Abstract
Tpt1 is an essential 230-amino-acid enzyme that catalyzes the final step in yeast tRNA splicing: the transfer of the 2′-PO4 from the splice junction to NAD+ to form ADP-ribose 1″-2″cyclic phosphate and nicotinamide. To understand the structural requirements for Saccharomyces cerevisiae Tpt1 activity, we performed an alanine-scanning mutational analysis of 14 amino acids that are conserved in homologous proteins from fungi, metazoa, protozoa, bacteria, and archaea. We thereby identified four residues—Arg23, His24, Arg71, and Arg138—as essential for Tpt1 function in vivo. Structure–activity relationships at these positions were clarified by introducing conservative substitutions. The activity of the Escherichia coli ortholog KptA in complementing tpt1Δ was abolished by alanine substitutions at the equivalent side chains, Arg21, His22, Arg69, and Arg125. Deletion analysis of Tpt1 shows that the C-terminal 20 amino acids, which are not conserved, are not essential for activity in vivo at 30°C. These findings attest to the structural and functional conservation of Tpt1-like 2′-phosphotransferases and identify likely constituents of the active site.
Keywords: tRNA splicing, phosphoryl transfer, nicotinamide adenine dinucleotide, mutagenics
INTRODUCTION
A subset of archaeal and eukaryal pre-tRNAs contain an intron in the anticodon loop that must be removed seamlessly by splicing before the tRNA can function in protein synthesis (Abelson et al. 1998). tRNA splicing in the budding yeast Saccharomyces cerevisiae occurs in three genetically and biochemically distinct stages. First, a splicing en-donuclease breaks the phosphodiester backbone of pre-tRNA at the exon–intron boundaries to yield 2′,3′cyclic phosphate and 5′-OH termini at both incision sites (Peebles et al. 1983; Trotta et al. 1997). Second, the ends of the tRNA halves are healed and then sealed by a polyfunctional yeast tRNA ligase (Trl1) to form a spliced tRNA containing a 2′-PO4, 3′-5′phosphodiester at the splice junction (Greer et al. 1983; Apostol et al. 1991; Sawaya et al. 2003). Third, the 2′-PO4 at the splice junction is removed by the NAD+-dependent 2′-phosphotransferase Tpt1 (Culver et al. 1993, 1997; Spinelli et al. 1997).
Tpt1 catalyzes the transfer of the tRNA 2′-PO4 to NAD+ to form ADP-ribose 1″-2″cyclic phosphate and nicotinamide. The Tpt1 mechanism entails two component steps (Spinelli et al. 1999; Steiger et al. 2005). First, NAD+ reacts with the tRNA 2′-phosphate to expel nicotinamide and generate a 2′ phospho-ADP-ribosylated RNA intermediate. Then, transesterification of the ADP-ribose 2′-O to the tRNA 2′-phosphate displaces the tRNA product and generates ADP-ribose 1″-2″cyclic phosphate (Fig. 1). Tpt1 exemplifies a family of structurally homologous proteins found in eukaryal, archaeal, and bacterial proteomes (Spinelli et al. 1998). Because Tpt1 homologs are found in bacterial species (e.g., E. coli) that have no known intron-containing tRNAs, it has been suggested that members of this enzyme family might dephosphorylate other substrates in vivo via a shared mechanism of phosphoryl transfer to NAD+ to form ADP-ribose 1″-2″ cyclic phosphate (Spinelli et al. 1998). One speculation was that the ADP-ribose 1″-2″ cyclic phosphate product of the Tpt1 reaction could serve as a signaling molecule as part of a metabolic or regulatory pathway, as do other nucleotide cyclic phosphates (Culver et al. 1997). However, the finding that the bacteriophage T4 RNA repair enzymes Rnl1 and Pnkp (which seal broken tRNAs without generating a 2′-phosphate intermediate) can fulfill the splicing functions of yeast Trl1 in vivo and bypass the requirement for Tpt1 (Schwer et al. 2004) implies that Tpt1 and its unique metabolite ADP-ribose 1″-2″ cyclic phosphate do not play essential roles in yeast independent of the tRNA splicing reaction.
FIGURE 1.
Mechanism and primary structure of NAD+-dependent 2′-phosphotransferase. (Left ) Two-step mechanism of tRNA 2′-phosphate removal catalyzed by S. cerevisiae Tpt1 and its E. coli ortholog KptA (Spinelli et al. 1999). See text for details. (Right) The amino acid sequence of S. cerevisiae (Sce) Tpt1 from residues 14–185 is aligned to the sequences of the homologous proteins of E. coli (Eco), Schizosaccharomyces pombe (Spo), Homo sapiens (Hsa), Drosophila melanogaster (Dme), Leishmania major (Lma), Trypanosoma cruzi (Tcr), Pyrococcus horikoshii (Pho), Nostoc punctiforme (Npu), Clostridium perfringens (Cpe), Aeropyrum pernix (Ape), and Archaeoglobus fulgidus (Afu). Positions of side chain identity or similarity in all six proteins are denoted by an arrowhead under the alignment. Residues of Tpt1 that were subjected to alanine scanning are indicated by shaded boxes. Amino acids found to be essential for Tpt1 function in vivo are indicated by vertical bars. Nonessential residues are indicated by +. The V8 protease-sensitive site of Tpt1 (Glu107–Ala108) is indicated by an arrow.
