Abstract
The RimM protein in Escherichia coli is associated with free 30S ribosomal subunits but not with 70S ribosomes. A ΔrimM mutant is defective in 30S maturation and accumulates 17S rRNA. To study the interaction of RimM with the 30S and its involvement in 30S maturation, RimM amino acid substitution mutants were constructed. A mutant RimM (RimM-YY→AA), containing alanine substitutions for two adjacent tyrosines within the PRC β-barrel domain, showed a reduced binding to 30S and an accumulation of 17S rRNA compared to wild-type RimM. The (RimM-YY→AA) and ΔrimM mutants had significantly lower amounts of polysomes and also reduced levels of 30S relative to 50S compared to a wild-type strain. A mutation in rpsS, which encodes r-protein S19, suppressed the polysome- and 16S rRNA processing deficiencies of the RimM-YY→AA but not that of the ΔrimM mutant. A mutation in rpsM, which encodes r-protein S13, suppressed the polysome deficiency of both rimM mutants. Suppressor mutations, found in either helices 31 or 33b of 16S rRNA, improved growth of both the RimM-YY→AA and ΔrimM mutants. However, they suppressed the 16S rRNA processing deficiency of the RimM-YY→AA mutant more efficiently than that of the ΔrimM mutant. Helices 31 and 33b are known to interact with S13 and S19, respectively, and S13 is known to interact with S19. A GST-RimM but not a GST-RimM(YY→AA) protein bound strongly to S19 in 30S. Thus, RimM likely facilitates maturation of the region of the head of 30S that contains S13 and S19 as well as helices 31 and 33b.
Keywords: RimM, 30S maturation, 16S rRNA processing, S19, S13, helices 31 and 33b
INTRODUCTION
Up to 40% of the total energy production in Escherichia coli is consumed by ribosomal biogenesis emphasizing the importance of a coordinated synthesis and assembly of ribosomal components. The 50S and 30S ribosomal subunits can be reconstituted into fully active ribosomes from the isolated components in vitro. However, nonphysiological conditions like heat-activation steps and different Mg2+ concentrations are required to achieve a reasonable output of mature ribosomal subunits. The maturation of the 50S and 30S subunits in vivo requires auxiliary proteins that are not part of mature ribosomes. Apart from rRNA processing enzymes and rRNA/r-protein modifying enzymes, a number of other proteins assist in the assembly of the 50S and 30S subunits in E. coli. The DEAD box RNA helicases (Fuller-Pace 1994), SrmB (Nashimoto et al. 1985; Nishi et al. 1988; Charollais et al. 2003), DbpA (Fuller-Pace et al. 1993; Tsu and Uhlenbeck 1998), and CsdA (formerly DeaD) (Toone et al. 1991; Charollais et al. 2004) are assumed to open up RNA structures and allow proper folding of the ribosome. The DnaK chaperone system (Alix and Guérin 1993; Maki et al. 2003), additional heat-shock proteins including GroEL (El Hage et al. 2001), as well as the essential GTPase Era (Nashimoto et al. 1985; Nashimoto 1993), the cold-shock protein RbfA (Dammel and Noller 1995; Jones and Inouye 1996), and the YrdC protein (Kaczanowska and Rydén-Aulin 2004) are likely involved in ribosome assembly.
The RimM protein is associated with free 30S subunits but not with 70S ribosomes, suggesting an involvement in maturation of the 30S subunits or in translation initiation (Bylund et al. 1997). RimM is present in most eubacterial species whose genomes have been sequenced; however, it is not found in any species of the domain Archaea. In addition, RimM-like proteins are reported from at least four eukaryotic species: the malaria parasites Plasmodium falciparum and Plasmodium yoelii, the malaria mosquito Anopheles gambiae, and the chloroplast of the plant Arabidopsis thaliana. An in-frame deletion of rimM (ΔrimM), removing all except the first five and last five codons, decreases the growth rate sevenfold and reduces the translational efficiency at 37°C (Bylund et al. 1997). Moreover, the ΔrimM mutant accumulates 17S rRNA, suggesting a role for RimM in maturation of the 30S subunits (Bylund et al. 1998). Extragenic suppressor mutations to the ΔrimM mutation increase the growth rate and translational efficiency of the ΔrimM mutant (Bylund et al. 1997, 1998, 2001). Some of these mutations alter the carboxy-terminal 16S rRNA-binding domain of r-protein S13 (Bylund et al. 1997). The remaining suppressor mutations increase the expression of rbfA (Bylund et al. 1998, 2001). The carboxy-terminal half of RimM was proposed to contain a PRC β-barrel domain (Anantharaman and Aravind 2002). Previously, a mutant RimM protein (RimM-YY→AA) containing alanine substitutions for two adjacent tyrosines within the PRC β-barrel domain showed a reduced binding to the 30S ribosomal subunits (Lövgren and Wikström 2001).
In this paper, we show that the RimM-YY→AA mutant accumulated 17S rRNA relative to mature 16S, had lower amounts of 30S relative to 50S subunits, and had reduced levels of polysomes in comparison to a wild-type strain. Mutations in two adjacent helices (31 and 33b) of 16S rRNA and in the genes for r-proteins S13 and S19 were found to suppress the slow growth of the RimM-YY→AA mutant. Furthermore, a GST-RimM wild-type protein bound strongly to S19 in the 30S subunits, whereas a GST-RimM(YY→AA) mutant protein did not. These findings suggest that RimM is important for the maturation of the head region of the 30S subunits in which helices 31 and 33b as well as r-proteins S13 and S19 are located.
RESULTS
Identification of functionally important amino acid residues in RimM
Functionally important amino acids in RimM were identified by using two different mutagenic approaches. First, to obtain random mutations in rimM, plasmid pMW279, carrying rimM under the control of an IPTG-inducible promoter, was mutagenized with hydroxylamine. Derivatives of pMW279 that contained rimM alleles that could not completely complement the slow growth of a ΔrimM mutant were identified. Two rimM alleles complemented the slow growth at 37°C but not at 42°C and expressed RimM proteins with the single amino acid substitutions G17E and G27R, respectively. Another rimM allele encoded a RimM-G121D protein that showed less complementation than wild-type RimM, when the expression from the IPTG-inducible promoter was limited. The amounts of RimM-G17E and RimM-G27R were 10-fold higher at both 37°C and 42°C than the amount of RimM in a wild-type strain (data not shown). Thus, the G17E and G27R substitutions severely affected the function of RimM, and the poor complementation by the two RimM mutant proteins at 42°C did not result from a decreased stability at higher temperatures.
Second, to identify conserved amino acids that might be of functional importance, the RimM amino acid sequences from 17 evolutionary distant species were aligned (Fig. 1 ▶). Some positions in the amino terminal part of RimM are highly conserved, including G17 and G27, which were identified as important for RimM function in the random mutagenesis screen above. In RimM from E. coli, these glycines are part of the sequences GKMG and GIRG, which are similar to the GXXG motif of the KH domain in RNA-binding proteins (Gibson et al. 1993; Liu et al. 1995). To investigate whether these sequences were important for the binding of RimM to the 30S subunits, we replaced the four glycines and the two basic amino acids of the two GXXG motifs with alanines. Strains with these alanine substitutions (G17A, K18A, G20A, G24A, R26A, and G27A) in chromosomally encoded RimM showed growth rates that were similar to that of a wild-type strain at both 37°C and 42°C (data not shown).
FIGURE 1.
Amino acid sequence alignment of RimM from different eubacterial species, the malaria mosquito Anopheles gambiae, and Arabidopsis thaliana. Eco, Escherichia coli; Bsu, Bacillus subtilis; Vpa, Vibrio parahaemolyticus; Nme, Neisseria meningitidis; Ath, Arabidopsis thaliana; Syn, Synechocystis sp. Strain PCC6803; Cte, Clostridium tetani; Aae, Aquifex aeolicus; Cht, Chlorobium tepidum; Fnu, Fusobacterium nucleatum; Mlo, Mesorhizobium loti; Mle, Mycobacterium leprae; Dra, Deinococcus radiodurans; Aga, Anopheles gambiae; Bbu, Borrelia burgdorferi; Hpy, Helicobacter pylori. The numbering to the left of the top row indicates the position in the respective sequence of the first amino acid shown. Amino acid substitutions were isolated for the positions indicated by arrows. The * indicates the position of the N84K substitution suppressing RimM-YY→AA.
In the proposed PRC β-barrel domain of RimM, the conserved aromatic amino acids in positions 106–108, together with the conserved aspartate in position 137 are suggested to bind protein (Anantharaman and Aravind 2002). The two tyrosines in positions 106 and 107 and the aspartate in position 137 were replaced with alanines. The D137A substitution reduced the growth rate at temperatures from 21°C to 44°C, and the effect was more pronounced at the higher temperatures. The growth deficiency was likely due to a decreased stability of the mutant protein, because the RimM levels were more than fivefold lower in the mutant compared to the wild type (data not shown). In contrast, the double substitution Y106A/Y107A reduced the function of RimM, because it lowered the growth rate threefold (Fig. 2 ▶) without affecting protein stability (Lövgren and Wikström 2001). The single substitution in position 106 had no effect on the growth rate, whereas that in position 107 slightly reduced the growth rate, especially at 42°C (data not shown).
