. Escherichia coli DNA polymerase IV mutator activity: Genetic requirements and mutational specificity. J. Bacteriol. 182: 4587–4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner, J., Gruz, P., Kim, S.R., Yamada, M., Matsui, K., Fuchs, R.P., and Nohmi, T. 1999. The dinB gene encodes a novel E. coli DNA polymerase, DNA pol IV, involved in mutagenesis. Mol. Cell 4: 281–286. [DOI] [PubMed] [Google Scholar]
- Winkler, M.E. 1996. Biosynthesis of histidine. In Escherichia coli and Salmonella: Cellular and molecular biology, 2nd ed. (eds. F.C. Neidhardt et al.), pp. 485–505. ASM Press, Washington, DC.
- Yokoyama, S., Watanabe, T., Murao, K., Ishikura, H., Yamaizumi, Z., Nishimura, S., and Miyazawa, T. 1985. Molecular mechanism of codon recognition by tRNA species with modified uridine in the first position of the anticodon. Proc. Natl. Acad. Sci. 82: 4905–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]