The exploitation of NAD+ as an acceptor for small-group transfer reactions, which was described for phosphoryl transfer by Phizicky and colleagues (Culver et al. 1993; Spinelli et al. 1999; Steiger et al. 2001), has since been extended to the Sir2 family of NAD+-dependent protein deacetylases (Sauve et al. 2001; Avalos et al. 2002, 2004; Zhao et al. 2003, 2004). Sir2 enzymes have attracted considerable attention because they are implicated in cellular aging and in deacetylation of the p53 tumor suppressor protein. The similarities in reaction chemistry raise interesting questions about the structural and evolutionary relatedness of the Tpt1 and Sir2 enzyme families and between Tpt1 and other medically relevant enzymes that use NAD+ as a substrate for transfer of ADP-ribose to a macromolecule acceptor, e.g., diptheria toxin, cholera toxin, and pertussis toxin, which catalyze ADP-ribosylation of essential cellular proteins (Bell and Eisenberg 1996).
Here, we begin to address this question by defining the essential structural features of yeast Tpt1 and its E. coli ortholog KptA by site-directed mutagenesis. We also analyze the native size of Tpt1 and probe its tertiary structure by limited proteolysis.
RESULTS AND DISCUSSION
Mutagenesis strategy
Figure 1 shows an alignment of the amino acid sequences of S. cerevisiae Tpt1 and its E. coli ortholog KptA with homologous proteins encoded by selected eukarya (Schizosaccharomyces pombe, Homo sapiens, Drosophila melanogaster, Trypanosoma cruzi, Leishmania major), bacteria (Nostoc punctiforme, Clostridium perfringens), and archaea (Pyrococcus horikoshii, Archaeoglobus fulgidus, Aeropyrum pernix). We used this alignment as a blueprint for mutational analysis of Tpt1. The strategy was to replace individual residues of interest with alanine and then test the TPT1-Ala alleles for in vivo activity by complementation of S. cerevisiae tpt1Δ. The positions at which alanine substitution elicited a growth defect were then targeted for further analysis entailing the introduction of conservative side-chain substitutions.
We selected 14 residues of yeast Tpt1 for alanine scanning. We focused on conserved basic residues such as Arg, Lys, and His that we regarded as candidates for a direct role in catalysis of phosphoryl transfer. We also mutated conserved Ser, Thr, Tyr, and Phe side chains that might engage in hydrogen bonding or base stacking interactions with NAD+. This suite of 14 mutated residues embraces all of the charged and polar amino acids that are strictly conserved among the Tpt1-like proteins.
Arg23, His24, Arg71, and Arg138 are essential for Tpt1 function in vivo
TPT1-Ala alleles were cloned into a CEN TRP1 plasmid in which their expression is under the control of the native TPT1 promoter. The plasmids were then transformed into a S. cerevisiae tpt1Δ strain in which the chromosomal TPT1 gene was deleted. Growth of tpt1Δ is contingent on maintenance of a wild-type TPT1 allele on a CEN URA3 plasmid (Schwer et al. 2004). Therefore, the tpt1Δ strain is unable to grow on agar medium containing 5-FOA (5-fluoroorotic acid, a drug which selects against the URA3 plasmid) unless it is first transformed with a gene encoding a biologically active 2′-phosphotransferase.
Four of the TPT1-Ala transformants (R23A, H24A, R71A, and R138A) failed to give rise to 5-FOA-resistant colonies after 7 d of incubation at 18°C, 25°C, 30°C, or 37°C; thus, these four alanine mutations were lethal in vivo (Table 1). The four essential Tpt1 residues—Arg23, His24, Arg71, and Arg138—are strictly conserved in the other Tpt1-like proteins shown in Figure 1. Ten other TPT1-Ala mutants supported colony formation during selection on 5-FOA at either 25°C or 30°C. The viable TPT1-Ala strains were tested for growth on rich medium (YPD agar) at 18°C, 25°C, 30°C, and 37°C. One of the mutants, F72A, displayed a temperature-sensitive phenotype, whereby F72A cells grew as well as wild-type TPT1 cells at 18°C, 25°C, and 30°C, but failed to grow at 37°C. Nine other TPT1-Ala strains (K16A, Y38A, K69A, H90A, S91A, H117A, T119A, H142A, and R158A) grew at all temperatures and their colony sizes were similar to that of wild-type TPT1 cells (Table 1). Thus, we surmise that Lys16, Tyr38, Lys69, His90, Ser91, His117, Thr119, His142, and Arg158 are not essential for 2′-phosphotrans-ferase activity in vivo.
TABLE 1.