FIGURE 2.
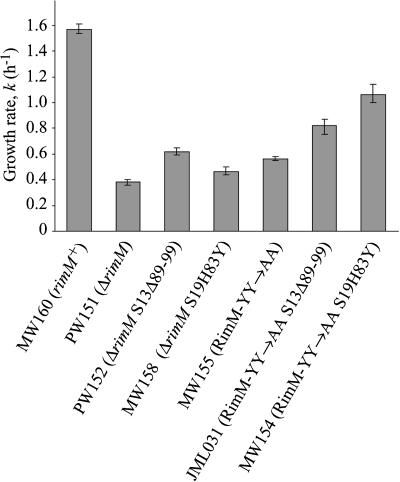
The effect of S13Δ89-99 and S19H83Y on the growth rate of the ΔrimM and RimM-YY→AA mutants grown in LB medium at 37°C. The growth rate was determined in four independent experiments and is expressed as k (= ln2/g, where g is the mass doubling time in h). The variation between the experiments is shown as error bars.
Charged amino acid side chains are likely exposed on the surface of proteins and might, in the case of RimM, interact with the 30S subunits. Therefore, charged amino acid residues adjacent to each other in RimM (E36-D37, E102-E103, K109-D110) were replaced by alanines. None of the double substitutions E36A/D37A, E102A/E103A, and K109A/D110A affected the growth rate of the cells on minimal medium plates at 25°C–41.5°C or on rich medium plates at 25°C–44°C (data not shown).
Isolation of suppressor mutations that increase the growth rate of the RimM-YY→AA mutant
The RimM-YY→AA protein binds less well to the 30S subunits than does the wild-type RimM protein (Lövgren and Wikström 2001). The RimM-YY→AA mutant was used to select for suppressor mutations that restored binding of RimM-YY→AA to the 30S subunits resulting in faster growth. One suppressor strain (JML025) contained an N84K substitution in RimM, which increased the growth rate of the RimM-YY→AA mutant twofold (the specific growth rate, k = ln2/g, where g is the mass doubling time in h, was 1.17 and 0.57, respectively). The growth rate of the wild-type strain MW100 was 1.61 in the same experiment. To examine whether the N84K substitution also restored the binding of RimM to the 30S subunits, a cell extract of the RimM-YY→AA mutant containing the N84K substitution (JML025) was fractionated by sucrose gradient centrifugation and screened for the presence of RimM with an anti-RimM antiserum. No higher amounts of RimM were observed in fractions containing the 30S subunits from the RimM-YY→AA/N84K suppressor strain compared to those from the RimM-YY→AA mutant (data not shown). Thus, the N84K substitution did not restore the binding to the 30S subunits, although it improved the function of the 30S subunits (see below).
Two of the suppressor strains (JML001, JML026) contained extragenic mutations that were similar to previously isolated mutations in the metY-nusA-infB operon that increase expression of rbfA (Bylund et al. 2001). Another mutation (spr-8, strain MW145) changed the histidine codon in position 83 of rpsS, for r-protein S19, to a tyrosine codon (data not shown). To investigate whether the S19H83Y protein also suppressed the slow growth of a strain lacking RimM, the rpsS mutation was combined with the ΔrimM mutation. S19H83Y was found to be a weaker suppressor of the ΔrimM mutant than were mutations that increased expression of rbfA, although they all suppressed the slow growth of the RimM-YY→AA mutant similarly (data not shown). These results indicated that the H83Y substitution in S19 might have restored a physical interaction between the RimM-YY→AA protein and S19. Therefore, a cellular extract of an S19H83Y RimM-YY→AA double mutant was fractionated by sucrose gradient centrifugation and screened for the presence of RimM with an anti-RimM antiserum. However, S19H83Y did not increase the amount of the RimM-YY→AA protein associated with the 30S subunits (data not shown).
Effect of alterations in r-proteins S13 and S19 on polysome profiles of the RimM-YY→AA and ΔrimM mutants
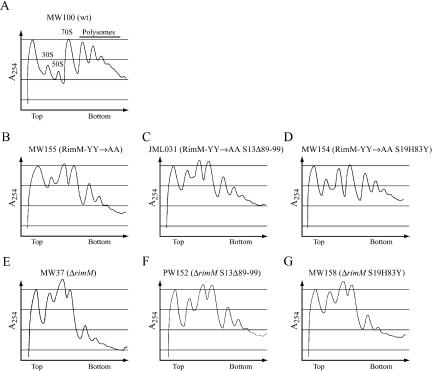
To estimate the translational defects of the RimM-YY→AA and ΔrimM mutants and to examine the ability of alterations in S13 and S19 to suppress these defects, we analyzed polysome profiles of the different strains by sucrose gradient centrifugation of cellular extracts. The ΔrimM mutant (MW37) showed a significantly lower amount of polysomes than the wild type (MW100) and, hence, an accumulation of free 30S and 50S subunits (Fig. 3A,E ▶). The RimM-YY→AA mutant showed a similar translational defect, although it was not as severe as that for the ΔrimM mutant (Fig. 3 ▶, cf. B and E). Interestingly, both rimM mutants had significantly lower amounts of free 30S subunits relative to 50S subunits compared to the wild type, indicating that a deficiency in the maturation of the 30S subunits could explain the low amounts of polysomes. Previously obtained mutations in rpsM, which encodes r-protein S13, improved translation in a ΔrimM mutant (Bylund et al. 1997). The rpsM873 mutation removed amino acids 89 to 99 of S13. The S13Δ89-99 protein partially corrected the imbalance in the amount of 30S to 50S subunits and increased the amount of polysomes of both the RimM-YY→AA (Fig. 3C ▶) and the ΔrimM mutant (Fig. 3F ▶). Also, the S19H83Y protein increased the amount of polysomes of both rimM mutants (Fig. 3D,G ▶). In the RimM-YY→AA mutant, the effect of S19H83Y was greater than that of S13Δ89-99 (Fig. 3B–D ▶), whereas in the ΔrimM mutant, the effect of S19H83Y was much less than that of S13Δ89-99 (Fig. 3E–G ▶). For the two rimM mutants as well as their S13Δ89-99 and S19H83Y derivatives, the relative growth rate (Fig. 2 ▶) correlated very well with the amount of polysomes detected, demonstrating that the slow growth of rimM mutants is due to poor translation.
FIGURE 3.
The effect of S13Δ89-99 and S19H83Y on polysome profiles of the ΔrimM and RimM-YY→AA mutants grown in LB medium at 37°C. Different ribosome particles are marked above corresponding peaks in A.
Mutations in helices 31 and 33b of 16S rRNA suppress the slow growth of the RimM-YY→AA and ΔrimM mutants
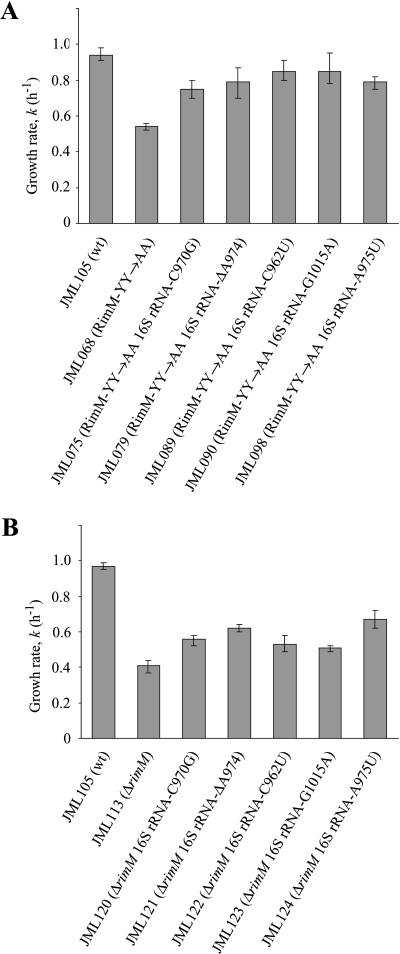
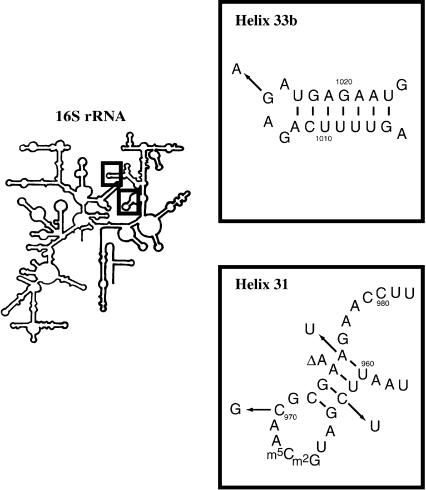
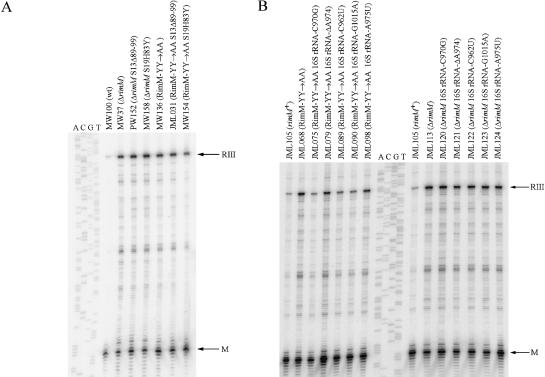
Conceivably, if RimM interacts with 16S rRNA of the 30S subunits, compensatory mutations in 16S rRNA could be isolated that would restore the interaction between the RimM-YY→AA protein and 16S rRNA. However, because E. coli contains seven chromosomal rrn operons (for rRNA), a single mutation may not give any observable suppression. Therefore, we took advantage of an E. coli strain in which all chromosomal rrn operons have been inactivated (Asai et al. 1999a) and the production of ribosomes is dependent on an rrn operon present on plasmid pHK-rrnC+ (Asai et al. 1999b). The rimM mutant gene (rimM120) for RimM-YY→AA was introduced into this strain. In this genetic background, RimM-YY→AA reduced the growth rate of the cells twofold (Fig. 4A ▶) compared to a threefold reduction in the growth rate of strains containing all chromosomal rrn operons (Fig. 2 ▶). Several faster-growing suppressor mutants of the RimM-YY→AA mutant (JML068) were isolated, the fastest of which had growth rates that were close to 90% of the rimM+ strain (Fig. 4A ▶). Twelve suppressor mutations were localized to plasmid pHK-rrnC+ and identified by DNA sequencing. Ten of the mutations were in four different positions of helix 31, and two were in the identical position of helix 33b of 16S rRNA (Fig. 5 ▶). To investigate whether the 16S rRNA mutations restored the binding of the RimM-YY→AA protein to 30S subunits, total cell extracts of three of the suppressor strains (JML075, JML079, and JML098) and of appropriate control strains were subjected to sucrose gradient centrifugation. Fractions were analyzed for the presence of the RimM-YY→AA protein with an anti-RimM antiserum by Western blotting. The 16S rRNA mutations did not restore binding of the RimM-YY→AA protein to the 30S subunits (data not shown). To determine whether the 16S rRNA mutations could also suppress a ΔrimM mutant, the suppressor plasmids were substituted for the suppressor-free rrn-plasmid pKK1192U in strain JML114 (Δ7rrn ΔrimM102/pKK1192U + p70). All of the mutant plasmids partially suppressed the slow growth of the ΔrimM mutant, indicating that the suppressor mutations were not allele-specific (Fig. 4B ▶).
FIGURE 4.
The effect of suppressor mutations in helices 31 and 33b of 16S rRNA on the growth rate of the RimM-YY→AA (A) and ΔrimM (B) mutants in LB medium at 37°C. The growth rate was determined in three independent experiments and is expressed as k (= ln2/g, where g is the mass doubling time in h). The variation between the experiments is shown as error bars.
FIGURE 5.
Mutations in helices 31 and 33b of 16S rRNA that suppress the slow growth of the RimM-YY→AA and ΔrimM mutants JML068 and JML114.
RimM is not directly responsible for the processing of 16S rRNA
The processing of 16S rRNA is initiated by RNase III cleavages in a double-stranded region composed of the sequences flanking mature 16S rRNA (Young and Steitz 1978). The generated 17S rRNA contains 115 extra nucleotides (nt) at the 5′ end and 33 at the 3′ end compared to mature 16S rRNA. In wild-type cells, RNase E and G remove the extra 115 nt at the 5′ end, whereas the removal of the 33 nt at the 3′ end occurs in one step by an unknown RNase and is dependent on the processing by RNase E at the 5′ end (Li et al. 1999). Because a ΔrimM mutant accumulates 17S rRNA (Bylund et al. 1998), we investigated whether the RimM-YY→AA mutant was deficient in processing of 16S rRNA. The ability of the RimM-YY→AA mutant to convert 17S to 16S rRNA was determined by primer extension analysis of the 5′-end of 16S rRNA. The RimM-YY→AA mutant showed five-to-sevenfold increased levels of 17S rRNA compared to the wild type; however, the processing deficiency was not as pronounced as for the ΔrimM mutant (Fig. 6A ▶; Table 1 ▶). To examine whether RimM could be the unknown RNase processing the 3′-end of 17S rRNA, total RNA, from a wild-type strain and from the ΔrimM and RimM-YY→AA mutants, was analyzed by Northern blotting with oligonucleotides specific for either the region just upstream of the 3′-end of mature 16S rRNA or the extra nucleotides present in 17S rRNA. The two rimM mutants showed only a partial accumulation of unprocessed 3′ ends (data not shown), mimicking the results for the processing of the 5′ end in the same mutants. Thus, RimM cannot be the unknown RNase that processes the 3′ end of 16S rRNA, and the observed partial processing deficiency was probably an indirect effect of poor processing by RNase E at the 5′ end.
FIGURE 6.
The effect of different suppressor mutations on the 16S rRNA processing deficiency of rimM mutants. The 5′-end of 16S rRNA was determined by primer extension analysis. RIII indicates a primer extension product of 179 nt corresponding to 17S rRNA processed at the RNase III site 115 nt upstream of the 5′-end of mature 16S rRNA. M indicates a product of 64 nt corresponding to the 5′-end of mature 16S rRNA. The sequencing ladder shown is of rrnC in plasmid pHK-rrnC+ and was obtained by using the same primer as for the primer extension reactions. (A) The effect of S13Δ89-99 and S19H83Y on the 16S rRNA processing in ΔrimM and RimM-YY→AA mutants. (B) The effect of 16S rRNA mutations on 16S rRNA processing in ΔrimM and RimM-YY→AA mutants.
TABLE 1.
The effect of S19H83Y, S13Δ89-99, or RimM-N84K on the processing of 16S rRNA in rimM mutants
| Accumulation of 17S rRNAa | |||||
| Strain | Relevant traits | Exp. I | Exp. II | Exp. III | Exp. IV |
| MW100 | wt | 0.074 | 0.029 | 0.051 | 0.061 |
| MW136 | RimM-YY→AA | 0.39 | 0.18 | 0.38 | 0.39 |
| MW154 | RimM-YY→AA S19H83Y | 0.26 | 0.087 | n.d. | n.d. |
| JML031 | RimM-YY→AA S13Δ89-99 | 0.43 | 0.21 | n.d. | n.d. |
| JML025 | RimM-YY→AA/N84K | n.d. | n.d. | 0.12 | 0.14 |
| MW37 | ΔrimM | 0.54 | 0.33 | n.d. | n.d. |
| MW158 | ΔrimM S19H83Y | 0.64 | 0.32 | n.d. | n.d. |
| PW152 | ΔrimM S13Δ89-99 | 0.52 | 0.34 | n.d. | n.d. |
aThe amount of 17S rRNA to the total amount of 16S rRNA [RIII / (M + RIII)] was calculated from the amount of radioactivity in the primer extension products obtained in experiments like that shown in Figure 6A ▶.
The effect of the suppressor mutations on the processing of 16S rRNA
The 16S rRNA processing deficiency of rimM mutants is likely the result of a defect in a step in the maturation of the 30S subunits that precedes the final processing of 17S into 16S rRNA (Bylund et al. 1998). To examine whether mutations suppressing the two rimM mutations improved the processing of 16S rRNA, we determined the processing efficiency of different suppressor strains by primer extension analysis of the 5′-end of 16S rRNA. Interestingly, S19H83Y suppressed partially the processing deficiency of the RimM-YY→AA mutant but not that of the ΔrimM mutant (Fig. 6A ▶; Table 1 ▶). S13Δ89-99, on the other hand, did not suppress the processing deficiency of either of the rimM mutants (Fig. 6A ▶; Table 1 ▶). To our surprise, the N84K substitution in the RimM-YY→AA protein improved the processing efficiency threefold (Table 1 ▶), although it did not improve the binding of RimM-YY→AA to the 30S subunits (see above). The five different suppressor mutations in the plasmid-carried 16S rRNA gene improved the processing of 16S rRNA in the RimM-YY→AA mutant 1.5-to-fourfold, whereas they only improved the processing 1.6-fold or less in the ΔrimM mutant (Fig. 6B ▶; Table 2 ▶). Note that the mutation with the least effect (A975U) in the RimM-YY→AA mutant (JML098) was the one that had the greatest effect in the ΔrimM mutant (JML124).
TABLE 2.
The effect of suppressor mutations in 16S rRNA on the processing of 16S rRNA in rimM mutants
| Accumulation of 17S rRNAa | |||||
| Strain | Relevant traits | Exp. I | Exp. II | Exp. III | Exp. IV |
| JML105 | RimM-wt 16S rRNA-wt | 0.066 | 0.016 | 0.039 | 0.075 |
| JML068 | RimM-YY→AA 16S rRNA-wt | 0.32 | 0.14 | 0.23 | n.d. |
| JML075 | RimM-YY→AA 16S rRNA-C970G | 0.12 | 0.047 | 0.058 | n.d. |
| JML079 | RimM-YY→AA 16S rRNA-ΔA974 | 0.18 | 0.051 | 0.052 | n.d. |
| JML089 | RimM-YY→AA 16S rRNA-C962U | 0.13 | 0.046 | 0.092 | n.d. |
| JML090 | RimM-YY→AA 16S rRNA-G1015A | 0.12 | 0.037 | 0.098 | n.d. |
| JML098 | RimM-YY→AA 16S rRNA-A975U | 0.23 | 0.075 | 0.15 | n.d. |
| JML113 | ΔrimM 16S rRNA-wt | 0.46 | n.d. | n.d. | 0.54 |
| JML120 | ΔrimM 16S rRNA-C970G | 0.40 | n.d. | n.d. | 0.45 |
| JML121 | ΔrimM 16S rRNA-ΔA974 | 0.38 | n.d. | n.d. | 0.32 |
| JML122 | ΔrimM 16S rRNA-C962U | 0.42 | n.d. | n.d. | 0.36 |
| JML123 | ΔrimM 16S rRNA-G1015A | 0.45 | n.d. | n.d. | 0.40 |
| JML124 | ΔrimM 16S rRNA-A975U | 0.30 | n.d. | n.d. | 0.32 |
aThe amount of 17S rRNA to the total amount of 16S rRNA [RIII / (M + RIII)] was calculated from the amount of radioactivity in the primer extension products obtained in experiments like that shown in Figure 6B ▶.