Effect of missense mutations on Tpt1 activity in vivo
| Tpt1 mutation | tpt1Δ complementation |
| K16A | +++ |
| R23A | lethal |
| R23K | lethal |
| R23Q | lethal |
| H24A | lethal |
| H24N | lethal |
| H24Q | lethal |
| Y38A | +++ |
| K69A | +++ |
| R71A | lethal |
| R71K | lethal |
| R71Q | ts |
| F72A | ts |
| H90A | +++ |
| S91A | +++ |
| H117A | +++ |
| T119A | +++ |
| H117A–T119A | lethal |
| R138A | lethal |
| R138K | +++ |
| R138Q | lethal |
| H142A | +++ |
| R158A | +++ |
Structure–activity relationships at essential residues of Tpt1
We tested the effects of conservative substitutions at the four Tpt1 positions defined as essential by alanine scanning. Arginine was replaced by lysine and glutamine; histidine was replaced by asparagine and glutamine. The eight conservative Tpt1 mutants were tested by plasmid shuffle for tpt1Δ complementation; the results are shown in Table 1.
R23K and R23Q failed to give rise to FOA-resistant colonies after 7 d of incubation at 18°C, 25°C, 30°C, or 37°C. Thus, an arginine is strictly essential at position 23, suggesting that a bidentate interaction of the Arg23 guanidinium nitrogens is critical for function. Different activity profiles were seen at Arg71 and Arg138. Substituting a lysine for Arg138 restored viability, whereas the glutamine substitution was lethal. Thus, the positive charge at position 138 is essential for Tpt1 activity. R138K cells grew as well as the parental wild-type strain on YPD at all temperatures tested. The lethality of the R71A mutation was not reversed by conservative replacement with lysine, but a partial gain of function occurred when Arg71 was changed to glutamine. The R71Q strain grew on YPD agar at 18°C, 25°C, and 30°C, albeit slower than wild-type TPT1, as gauged by smaller colony size (scored as ++ growth). The R71Q strain failed to grow on YPD agar at 37°C. We surmise that positive charge at Arg71 is important for Tpt1 function, but not sufficient, and that Arg71 probably makes an essential hydrogen bond that can be mimicked in part by the neutral glutamine side chain. The conservative changes of His24 to either Asn or Gln were lethal in vivo. This result is consistent with His24 functioning as a general acid-base catalyst, rather than a neutral hydrogen bond donor or acceptor.
His117 and Thr119 of Tpt1 are functionally redundant constituents of an HGT motif found in the NAD+ binding pocket of diphtheria toxin
Culver et al. (1997) noted that Tpt1 contains a tripeptide motif 117HGT119 identical to the 21HGT23 tripeptide found in diphtheria toxin and Pseudomonas exotoxin A. Diphtheria toxin catalyzes the transfer of ADP-ribose from NAD+ to translation elongation factor 2, thereby inhibiting protein synthesis. The crystal structure of diphtheria toxin bound to NAD+ revealed that His21 and Thr23 reside within a beta strand that comprises part of the NAD+ binding pocket (Bell and Eisenberg 1996). His21 and Thr23 coordinate the ribose 2′-O atom of the adenosine component of NAD+ via hydrogen bonds to their Nε and Oγ atoms, respectively. Our finding that single alanine mutations at His117 and Thr119 of Tpt1 had no obvious effect on Tpt1 function in vivo hinted that either: (1) The presence of the HGT motif in Tpt1 was a red herring and the NAD+ binding site of Tpt1 bears no similarity to that of diphtheria toxin, or (2) His117 and Thr119 do comprise part of a diphtheria toxin-like NAD+ binding site, but the side chains are functionally redundant because they contact the same atom of the NAD+ substrate. Consistent with the latter hypothesis, we found that a Tpt1 double-alanine mutation H117A-T119A was lethal in vivo at all temperatures tested (Table 1).
Deletion analysis of S. cerevisiae Tpt1
C-terminal truncation mutants TPT1(1–220), TPT1(1–210), and TPT1(1–200) were tested by plasmid shuffle for tpt1Δ complementation. The truncation mutants were expressed from CEN plasmids under the control of the native TPT1 promoter. The TPT1(1–220) and TPT1(1–210) alleles supported growth of the tpt1Δ strain on 5-FOA at 25°C and 30°C, whereas TPT1(1–200) was lethal at 18°C, 25°C, 30°C, and 37°C (not shown). The TPT1(1–220) strain grew as well as TPT1 cells on YPD agar at 18°C, 25°C, and 30°C, as gauged by colony size, but failed to grow at 37°C. The TPT1(1–210) strain formed smaller colonies than wild-type TPT1 cells on YPD agar at 18°C, 25°C, and 30°C (++ growth), and did not grow at 37°C. We surmise that (1) the C-terminal 10-amino-acid segment promotes Tpt1 function at elevated temperatures and (2) the segment from amino acids 201–210 is essential for Tpt1 activity in vivo at all growth temperatures.