The tyrosines of the PRC-barrel domain of RimM bind specifically to r-protein S19 in the 30S subunits
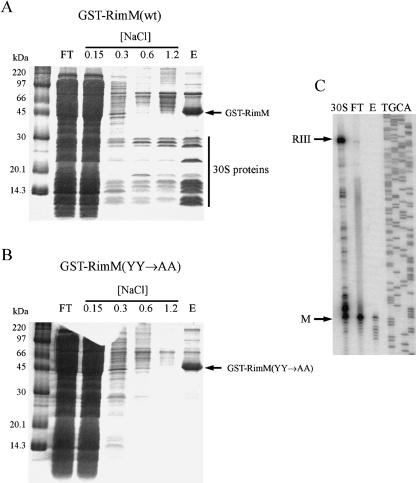
RimM is found associated with free 30S subunits but not with 70S ribosomes (Bylund et al. 1997). To identify the binding site for RimM in the 30S subunits, a hybrid protein between glutathione S-transferase (GST) and RimM was used to pull out proteins interacting with RimM. The GST-RimM protein expressed from plasmid pJML005 complemented the slow growth of a ΔrimM mutant (GOB191), suggesting that the RimM part of the hybrid protein was functional (data not shown). The GST-RimM protein from total cell extracts of strain GOB191/pJML005 was bound to a glutathione column, which was then washed with increasing concentrations of NaCl. Finally, the GST-RimM protein plus proteins bound to RimM were eluted from the column. When extracts were prepared and analyzed at 4°C, several proteins smaller than 30 kDa in size were found in the stringent wash fractions and in the eluate (Fig. 7A ▶). The eluate contained all small subunit r-proteins except S3, S14, S16, and S21 as determined by electro-spray ionization tandem mass spectrometry (ESI-MS/MS) (data not shown). In addition, r-protein L1 and some proteins not involved in translation were found in the eluate. Further, the eluate was shown by primer extension analysis to contain 16S rRNA (Fig. 7C ▶), indicating that the GST-RimM protein bound to 30S subunits and not to free r-proteins. In contrast, when extracts of a strain expressing a GST-RimM(YY→AA) mutant protein were analyzed, no r-proteins were present in the eluate or in the stringent wash fractions (Fig. 7B ▶). Thus, these results are consistent with the previous findings that the two conserved tyrosines are important for the binding of RimM to the 30S subunits (Lövgren and Wikström 2001).
FIGURE 7.
Analysis of cellular components copurifying with GST-RimM hybrid proteins at 4°C. (A) A cell extract of strain GOB191/ pJML005 (ΔrimM/GST-RimM) was prepared by freeze-thawing, and the GST-RimM hybrid protein in the extract was adsorbed to Glutathione Sepharose 4B, washed at the indicated molar concentrations of NaCl and finally eluted by reduced glutathione, all at 4°C. FT, flow-through; E, eluate. (B) As in A, but the strain used was GOB191/ pJML051 [ΔrimM/GST-RimM(YY→AA)]. (C) Primer extension analysis of 16S rRNA prepared from the flow-through and eluate from A, and from 30S subunits obtained after sucrose gradient centrifugation of a total cell extract of the wild-type strain MW100.
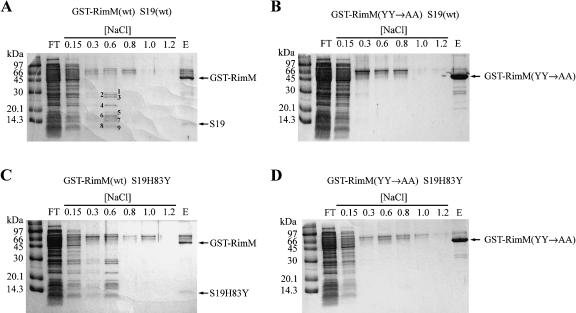
When extracts that contained the GST-RimM wild-type protein were prepared and analyzed at room temperature (~21°C), most 30S subunit r-proteins were released from the column at washes with up to 0.6 M NaCl (Fig. 8A ▶). All 30S structural proteins except S6, S12, S15, S17, S18, S19, S20, and S21 were identified when total protein in the 0.6 M NaCl wash fraction was analyzed by ESI-MS/MS (data not shown). The most intense protein bands (≤ 30 kDa in Fig. 8A ▶) were identified as r-proteins S2, S3, S4, S5, S7, S9, S10, S11, S13, and S17 by MALDI mass spectrometry. Moreover, 16S rRNA was present in the 0.6 M NaCl wash fraction as shown by primer extension analysis (data not shown), suggesting that intact 30S subunits were initially bound to the GST-RimM protein not only at 4°C but also at room temperature. No r-proteins were found in the 0.6 M NaCl wash fraction when an extract of the strain expressing the GST-RimM(YY→AA) mutant protein was analyzed by ESI-MS/ MS (Fig. 8B ▶; data not shown). Interestingly, r-protein S19, as identified by MALDI mass spectrometry (data not shown), copurified with the GST-RimM wild-type protein but not with the GST-RimM(YY→AA) mutant protein (Fig. 8 ▶, cf. A and B). These findings demonstrate that the tyrosines present in positions 106 and 107 of wild-type RimM but absent in the RimM-YY→AA protein are crucial for the binding of RimM to S19 in the 30S subunits. Note also that S19 was not present among those r-proteins that were identified in the 0.6 M NaCl wash fraction when the GST-RimM wild-type protein was purified, suggesting that S19 bound strongly to the GST-RimM protein. Thus, the interaction between RimM and S19 at room temperature seems to be stronger than that between S19 and other components of the 30S subunits, at least at higher NaCl concentrations.
FIGURE 8.
Analysis of cellular components copurifying with GST-RimM hybrid proteins at 21°C. Cellular extracts were prepared by sonication and the GST-RimM hybrid proteins were purified as described in the Figure 7 ▶ legend. Similar results were obtained when extracts were prepared by freeze-thawing. (A) Strain GOB191/pJML005 (ΔrimM S19-wt/GST-RimM). The proteins indicated in the lane with the 0.6 M NaCl wash fraction were identified as 30S subunit r-proteins by MALDI mass spectrometry: 1, S2; 2, S3; 3, S4; 4, S7; 5, S5; 6, S9; 7, S11 and S13; 8, S10; and 9, S17. In addition, one weak band above that containing S2 was identified as r-protein L2. (B) Strain GOB191/pJML051 [ΔrimM S19-wt/GST-RimM(YY→AA)]. (C) Strain MW158/pJML005 (ΔrimM S19H83Y/GST-RimM). (D) Strain MW158/pJML051 [ΔrimM S19H83Y/GST-RimM(YY→AA)].
The 30S subunits bound to the GST-RimM wild-type protein both at 4°C and at room temperature contained mature 16S rRNA and almost no 17S rRNA. This was unexpected, because a ΔrimM mutant accumulates 17S rRNA and therefore RimM has been suggested to bind 30S subunits that contain 17S rRNA rather than fully processed 16S rRNA.
To investigate whether S19H83Y, which improved the processing of 16S rRNA of the RimM-YY→AA mutant but not that of the ΔrimM mutant, could restore the binding of the RimM-YY→AA protein to the 30S subunits, the GST-RimM and GST-RimM(YY→AA) proteins were purified from strains expressing the S19H83Y protein. The S19H83Y protein copurified with the GST-RimM protein only (Fig. 8C ▶) and not with the GST-RimM(YY→AA) protein (Fig. 8D ▶) when extracts were prepared and analyzed at room temperature. Further, no larger amounts of r-proteins were found in the wash fractions of the S19H83Y GST-RimM(YY→AA) strain than in those of the S19-wt GST-RimM(YY→AA) strain. Thus, S19H83Y did not seem to restore the binding of the RimM-YY→AA protein to the 30S subunits.