Physical characterization of recombinant Tpt1 protein
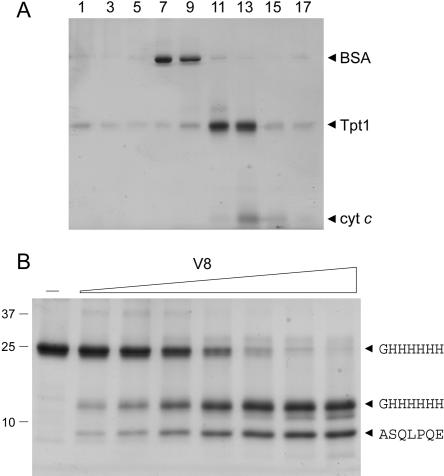
Phizicky and colleagues have conducted a thorough biochemical characterization of the 2′-phosphatase reaction using customized synthetic RNA substrates (Spinelli et al. 1999; Steiger et al. 2001). However, the physical properties of the protein have not been examined in detail. Here we produced Tpt1 in bacteria as an N-terminal His10 fusion and purified the recombinant protein from a soluble bacterial lysate by adsorption to Ni-agarose and elution with buffer containing imidazole. SDS-PAGE showed that the Tpt1 polypeptide was the predominant species recovered in the imidazole eluate fraction (Fig. 2B, lane –). N-terminal sequencing of the 25-kDa Tpt1 polypeptide by automated Edman chemistry after transfer from an SDS-gel to a PVDF membrane confirmed that the N-terminal sequence (GH HHHHH) corresponded to that of the His-tag. The native size of Tpt1 was examined by zonal velocity sedimentation in a 15%–30% glycerol gradient (Fig. 2A). Marker proteins BSA (66 kDa) and cytochrome c (12 kDa) were included as internal standards in the gradient. After centrifugation, the polypeptide compositions of the odd numbered gradient fractions were analyzed by SDS-PAGE. His10-Tpt1 sedimented as a discrete peak between BSA and cytochrome c. An S value of 2.7 was determined for Tpt1 by interpolation to the internal standards. These results are consistent with a monomeric quaternary structure for Tpt1.
FIGURE 2.
Sedimentation and limited proteolysis of Tpt1. (A) Glycerol gradient sedimentation. An aliquot (80 μg) of the Ni-agarose preparation of His10-Tpt1 was mixed with BSA (40 μg) and cytochrome c (50 μg). The protein mixture was applied to a 4.8-mL 15%–30% glycerol gradient containing 50 mM Tris-HCl (pH 8.0), 300 mM NaCl, 1 mM EDTA, 2 mM DTT, 0.025% Triton X-100. The gradient was centrifuged in a Beckman SW50 rotor at 50,000 rpm for 16 h at 4°C. Fractions (~0.26 mL) were collected from the bottom of the tubes. Aliquots (12 μL) of odd-numbered fractions were analyzed by SDS-PAGE. (B) Proteolysis. Reaction mixtures (20 μL) containing 10 mM Tris-HCl (pH 8.0), 5 μg of Tpt1, and 1.6, 3.1, 6.2, 12.5, 25, 50, or 100 ng of V8 protease (from left to right) were incubated for 15 min at 22°C. V8 protease was omitted from the control mixture shown in lane –. The reactions were quenched by adding SDS and the digests were resolved by SDS-PAGE. The Coomassie Blue-stained gel is shown. The positions and sizes (kDa) of marker polypeptides are indicated on the left. Duplicate samples were resolved by SDS-PAGE and the gel contents were transferred electrophoretically to a polyvi-nylidene difluoride membrane. Polypeptides were visualized by staining the membrane with Coomassie Blue dye. Membrane slices containing intact His10-Tpt1 and individual proteolytic products (denoted by arrow-heads on the right) were excised and subjected to automated Edman sequencing. The sequences are indicated in single-letter code (right).
The tertiary structure of Tpt1 was probed by limited proteolysis (Fig. 2B). The recombinant protein was incubated with increasing amounts of V8 protease. Initial scission of the Tpt1 by V8 yielded two major products, of ~13 and 9 kDa, respectively. The smaller fragment had N-terminal sequence (108ASQPLPQE), arising via V8 cleavage at residue Glu107 (denoted by an arrowhead in the Tpt1 sequence alignment in Fig. 1). The larger species retained the intact N terminus (GHHHHHH). Thus, V8 cleavage at a single site split the enzyme into a ~9-kDa C-terminal fragment and a ~13-kDa carboxyl domain. We surmise that the amino and carboxyl domain fragments are themselves tightly folded insofar as they were resistant to digestion by concentrations of V8 sufficient to cleave all the input Tpt1 (Fig. 2B).
Mutational analysis of E.coli KptA highlights a conserved tetrad of essential amino acids
Spinelli et al. (1998) reported that the E. coli Tpt1 homolog (named KptA) is capable of complementing Trl1 function when expressed in yeast. We have confirmed that kptA complements the yeast tpt1Δ mutation in the plasmid-shuffle assay and extended the analysis to address whether Tpt1 and KptA rely on a common ensemble of putative catalytic functional groups. KptA residues Arg21, His22, Arg69, and Arg125 are the presumptive counterparts of the four amino acids of Tpt1 identified as essential by alanine scanning (Fig. 1). These four residues of KptA were replaced by alanine and the kptA-Ala alleles were tested for tpt1Δ complementation; R21A, H22A, R69A, and R125A were lethal at all temperatures tested (Table 2).
TABLE 2.
Effect of mutations on KptA activity in yeast
| kptA allele | tpt1Δ complementation |
| WT | +++ |
| R21A | lethal |
| H22A | lethal |
| R69A | lethal |
| R125A | lethal |
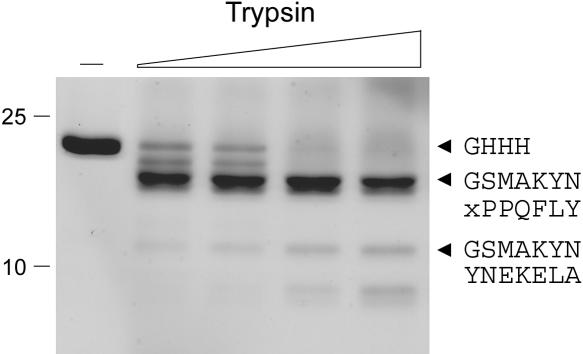
We produced KptA as an N-terminal His-10 fusion and purified the recombinant protein from a soluble bacterial lysate by Ni-agarose chromatography. SDS-PAGE showed that the KptA polypeptide was the predominant species recovered in the imidazole eluate fractions (Fig. 3, lane –). N-terminal sequencing of the ~22-kDa KptA polypeptide by automated Edman chemistry after transfer from an SDS-gel to a PVDF membrane confirmed that the N-terminal sequence (GHHH) corresponded to that of the His-tag. Treatment of KptA with limiting concentrations of trypsin yielded a doublet that was relatively resistant to digestion by trypsin concentrations at which all of the input KptA had decayed (Fig. 3). The doublet consisted of a mixture of two peptides. The more abundant component had an N-terminal sequence GSMAKYN arising via tryptic cleavage within the His-tag at a basic residue located two amino acids upstream of the 1MAKYN5 sequence at the N-terminus of the native KptA polypeptide. A less abundant component contained the terminal sequence xPPQFLY arising via tryptic cleavage at Lys97 (in the sequence 97KTPPQFLY). This internal tryptic site of KptA is located five residues upstream of the V8-sensitive site in S. cerevisiae Tpt1 (see Fig. 1), suggesting that the poorly conserved central segment might comprise an interdomain linker or a surface-exposed loop. An ~12-kDa tryptic cleavage product appearing at higher protease concentrations consisted of a mixture of two poly-peptides with overlapping N-termini, one arising form cleavage within the His-tag (GSMAKYN) and another resulting from cleavage at the Lys3-Tyr4 dipeptide of the KptA protein (YNEKELA).
FIGURE 3.
Limited proteolysis of purified KptA. Reaction mixtures (20 μL) containing 10 mM Tris-HCl (pH 8.0), 5 μg of KptA, and 1.6, 3.2, 6.2, or 12.5 ng of trypsin (from left to right) were incubated for 15 min at 22°C. Trypsin was omitted from the control mixture shown in lane –. The reactions were quenched by adding SDS and the digests were resolved by SDS-PAGE. The Coomassie Blue-stained gel is shown. A duplicate gel was electroblotted to PVDF; intact His10-Tpt1 and individual tryptic products were subjected to automated Edman sequencing; their sequences are indicated in single-letter code (right).
Mechanistic implications
We infer that members of the Tpt1-like phosphotransferase family have a common active site that includes the conserved Arg-His-Arg-Arg tetrad. The observed structure–activity relationships at these essential residues in Tpt1 engender plausible models for their catalytic roles in one or both component steps of the 2′-phosphotransferase reaction. For example, the mutational data are consistent with His24 functioning as a general acid-base catalyst, rather than a neutral hydrogen bond donor or acceptor. There is a clear need for a general base catalyst to deprotonate the ribose 2′-O of ADP-ribose and thereby activate it for nucleophilic attack on the phosphorus during the second cyclization step of the Tpt1 reaction. There is also a need for a general acid catalyst to donate a proton to the ribose 2′-O leaving group of the tRNA (Fig. 1). Two histidines serve as the proton donor and acceptor, respectively, in the analogous transesterification reactions catalyzed by the RNAse A superfamily (Raines 1998). A histidine is proposed to assist in deprotonating the 2′-O of ADP-ribose during the transacetylation reaction catalyzed by Sir2 (Zhao et al. 2003; Avalos et al. 2004). Although we invoke His21 as an acid-base catalyst, we do not exclude the possibility that it might instead coordinate one of the nonbridging oxygens of the tRNA 2′-phosphodiester and thereby stabilize the pentaco-ordinate transition state during the cyclization step. Indeed, a histidine plays just such a role in transition state stabilization during the DNA transesterification reaction catalyzed by topoisomerase IB (Krogh and Shuman 2000).