DISCUSSION
Identification of functionally important positions in RimM
The introduction of charged amino acids in two of the most conserved positions of RimM (G17E and G27R) dramatically reduced the stability of the protein (data not shown), but also reduced its function at high temperature without a further decrease in stability, implying a structural role of the conserved glycines. More subtle substitutions in the same positions (G17A and G27A) had no significant effects on the function of RimM. Because alanine for glycine substitutions are believed to affect the function of a protein mainly when glycines are important for the flexibility of a polypeptide chain, our results suggest other structural roles for these glycines in RimM. The double substitution Y106A/ Y107A reduced the affinity of RimM for the 30S subunits, implying that the two tyrosines are important for the binding of RimM to the 30S subunits (this paper; Lövgren and Wikström 2001). These tyrosines together with the highly conserved aspartate in position 137 of a proposed PRC β-barrel domain are suggested to line a cleft into which a peptide of an interacting protein could be accommodated (Anantharaman and Aravind 2002). The D137A substitution severely reduced the stability of RimM, which precluded the possibility to assess whether or not this position in RimM interacts with the 30S subunits. However, the effect on the stability suggests an important role of this aspartate for the structure and stability of the PRC domain. Another substitution (G121D) in the PRC domain also affected the structure and/or function of RimM, as plasmid-encoded RimM variants with this substitution showed a reduced ability to complement chromosomal deletions of rimM.
Identification of the target region for RimM in the 30S subunits
Alterations in r-proteins S13 and S19 as well as mutations in helices 31 and 33b of 16S rRNA suppress the slow growth of a RimM-YY→AA mutant and to different degrees that of a ΔrimM mutant. These alterations are all located in a distinct region of the head of the 30S subunit (Fig. 9 ▶) in which helix 33b interacts with S19, helix 31 contacts S13, and S13 and S19 interact with each other (Wimberly et al. 2000). In addition, wild-type RimM, in contrast to the RimM-YY→AA protein, bound strongly to S19 in the 30S subunits, suggesting that the tyrosines in positions 106–107 of RimM mediate the binding to S19. One possible role for RimM in ribosome maturation might be to facilitate the interaction of S19 with helix 33b, and perhaps also the interaction between S13 and helix 31. In the maturation of the 30S subunits, the 5′-end processing of 16S rRNA has been suggested to be one of the last steps, which would also activate the 30S subunits (see Nierhaus 1991). Conceivably, a defect in any step in the 30S maturation process that precedes the processing of the 5′-end of 16S rRNA might result in a 16S rRNA processing deficiency and also a translational deficiency. Thus, the 16S rRNA processing deficiency observed for the ΔrimM and RimM-YY→AA mutants (this paper; Bylund et al. 1998) could stem from poor or inappropriate interactions in the S13-S19 region of the 30S subunits. Interestingly, S19 plays a key role in r-protein-dependent conformational rearrangements of 16S rRNA during 30S maturation in vitro (Holmes and Culver 2004).
FIGURE 9.
Alterations in the E. coli 30S subunit (indicated in the model for the 30S subunit of Thermus thermophilus) that suppress rimM mutations. The structure of the 30S subunit is from Wimberly et al. (2000) and was retrieved from the Protein Data Bank (PDB no. 1FJF). Only part of the structure is shown. Helices 31 and 33b of 16S rRNA are presented as stick models in green except for the positions with suppressor mutations, which are in space-fill models. R-protein S13 is shown in a blue ribbon model except for a region in gray, which contains the alterations that suppress rimM mutations. S19 is shown in a yellow ribbon model with the most carboxy terminal amino acid, R81, of the determined structure, colored red. Thus, the alteration, H83Y, in S19 that suppressed RimM-YY→AA is in a position just outside of the determined structure.
RimM-YY→AA-dependent and RimM-YY→AA-independent suppression
The S19H83Y protein partially restored the ability of the RimM-YY→AA mutant to process 16S rRNA and to form polysomes. However, S19H83Y had less effect on the growth and polysome deficiencies and no effect on the 16SrRNA processing deficiency of the ΔrimM mutant. Thus, to mediate efficient suppression, S19H83Y is dependent on the presence of the RimM-YY→AA protein, especially for the processing of 16S rRNA. Four of the five different mutations in helices 31 and 33b of 16S rRNA were more efficient suppressors of the 16S rRNA processing deficiency of the strain containing the RimM-YY→AA protein compared to that of a mutant lacking RimM, although the 16S rRNA mutations had similar effects on the growth rate of the two rimM mutants. The fifth 16S rRNA mutation (A975U) suppressed the processing deficiency similarly in the two rimM mutants; however, it was the weakest of the five suppressor mutations in the RimM-YY→AA mutant. Interestingly, the effect of the 16S rRNA suppressor mutations on the growth rate did not correlate with their effect on the processing of 16S rRNA. Further, S13Δ89-99 increased the growth rate of and amount of polysomes in the ΔrimM and RimM-YY→AA mutants, although it did not correct the 16S rRNA processing deficiency. Taken together, these findings suggest that for the suppression of the translational defects of the rimM mutants, there are two different mechanisms. One mechanism improves the function of 30S subunits containing fully processed 16S rRNA. This is corroborated by earlier results showing that mature ribosomes in a strain lacking RimM are not as efficient as wild-type ribosomes in translating the lacZ mRNA (Bylund et al. 1997). A second mechanism improves the maturation of the 30S subunits, resulting in more efficient processing of 16S rRNA. Evidently, the ability of the different ribosomal suppressor mutations to mediate efficient processing of 16S rRNA requires the presence of the RimM-YY→AA (or wild-type RimM) protein. This suggests that the poor interaction of the RimM-YY→AA protein with the 30S subunits in combination with structural changes in the 30S subunits brought about by the suppressor mutations can promote efficient processing of 16S rRNA. Alternatively, the ribosomal suppressor mutations might partially restore the interaction of the RimM-YY→AA protein with the 30S subunits, despite the fact that we have been unable to demonstrate any such effects. We have assumed that the interaction detected between RimM and 30S subunits after sucrose gradient centrifugation of cellular extracts involves immature 30S subunits that contain partially processed 16S rRNA, because the ΔrimM and RimM-YY→AA mutants accumulate 17S rRNA, and because RimM is not associated with 30S subunits in 70S ribosomes (containing only fully processed 16S rRNA). However, the analysis of 30S subunits copurifying with a GST-RimM protein suggests that the binding detected is with 30S subunits containing fully processed 16S rRNA. Possibly, two types of interactions between RimM and the 30S subunits take place: a weak interaction, which we have been unable to detect, occurs initially between RimM and the 30S subunits that contain 17S rRNA, followed by a stronger binding, which we have observed in this study, once the 30S subunits have matured further and contain fully processed 16S rRNA. Interestingly, the N84K substitution in RimM-YY→AA improved processing of 16S rRNA, suggesting that it did improve binding of this RimM protein to the 30S subunits although it did not affect binding in our assay. The simplest explanation of these contradictory results, assuming that RimM is not required for the synthesis of a protein involved in 16S rRNA processing, is that the N84K substitution, and possibly some of the ribosomal suppressors, such as S19H83Y, improve the proposed weak interaction between the RimM-YY→AA protein and the 30S subunits.
Are RimM and RbfA involved in 30S maturation steps both before and after processing of 16S rRNA?
RimM and RbfA bind to free 30S subunits in vivo (Dammel and Noller 1995; Bylund et al. 1997), and the GTPase Era binds to 16S rRNA and 30S subunits in vitro (Sayed et al. 1999). Cells lacking RimM or RbfA or cells depleted for Era accumulate 17S rRNA (Bylund et al. 1998; Inoue et al. 2003; Xia et al. 2003), and the levels of polysomes decrease with a concomitant increase in free 30S and 50S subunits (this paper; Dammel and Noller 1995; Inoue et al. 2003). Thus, these three proteins seem to take part in late steps of the maturation of the 30S subunits that precede the conversion of 17S to 16S rRNA. In addition, increased expression of RbfA suppresses a cold-sensitive mutation in the 5′-terminal helix of 16S rRNA, and RbfA was proposed to bind to this helix (Dammel and Noller 1995). The 5′-terminal helix is found only in mature 16S rRNA and not in 17S rRNA (Srivastava and Schlessinger 1989), suggesting that RbfA is involved in maturation after formation of 16S rRNA. Interestingly, a mutant RbfA protein missing the 25 most carboxy-terminal amino acids does not associate stably with 30S subunits and does not suppress the cold sensitivity of the helix mutation. However, the mutant RbfA protein complemented the 16S rRNA processing defect of the rbfA null mutant (Xia et al. 2003). Thus, RbfA may be involved in steps of 30S maturation both before and after the conversion of 17S to 16S rRNA. Increased expression of RbfA partially suppresses translational defects of the ΔrimM mutant (Bylund et al. 1998). Previously, we suggested that RimM participates before RbfA in 30S maturation and that RbfA cannot bind efficiently to the 30S subunits in the absence of RimM as the 30S subunits have not matured correctly. Conceivably, an increased level of RbfA would suppress a weak binding. However, in a ΔrimM mutant grown at 37°C, most RbfA molecules are associated with the 30S subunits and almost no free RbfA is found (J.M. Lövgren and P.M. Wikström, unpubl.). Thus, the 30S maturation deficiency of the ΔrimM mutant might be partly explained by limiting levels of free RbfA. This explanation would also account for why an increased amount of RbfA partially suppresses the defects of rimM mutants. Thus, if RimM acts before RbfA, then the reason for RbfA not being released from the 30S subunits in the ΔrimM mutant could be that there is a block in the next step of the maturation process. Such a step could be the binding of Era, because increased levels of Era suppress an rbfA mutant (Inoue et al. 2003). However, increased levels of Era do not suppress the slow growth of the ΔrimM mutant, as would have been expected. Alternatively, RbfA might act before RimM and will normally only be released from the 30S subunits when RimM has participated in the maturation process, which would explain the out-titration of RbfA in the ΔrimM mutant. Consequently, RbfA and RimM might bind, in that order, to immature 30S subunits and facilitate maturation steps that precede and are required for efficient conversion of 17S to 16S rRNA. Then, RbfA could also participate in the formation of the 5′ terminal helix of mature 16S rRNA before being released from the 30S subunits. Possibly, RimM could be involved in maturation steps after the final processing of 16S rRNA, as indicated by the presence of mature 16S in the 30S subunits that copurified with the GST-RimM hybrid protein.
Here we have shown that the role of RimM in ribosome maturation involves the binding to S19 in the head of the 30S subunits. Our future strategies will aim at revealing any interplay between RimM and RbfA, and it will be interesting to determine whether RbfA takes part in maturation of the S19 region of the 30S subunits.
MATERIALS AND METHODS
Strains, phages, plasmids, and growth conditions
Relevant strains, phages, and plasmids used are listed in Table 3 ▶. Strain JML068 (Δ7rrn rimM120/pHK-rrnC+ p70) was constructed in several steps: First, pheA18::Tn10 from CAG12158 (Singer et al. 1989) was introduced into strain MW136 (rimM120) by P1 transduction selecting for TcR. From the resulting strain, MW148 (rimM120 pheA18::Tn10), the rimM120 mutation was transferred to strain TA527 (Δ7rrn/pHK-rrnC+ pTRNA66) by selecting for the linked pheA18::Tn10, yielding strain JML042 (Δ6rrn rrnG+ rimM120 pheA18::Tn10/pHK-rrnC+ pTRNA66). Then, Δ (rrsG-gltW-rrlG)30::lacZ+ from strain TA527 was reintroduced by P1 transduction of strain JML042 selecting for Phe+, yielding strain JML067 (Δ7rrn rimM120/pHK-rrnC+ pTRNA66). Finally, pTRNA66 (SpR) of strain JML067 was replaced by the similar p70 (TcR) plasmid from strain AVS69009 (Vila-Sanjurjo et al. 1999) by transformation, selecting for TcR and screening for SpS. A derivative of strain JML068, JML071 (Δ7rrn rimM120/pKK1192U p70), was constructed by transformation of JML068 with plasmid pKK1192U (CbR) (Makosky and Dahlberg 1987), which carries an rrnB operon with a mutation in 16S rRNA (C1192U) conferring SpR, selecting for SpR and CbR, and screening for KmS (i.e., the loss of pHK-rrnC+). Strains JML113 and JML114 containing ΔrimM102 instead of rimM120 were constructed in a similar way as JML068 and JML071, respectively.
TABLE 3.
Bacterial strains, bacteriophages, and plasmids
| Strain, phage, or plasmid | Genotype | Origin or referencea |
| Strains | ||
| CAG12158 | pheA18::Tn10 | (Singer et al. 1989) |
| GOB191 | Hfr P4X ΔrimM102 pheA18::Tn10 | P1(CAG12158) X MW37 |
| JML001 | MW136 spr-9 (ΔA in codon 412 of nusA) | |
| JML025 | Hfr P4X rimM131 (AAC to AAA in codon 84, TAC to GCT in codon 106, and TAC to GCG in codon 107) | |
| JML026 | MW136 spr-19 (ΔTinfβ) | |
| JML031 | MW136 rpsM873 zhc-2421::Tn10 | |
| JML037 | Hfr P4X rimM121 (GAA to GCA and GAC to GCG in codons 36 and 37, respectively) | |
| JML039 | Hfr P4X rimM122 (GAA to GCA and GAG to GCG in codons 102 and 103, respectively) | |
| JML040 | Hfr P4X rimM123 (AAA to GCA and GAC to GCG in codons 109 and 110, respectively) | |
| JML068 | TA527 rimM120/pHK-rrnC+ p70 (substituted for pTRNA66) | |
| JML071 | JML068 pKK1192U (substituted for pHK-rrnC+) | |
| JML075 | JML068 spr-41 (C970G in rrsC) | |
| JML079 | JML068 spr-45 (ΔA975 in rrsC) | |
| JML089 | JML068 spr-53 (C962U in rrsC) | |
| JML090 | JML068 spr-54 (G1015A in rrsC) | |
| JML098 | JML068 spr-62 (A975U in rrsC) | |
| JML105 | TA527 p70 (substituted for pTRNA66) | |
| JML113 | TA527 ΔrimM102/pHK-rrnC+ p70 (substituted for pTRNA66) | |
| JML114 | TA527 ΔrimM102/pKK1192U and p70 (substituted for pHK-rrnC+ and pTRNA66, respectively) | |
| JML120 | JML113 spr-41 (C970G in rrsC) | |
| JML121 | JML113 spr-45 (ΔA975 in rrsC) | |
| JML122 | JML113 spr-53 (C962U in rrsC) | |
| JML123 | JML113 spr-54 (G1015A in rrsC) | |
| JML124 | JML113 spr-62 (A975U in rrsC) | |
| JML145 | Hfr P4X rimM134 (GAC to GCA in codon 137) | |
| MW37 | Hfr P4X ΔrimM102 yfiB::nptI | (Persson et al. 1995) |
| MW100 | Hfr P4X | (Wikström et al. 1988) |
| MW122 | Hfr P4X rimM114 (GGT to GCT in codon 20) | |
| MW124 | Hfr P4X rimM115 (GGT to GCT in codon 24) | |
| MW130 | Hfr P4X rimM113 (AAA to GCA in codon 18) | |
| MW134 | Hfr P4X rimM116 (CGT to GCT in codon 26) | |
| MW136 | Hfr P4X rimM120 (TAC to GCT in codon 106 and TAC to GCG in codon 107) | |
| MW138 | Hfr P4X rimM112 (GGA to GCA in codon 17) | |
| MW139 | Hfr P4X rimM117 (GGG to GCG in codon 27) | |
| MW145 | MW136 rpsS876 (spr-8) | |
| MW154 | MW145 zhc-2421::Tn10 | |
| MW155 | MW136 zhc-2421::Tn10 | |
| MW158 | MW37 rpsS876 zhc-2421::Tn10 | |
| MW160 | Hfr P4X yfiB::nptI rpsS876 zhc-2421::Tn10 | |
| MW162 | Hfr P4X rimM118 (TAC to GCT in codon 106) | |
| MW163 | Hfr P4X rimM119 (TAC to GCG in codon 107) | |
| PW151 | MW37 zhc-2421::Tn10 | |
| PW152 | MW37 rpsM873 (deletion of codons 89 to 99) zhc-2421::Tn10 | |
| TA527 | Δ (rrsA-ileT-alaT-rrlA)34 Δ (rrsB-gltT-rrlB)101 | (Asai et al. 1999a) |
| Δ (rrsC-gltU-rrlC)15::cat Δ (rrsD-ileU-alaU-rrlD)25::cat | ||
| Δ (purDH-rrnE-metA) Δ (rrsG-gltW-rrlG)30:: lacZ+ | ||
| Δ (rrsH-ileV-alaV-rrlH)103 ara Δlac thi/pTRNA66 | ||
| pHK-rrnC+ | ||
| Bacteriophages | ||
| λ439ΔrimM-2 | ΔrimM102 yfiB::nptI | (Persson et al. 1995) |
| P1vir | Laboratory stock | |
| Plasmids | ||
| pGEX-4T-2 | bla lacIq Ptac-gst | Amersham Biosciences |
| pJML005 | bla lacIq Ptac-gst-rimM+ | |
| pJML051 | bla lacIq Ptac-gst-rimM120 | |
| pMAK705 | cat | (Hamilton et al. 1989) |
| pMW279 | Plac-rimM+-cat-rrnBT1bla | (Persson et al. 1995) |
| pMW296 | ffh+rpsP+-rimM+-trmD+-rplS+yfiB+bla | |
| pMW316 | pMW279 rimM107 (GGA to GAA in codon 17 and GGC to GAC in codon 121) | |
| pMW317 | pMW279 rimM108 (CCC to CCT in codon 13 and GGG to AGG in codon 27) | |
| pMW324 | pMW279 rimM109 (GGA to GAA in codon 17) | |
| pMW325 | pMW279 rimM111 (GGG to AGG in codon 27) | |
| pMW334 | pMW279 rimM110 (GGC to GAC in codon 121) |
aUnless otherwise noted, the origin was this study.
Plasmid pJML005 that carries a fusion between the glutathione S-transferase gene (gst) and rimM (codons 2–182) was constructed by PCR-amplifying the wild-type rimM with oligonucleotides 5′-TTTTGGATCCAAACAACTCACCGCGCAAG-3′ and 5′-TTTT GAATTCTTAAAAACCAGGGTCCCAATC-3′, digesting the PCR fragment with BamHI and EcoRI, and inserting it into similarly digested pGEX-4T-2. The latter oligonucleotide destroyed the BamHI site in the 3′-end of rimM without changing the encoded amino acid sequence. Plasmid pJML051 expressing a GST-RimM(YY→AA) hybrid protein was constructed in the same way using chromosomal DNA from the RimM-YY→AA mutant MW136 as the template for PCR amplification.