The mutational data are consistent with a role for Arg23 and/or Arg138 in coordinating the tRNA 2′-phosphate, which could confer substrate specificity in step 1 and stabilize the transition state during the second cyclization step. Whereas Arg23 is strictly essential, suggesting bidentate interactions of this side chain with the phosphate oxygens, Arg138 can be replaced by lysine, implying that a mono-dentate contact suffices. This model echoes the situation is found at the active site of topoisomerase IB, where two essential arginines catalyze transesterification, one of which is strictly essential while the other can be replaced by lysine (Cheng et al. 1997; Krogh and Shuman 2002). Alternatively, Arg23 or Arg138 might interact with the pyrophosphate bridge of NAD+.
The essential role played by Arg71 can be partially sustained by glutamine, but not by lysine. We speculate that Arg71 may engage in hydrogen bonding interactions with the ribose oxygens of NAD+, analogous to the contacts between an arginine and the ribose hydroxyl of ATP or GTP seen in the crystal structures of DNA ligase and RNA gua-nylyltransferase (Häkansson et al. 1997; Odell et al. 2001). Alternatively, Arg71 might coordinate the amide moiety of nicotinamide.
MATERIALS AND METHODS
Plasmids for expression of TPT1 in yeast
A 1.3-kbp DNA fragment containing the TPT1 ORF plus 505-bp of upstream (5′) and 105-bp of downstream (3′) chromosomal DNA was amplified by PCR from total yeast DNA using primers that introduced an EcoRI site at the 5′ end and a BamHI site at the 3′ end. The PCR product was digested with EcoRI and BamHI and then inserted into yeast expression vectors pSE360 (CEN URA3) to yield p360-TPT1. The TPT1 cassette was modified by introducing an NdeI restriction site (CATATG) at the translation initiation codon; the modified cassette was then inserted into yeast vector pSE358 (CEN TRP1) to yield p358-TPT1. The TPT1 ORF was sequenced completely in the p360 and p358 plasmids to confirm that no coding changes were introduced during amplification and cloning.
Missense and deletion mutations of TPT1
Missense mutations and overlapping diagnostic restriction sites were introduced into the full-length TPT1 gene via the two-stage PCR overlap extension method. The mutated PCR products were digested with NdeI and BamHI and inserted into NdeI/BamHI-cut p358-TPT1 in lieu of the wild-type gene. Deleted versions of TPT1 were constructed by PCR amplification using oligonucleotide primers that introduced a stop codon and downstream BamHI site at the desired positions in the TPT1 ORF. The PCR products were digested with NdeI and BamHI and inserted into p358-TPT1 in place of the wild-type gene fragment. The inserts were sequenced completely to confirm the presence of the desired mutations and to exclude the acquisition of unwanted coding changes during PCR amplification and cloning.
Test of Tpt1 function by plasmid shuffle
The tpt1Δ haploid strain YBS501 (MATa ura3–1 ade2–1 trp1–1 his3–11,15 leu2–3,11–2 can1–100 tpt1:: LEU2 p360-TPT1), in which the TPT1 ORF was deleted and replaced by LEU2, is dependent for viability on the CEN URA3 TPT1 plasmid p360-TPT1 (Schwer et al. 2004). Whereas YBS501 is unable to form colonies on agar medium containing 5-FOA, a drug that selects against the URA3 gene, transformation of YBS501 with a CEN TRP1 TPT1 plasmid permits growth on medium containing 5-FOA.
YBS501 was transformed with CEN TRP1 plasmids bearing wild-type or mutant alleles of TPT1. Transformants were selected on Trp− agar media. Two individual colonies were transferred to Trp− agar medium and cells from each isolate were then streaked on agar containing 0.75 mg/mL 5-FOA. The plates were incubated at 18°C, 25°C, 30°C, and 37°C. Lethal mutations were those that did not allow formation of 5′-FOA-resistant colonies after 7 d at any of the temperatures tested. Other mutated alleles supported 5′-FOA-resistant colony formation within 4 d at one or more of the growth temperatures. Two individual colonies from each streak were picked from the 5-FOA plate, transferred to yeast extract/peptone/dextrose (YPD) medium and then tested for growth on YPD agar at 18°C, 25°C, 30°C and 37°C. Growth was assessed by colony size and scored as follows: +++ indicates colony size indistinguishable from that of a wild-type TPT1 strain; ++ indicates colony size smaller than that of wild-type yeast; and + denotes pinpoint colonies. Temperature-sensitive (ts) mutants were those that did not form colonies at 37°C after 7 d.
Mutational analysis of E. coli KptA function in yeast
The coding sequence of the E. coli kptA gene was amplified by PCR from total bacterial DNA with primers designed to introduce a BamHI site immediately 5′of the translation initiation codon and a SacI site immediately 3′ of the stop codon. The PCR product was digested with BamHI and SacI and then inserted into a yeast CEN TRP1 vector to yield pYX-KptA, in which expression of the bacterial gene is driven by the constitutive yeast TPI1 promoter. Ala-nine-substitution mutations and overlapping diagnostic restriction sites were introduced into the kptA gene by overlap PCR; the mutated genes were digested with BamHI and SacI and inserted into BamHI/SacI-cut pYX-KptA in lieu of the wild-type kptA gene. The inserts were sequenced completely to confirm the presence of the desired mutations and to exclude the acquisition of unwanted coding changes. The S. cerevisiae tpt1Δ strain was transformed with pYX plasmids bearing wild-type or mutant alleles of kptA. Plasmid shuffle by counterselection on 5-FOA and scoring of the mutant phenotypes were conducted as described above.
Recombinant Trl1 and KptA proteins
The full-length TPT1 ORF was excised from p358-TPT1 with NdeI and BamHI and inserted into pET16b to yield pET16-Tpt1. The kptA ORF was excised from pYX-KptA with BamHI and SacI and inserted into pET28-His10 to yield pET28-KptA. Cultures (500 mL) of E. coli BL21(DE3) containing pET28-Tpt1 or pET28-KptA were grown at 37°C in Luria-Bertani medium containing 0.1 mg/ mL ampicillin or 0.06 mg/mL kanamycin, respectively, until the A600 reached 0.5. The cultures were adjusted to 0.4 mM isopropyl-β-D-thiogalactopyroanoside and incubation was continued at 17°C overnight. Cells were harvested by centrifugation and the pellet was stored at −80°C. All subsequent procedures were performed at 4°C. Thawed bacterial pellets were resuspended in 10 mL of buffer A (50 mM Tris-HCl, pH 7.5, 0.2 M NaCl, 10% sucrose) and left on ice for 10 min. The suspensions were then sonicated for 30 sec. Lysozyme was added to a final concentration of 50 μg/mL and the suspensions were further incubated on ice for 30 min. Triton X-100 was added to a final concentration of 0.1% and sonication was repeated for 1 min to reduce viscosity. Insoluble material was removed by centrifugation in a Sorvall SS34 rotor at 18,000 rpm for 45 min. The soluble extracts were applied to 1.5-mL columns of Ni-NTA agarose (Qiagen) equilibrated with buffer A containing 0.1% Triton X-100. The column was washed with the same buffer and then eluted step-wise with buffer B (50 mM Tris-HCl, pH 8.0, 0.1 M NaCl, 10% glycerol) containing 50, 100, 200, 500, and 1000 mM imidazole. The polypeptide compositions of the column fractions were monitored by SDS-PAGE. The recombinant Tpt1 protein was retained on the column and recovered in the 200 mM imidazole eluate; recombinant KptA was retained and eluted in the 500 mM imidazole fraction. The eluates were dialyzed against buffer C (50 mM Tris-HCl, pH 8.0, 50 mM NaCl, 2 mM DTT, 10% glycerol, 0.05% Triton X-100) and then stored at −80°C. Protein concentrations were determined using the BioRad dye-binding assay with bovine serum albumin as a standard. The yield of recombinant protein was 4 mg for Tpt1 and 4.8 mg for KptA.
Article and publication are at http://www.rnajournal.org/cgi/doi/10.1261/rna.7193705.
REFERENCES
- Abelson, J., Trotta, C.R., and Li, H. 1998. tRNA splicing. J. Biol. Chem. 273: 12685–12688. [DOI] [PubMed] [Google Scholar]
- Apostol, B.L., Westaway, S.K., Abelson, J., and Greer, C.L. 1991. Deletion analysis of a multifunctional yeast tRNA ligase polypeptide: Identification of essential and dispensable functional domains. J. Biol. Chem. 266: 7445–7455. [PubMed] [Google Scholar]
- Avalos, J.L., Celic, I., Muhammad, S., Cosgrove, M.S., Boeke, J.D., and Wolberger, C. 2002. Structure of a Sir2 enzyme bound to an acetylated p53 peptide. Mol. Cell 10: 523–535. [DOI] [PubMed] [Google Scholar]
- Avalos, J.L., Boeke, J.D., and Wolberger, C. 2004. Structural basis for the mechanism and regulation of Sir2 enzymes. Mol. Cell 13: 639–648. [DOI] [PubMed] [Google Scholar]
- Bell, C.E. and Eisenberg, D. 1996. Crystal structure of diphtheria toxin bound to nicotinamide adenine dinucleotide. Biochemistry 35: 1137–1149. [DOI] [PubMed] [Google Scholar]
- Cheng, C., Wang, L.K., Sekiguchi, J., and Shuman, S. 1997. Mutational analysis of 39 residues of vaccinia DNA topoisomerase identifies Lys-220, Arg-223, and Asn-228 as important for covalent catalysis. J. Biol. Chem. 272: 8263–8269. [DOI] [PubMed] [Google Scholar]
- Culver, G.M., McCraith, S.M., Zillman, M., Kierzek, R., Michaud, N., LaReau, R.D., Turner, D.H., and Phizicky, E.M. 1993. An NAD derivative produced during transfer RNA splicing: ADP-ribose 1″-2″ cyclic phosphate. Science 261: 206–208. [DOI] [PubMed] [Google Scholar]
- Culver, G.M., McCraith, S.M., Consaul, S.A., Stanford, D.R., and Phizicky, E.M. 1997. A 2′-phosphotransferase implicated in tRNA splicing is essential in Saccharomyces cerevisiae. J. Biol. Chem. 272: 13203–13210. [DOI] [PubMed] [Google Scholar]