Rich medium used was LB (Bertani 1951). Cultures were grown at indicated temperatures, and the growth was monitored at 600 nm using a Shimadzu UV-1601 spectrophotometer.
Isolation of rimM mutants
Random mutagenesis of rimM was done using hydroxylamine. Ten μg of plasmid pMW279, which carries the rimM gene, was mutagenized at 75°C with 1 M of hydroxylamine for 90 min, and then dialyzed against water. The wild-type strain MW100 was transformed with the mutagenized pMW279, and transformants were transduced to kanamycin resistance using λ439ΔrimM-2 (Persson et al. 1995). Two rimM alleles complemented the slow growth at 37°C but not at 42°C; however, they contained two mutations each. One of these alleles, in plasmid pMW316, encoded a RimM protein with two amino acid substitutions, G17E and G121D, while the other allele, in plasmid pMW317, encoded a RimM protein with a single substitution, G27R, and contained a silent mutation in codon 13. The two mutations in pMW316 were cloned apart, yielding plasmids pMW324 (RimM-G17E) and pMW334 (RimM-G121D). The silent mutation in pMW317 was removed by PCR, yielding plasmid pMW325 (RimM-G27R).
Oligonucleotide-directed mutagenesis by using either single-stranded M13 DNA templates (Kunkel 1985; Kunkel et al. 1987) or overlap extension PCR (Ho et al. 1989) was done to create specific amino acid substitutions in RimM. The mutations were cloned into plasmid pMW296 carrying a 4.6-kb chromosomal fragment that contains the rimM region. This fragment was then transferred to plasmid pMAK705, which was used to introduce the mutant alleles into the E. coli chromosome as described (Hamilton et al. 1989).
Isolation of suppressor mutations
To isolate faster-growing derivatives of the RimM-YY→AA mutants MW136 and JML068, several cultures were inoculated from independent colonies and grown overnight in rich liquid medium. An aliquot of each overnight culture was streaked out on rich medium plates and subinoculated into fresh liquid medium. This procedure was repeated until revertants appeared on the plates. Only one revertant from each original culture was saved for further analyses. To investigate whether the suppressor mutations in strain JML068 were plasmid-carried, plasmid DNA from the suppressor mutants was used to transform strain JML071 selecting for KmR (pHK-rrnC) and screening for SpS. Plasmid preparations that conferred fast growth were used to transform strain DH5α (Hanahan 1983) selecting for KmR and screening for TcS, to obtain clones that only contained the pHK-rrnC plasmid and not the p70 tRNA plasmid. Plasmid DNA from such KmR TcS DH5α derivatives was used to transform strain JML071, selecting for KmR, to investigate whether the suppressor mutations were on the rrn-plasmid.
Ribosome profiles after sucrose gradient centrifugation
Cell cultures were grown in LB at 37°C to 70 Klett units. Poly-somes were stabilized by addition of chloramphenicol as described (Brow and Noller 1983). Cells were harvested by centrifugation, and polysome extracts were prepared by a previously described freeze-thaw and lysozyme method (Ron et al. 1966) and fractionated by sucrose gradient centrifugation according to Powers and Noller (1990), with the exception that the sucrose was prepared in 10 mM Tris-HCl, pH 7.5, 50 mM KCl, 6 mM β-mercaptoethanol, 10 MgCl2. For ribosome profiles under conditions that dissociate the ribosomal subunits, S30 extracts were prepared and fractionated by sucrose gradient centrifugation as previously described (Bylund et al. 1997).
Primer extension on rRNA
Total RNA was prepared using the Qiagen RNA/DNA and Qiagen RNeasy Kits (QIAGEN). RNA from ribosomal particles purified by sucrose gradient centrifugation was prepared by three successive extractions with phenol/chloroform/isoamyl alcohol (25:24:1). The RNA was then precipitated by ethanol and dissolved in 50 μL of water. Primer extension was performed as described earlier (Bylund et al. 1998).
Preparation of cell extracts and purification of GST-RimM proteins
One-hundred-mL cultures were grown in LB medium to 70 Klett Units, harvested by centrifugation, and resuspended in 0.5 mL of 20 mM Tris-HCl pH 7.0, 150 mM NaCl. Cells were disrupted either by 10–15 cycles of 5-sec sonication at setting 20% on a Vibra cell VCX400 (Sonics and Materials), or by freeze-thawing three times in the presence of lysozyme (1 μg/mL). RNase-free DNase I (10 U) was added, cell debris was removed by centrifugation, and the cell extracts were adsorbed to 0.1 mL of Glutathione Sepharose 4B (Amersham Biosciences). The Sepharose was packed into MicroSpin columns (Amersham Biosciences) and washed with 0.5 mL volumes of 20 mM Tris-HCl, pH 7.0, containing increasing concentrations of NaCl. Finally, the GST-RimM fusions were eluted from the columns with 0.5 mL of 10 mM reduced glutathione (Sigma-Aldrich) in 50 mM Tris-HCl, pH 8.0.
Mass spectrometric analyses
Total protein in fractions from purification of GST-RimM proteins was precipitated with acetone, dissolved, digested with trypsin according to the manufacturer’s directions (Trypsin Gold; Promega), and purified on a ZipTip (Millipore). Peptide separation and ESI-MS/MS (Q-TOF Ultima Waters-Micromass, MS Technologies) were performed as described (Szkanderova et al. 2003) by using a reverse-phase C18 column (Atlantis, NanoEase 75 μm × 150 mm) and analyzed with ProteinLynx GlobalSERVER version 2.05 (Waters-Micromass, MS Technologies). Peptides were identified using the Swiss-Prot database (Swiss Institute of Bioinformatics, Geneva, Switzerland).
Protein bands were cut out from colloidal Coomassie-stained (Neuhoff et al. 1990) polyacrylamide gels and digested according to Shevchenko et al. (1996). The extracted peptide solution was purified on a ZipTip and analyzed by MALDI mass spectrometry (Voyager-DE STR, Applied Biosystems) according to Szkanderova et al. (2003). Proteins were identified by using the Swiss-Prot database and the Mascot search engine (www.matrixscience.com).
Acknowledgments
We thank Dr. Catherine L. Squires for kindly providing strain TA527, Dr. Antón Vila-Sanjurjo for kindly providing strains AVS69009 and AVS69009/pKK1192U, and Dr. Debra Milton for helpful comments on the manuscript. O.P.P. was supported by the Swedish Natural Science Research Council (B-BU 10664). P.M.W. was supported by the Swedish Natural Science Research Council (B-BU 9911), the Swedish Research Council (621–2001–2171), the Magnus Bergvall Foundation, and the Kempe Foundations. O.P.P. and P.M.W. were jointly supported by the Carl Trygger Foundation. G.W. was supported by the Kempe Foundations and the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning.
Article and publication are at http://www.rnajournal.org/cgi/doi/10.1261/rna.7720204.