- Greer, C.L., Peebles, C.L., Gegenheimer, P., and Abelson J. 1983. Mechanism of action of a yeast RNA ligase in tRNA splicing. Cell 32: 537–546. [DOI] [PubMed] [Google Scholar]
- Håkansson, K., Doherty, A.J., Shuman, S., and Wigley, D.B. 1997. X-ray crystallography reveals a large conformational change during guanyl transfer by mRNA capping enzymes. Cell 89: 545–553. [DOI] [PubMed] [Google Scholar]
- Krogh, B.O. and Shuman, S. 2000. Catalytic mechanism of DNA to-poisomerase IB. Mol. Cell 5: 1035–1041. [DOI] [PubMed] [Google Scholar]
- ———. 2002. Proton relay mechanism of general acid catalysis by DNA topoisomerase IB. J. Biol. Chem. 277: 5711–5714. [DOI] [PubMed] [Google Scholar]
- Odell, M., Sriskanda, V., Shuman, S., and Nikolov, D. 2000. Crystal structure of eukaryotic DNA ligase-adenylate illuminates the mechanism of nick sensing and strand joining. Mol. Cell 6: 1183–1193. [DOI] [PubMed] [Google Scholar]
- Peebles, C.L., Gegenheimer, P., and Abelson, J. 1983. Precise excision of intervening sequences from precursor tRNAs by a membrane-associated yeast endonuclease. Cell 32: 525–536. [DOI] [PubMed] [Google Scholar]
- Raines, R.T. 1998. Ribonuclease A. Chem. Rev. 98: 1045–1065. [DOI] [PubMed] [Google Scholar]
- Sauve, A.A., Celic, I., Avalos, J., Deng, H., Boeke, J.D., and Schramm, V. 2001. Chemistry of gene silencing: The mechanism of NAD+-dependent deacetylation reactions. Biochemistry 40: 15456–15463. [DOI] [PubMed] [Google Scholar]
- Sawaya, R., Schwer, B., and Shuman, S. 2003. Genetic and biochemical analysis of the functional domains of yeast tRNA ligase. J. Biol. Chem. 278: 43298–43398. [DOI] [PubMed] [Google Scholar]
- Schwer, B., Sawaya, R., Ho, C.K., and Shuman, S. 2004. Portability and fidelity of RNA-repair systems. Proc Natl. Acad. Sci. 101: 2788–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli, S.L., Consaul, S.A., and Phizicky, E.M. 1997. A conditional lethal yeast phosphotransferase mutant accumulates tRNA with a 2′-phosphate and an unmodified base at the splice junction. RNA 3: 1388–1400. [PMC free article] [PubMed] [Google Scholar]
- Spinelli, S.L., Malik, H.S., Consaul, S.A., and Phizicky, E.M. 1998. A functional homolog of a yeast tRNA splicing enzyme is conserved in higher eukaryotes and in Escherichia coli. Proc. Natl. Acad. Sci. 95: 14136–14141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli, S.L., Kierzek, R., Turner, D.H., and Phizicky, E.M. 1999. Transient ADP-ribosylation of a 2′-phosphate implicated in its removal from ligated tRNA during splicing in yeast. J. Biol. Chem. 274: 2637–2644. [DOI] [PubMed] [Google Scholar]
- Steiger, M.A., Kierzek, R., Turner, D.H., and Phizicky, E.M. 2001. Substrate recognition by a yeast 2′-phosphotransferase involved in tRNA splicing and its Escherichia coli homolog. Biochemistry 40: 14098–14105. [DOI] [PubMed] [Google Scholar]
- Steiger, M.A., Jackman, J.E., and Phizicky, E.M. 2005. Analysis of 2′-phosphotransferase (Tpt1p) from Saccharomyces cerevisiae: Evidence for a conserved two-step reaction mechanism. RNA (this issue). [DOI] [PMC free article] [PubMed]
- Trotta, C.R., Miao, F., Arn, E.A., Stevens, S.W., Ho, C.K., Rauhut, R., and Abelson, J.N. 1997. The yeast tRNA splicing endonuclease: A tetrameric enzyme with two active site subunits homologous to the archaeal tRNA endonucleases. Cell 89: 849–858. [DOI] [PubMed] [Google Scholar]
- Zhao, K., Chai, X., and Marmorstein, R. 2003. Structure of the yeast Hst2 protein deacetylase in ternary complex with 2′-O-acetyl ADP ribose and histone peptide. Structure 11: 1403–1411. [DOI] [PubMed] [Google Scholar]
- Zhao, K., Harshaw, R., Chai, X., and Marmorstein, R. 2004. Structural basis for nicotinamide cleavage and ADP-ribose transfer by NAD+-dependent Sir2 histone/protein deacetylases. Proc. Natl. Acad. Sci. 101: 8563–8568. [DOI] [PMC free article] [PubMed] [Google Scholar]