REFERENCES
- Alix, J.-H. and Guérin, M.-F. 1993. Mutant DnaK chaperones cause ribosome assembly defects in Escherichia coli. Proc. Natl. Acad. Sci. 90: 9725–9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharaman, V. and Aravind, L. 2002. The PRC-barrel: A widespread, conserved domain shared by photosynthetic reaction center subunits and proteins of RNA metabolism. Genome Biol. 3: research0061.0061–research0061.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai, T., Condon, C., Voulgaris, J., Zaporojets, D., Shen, B., Al-Omar, M., Squires, C., and Squires, C.L. 1999a. Construction and initial characterization of Escherichia coli strains with few or no intact chromosomal rRNA operons. J. Bacteriol. 181: 3803–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai, T., Zaporojets, D., Squires, C., and Squires, C.L. 1999b. An Escherichia coli strain with all chromosomal rRNA operons inactivated: Complete exchange of rRNA genes between bacteria. Proc. Natl. Acad. Sci. 96: 1971–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertani, G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62: 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brow, D.A. and Noller, H.F. 1983. Protection of ribosomal RNA from kethoxal in polyribosomes. Implication of specific sites in ribosome function. J. Mol. Biol. 163: 27–46. [DOI] [PubMed] [Google Scholar]
- Bylund, G.O., Persson, B.C., Lundberg, L.A.C., and Wikström, P.M. 1997. A novel ribosome-associated protein is important for efficient translation in Escherichia coli. J. Bacteriol. 179: 4567–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund, G.O., Wipemo, L.C., Lundberg, L.A.C., and Wikström, P.M. 1998. RimM and RbfA are essential for efficient processing of 16S rRNA in Escherichia coli. J. Bacteriol. 180: 73–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylund, G.O., Lövgren, J.M., and Wikström, P.M. 2001. Characterization of mutations in the metY-nusA-infB operon that suppress the slow growth of a ΔrimM mutant. J. Bacteriol. 183: 6095–6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charollais, J., Pflieger, D., Vinh, J., Dreyfus, M., and Iost, I. 2003. The DEAD-box RNA helicase SrmB is involved in the assembly of 50S ribosomal subunits in Escherichia coli. Mol. Microbiol. 48: 1253–1265. [DOI] [PubMed] [Google Scholar]
- Charollais, J., Dreyfus, M., and Iost, I. 2004. CsdA, a cold-shock RNA helicase from Escherichia coli, is involved in the biogenesis of 50S ribosomal subunit. Nucleic Acids Res. 32: 2751–2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammel, C.S. and Noller, H.F. 1995. Suppression of a cold-sensitive mutation in 16S rRNA by overexpression of a novel ribosome-binding factor, RbfA. Genes & Dev. 9: 626–637. [DOI] [PubMed] [Google Scholar]
- El Hage, A., Sbaï, M., and Alix, J.H. 2001. The chaperonin GroEL and other heat-shock proteins, besides DnaK, participate in ribosome biogenesis in Escherichia coli. Mol. Gen. Genet. 264: 796–808. [DOI] [PubMed] [Google Scholar]
- Fuller-Pace, F.V. 1994. RNA helicases: Modulators of RNA structure. Trends Cell Biol. 4: 271–274. [DOI] [PubMed] [Google Scholar]
- Fuller-Pace, F.V., Nicol, S.M., Reid, A.D., and Lane, D.P. 1993. DbpA: A DEAD box protein specifically activated by 23S rRNA. EMBO J. 12: 3619–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson, T.J., Thompson, J.D., and Heringa, J. 1993. The KH domain occurs in a diverse set of RNA-binding proteins that include the antiterminator NusA and is probably involved in binding to nucleic acid. FEBS Lett. 324: 361–366. [DOI] [PubMed] [Google Scholar]
- Hamilton, C.M., Aldea, M., Washburn, B.K., Babitzke, P., and Kushner, S.R. 1989. New method for generating deletions and gene replacements in Escherichia coli. J. Bacteriol. 171: 4617–4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166: 557–580. [DOI] [PubMed] [Google Scholar]
- Ho, S.N., Hunt, H.D., Horton, R.M., Pullen, J.K., and Pease, L.R. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77: 51–59. [DOI] [PubMed] [Google Scholar]
- Holmes, K.L. and Culver, G.M. 2004. Mapping structural differences between 30S ribosomal subunit assembly intermediates. Nat. Struct Mol. Biol. 11: 179–186. [DOI] [PubMed] [Google Scholar]
- Inoue, K., Alsina, J., Chen, J., and Inouye, M. 2003. Suppression of defective ribosome assembly in a rbfA deletion mutant by overexpression of Era, an essential GTPase in Escherichia coli. Mol. Microbiol 48: 1005–1016. [DOI] [PubMed] [Google Scholar]
- Jones, P.G. and Inouye, M. 1996. RbfA, a 30S ribosomal binding factor, is a cold-shock protein whose absence triggers the cold-shock response. Mol. Microbiol. 21: 1207–1218. [DOI] [PubMed] [Google Scholar]
- Kaczanowska, M. and Rydén-Aulin, M. 2004. Temperature sensitivity caused by mutant release factor 1 is suppressed by mutations that affect 16S rRNA maturation. J. Bacteriol. 186: 3046–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel, T.A. 1985. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc. Natl. Acad. Sci. 82: 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel, T.A., Roberts, J.D., and Zakour, R.A. 1987. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 154: 367–382. [DOI] [PubMed] [Google Scholar]
- Li, Z., Pandit, S., and Deutscher, M.P. 1999. RNase G (CafA protein) and RNase E are both required for the 5′ maturation of 16S ribosomal RNA. EMBO J. 18: 2878–2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, M.Y., Yang, H., and Romeo, T. 1995. The product of the pleiotropic Escherichia coli gene csrA modulates glycogen biosynthesis via effects on mRNA stability. J. Bacteriol. 177: 2663–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lövgren, J.M. and Wikström, P.M. 2001. Hybrid protein between ribosomal protein S16 and RimM of Escherichia coli retains the ribosome maturation function of both proteins. J. Bacteriol. 183: 5352–5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki, J.A., Southworth, D.R., and Culver, G.M. 2003. Demonstration of the role of the DnaK chaperone system in assembly of 30S ribosomal subunits using a purified in vitro system. RNA 9: 1418–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makosky, P.C. and Dahlberg, A.E. 1987. Spectinomycin resistance at site 1192 in 16S ribosomal RNA of E. coli: An analysis of three mutants. Biochimie 69: 885–889. [DOI] [PubMed] [Google Scholar]
- Nashimoto, H. 1993. Non-ribosomal proteins affecting the assembly of ribosomes in Escherichia coli. In The translational apparatus (eds. K.H. Nierhaus et al.), pp. 185–195. Plenum Press, New York.
- Nashimoto, H., Miura, A., Saito, H., and Uchida, H. 1985. Suppressors of temperature-sensitive mutations in a ribosomal protein gene, rpsL (S12), of Escherichia coli K12. Mol. Gen. Genet. 199: 381–387. [DOI] [PubMed] [Google Scholar]
- Neuhoff, V., Stamm, R., Pardowitz, I., Arold, N., Ehrhardt, W., and Taube, D. 1990. Essential problems in quantification of proteins following colloidal staining with coomassie brilliant blue dyes in polyacrylamide gels, and their solution. Electrophoresis 11: 101–117. [DOI] [PubMed] [Google Scholar]
- Nierhaus, K.H. 1991. The assembly of prokaryotic ribosomes. Biochimie 73: 739–755. [DOI] [PubMed] [Google Scholar]
- Nishi, K., Morel-Deville, F., Hershey, J.W.B., Leighton, T., and Schnier, J. 1988. An eIF-4A-like protein is a suppressor of an Escherichia coli mutant defective in 50S ribosomal subunit assembly. Nature 336: 496–498. [DOI] [PubMed] [Google Scholar]
- Persson, B.C., Bylund, G.O., Berg, D.E., and Wikström, P.M. 1995. Functional analysis of the ffh-trmD region of the Escherichia coli chromosome by using reverse genetics. J. Bacteriol. 177: 5554–5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers, T. and Noller, H.F. 1990. Dominant lethal mutations in a conserved loop in 16S rRNA. Proc. Natl. Acad. Sci. 87: 1042–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron, E.Z., Kohler, R.E., and Davis, B.D. 1966. Polysomes extracted from Escherichia coli by freeze-thaw-lysozyme lysis. Science 153: 1119–1120. [DOI] [PubMed] [Google Scholar]
- Sayed, A., Matsuyama, S., and Inouye, M. 1999. Era, an essential Escherichia coli small G-protein, binds to the 30S ribosomal subunit. Biochem. Biophys. Res. Comm. 264: 51–54. [DOI] [PubMed] [Google Scholar]
- Shevchenko, A., Wilm, M., Vorm, O., and Mann, M. 1996. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68: 850–858. [DOI] [PubMed] [Google Scholar]
- Singer, M., Baker, T.A., Schnitzler, G., Deischel, S.M., Goel, M., Dove, W., Jaacks, K.J., Grossman, A.D., Erickson, J.W., and Gross, C.A. 1989. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol. Rev. 53: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava, A.K. and Schlessinger, D. 1989. Processing pathway of Escherichia coli 16S precursor rRNA. Nucleic Acids Res. 17: 1649–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkanderova, S., Hernychova, L., Kasalova, I., Vavrova, J., Stulik, J., Abend, M., and van Beuningen, D. 2003. Proteomic analysis of radiation-induced alterations in L929 cells. Folia Biol. (Praha) 49: 15–25. [PubMed] [Google Scholar]
- Toone, W.M., Rudd, K.E., and Friesen, J.D. 1991. deaD, a new Escherichia coli gene encoding a presumed ATP-dependent RNA helicase, can suppress a mutation in rpsB, the gene encoding ribosomal protein S2. J. Bacteriol. 173: 3291–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsu, C.A. and Uhlenbeck, O.C. 1998. Kinetic analysis of the RNA-dependent adenosinetriphosphatase activity of DbpA, an Escherichia coli DEAD protein specific for 23S ribosomal RNA. Biochemistry 37: 16989–16996. [DOI] [PubMed] [Google Scholar]
- Vila-Sanjurjo, A., Squires, C.L., and Dahlberg, A.E. 1999. Isolation of kasugamycin resistant mutants in the 16 S ribosomal RNA of Escherichia coli. J. Mol. Biol. 293: 1–8. [DOI] [PubMed] [Google Scholar]
- Wikström, P.M., Byström, A.S., and Björk, G.R. 1988. Non-autogenous control of ribosomal protein synthesis from the trmD operon in Escherichia coli. J. Mol. Biol. 203: 141–152. [DOI] [PubMed] [Google Scholar]
- Wimberly, B.T., Brodersen, D.E., Clemons, W.M., Morgan-Warren, R.J., Carter, A.P., Vonrhein, C., Hartsch, T., and Ramakrishnan, V. 2000. Structure of the 30S ribosomal subunit. Nature 407: 327–339. [DOI] [PubMed] [Google Scholar]
- Xia, B., Ke, H., Shinde, U., and Inouye, M. 2003. The role of RbfA in 16S rRNA processing and cell growth at low temperature in Escherichia coli. J. Mol. Biol. 332: 575–584. [DOI] [PubMed] [Google Scholar]
- Young, R.A. and Steitz, J.A. 1978. Complementary sequences 1700 nucleotides apart form a ribonuclease III cleavage site in Escherichia coli ribosomal precursor RNA. Proc. Natl. Acad. Sci. 75: 3593–3597. [DOI] [PMC free article] [PubMed] [Google Scholar]